Biological Effects of Tricalcium Silicate Nanoparticle-Containing Cement on Stem Cells from Human Exfoliated Deciduous Teeth
Abstract
:1. Introduction
2. Materials and Methods
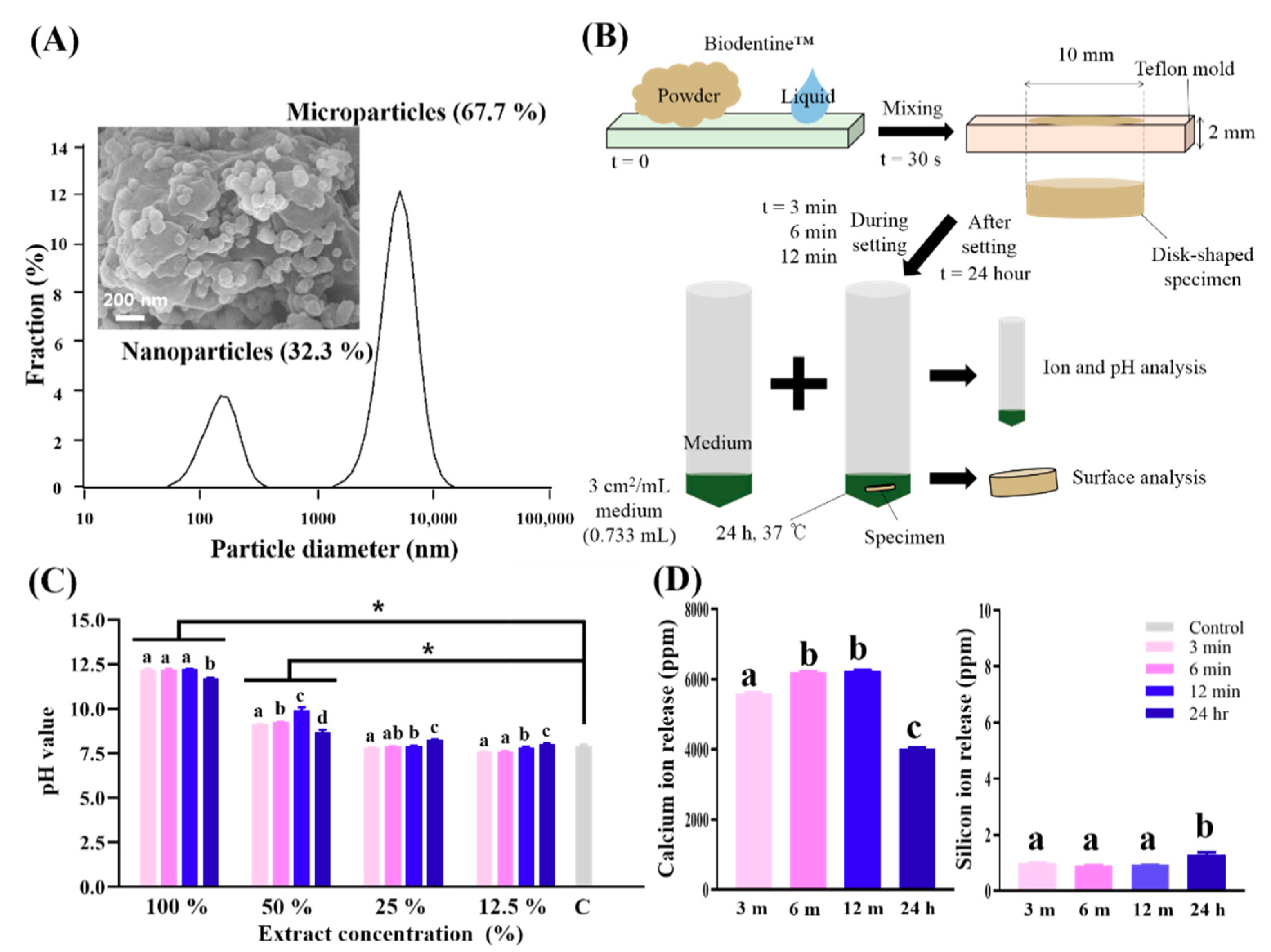
2.1. Preparation of Biodentine™ Specimens and Their Extracts
2.2. Physicochemical Analysis in a Cell-Free Culture Environment
2.2.1. Powder Grain Size
2.2.2. pH Measurements
2.2.3. Ion Release by ICP-AES
2.3. Physical Analysis of Surfaces in a Cell-Free Culture Environment
2.3.1. Scanning Electron Microscopy (SEM)
2.3.2. Energy-Dispersive Spectroscopy (EDS)
2.3.3. X-ray Diffraction (XRD)
2.4. Primary Culture of SHED
2.5. In Vitro Study of Biodentine™ on SHED
2.5.1. Cytotoxicity by CCK-8 Assay and Staining by Phalloidin and 4′,6-Diamidine-2′-phenylindole Dihydrochloride (DAPI)
2.5.2. Odontogenic Differentiation and Biomineralization Evaluated by Alizarin Red S (ARS) Staining
2.6. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Analysis in a Cell-Free Culture Environment
3.2. Physical Analysis of Surfaces in a Cell-Free Culture Environment
3.3. In Vitro Study of Biodentine™ on SHED
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Priyadarsini, S.; Mukherjee, S.; Mishra, M. Nanoparticles used in dentistry: A review. J. Oral Biol. Craniofacial Res. 2018, 8, 58–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dashnyam, K.; Lee, J.-H.; Mandakhbayar, N.; Jin, G.-Z.; Lee, H.-H.; Kim, H.-W. Intra-articular biomaterials-assisted delivery to treat temporomandibular joint disorders. J. Tissue Eng. 2018, 9, 2041731418776514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.-H.; Lee, M.Y.; Lim, Y.; Knowles, J.; Kim, H.-W. Auditory disorders and future therapies with delivery systems. J. Tissue Eng. 2018, 9, 2041731418808455. [Google Scholar] [CrossRef] [Green Version]
- Park, I.-S.; Mahapatra, C.; Park, J.S.; Dashnyam, K.; Kim, J.-W.; Ahn, J.C.; Chung, P.-S.; Yoon, D.S.; Mandakhbayar, N.; Singh, R.K.; et al. Revascularization and limb salvage following critical limb ischemia by nanoceria-induced Ref-1/APE1-dependent angiogenesis. Biomaterials 2020, 242, 119919. [Google Scholar] [CrossRef]
- Lee, J.-H.; El-Fiqi, A.; Mandakhbayar, N.; Lee, H.-H.; Kim, H.-W. Drug/ion co-delivery multi-functional nanocarrier to regenerate infected tissue defect. Biomaterials 2017, 142, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Mandakhbayar, N.; El-Fiqi, A.; Kim, H.-W. Intracellular co-delivery of Sr ion and phenamil drug through mesoporous bioglass nanocarriers synergizes BMP signaling and tissue mineralization. Acta Biomater. 2017, 60, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.T.; Ahn, H.T.; Kim, M.I. Reagent-free colorimetric assay for galactose using agarose gel entrapping nanoceria and galactose oxidase. Nanomaterials 2020, 10, 895. [Google Scholar] [CrossRef] [PubMed]
