Femtosecond Pulse Ablation Assisted Mg-ZnO Nanoparticles for UV-Only Emission
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Characterization
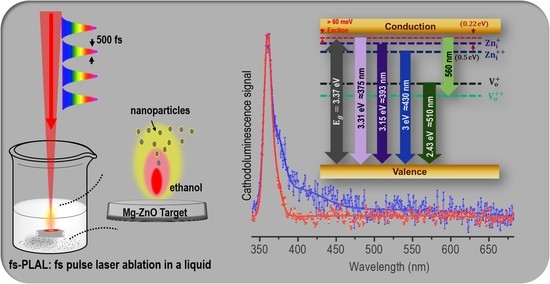
2.2. UV Emission and Spectroscopy
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lorenz, M.; Rao, M.R.; Venkatesan, T.; Fortunato, E.; Barquinha, P.; Branquinho, R.; Salgueiro, D.; Martins, R.; Carlos, E.; Liu, A.; et al. The 2016 oxide electronic materials and oxide interfaces roadmap. J. Phys. D Appl. Phys. 2016, 49, 433001. [Google Scholar] [CrossRef]
- Lim, J.H.; Kang, C.K.; Kim, K.K.; Park, I.K.; Hwang, D.K.; Park, S.J. UV Electroluminescence Emission from ZnO Light-Emitting Diodes Grown by High-Temperature Radiofrequency Sputtering. Adv. Mater. 2006, 18, 2720–2724. [Google Scholar] [CrossRef]
- Zeng, H.; Duan, G.; Li, Y.; Yang, S.; Xu, X.; Cai, W. Blue Luminescence of ZnO Nanoparticles Based on Non-Equilibrium Processes: Defect Origins and Emission Controls. Adv. Funct. Mater. 2010, 20, 561–572. [Google Scholar] [CrossRef]
- Özgür, Ü.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Doğan, S.; Avrutin, V.; Cho, S.J.; Morkoç, H. A comprehensive review of ZnO materials and devices. J. Appl. Phys. 2005, 98, 041301. [Google Scholar] [CrossRef] [Green Version]
- Gimenez, A.J.; Yáñez-Limón, J.M.; Seminario, J.M. ZnO Paper Based Photoconductive UV Sensor. J. Phys. Chem. C 2011, 115, 282–287. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Leung, Y.H. Optical Properties of ZnO Nanostructures. Small 2018, 2, 944–961. [Google Scholar] [CrossRef]
- Keis, K.; Magnusson, E.; Lindström, H.; Lindquist, S.E.; Hagfeldt, A. A 5% efficient photoelectrochemical solar cell based on nanostructured ZnO electrodes. Sol. Energy Mater. Sol. Cells 2002, 73, 51–58. [Google Scholar] [CrossRef]
- Mishra, Y.K.; Adelung, R. ZnO tetrapod materials for functional applications. Mater. Today 2018, 21, 631–651. [Google Scholar] [CrossRef]
- Jiang, J.; Pi, J.; Cai, J. The Advancing of Zinc Oxide Nanoparticles for Biomedical Applications. Bioinorg. Chem. Appl. 2018, 2018, 1565–3633. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori Mohd, S.K.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activityand Toxicity Mechanism. Nano Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [Green Version]
- Abdalkarim, S.Y.H.; Yu, H.Y.; Wang, C.; Yang, L.; Guan, Y.; Huang, L.; Yao, J. Sheet-like Cellulose Nanocrystal-ZnO Nanohybrids as Multifunctional Reinforcing Agents in Biopolyester Composite Nanofibers with Ultrahigh UV-Shielding and Antibacterial Performances. ACS Appl. Bio Mater. 2018, 1, 714–727. [Google Scholar] [CrossRef]
- Agrawal, J.; Dixit, T.; Palani, A.I.; Rao, M.S.R.; Singh, V. Zinc Interstitial Rich ZnO Honeycomb Nanostructures for Deep UV Photodetection. Phys. Status Solidi Rapid Res. Lett. 2018, 12, 1800241. [Google Scholar] [CrossRef]
- Raji, R.; Gopchandran, K. ZnO nanostructures with tunable visible luminescence: Effects of kinetics of chemical reduction and annealing. J. Sci. Adv. Mater. Devices 2017, 2, 51–58. [Google Scholar] [CrossRef]
- Wang, S.P.; Zhong, S.L.; Xu, H.L. Shape tuning of ZnO with ammonium molybdate and their morphology-dependent photoluminescence properties. J. Phys. Conf. Ser. 2009, 188, 012034. [Google Scholar] [CrossRef]
