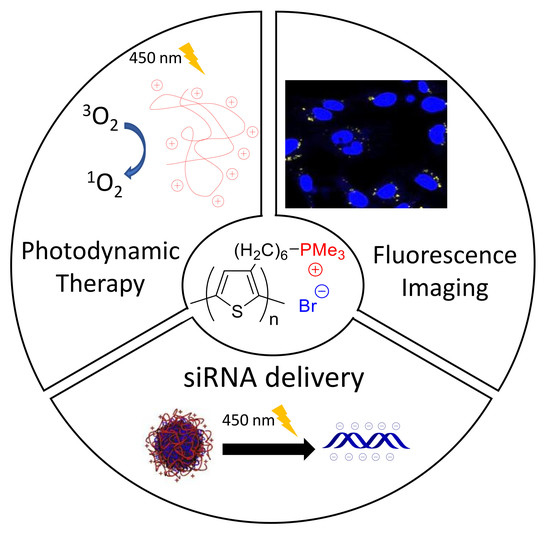
Polythiophenes with Cationic Phosphonium Groups as Vectors for Imaging, siRNA Delivery, and Photodynamic Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Polymer Synthesis and Characterisation
2.2. UV-Visible Absorption and Emission Properties
2.3. Singlet Oxygen Measurements
2.4. Dynamic Light Scattering (DLS) and Zeta Potential
2.5. Cell Culture Conditions
2.6. In Vitro Cytotoxicity Assay
2.7. In Vitro Phototoxicity Assay
2.8. Confocal Fluorescent Imaging on Living Cells
2.9. Gel Electrophoresis with siRNA
2.10. In Vitro Combined PDT and siRNA Delivery
2.11. Statistical Studies
3. Results and Discussion
3.1. Polymer Synthesis
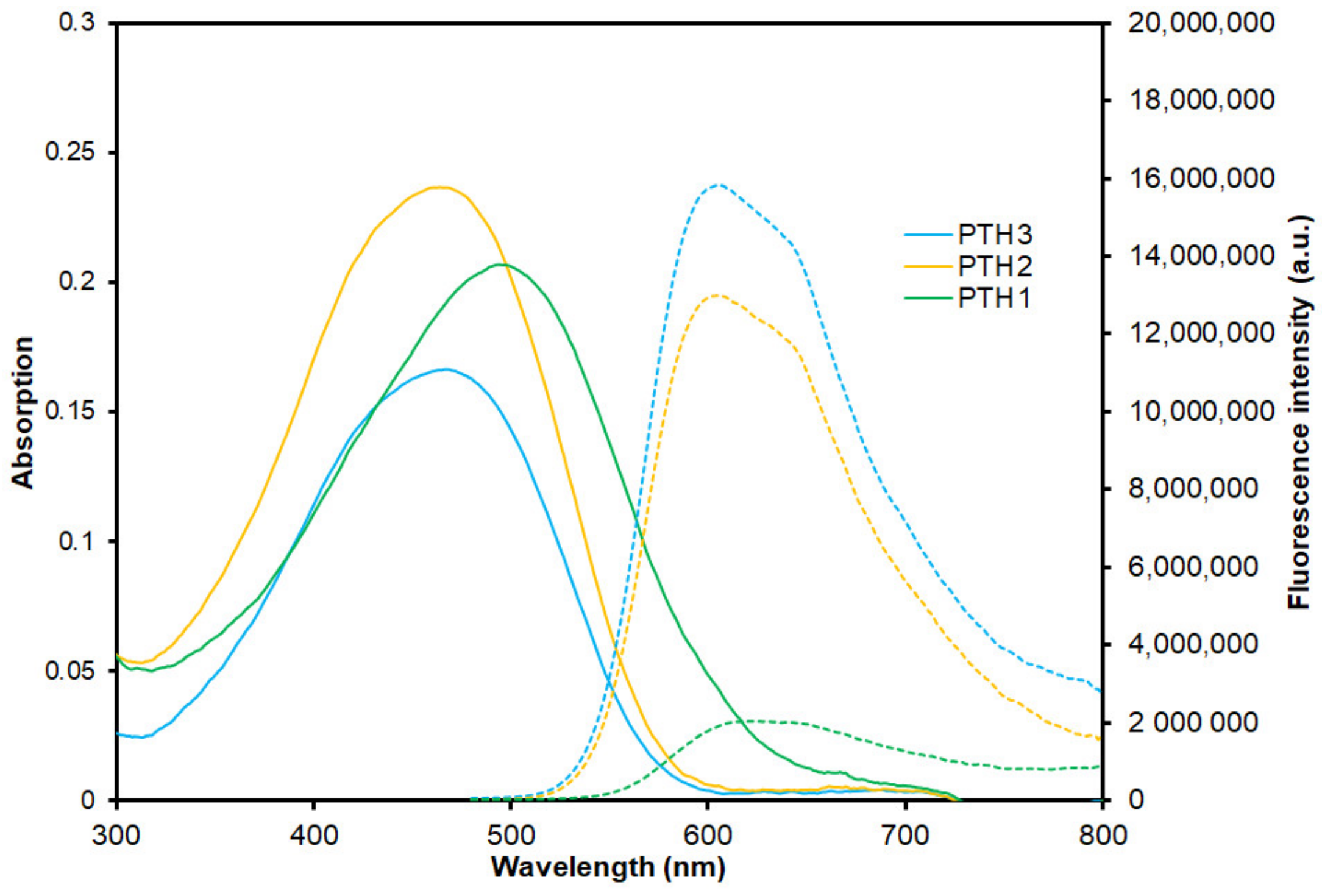
3.2. Optical Properties of the Phosphonium-Based Polythiophene CPEs
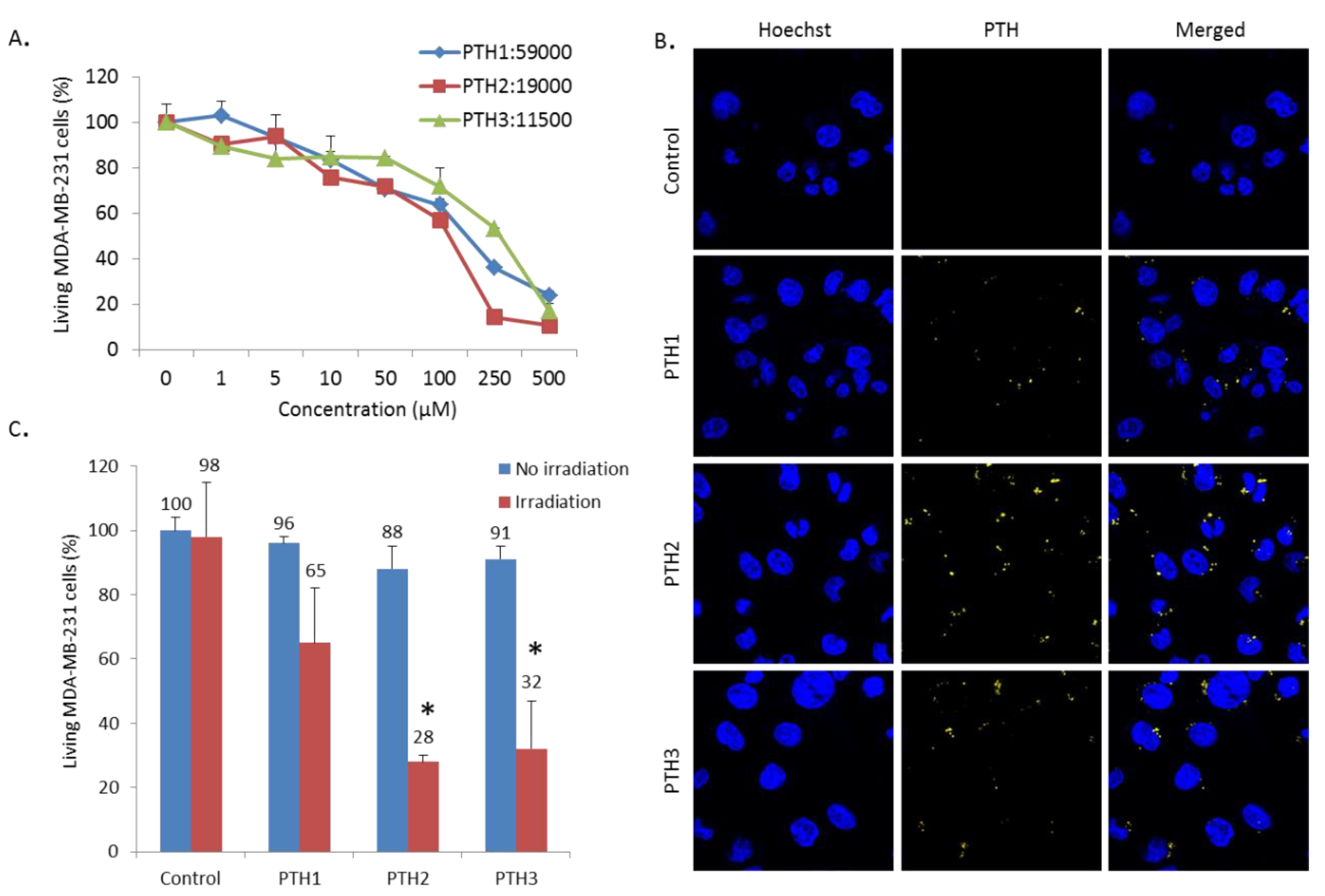
3.3. Cytotoxicity, Imaging and Photodynamic Therapy Activity
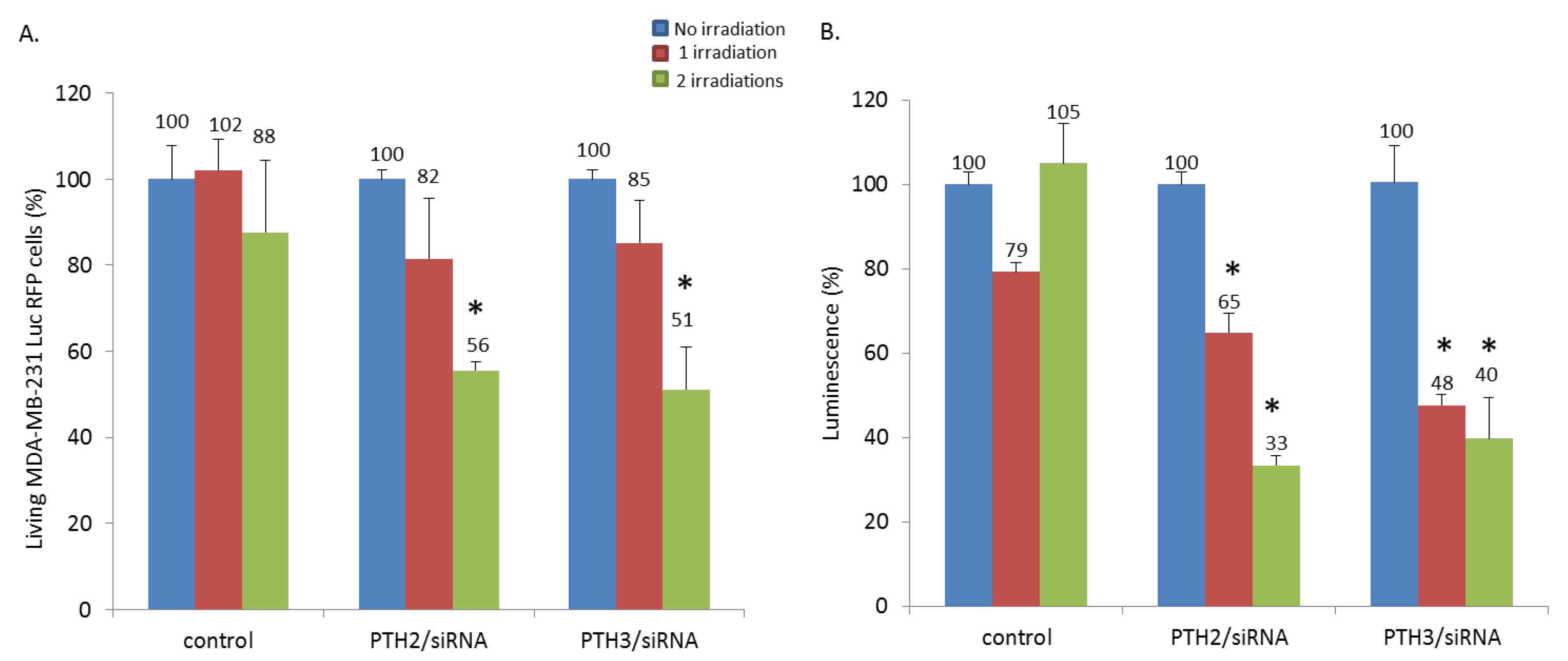
3.4. In Vitro Combined PDT and siRNA Delivery
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- David, A.R.; Zimmerman, M.R. Cancer: An old disease, a new disease or something in between? Nat. Rev. Cancer 2010, 10, 728–733. [Google Scholar] [CrossRef]
- Schirrmacher, V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment. Int. J. Oncol. 2019, 54, 407–419. [Google Scholar] [CrossRef]
- Nussbaumer, S.; Bonnabry, P.; Veuthey, J.-L.; Fleury-Souverain, S. Analysis of anticancer drugs: A review. Talanta 2011, 85, 2265–2289. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Ju, D.-T.; Chang, C.-F.; Muralidhar Reddy, P.; Velmurugan, B.K. A review on the effects of current chemotherapy drugs and natural agents in treating non-small cell lung cancer. Biomed. Taipei 2017, 7, 23. [Google Scholar] [CrossRef] [Green Version]
- Idris, N.M.; Gnanasammandhan, M.K.; Zhang, J.; Ho, P.C.; Mahendran, R.; Zhang, Y. In vivo photodynamic therapy using upconversion nanoparticles as remote-controlled nanotransducers. Nat. Med. 2012, 18, 1580–1585. [Google Scholar] [CrossRef]
- Henderson, B.W.; Dougherty, T.J. How does photodynamic therapy work? Photochem. Photobiol. 1992, 55, 145–157. [Google Scholar] [CrossRef]
- Naik, A.; Rubbiani, R.; Gasser, G.; Spingler, B. Visible-Light-Induced Annihilation of Tumor Cells with Platinum–Porphyrin Conjugates. Angew. Chem. Int. Ed. 2014, 53, 6938–6941. [Google Scholar] [CrossRef]
- Sanoj Rejinold, N.; Choi, G.; Choy, J.-H. Recent trends in nano photo-chemo therapy approaches and future scopes. Coord. Chem. Rev. 2020, 411, 213252. [Google Scholar] [CrossRef]