- kostevšek, N.; Cheung, C.; Serša, I.; Kreft, M.; Monaco, I.; Comes-Franchini, M.; Vidmar, J.; Al-Jamal, W. Magneto-liposomes as MRI contrast agents: A systematic study of different liposomal formulations. Nanomaterials 2020, 10, 889. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Ma, P.X. Nanostructured biomaterials for regeneration. Adv. Funct. Mater. 2008, 18, 3566–3582. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-H.; Kim, D.-H.; Lee, H.-H.; Kim, H.-W. Role of nuclear mechanosensitivity in determining cellular responses to forces and biomaterials. Biomaterials 2019, 197, 60–71. [Google Scholar] [CrossRef]
- Abou-Saleh, H.; Younes, N.; Rasool, K.; Younis, M.H.; Prieto, R.M.; Yassine, H.M.; Mahmoud, K.A.; Pintus, G.; Nasrallah, G.K. Impaired liver size and compromised neurobehavioral activity are elicited by chitosan nanoparticles in the zebrafish embryo model. Nanomaterials 2019, 9, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, C.; Kim, S.; Sun, T.; Cho, Y.-B.; Song, M. Pulp-dentin regeneration: Current approaches and challenges. J. Tissue Eng. 2019, 10, 2041731418819263. [Google Scholar] [CrossRef] [Green Version]
- Stanley, H.R. Pulp capping: Conserving the dental pulp—Can it be done? Is it worth it? Oral Surg. Oral Med. Oral Pathol. 1989, 68, 628–639. [Google Scholar] [CrossRef]
- Dammaschke, T.; Galler, K.; Krastl, G. Current recommendations for vital pulp treatment. Dtsch. Zahnärztl. Z. Int. 2019, 1, 43–52. [Google Scholar] [CrossRef]
- Cooper, P.R.; Takahashi, Y.; Graham, L.W.; Simon, S.; Imazato, S.; Smith, A.J. Inflammation-regeneration interplay in the dentine-pulp complex. J. Dent. 2010, 38, 687–697. [Google Scholar] [CrossRef]
- Dammaschke, T. The history of direct pulp capping. J. Hist. Dent. 2008, 56, 9–23. [Google Scholar]
- Siqueira, J.F., Jr.; Lopes, H.P. Mechanisms of antimicrobial activity of calcium hydroxide: A critical review. Int. Endod. J. 1999, 32, 361–369. [Google Scholar] [CrossRef]
- Koike, T.; Polan, M.A.; Izumikawa, M.; Saito, T. Induction of reparative dentin formation on exposed dental pulp by dentin phosphophoryn/collagen composite. BioMed Res. Int. 2014, 2014, 745139. [Google Scholar] [CrossRef] [Green Version]
- Komabayashi, T.; Zhu, Q.; Eberhart, R.; Imai, Y. Current status of direct pulp-capping materials for permanent teeth. Dent. Mater. J. 2016, 35, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Faraco, I.M., Jr.; Holland, R. Response of the pulp of dogs to capping with mineral trioxide aggregate or a calcium hydroxide cement. Dent. Traumatol. Off. Publ. Int. Assoc. Dent. Traumatol. 2001, 17, 163–166. [Google Scholar] [CrossRef]
- Parirokh, M.; Torabinejad, M. Mineral trioxide aggregate: A comprehensive literature review—Part III: Clinical applications, drawbacks, and mechanism of action. J. Endod. 2010, 36, 400–413. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Hurt, A.P.; Coleman, N.J. The application of 29Si nmr spectroscopy to the analysis of calcium silicate-based cement using Biodentine™ as an example. J. Funct. Biomater. 2019, 10, 25. [Google Scholar] [CrossRef] [Green Version]
