Improved Charge Transfer Contribution by Cosputtering Ag and ZnO
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Preparation of the Ag/ZnO 2D Ordered Array
2.3. Characterization of the Ordered Arrays
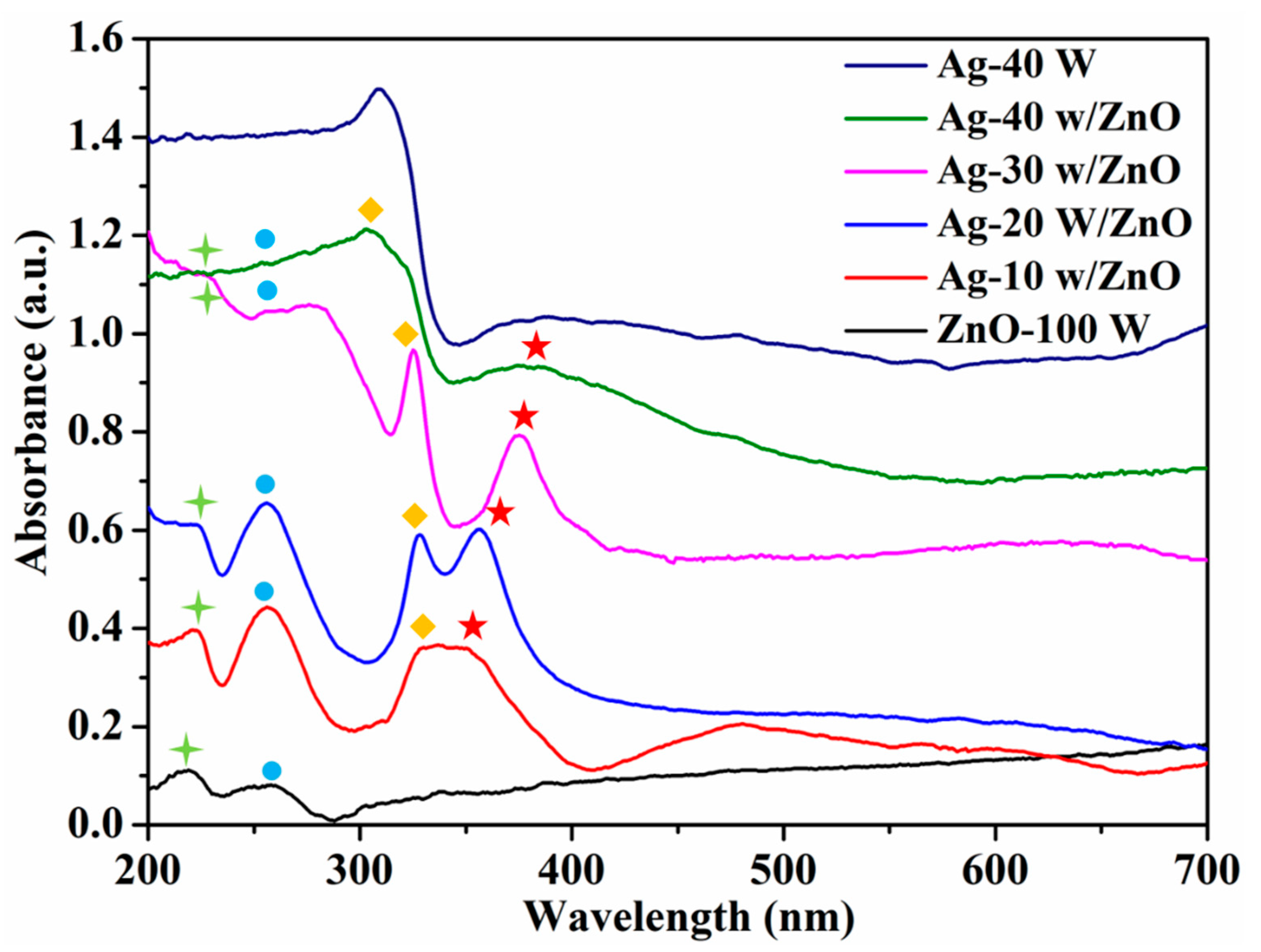
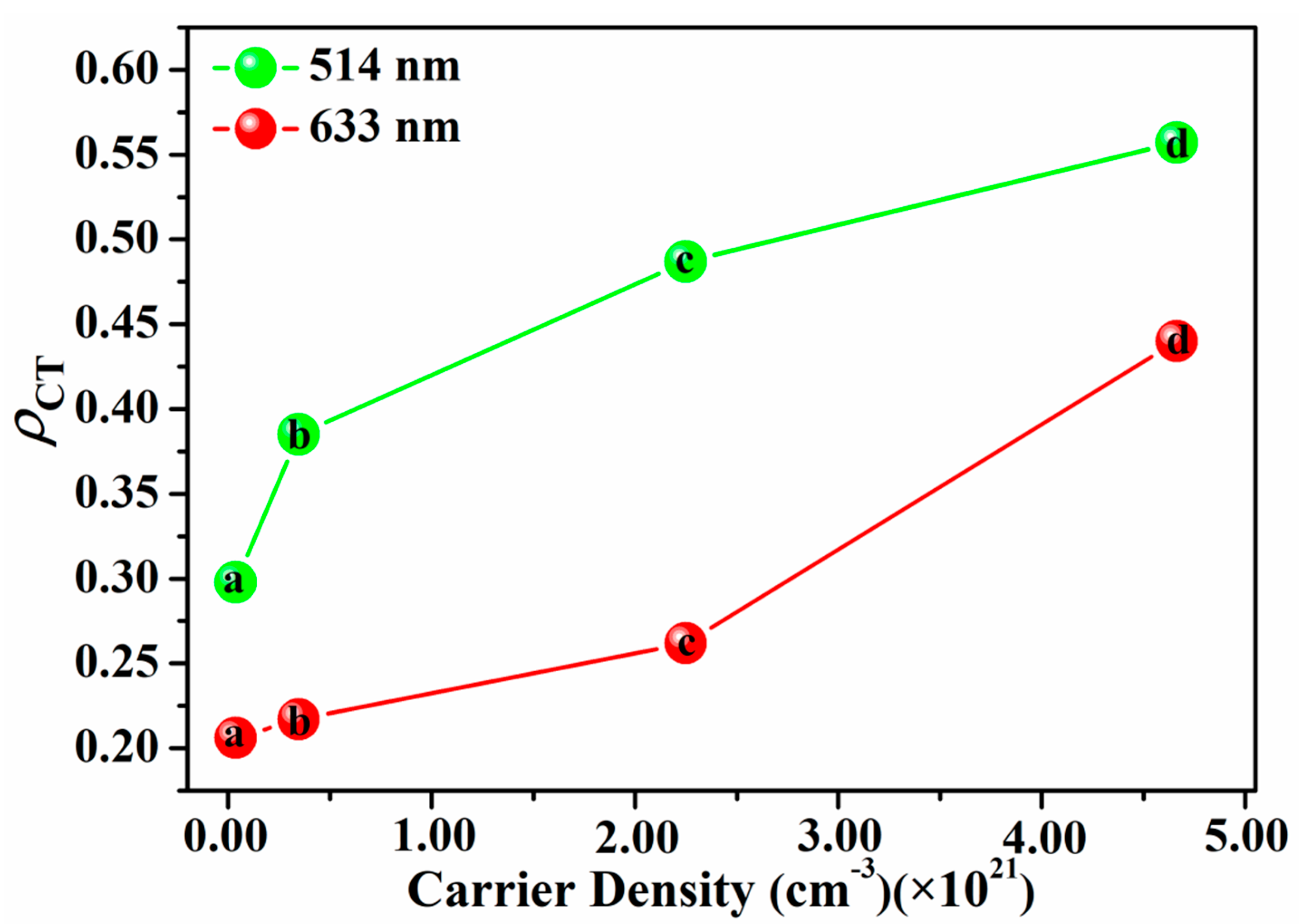
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lombardi, J.R.; Birke, R.L. A Unified View of Surface-Enhanced Raman Scattering. Acc. Chem. Res. 2009, 42, 734–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halas, N.J.; Moskovits, M. Surface-Enhanced Raman Spectroscopy: Substrates and Materials for Research and Applications. MRS Bull. 2013, 38, 607–611. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Huang, S.C.; Hu, S.; Yan, S.; Ren, B. Fundamental understanding and applications of plasmon-enhanced Raman spectroscopy. Nat. Rev. Phys. 2020, 2, 253–271. [Google Scholar] [CrossRef]
- Siebert, F.; Hildebrandt, P. Vibrational Spectroscopy in Life Science; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008. [Google Scholar]
- Aroca, R. Surface-Enhanced Vibrational Spectroscopy; John Wiley & Sons, Ltd.: Chichester, UK, 2006. [Google Scholar]
- Kneipp, K.; Ozaki, Y.; Tian, Z.Q. Recent Developments in Plasmon-Supported Raman Spectroscopy; World Scientific Publishing Europe Ltd.: London, UK, 2017. [Google Scholar]
- Ding, S.Y.; You, E.M.; Tian, Z.Q.; Moskovits, M. Electromagnetic Theories of Surface-enhanced Raman Spectroscopy. Chem. Soc. Rev. 2017, 46, 4042–4076. [Google Scholar] [CrossRef]
- Baffou, G.; Quidant, R. Nanoplasmonics for Chemistry. Chem. Soc. Rev. 2014, 43, 3898–3907. [Google Scholar] [CrossRef]
- Wu, D.Y.; Li, J.F.; Ren, B.; Tian, Z.Q. Electrochemical surface-enhanced Raman spectroscopy of nanostructures. Chem. Soc. Rev. 2008, 37, 1025–1041. [Google Scholar] [CrossRef] [Green Version]
- Aroca, R.F. Plasmon enhanced spectroscopy. Phys. Chem. Chem. Phys. 2013, 15, 5355–5363. [Google Scholar] [CrossRef]