- Cheng, Y.-J.; Hu, J.-J.; Qin, S.-Y.; Zhang, A.-Q.; Zhang, X.-Z. Recent advances in functional mesoporous silica-based nanoplatforms for combinational photo-chemotherapy of cancer. Biomaterials 2020, 232, 119738. [Google Scholar] [CrossRef]
- Hu, C.; Zhuang, W.; Yu, T.; Chen, L.; Liang, Z.; Li, G.; Wang, Y. Multi-stimuli responsive polymeric prodrug micelles for combined chemotherapy and photodynamic therapy. J. Mater. Chem. B 2020, 8, 5267–5279. [Google Scholar] [CrossRef]
- Hu, X.; Lu, Y.; Dong, C.; Zhao, W.; Wu, X.; Zhou, L.; Chen, L.; Yao, T.; Shi, S. A RuII Polypyridyl Alkyne Complex Based Metal–Organic Frameworks for Combined Photodynamic/Photothermal/Chemotherapy. Chem. Eur. J. 2020, 26, 1668–1675. [Google Scholar] [CrossRef]
- Meng, L.-B.; Zhang, W.; Li, D.; Li, Y.; Hu, X.-Y.; Wang, L.; Li, G. pH-Responsive supramolecular vesicles assembled by water-soluble pillar [5] arene and a BODIPY photosensitizer for chemo-photodynamic dual therapy. Chem. Commun. 2015, 51, 14381–14384. [Google Scholar] [CrossRef]
- Wang, D.; Wang, T.; Liu, J.; Yu, H.; Jiao, S.; Feng, B.; Zhou, F.; Fu, Y.; Yin, Q.; Zhang, P.; et al. Acid-Activatable Versatile Micelleplexes for PD-L1 Blockade-Enhanced Cancer Photodynamic Immunotherapy. Nano Lett. 2016, 16, 5503–5513. [Google Scholar] [CrossRef]
- Wang, X.; Liu, K.; Yang, G.; Cheng, L.; He, L.; Liu, Y.; Li, Y.; Guo, L.; Liu, Z. Near-infrared light triggered photodynamic therapy in combination with gene therapy using upconversion nanoparticles for effective cancer cell killing. Nanoscale 2014, 6, 9198–9205. [Google Scholar] [CrossRef]
- Tseng, S.J.; Liao, Z.-X.; Kao, S.-H.; Zeng, Y.-F.; Huang, K.-Y.; Li, H.-J.; Yang, C.-L.; Deng, Y.-F.; Huang, C.-F.; Yang, S.-C.; et al. Highly specific in vivo gene delivery for p53-mediated apoptosis and genetic photodynamic therapies of tumour. Nat. Commun. 2015, 6, 6456. [Google Scholar] [CrossRef]
- Sun, S.; Xu, Y.; Fu, P.; Chen, M.; Sun, S.; Zhao, R.; Wang, J.; Liang, X.; Wang, S. Ultrasound-targeted photodynamic and gene dual therapy for effectively inhibiting triple negative breast cancer by cationic porphyrin lipid microbubbles loaded with HIF1α-siRNA. Nanoscale 2018, 10, 19945–19956. [Google Scholar] [CrossRef]
- Chen, W.-H.; Lecaros, R.L.G.; Tseng, Y.-C.; Huang, L.; Hsu, Y.-C. Nanoparticle delivery of HIF1α siRNA combined with photodynamic therapy as a potential treatment strategy for head-and-neck cancer. Cancer Lett. 2015, 359, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Zhao, R.; Liang, X.; Zhao, B.; Chen, M.; Liu, R.; Sun, S.; Yue, X.; Wang, S. Ultrasound assisted gene and photodynamic synergistic therapy with multifunctional FOXA1-siRNA loaded porphyrin microbubbles for enhancing therapeutic efficacy for breast cancer. Biomaterials 2018, 173, 58–70. [Google Scholar] [CrossRef]