- Malkondu, Ö.; Kazandağ, M.K.; Kazazoğlu, E. A review on biodentine, a contemporary dentine replacement and repair material. BioMed Res. Int. 2014, 2014, 160951. [Google Scholar] [CrossRef] [Green Version]
- Jang, Y.E.; Lee, B.N.; Koh, J.T.; Park, Y.J.; Joo, N.E.; Chang, H.S.; Hwang, I.N.; Oh, W.M.; Hwang, Y.C. Cytotoxicity and physical properties of tricalcium silicate-based endodontic materials. Restor. Dent. Endod. 2014, 39, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Marconyak, L.J., Jr.; Kirkpatrick, T.C.; Roberts, H.W.; Roberts, M.D.; Aparicio, A.; Himel, V.T.; Sabey, K.A. A comparison of coronal tooth discoloration elicited by various endodontic reparative materials. J. Endod. 2016, 42, 470–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, X.; Mao, J.; Liu, Y. Pulp stem cells derived from human permanent and deciduous teeth: Biological characteristics and therapeutic applications. Stem Cells Transl. Med. 2020, 9, 445–464. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Kim, D.S.; Jang, H.; Kim, H.-R.; Kang, H.-W. Bioprinting of three-dimensional dentin–pulp complex with local differentiation of human dental pulp stem cells. J. Tissue Eng. 2019, 10, 2041731419845849. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef] [Green Version]
- Modena, K.C.; Casas-Apayco, L.C.; Atta, M.T.; Costa, C.A.; Hebling, J.; Sipert, C.R.; Navarro, M.F.; Santos, C.F. Cytotoxicity and biocompatibility of direct and indirect pulp capping materials. J. Appl. Oral Sci. Rev. FOB 2009, 17, 544–554. [Google Scholar] [CrossRef]
- Kim, D.-A.; Lee, J.-H.; Jun, S.-K.; Kim, H.-W.; Eltohamy, M.; Lee, H.-H. Sol–gel-derived bioactive glass nanoparticle-incorporated glass ionomer cement with or without chitosan for enhanced mechanical and biomineralization properties. Dent. Mater. 2017, 33, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Victoria-Escandell, A.; Ibañez-Cabellos, J.S.; De Cutanda, S.B.S.; Berenguer-Pascual, E.; Beltrán-García, J.; García-López, E.; Pallardó, F.V.; García-Giménez, J.L.; Pallarés-Sabater, A.; Zarzosa-López, I.; et al. Cellular responses in human dental pulp stem cells treated with three endodontic materials. Stem Cells Int. 2017, 2017, 8920356. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.; Mahapatra, C.; Lee, H.; Kim, H.; Lee, J. Biological effects of provisional resin materials on human dental pulp stem cells. Oper. Dent. 2017, 42, E81–E92. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Kohli, M.R.; Yu, Q.; Kim, S.; Qu, T.; He, W.X. Biodentine induces human dental pulp stem cell differentiation through mitogen-activated protein kinase and calcium-/calmodulin-dependent protein kinase II pathways. J. Endod. 2014, 40, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.W.; Lee, S.Y.; Ann, H.J.; Kum, K.Y.; Kim, E.C. Effects of calcium silicate endodontic cements on biocompatibility and mineralization-inducing potentials in human dental pulp cells. J. Endod. 2014, 40, 1194–1200. [Google Scholar] [CrossRef] [PubMed]