- Shalish, I.; Temkin, H.; Narayanamurti, V. Size-dependent surface luminescence in ZnO nanowires. Phys. Rev. B 2004, 69, 245401. [Google Scholar] [CrossRef]
- Ghosh, M.; Raychaudhuri, A.K. Shape transition in ZnO nanostructures and its effect on blue-green photoluminescence. Nanotechnology 2008, 19, 445704. [Google Scholar] [CrossRef] [Green Version]
- Yadav, M.K.; Ghosh, M.; Biswas, R.; Raychaudhuri, A.K.; Mookerjee, A.; Datta, S. Band-gap variation in Mg- and Cd-doped ZnO nanostructures and molecular clusters. Phys. Rev. B 2007, 76, 195450. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Fang, T.; Hung, F.; Ji, L.; Chang, S.; Young, S.; Hsiao, Y. The crystallization and physical properties of Al-doped ZnO nanoparticles. Appl. Surf. Sci. 2008, 254, 5791–5795. [Google Scholar] [CrossRef]
- Janotti, A.; de Walle, C.G.V. Fundamentals of zinc oxide as a semiconductor. Rep. Prog. Phys. 2009, 72, 126501. [Google Scholar] [CrossRef] [Green Version]
- Fabitha, K.; Rao, M.R. Ho3+-doped ZnO nano phosphor for low-threshold sharp red light emission at elevated temperatures. JOSA B 2017, 34, 2485–2492. [Google Scholar] [CrossRef]
- Singh, S.; Rama, N.; Ramachandra Rao, M. Influence of d-d transition bands on electrical resistivity in Ni doped polycrystalline ZnO. Appl. Phys. Lett. 2006, 88, 222111. [Google Scholar] [CrossRef]
- Singh, S.; Rao, M.R. Optical and electrical resistivity studies of isovalent and aliovalent 3 d transition metal ion doped ZnO. Phys. Rev. B 2009, 80, 045210. [Google Scholar] [CrossRef]
- Pradeev raj, K.; Sadaiyandi, K.K.; Kennedy, A.; Sagadevan, S.; Chowdhury, Z.Z.; Johan, M.R.B.; Aziz, F.A.; Rafique, R.F.; Thamiz Selvi, R.; Rathina bala, R. Influence of Mg Doping on ZnO Nanoparticles for Enhanced Photocatalytic Evaluation and Antibacterial Analysis. Nanoscale Res. Lett. 2018, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Manaia, E.B.; Kaminski, R.C.K.; Caetano, B.L.; Briois, V.; Chiavacci, L.A.; Bourgaux, C. Surface modified Mg-doped ZnO QDs for biological imaging. Eur. J. Nanomed. 2015, 7, 109–120. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.; Sakurai, M.; Aono, M. ZnO-based ultraviolet photodetectors. Sensors 2010, 10, 8604–8634. [Google Scholar] [CrossRef] [Green Version]
- Oguma, K.; Kita, R.; Sakai, H.; Murakami, M.; Takizawa, S. Application of UV light emitting diodes to batch and flow-through water disinfection systems. Desalination 2013, 328, 24–30. [Google Scholar] [CrossRef]
- Dimapilis, E.A.S.; Hsu, C.S.; Mendoza, R.M.O.; Lu, M.C. Zinc oxide nanoparticles for water disinfection. Sustain. Environ. Res. 2018, 28, 47–56. [Google Scholar] [CrossRef]
- Yousefi, R.; Zak, A.K.; Jamali-Sheini, F. Growth, X-ray peak broadening studies, and optical properties of Mg-doped ZnO nanoparticles. Mater. Sci. Semicon Proc. 2013, 16, 771–777. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Zhu, K.R.; Lin, Z.Q.; Jin, S.W.; Li, G. Structure and Raman scattering of Mg-doped ZnO nanoparticles prepared by sol–gel method. Rare Met. 2018, 37, 881–885. [Google Scholar] [CrossRef]
- Fujihara, S.; Ogawa, Y.; Kasai, A. Tunable Visible Photoluminescence from ZnO Thin Films through Mg-Doping and Annealing. ACS Chem. Mater. 2004, 16, 2965–2968. [Google Scholar] [CrossRef]