- Huang, C.; Zheng, J.; Ma, D.; Liu, N.; Zhu, C.; Li, J.; Yang, R. Hypoxia-triggered gene therapy: A new drug delivery system to utilize photodynamic-induced hypoxia for synergistic cancer therapy. J. Mater. Chem. B 2018, 6, 6424–6430. [Google Scholar] [CrossRef]
- Laroui, N.; Coste, M.; Lichon, L.; Bessin, Y.; Gary-Bobo, M.; Pratviel, G.; Bonduelle, C.; Bettache, N.; Ulrich, S. Combination of photodynamic therapy and gene silencing achieved through the hierarchical self-assembly of porphyrin-siRNA complexes. Int. J. Pharm. 2019, 569, 118585. [Google Scholar] [CrossRef]
- Mauriello Jimenez, C.; Aggad, D.; Croissant, J.G.; Tresfield, K.; Laurencin, D.; Berthomieu, D.; Cubedo, N.; Rossel, M.; Alsaiari, S.; Anjum, D.H.; et al. Porous Porphyrin-Based Organosilica Nanoparticles for NIR Two-Photon Photodynamic Therapy and Gene Delivery in Zebrafish. Adv. Funct. Mater. 2018, 28, 1800235. [Google Scholar] [CrossRef]
- Vankayala, R.; Kuo, C.-L.; Nuthalapati, K.; Chiang, C.-S.; Hwang, K.C. Nucleus-Targeting Gold Nanoclusters for Simultaneous In Vivo Fluorescence Imaging, Gene Delivery, and NIR-Light Activated Photodynamic Therapy. Adv. Funct. Mater. 2015, 25, 5934–5945. [Google Scholar] [CrossRef]
- Vijayaraghavan, P.; Vankayala, R.; Chiang, C.-S.; Sung, H.-W.; Hwang, K.C. Complete destruction of deep-tissue buried tumors via combination of gene silencing and gold nanoechinus-mediated photodynamic therapy. Biomaterials 2015, 62, 13–23. [Google Scholar] [CrossRef]
- Schumann, C.; Taratula, O.; Khalimonchuk, O.; Palmer, A.L.; Cronk, L.M.; Jones, C.V.; Escalante, C.A.; Taratula, O. ROS-induced nanotherapeutic approach for ovarian cancer treatment based on the combinatorial effect of photodynamic therapy and DJ-1 gene suppression. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1961–1970. [Google Scholar] [CrossRef]
- Liang, R.; Tian, R.; Ma, L.; Zhang, L.; Hu, Y.; Wang, J.; Wei, M.; Yan, D.; Evans, D.G.; Duan, X. A Supermolecular Photosensitizer with Excellent Anticancer Performance in Photodynamic Therapy. Adv. Funct. Mater. 2014, 24, 3144–3151. [Google Scholar] [CrossRef]
- Chen, H.; Xiao, L.; Anraku, Y.; Mi, P.; Liu, X.; Cabral, H.; Inoue, A.; Nomoto, T.; Kishimura, A.; Nishiyama, N.; et al. Polyion Complex Vesicles for Photoinduced Intracellular Delivery of Amphiphilic Photosensitizer. J. Am. Chem. Soc. 2014, 136, 157–163. [Google Scholar] [CrossRef]
- Jiang, H.; Taranekar, P.; Reynolds, J.R.; Schanze, K.S. Conjugated Polyelectrolytes: Synthesis, Photophysics, and Applications. Angew. Chem. Int. Ed. 2009, 48, 4300–4316. [Google Scholar] [CrossRef]