- Athanasiadou, E.; Paschalidou, M.; Theocharidou, A.; Kontoudakis, N.; Arapostathis, K.; Bakopoulou, A. Biological interactions of a calcium silicate based cement (Biodentine™) with Stem Cells from Human Exfoliated Deciduous teeth. Dent. Mater. 2018, 34, 1797–1813. [Google Scholar] [CrossRef]
- Neuhaus, K.; Lussi, A. Management of Dental Emergencies in Children and Adolescents; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2019. [Google Scholar]
- Nakamura, S.; Yamada, Y.; Katagiri, W.; Sugito, T.; Ito, K.; Ueda, M. Stem cell proliferation pathways comparison between human exfoliated deciduous teeth and dental pulp stem cells by gene expression profile from promising dental pulp. J. Endod. 2009, 35, 1536–1542. [Google Scholar] [CrossRef]
- Bardellini, E.; Amadori, F.; Santoro, A.; Conti, G.; Orsini, G.; Majorana, A. Odontoblastic cell quantification and apoptosis within pulp of deciduous teeth versus pulp of permanent teeth. J. Clin. Pediatr. Dent. 2016, 40, 450–455. [Google Scholar] [CrossRef] [Green Version]
- Laurent, P.; Camps, J.; De Méo, M.; Déjou, J.; About, I. Induction of specific cell responses to a Ca3SiO5-based posterior restorative material. Dent. Mater. 2008, 24, 1486–1494. [Google Scholar] [CrossRef]
- Araújo, L.B.; Cosme-Silva, L.; Fernandes, A.P.; Oliveira, T.M.D.; Cavalcanti, B.D.N.; Gomes Filho, J.E.; Sakai, V.T. Effects of mineral trioxide aggregate, Biodentine™ and calcium hydroxide on viability, proliferation, migration and differentiation of stem cells from human exfoliated deciduous teeth. J. Appl. Oral Sci. Rev. FOB 2018, 26, e20160629. [Google Scholar] [CrossRef] [Green Version]
- International Organization for Standardization. 10993-12. Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference Materials; International Organization for Standardization: Geneve, Switzerland, 2012. [Google Scholar]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. SHED: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812. [Google Scholar] [CrossRef] [Green Version]
- International Organization for Standardization. 10993-5. Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity; International Organization for Standardization: Geneve, Switzerland, 1999. [Google Scholar]
- Mahapatra, C.; Kim, J.-J.; Lee, J.-H.; Jin, G.-Z.; Knowles, J.C.; Kim, H.-W. Differential chondro- and osteo-stimulation in three-dimensional porous scaffolds with different topological surfaces provides a design strategy for biphasic osteochondral engineering. J. Tissue Eng. 2019, 10, 2041731419826433. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.D.; El-Fiqi, A.; Lee, H.-Y.; Singh, R.K.; Kim, D.-A.; Lee, H.-H.; Kim, H.-W. Chitosan–nanobioactive glass electrophoretic coatings with bone regenerative and drug delivering potential. J. Mater. Chem. 2012, 22, 24945–24956. [Google Scholar] [CrossRef]
- Atmeh, A.R.; Chong, E.Z.; Richard, G.; Festy, F.; Watson, T.F. Dentin-cement interfacial interaction: Calcium silicates and polyalkenoates. J. Dent. Res. 2012, 91, 454–459. [Google Scholar] [CrossRef]