- Albrecht, M.G.; Creighton, J.A. Anomalously Intense Raman Spectra of Pyridine at a Silver Electrode. J. Am. Chem. Soc. 1977, 99, 5215. [Google Scholar] [CrossRef]
- Nie, S.M.; Emory, S.R. Probing Single Molecules and Single Nanoparticles by Surface-Enhanced Raman Scattering. Science 1997, 275, 1102–1106. [Google Scholar] [CrossRef]
- Panneerselvam, R.; Liu, G.K.; Wang, Y.-H.; Liu, J.Y.; Ding, S.Y.; Li, J.F.; Wu, D.Y.; Tian, Z.Q. Surface-enhanced Raman spectroscopy: Bottlenecks and future directions. Chem. Commun. 2018, 54, 10–25. [Google Scholar] [CrossRef]
- Pérez-Jiménez, A.I.; Lyu, D.; Lu, Z.; Liu, G.; Ren, B. Surface-enhanced Raman spectroscopy: Benefits, trade-offs and future developments. Chem. Sci. 2020, 11, 4563–4577. [Google Scholar] [CrossRef] [Green Version]
- Yamada, H.; Yamamoto, Y.; Tani, N. Surface-enhanced raman scattering (SERS) of adsorbed molecules on smooth surfaces of metals and a metal oxide. Chem. Phys. Lett. 1982, 86, 397–400. [Google Scholar] [CrossRef]
- Yamada, H.; Yamamoto, Y. Surface enhanced Raman scattering (SERS) of chemisorbed species on various kinds of metals and semiconductors. Surf. Sci. 1983, 134, 71–90. [Google Scholar] [CrossRef]
- Han, X.X.; Ji, W.; Zhao, B.; Ozaki, Y. Semiconductor-enhanced Raman Scattering: Active Nanomaterials and Applications. Nanoscale 2017, 9, 4847–4861. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Zhao, B.; Ozaki, Y. Semiconductor Materials in Analytical Applications of Surface-enhanced Raman Scattering. J. Raman Spectrosc. 2016, 47, 51–58. [Google Scholar] [CrossRef]
- Wu, K.; Chen, J.; McBride, J.R. Efficient Hot-Electron Transfer by a Plasmoninduced Interfacial Charge-Transfer Transition. Science 2015, 349, 632–635. [Google Scholar] [CrossRef] [Green Version]