- Feng, X.; Liu, L.; Wang, S.; Zhu, D. Water-soluble fluorescent conjugated polymers and their interactions with biomacromolecules for sensitive biosensors. Chem. Soc. Rev. 2010, 39, 2411–2419. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.E.; Woo, H.Y. Control of electrostatic interaction between a molecular beacon aptamer and conjugated polyelectrolyte for detection range-tunable ATP assay. Polym. Chem. 2017, 8, 6329–6334. [Google Scholar] [CrossRef]
- Xia, F.; Zuo, X.; Yang, R.; Xiao, Y.; Kang, D.; Vallée-Bélisle, A.; Gong, X.; Heeger, A.J.; Plaxco, K.W. On the Binding of Cationic, Water-Soluble Conjugated Polymers to DNA: Electrostatic and Hydrophobic Interactions. J. Am. Chem. Soc. 2010, 132, 1252–1254. [Google Scholar] [CrossRef]
- Rubio-Magnieto, J.; Thomas, A.; Richeter, S.; Mehdi, A.; Dubois, P.; Lazzaroni, R.; Clement, S.; Surin, M. Chirality in DNA-[small pi]-conjugated polymer supramolecular structures: Insights into the self-assembly. Chem. Commun. 2013, 49, 5483–5485. [Google Scholar] [CrossRef]
- Rubio-Magnieto, J.; Azene, E.G.; Knoops, J.; Knippenberg, S.; Delcourt, C.; Thomas, A.; Richeter, S.; Mehdi, A.; Dubois, P.; Lazzaroni, R.; et al. Self-assembly and hybridization mechanisms of DNA with cationic polythiophene. Soft Matter 2015, 11, 6460–6471. [Google Scholar] [CrossRef]
- Leclercq, M.; Rubio-Magnieto, J.; Mohammed, D.; Gabriele, S.; Leclercq, L.; Cottet, H.; Richeter, S.; Clément, S.; Surin, M. Supramolecular Self-Assembly of DNA with a Cationic Polythiophene: From Polyplexes to Fibers. ChemNanoMat 2019, 5, 703–709. [Google Scholar] [CrossRef]
- Liang, J.; Li, K.; Liu, B. Visual sensing with conjugated polyelectrolytes. Chem. Sci. 2013, 4, 1377–1394. [Google Scholar] [CrossRef]
- Feng, G.; Ding, D.; Liu, B. Fluorescence bioimaging with conjugated polyelectrolytes. Nanoscale 2012, 4, 6150–6165. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Bazan, G.C.; Liu, B. Conjugated-Polymer-Amplified Sensing, Imaging, and Therapy. Chem 2017, 2, 760–790. [Google Scholar] [CrossRef] [Green Version]
- Zhan, R.; Liu, B. Functionalized Conjugated Polyelectrolytes for Biological Sensing and Imaging. Chem. Rec. 2016, 16, 1715–1740. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Lv, F.; Liu, L.; Yang, Q.; Wang, S.; Bazan, G.C. A Highly Emissive Conjugated Polyelectrolyte Vector for Gene Delivery and Transfection. Adv. Mater. 2012, 24, 5428–5432. [Google Scholar] [CrossRef]
- Wang, G.; Yin, H.; Yin Ng, J.C.; Cai, L.; Li, J.; Tang, B.Z.; Liu, B. Polyethyleneimine-grafted hyperbranched conjugated polyelectrolytes: Synthesis and imaging of gene delivery. Polyme. Chem. 2013, 4, 5297–5304. [Google Scholar] [CrossRef]