- Camilleri, J. Hydration characteristics of Biodentine and Theracal used as pulp capping materials. Dent. Mater. 2014, 30, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Okabe, T.; Sakamoto, M.; Takeuchi, H.; Matsushima, K. Effects of pH on mineralization ability of human dental pulp cells. J. Endod. 2006, 32, 198–201. [Google Scholar] [CrossRef]
- Loison-Robert, L.S.; Tassin, M.; Bonte, E.; Berbar, T.; Isaac, J.; Berdal, A.; Simon, S.; Fournier, B.P.J. In vitro effects of two silicate-based materials, Biodentine and BioRoot RCS, on dental pulp stem cells in models of reactionary and reparative dentinogenesis. PLoS ONE 2018, 13, e0190014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajasekharan, S.; Vercruysse, C.; Martens, L.; Verbeeck, R. Effect of exposed surface area, volume and environmental pH on the calcium ion release of three commercially available tricalcium silicate based dental cements. Materials 2018, 11, 123. [Google Scholar] [CrossRef] [Green Version]
- Rashid, F.; Shiba, H.; Mizuno, N.; Mouri, Y.; Fujita, T.; Shinohara, H.; Ogawa, T.; Kawaguchi, H.; Kurihara, H. The effect of extracellular calcium ion on gene expression of bone-related proteins in human pulp cells. J. Endod. 2003, 29, 104–107. [Google Scholar] [CrossRef]
- Dashnyam, K.; Jin, G.Z.; Kim, J.H.; Perez, R.; Jang, J.H.; Kim, H.W. Promoting angiogenesis with mesoporous microcarriers through a synergistic action of delivered silicon ion and VEGF. Biomaterials 2017, 116, 145–157. [Google Scholar] [CrossRef]
- Jun, S.-K.; Lee, J.-H.; Lee, H.-H. The Biomineralization of a bioactive glass-incorporated light-curable pulp capping material using human dental pulp stem cells. BioMed Res. Int. 2017, 2017, 2495282. [Google Scholar] [CrossRef] [Green Version]
- Moon, H.-J.; Lee, J.-H.; Kim, J.-H.; Knowles, J.C.; Cho, Y.-B.; Shin, D.-H.; Lee, H.-H.; Kim, H.-W. Reformulated mineral trioxide aggregate components and the assessments for use as future dental regenerative cements. J. Tissue Eng. 2018, 9, 2041731418807396. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Wang, Q.; Dong, B.; Jiang, B.; Xing, F. Ion-triggered calcium hydroxide microcapsules for enhanced corrosion resistance of steel bars. RSC Adv. 2018, 8, 39536–39544. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, W.; Olek, J. Elucidating the accelerated carbonation products of calcium silicates using multi-technique approach. J. CO2 Util. 2018, 23, 61–74. [Google Scholar] [CrossRef]
- Bhaduri, G.A.; Alamiry, M.A.H.; Šiller, L. Nickel nanoparticles for enhancing carbon capture. J. Nanomater. 2015, 2015, 581785. [Google Scholar] [CrossRef] [Green Version]
- Mangla, O.; Roy, S. Monoclinic zirconium oxide nanostructures having tunable band gap synthesized under extremely non-equilibrium plasma conditions. Proceedings 2019, 3, 10. [Google Scholar] [CrossRef] [Green Version]