- Singh, J.; Kumar, P.; Hui, K.S.; Hui, K.N.; Ramam, K.; Tiwari, R.S.; Srivastava, O.N. Synthesis, band-gap tuning, structural and optical investigations of Mg doped ZnO nanowires. RSC CrystEngComm 2012, 14, 5898–5904. [Google Scholar] [CrossRef]
- Fazio, E.; Cacciola, A.; Mezzasalma, A.; Mondio, G.; Neri, F.; Saija, R. Modelling of the optical absorption spectra of PLAL prepared ZnO colloids. J. Quant. Spectrosc. Radiat. Transf. 2013, 124, 86–93. [Google Scholar] [CrossRef]
- Navas, M.; Soni, R.; Tarasenka, N.; Tarasenko, N. Temperature and solution assisted synthesis of anisotropic ZnO nanostructures by pulsed laser ablation. Appl. Surf. Sci. 2017, 414, 413–423. [Google Scholar] [CrossRef]
- Said, A.; Sajti, L.; Giorgio, S.; Marine, W. Synthesis of nanohybrid materials by femtosecond laser ablation in liquid medium. J. Phys. Conf. Ser. 2007, 59, 259–265. [Google Scholar] [CrossRef]
- Sajti, C.; Giorgio, S.; Khodorkovsky, V.; Marine, W. Femtosecond laser synthesized nanohybrid materials for bioapplications. Appl. Surf. Sci. 2007, 253, 8111–8114. [Google Scholar] [CrossRef]
- Chelnokov, E.; Rivoal, M.; Colignon, Y.; Gachet, D.; Bekere, L.; Thibaudau, F.; Giorgio, S.; Khodorkovsky, V.; Marine, W. Band gap tuning of ZnO nanoparticles via Mg doping by femtosecond laser ablation in liquid environment. Appl. Surf. Sci. 2012, 258, 9408–9411. [Google Scholar] [CrossRef]
- Ahn, C.H.; Kim, Y.Y.; Kim, D.C.; Mohanta, S.K.; Cho, H.K. A comparative analysis of deep level emission in ZnO layers deposited by various methods. J. Appl. Phys. 2009, 105, 013502. [Google Scholar] [CrossRef]
- Ohtomo, A.; Kawasaki, M.; Koida, T.; Masubuchi, K.; Koinuma, H.; Sakurai, Y.; Yoshida, Y.; Yasuda, T.; Segawa, Y. MgxZn1-xO as a II–VI widegap semiconductor alloy. Appl. Phys. Lett. 1998, 72, 2466–2468. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.I.; Page, K.; Seshadri, R. Synchrotron X-ray study of polycrystalline wurtzite Zn1-xMgxO (0≤ x ≤ 0.15): Evolution of crystal structure and polarization. Appl. Phys. Lett. 2007, 90, 101904. [Google Scholar] [CrossRef] [Green Version]
- De Prado, E.; Florian, C.; Sotillo, B.; Siegel, J.; Solis, J.; Fernández, P. Optical spectroscopy study of nano- and microstructures fabricated by femtosecond laser pulses on ZnO based systems. CrystEngComm 2018, 20, 2952–2960. [Google Scholar] [CrossRef]
- Jaramillo, A.; Baez-Cruz, R.; Montoya, L.; Medinam, C.; Pérez-Tijerina, E.; Salazar, F.; Rojas, D.; Melendrez, M. Estimation of the surface interaction mechanism of ZnO nanoparticles modified with organosilane groups by Raman Spectroscopy. Ceram. Int. 2017, 43, 11838–11847. [Google Scholar] [CrossRef]
- Šćepanović, M.; Grujić-Brojčin, M.; Vojisavljević, K.; Bernik, S.; Srećković, T. Raman study of structural disorder in ZnO nanopowders. J. Raman Spectrosc. 2010, 41, 914–921. [Google Scholar] [CrossRef]
- Gupta, J.; Bahadur, D. Defect-Mediated Reactive Oxygen Species Generation in Mg-Substituted ZnO Nanoparticles: Efficient Nanomaterials for Bacterial Inhibition and Cancer Therapy. ACS Omega 2018, 3, 2956–2965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coulter, J.B.; Birnie, D.P., III. Assessing Tauc Plot Slope Quantification: ZnO Thin Films as a Model System. Phys. Status Solidi B 2018, 255, 1700393. [Google Scholar] [CrossRef]