- Li, S.; Yuan, H.; Chen, H.; Wang, X.; Zhang, P.; Lv, F.; Liu, L.; Wang, S. Cationic Poly (p-phenylene vinylene) Materials as a Multifunctional Platform for Light-Enhanced siRNA Delivery. Chem. Asian J. 2016, 11, 2686–2689. [Google Scholar] [CrossRef]
- Jiang, R.; Lu, X.; Yang, M.; Deng, W.; Fan, Q.; Huang, W. Monodispersed Brush-Like Conjugated Polyelectrolyte Nanoparticles with Efficient and Visualized SiRNA Delivery for Gene Silencing. Biomacromolecules 2013, 14, 3643–3652. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Tao, H.; Hu, W.; Miao, X.; Tang, Y.; He, T.; Li, J.; Wang, Q.; Guo, L.; Lu, X.; et al. Two-Photon-Induced Charge-Variable Conjugated Polyelectrolyte Brushes for Effective Gene Silencing. ACS Appl. Bio Mater. 2019, 2, 1676–1685. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Wu, T.; Sun, J.; Wang, X.; Cao, L.; Feng, F. Cationic Polythiophenes as Gene Delivery Enhancer. ACS Appl. Mater. Interfaces 2017, 9, 16735–16740. [Google Scholar] [CrossRef]
- Zhang, C.; Ji, J.; Shi, X.; Zheng, X.; Wang, X.; Feng, F. Synthesis of Structurally Defined Cationic Polythiophenes for DNA Binding and Gene Delivery. ACS Appl. Mater. Interfaces 2018, 10, 4519–4529. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Hu, W.; Ma, H.; Jiang, R.; Tang, Y.; Ji, Y.; Lu, X.; Hou, B.; Deng, W.; Huang, W.; et al. Photo-Induced Charge-Variable Conjugated Polyelectrolyte Brushes Encapsulating Upconversion Nanoparticles for Promoted siRNA Release and Collaborative Photodynamic Therapy under NIR Light Irradiation. Adv. Funct. Mater. 2017, 27, 1702592. [Google Scholar] [CrossRef]
- Hu, R.; Li, S.-L.; Bai, H.-T.; Wang, Y.-X.; Liu, L.-B.; Lv, F.-T.; Wang, S. Regulation of oxidative stress inside living cells through polythiophene derivatives. Chin. Chem. Lett. 2016, 27, 545–549. [Google Scholar] [CrossRef]
- So, R.C.; Carreon-Asok, A.C. Molecular Design, Synthetic Strategies, and Applications of Cationic Polythiophenes. Chem. Rev. 2019, 119, 11442–11509. [Google Scholar] [CrossRef]
- Lan, M.; Zhao, S.; Xie, Y.; Zhao, J.; Guo, L.; Niu, G.; Li, Y.; Sun, H.; Zhang, H.; Liu, W.; et al. Water-Soluble Polythiophene for Two-Photon Excitation Fluorescence Imaging and Photodynamic Therapy of Cancer. ACS Appl. Mater. Interfaces 2017, 9, 14590–14595. [Google Scholar] [CrossRef]
- Guo, L.; Ge, J.; Liu, Q.; Jia, Q.; Zhang, H.; Liu, W.; Niu, G.; Liu, S.; Gong, J.; Hackbarth, S.; et al. Versatile Polymer Nanoparticles as Two-Photon-Triggered Photosensitizers for Simultaneous Cellular, Deep-Tissue Imaging, and Photodynamic Therapy. Adv. Healthc. Mater. 2017, 6, 1601431. [Google Scholar] [CrossRef]