- Camilleri, J. Hydration mechanisms of mineral trioxide aggregate. Int. Endod. J. 2007, 40, 462–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Setbon, H.M.; Devaux, J.; Iserentant, A.; Leloup, G.; Leprince, J.G. Influence of composition on setting kinetics of new injectable and/or fast setting tricalcium silicate cements. Dent. Mater. 2014, 30, 1291–1303. [Google Scholar] [CrossRef]
- Ashofteh Yazdi, K.; Ghabraei, S.; Bolhari, B.; Kafili, M.; Meraji, N. Microstructure and chemical analysis of four calcium silicate-based cements in different environmental conditions. Clin. Oral Investig. 2019, 23, 43–52. [Google Scholar] [CrossRef]
- Collado-González, M.; García-Bernal, D.; Oñate-Sánchez, R.E.; Ortolani-Seltenerich, P.S.; Álvarez-Muro, T.; Lozano, A.; Forner, L.; Llena, C.; Moraleda, J.M.; Rodríguez-Lozano, F.J. Cytotoxicity and bioactivity of various pulpotomy materials on stem cells from human exfoliated primary teeth. Int. Endod. J. 2017, 50 (Suppl. 2), e19–e30. [Google Scholar] [CrossRef]



| Product Name | Composition | Setting Time | Mixing and Placement Time | Manufacturer | ||
|---|---|---|---|---|---|---|
| Biodentine™ | Powder | Tri-calcium silicate | Main core material | 12 min | 6 min | Septodont |
| Di-calcium silicate | Second core material | |||||
| Calcium carbonate | Filler | |||||
| Iron oxide | Shade | |||||
| Zircornium oxide | Radiopacifier | |||||
| Liquid | Calcium chloride | Accelerator | ||||
| Hydrosoluble polymer | Water-reducing agent | |||||
| Extraction Starting Time after Start of Mixing | Mixing Time | Mixing Speed | Setting Reaction | |||
|---|---|---|---|---|---|---|
| During Setting | After Setting | |||||
| 3 min | 6 min | 12 min | 24 h | 30 s | 4000 rotations/min | Hydration |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, Y.; Yoon, J.-Y.; Dev Patel, K.; Ma, L.; Lee, H.-H.; Kim, J.; Lee, J.-H.; Shin, J. Biological Effects of Tricalcium Silicate Nanoparticle-Containing Cement on Stem Cells from Human Exfoliated Deciduous Teeth. Nanomaterials 2020, 10, 1373. https://doi.org/10.3390/nano10071373
Jung Y, Yoon J-Y, Dev Patel K, Ma L, Lee H-H, Kim J, Lee J-H, Shin J. Biological Effects of Tricalcium Silicate Nanoparticle-Containing Cement on Stem Cells from Human Exfoliated Deciduous Teeth. Nanomaterials. 2020; 10(7):1373. https://doi.org/10.3390/nano10071373
Chicago/Turabian StyleJung, Yoonsun, Ji-Young Yoon, Kapil Dev Patel, Lan Ma, Hae-Hyoung Lee, Jongbin Kim, Jung-Hwan Lee, and Jisun Shin. 2020. "Biological Effects of Tricalcium Silicate Nanoparticle-Containing Cement on Stem Cells from Human Exfoliated Deciduous Teeth" Nanomaterials 10, no. 7: 1373. https://doi.org/10.3390/nano10071373
APA StyleJung, Y., Yoon, J.-Y., Dev Patel, K., Ma, L., Lee, H.-H., Kim, J., Lee, J.-H., & Shin, J. (2020). Biological Effects of Tricalcium Silicate Nanoparticle-Containing Cement on Stem Cells from Human Exfoliated Deciduous Teeth. Nanomaterials, 10(7), 1373. https://doi.org/10.3390/nano10071373