- Adachi, S. Energy-Band Structure: Energy-Band Gaps. In Properties of Semiconductor Alloys; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009; Chapter 6; pp. 133–228. [Google Scholar]
- Makino, T.; Segawa, Y.; Kawasaki, M.; Ohtomo, A.; Shiroki, R.; Tamura, K.; Yasuda, T.; Koinuma, H. Band gap engineering based on MgxZn1-xO and CdyZn1-yO ternary alloy films. Appl. Phys. Lett. 2001, 78, 1237–1239. [Google Scholar] [CrossRef] [Green Version]
- Tsay, C.; Chen, S.T.; Fan, M. Solution-Processed Mg-Substituted ZnO Thin Films for Metal-Semiconductor- Metal Visible-Blind Photodetectors. Coatings 2019, 9, 277. [Google Scholar] [CrossRef] [Green Version]
- Lima, S.; Sigoli, F.; Jafelicci, M.J.; Davolos, M. Luminescent properties and lattice defects correlation on zinc oxide. J. Inorg. Mater. 2001, 3, 749–754. [Google Scholar] [CrossRef]
- De Oliveira, R.C.; Martins, D.E.; Bernardi, M.I.B.; Mesquita, A. Zn1-xMgxO nanoparticles prepared by the polymeric precursor method: Correlation between photoluminescence and local structure. Opt. Mater. 2018, 86, 71–78. [Google Scholar] [CrossRef]
- Vanheusden, K.; Seager, C.H.; Warren, W.L.; Tallant, D.R.; Voigt, J.A. Correlation between photoluminescence and oxygen vacancies in ZnO phosphors. Appl. Phys. Lett. 1996, 68, 403–405. [Google Scholar] [CrossRef]
- Trunk, M.; Venkatachalapathy, V.; Galeckas, A.; Kuznetsov, A.Y. Deep level related photoluminescence in ZnMgO. Appl. Phys. Lett. 2010, 97, 211901. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Jiang, Y.; Peng, H.; Wei, J.; Zhang, S.; Chen, S. Efficient quantum dot light-emitting diodes with a Zn0.85Mg0.15O interfacial modification layer. Nanoscale 2017, 9, 8962–8969. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, D.; Xue, Z.; Zhang, X. Mechanisms of green emission from ZnO films prepared by rf magnetron sputtering. Opt. Mater. 2004, 26, 23–26. [Google Scholar] [CrossRef]
- Lähnemann, J.; Flissikowski, T.; Wölz, M.; Geelhaar, L.; Grahn, H.T.; Brandt, O.; Jahn, U. Quenching of the luminescence intensity of GaN nanowires under electron beam exposure: Impact of C adsorption on the exciton lifetime. Nanotechnology 2016, 27, 455706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willander, M.; Nur, O.; Sadaf, J.R.; Qadir, M.I.; Zaman, S.; Zainelabdin, A.; Bano, N.; Hussain, I. Luminescence from Zinc Oxide Nanostructures and Polymers and their Hybrid Devices. Materials 2010, 3, 2643–2667. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahoo, A.; Miryala, M.; Dixit, T.; Klimkowicz, A.; Francis, B.; Murakami, M.; Rao, M.S.R.; Krishnan, S. Femtosecond Pulse Ablation Assisted Mg-ZnO Nanoparticles for UV-Only Emission. Nanomaterials 2020, 10, 1326. https://doi.org/10.3390/nano10071326
Sahoo A, Miryala M, Dixit T, Klimkowicz A, Francis B, Murakami M, Rao MSR, Krishnan S. Femtosecond Pulse Ablation Assisted Mg-ZnO Nanoparticles for UV-Only Emission. Nanomaterials. 2020; 10(7):1326. https://doi.org/10.3390/nano10071326
Chicago/Turabian StyleSahoo, Anubhab, Muralidhar Miryala, Tejendra Dixit, Alicja Klimkowicz, Bellarmine Francis, Masato Murakami, Mamidanna Sri Ramachandra Rao, and Sivarama Krishnan. 2020. "Femtosecond Pulse Ablation Assisted Mg-ZnO Nanoparticles for UV-Only Emission" Nanomaterials 10, no. 7: 1326. https://doi.org/10.3390/nano10071326