- Osaka, I.; McCullough, R.D. Advances in Molecular Design and Synthesis of Regioregular Polythiophenes. Acc. Chem. Res. 2008, 41, 1202–1214. [Google Scholar] [CrossRef]
- Amna, B.; Siddiqi, H.M.; Hassan, A.; Ozturk, T. Recent developments in the synthesis of regioregular thiophene-based conjugated polymers for electronic and optoelectronic applications using nickel and palladium-based catalytic systems. RSC Adv. 2020, 10, 4322–4396. [Google Scholar] [CrossRef] [Green Version]
- Layman, J.M.; Ramirez, S.M.; Green, M.D.; Long, T.E. Influence of Polycation Molecular Weight on Poly (2-dimethylaminoethyl methacrylate)-Mediated DNA Delivery In Vitro. Biomacromolecules 2009, 10, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Alameh, M.; Lavertu, M.; Tran-Khanh, N.; Chang, C.-Y.; Lesage, F.; Bail, M.; Darras, V.; Chevrier, A.; Buschmann, M.D. siRNA Delivery with Chitosan: Influence of Chitosan Molecular Weight, Degree of Deacetylation, and Amine to Phosphate Ratio on in Vitro Silencing Efficiency, Hemocompatibility, Biodistribution, and in Vivo Efficacy. Biomacromolecules 2018, 19, 112–131. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, K.; Nishikawa, M.; Kawakami, S.; Nakano, T.; Hattori, Y.; Fumoto, S.; Yamashita, F.; Hashida, M. Molecular weight-dependent gene transfection activity of unmodified and galactosylated polyethyleneimine on hepatoma cells and mouse liver. Mol. Ther. 2003, 7, 254–261. [Google Scholar] [CrossRef]
- Loczenski Rose, V.; Mastrotto, F.; Mantovani, G. Phosphonium polymers for gene delivery. Polym. Chem. 2017, 8, 353–360. [Google Scholar] [CrossRef]
- Ornelas-Megiatto, C.; Wich, P.R.; Fréchet, J.M.J. Polyphosphonium Polymers for siRNA Delivery: An Efficient and Nontoxic Alternative to Polyammonium Carriers. J. Am. Chem. Soc. 2012, 134, 1902–1905. [Google Scholar] [CrossRef]
- Hemp, S.T.; Allen, M.H.; Green, M.D.; Long, T.E. Phosphonium-Containing Polyelectrolytes for Nonviral Gene Delivery. Biomacromolecules 2012, 13, 231–238. [Google Scholar] [CrossRef]
- Clement, S.; Tizit, A.; Desbief, S.; Mehdi, A.; De Winter, J.; Gerbaux, P.; Lazzaroni, R.; Boury, B. Synthesis and characterisation of [small pi]-conjugated polymer/silica hybrids containing regioregular ionic polythiophenes. J. Mater. Chem. 2011, 21, 2733–2739. [Google Scholar] [CrossRef]
- Gary-Bobo, M.; Mir, Y.; Rouxel, C.; Brevet, D.; Basile, I.; Maynadier, M.; Vaillant, O.; Mongin, O.; Blanchard-Desce, M.; Morère, A.; et al. Mannose-Functionalized Mesoporous Silica Nanoparticles for Efficient Two-Photon Photodynamic Therapy of Solid Tumors. Angew. Chem. Int. Ed. 2011, 50, 11425–11429. [Google Scholar] [CrossRef]
- Marrocchi, A.; Lanari, D.; Facchetti, A.; Vaccaro, L. Poly (3-hexylthiophene): Synthetic methodologies and properties in bulk heterojunction solar cells. Energy Environ. Sci. 2012, 5, 8457–8474. [Google Scholar] [CrossRef]
- Brouwer, A. Standards for photoluminescence quantum yield measurements in solution (IUPAC Technical Report) *. Pure Appl. Chem. 2011, 83, 2213–2228. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Rodgers, M.A.J. Energy and Electron Transfer Reactions of the MLCT State of Ruthenium Tris (bipyridyl) with Molecular Oxygen: A Laser Flash Photolysis Study. J. Phys. Chem. 1995, 99, 12797–12803. [Google Scholar] [CrossRef]
- Trznadel, M.; Pron, A.; Zagorska, M.; Chrzaszcz, R.; Pielichowski, J. Effect of Molecular Weight on Spectroscopic and Spectroelectrochemical Properties of Regioregular Poly (3-hexylthiophene). Macromolecules 1998, 31, 5051–5058. [Google Scholar] [CrossRef] [PubMed]
- Gaylord, B.S.; Heeger, A.J.; Bazan, G.C. DNA Hybridization Detection with Water-Soluble Conjugated Polymers and Chromophore-Labeled Single-Stranded DNA. J. Am. Chem. Soc. 2003, 125, 896–900. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Pinto, M.R.; Schanze, K.S. Photophysics, aggregation and amplified quenching of a water-soluble poly (phenylene ethynylene). Chem. Commun. 2002, 446–447. [Google Scholar] [CrossRef]
- Zhao, X.; Pinto, M.R.; Hardison, L.M.; Mwaura, J.; Müller, J.; Jiang, H.; Witker, D.; Kleiman, V.D.; Reynolds, J.R.; Schanze, K.S. Variable Band Gap Poly (arylene ethynylene) Conjugated Polyelectrolytes. Macromolecules 2006, 39, 6355–6366. [Google Scholar] [CrossRef]
- Cook, S.; Furube, A.; Katoh, R. Analysis of the excited states of regioregular polythiophene P3HT. Energy Environ. Sci. 2008, 1, 294–299. [Google Scholar] [CrossRef]
- Kraabel, B.; Moses, D.; Heeger, A.J. Direct observation of the intersystem crossing in poly (3-octylthiophene). J. Chem. Phys. 1995, 103, 5102–5108. [Google Scholar] [CrossRef]
- Khatoon, S.S.; Chen, Y.; Zhao, H.; Lv, F.; Liu, L.; Wang, S. In situ self-assembly of conjugated polyelectrolytes for cancer targeted imaging and photodynamic therapy. Biomater. Sci. 2020, 8, 2156–2163. [Google Scholar] [CrossRef]
- Lovell, J.F.; Liu, T.W.B.; Chen, J.; Zheng, G. Activatable Photosensitizers for Imaging and Therapy. Chem. Rev. 2010, 110, 2839–2857. [Google Scholar] [CrossRef]
- Fernández, D.A.; Awruch, J.; Dicelio, L.E. Photophysical and Aggregation Studies of t-Butyl-Substituted Zn Phthalocyanines. Photochem. Photobiol. 1996, 63, 784–792. [Google Scholar] [CrossRef]
- Zhang, C.; Du, K.; Zhang, X.; Zheng, X.; Cao, G.; Zhang, F.; Feng, F. Optical properties of phosphonium-, quaternary ammonium- and imidazolium- substituted regioregular polythiophenes and application for imaging live cells. Dye. Pigm. 2019, 170, 107581. [Google Scholar] [CrossRef]
- Zhao, J.; Stenzel, M.H. Entry of nanoparticles into cells: The importance of nanoparticle properties. Polym. Chem. 2018, 9, 259–272. [Google Scholar] [CrossRef]
- Zhang, S.; Gao, H.; Bao, G. Physical Principles of Nanoparticle Cellular Endocytosis. ACS Nano 2015, 9, 8655–8671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, Z.-G.; Wei, W.; Lv, P.-P.; Yue, H.; Wang, L.-Y.; Su, Z.-G.; Ma, G.-H. Surface Charge Affects Cellular Uptake and Intracellular Trafficking of Chitosan-Based Nanoparticles. Biomacromolecules 2011, 12, 2440–2446. [Google Scholar] [CrossRef] [PubMed]
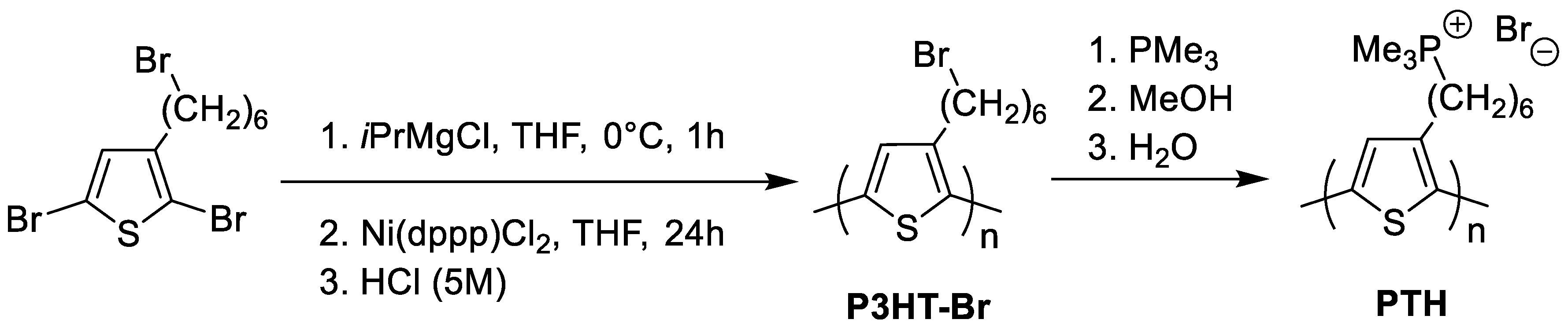

| Name | Yield (%) | Mn (g·mol−1) 1 | Ð | DP 2 |
|---|---|---|---|---|
| P3HT-Br1 | 55 | 40 600 | 1.16 | 165 |
| P3HT-Br2 | 72 | 14 500 | 1.28 | 59 |
| P3HT-Br3 | 62 | 8 500 | 1.31 | 36 |
| Compound | λabs max (nm) | λem max (nm) 1 | ΦF 2 | τF (ns) | ΦΔ |
|---|---|---|---|---|---|
| PTH1 | 495 | 624 | 0.01 | 0.5 | < 0.01 |
| PTH2 | 464 | 605 | 0.07 | 0.7 | 0.13 |
| PTH3 | 466 | 605 | 0.08 | 0.7 | 0.15 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lichon, L.; Kotras, C.; Myrzakhmetov, B.; Arnoux, P.; Daurat, M.; Nguyen, C.; Durand, D.; Bouchmella, K.; Ali, L.M.A.; Durand, J.-O.; et al. Polythiophenes with Cationic Phosphonium Groups as Vectors for Imaging, siRNA Delivery, and Photodynamic Therapy. Nanomaterials 2020, 10, 1432. https://doi.org/10.3390/nano10081432
Lichon L, Kotras C, Myrzakhmetov B, Arnoux P, Daurat M, Nguyen C, Durand D, Bouchmella K, Ali LMA, Durand J-O, et al. Polythiophenes with Cationic Phosphonium Groups as Vectors for Imaging, siRNA Delivery, and Photodynamic Therapy. Nanomaterials. 2020; 10(8):1432. https://doi.org/10.3390/nano10081432
Chicago/Turabian StyleLichon, Laure, Clément Kotras, Bauyrzhan Myrzakhmetov, Philippe Arnoux, Morgane Daurat, Christophe Nguyen, Denis Durand, Karim Bouchmella, Lamiaa Mohamed Ahmed Ali, Jean-Olivier Durand, and et al. 2020. "Polythiophenes with Cationic Phosphonium Groups as Vectors for Imaging, siRNA Delivery, and Photodynamic Therapy" Nanomaterials 10, no. 8: 1432. https://doi.org/10.3390/nano10081432
APA StyleLichon, L., Kotras, C., Myrzakhmetov, B., Arnoux, P., Daurat, M., Nguyen, C., Durand, D., Bouchmella, K., Ali, L. M. A., Durand, J.-O., Richeter, S., Frochot, C., Gary-Bobo, M., Surin, M., & Clément, S. (2020). Polythiophenes with Cationic Phosphonium Groups as Vectors for Imaging, siRNA Delivery, and Photodynamic Therapy. Nanomaterials, 10(8), 1432. https://doi.org/10.3390/nano10081432