- Papa, L.; Freitas, I.C.D.; Geonmonond, R.S.; Aquino, C.B.D.; Pieretti, J.C.; Domingues, S.H.; Ando, R.A.; Camargo, P.H.C. Supports matter: Unraveling the role of charge transfer in the plasmonic catalytic activity of silver nanoparticles. J. Mater. Chem. A 2017, 5, 11720–11729. [Google Scholar] [CrossRef]
- Liu, W.; Bai, H.; Li, X.; Li, W.; Zhai, J.; Li, J.; Xi, G. Improved Surface-Enhanced Raman Spectroscopy Sensitivity on Metallic Tungsten Oxide by the Synergistic Effect of Surface Plasmon Resonance Coupling and Charge Transfer. J. Phys. Chem. Lett. 2018, 9, 4096–4100. [Google Scholar] [CrossRef]
- Ji, W.; Li, L.; Song, W.; Wang, X.; Zhao, B.; Ozaki, Y. Enhanced Raman Scattering by ZnO Superstructures: Synergistic Effect of Charge Transfer and Mie Resonances. Angew. Chem. Int. Ed. 2019, 58, 14552–14556. [Google Scholar]
- Zhang, X.Y.; Han, D.; Pang, Z.; Sun, Y.; Wang, Y.; Zhang, Y.; Chen, L. Charge Transfer in an Ordered Ag/Cu2S/4-MBA System Based on Surface-Enhanced Raman Scattering. J. Phys. Chem. C 2018, 122, 5599–5605. [Google Scholar] [CrossRef]
- Dey, P.; Baumann, V.; Rodríguez-Fernández, J. Gold Nanorod Assemblies: The Roles of Hot-Spot Positioning and Anisotropy in Plasmon Coupling and SERS. Nanomaterials 2020, 10, 942. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Chen, L.; Wang, Y.; Zhang, Y.; Yang, J.; Choi, H.C.; Young, M.J. Design of tunable ultraviolet (UV) absorbance by controlling the Ag-Al co-sputtering deposition. Spectrochim. Acta Part A 2017, 197, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, F.; Deng, X.Y.; Xue, X.; Wang, L.; Sun, Y.; Feng, J.D.; Zhang, Y.; Wang, Y.; Jung, Y.M. SERS study of surface plasmon resonance induced carrier movement in Au@Cu2O core-shell nanoparticles. Spectrochim. Acta Part A 2018, 189, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhu, L.; Ma, C.; Zhang, L.; Guo, L.; Liu, Y.; Ma, H.; Zhao, B. Plasmonic Molybdenum Tungsten Oxide Hybrid with Noble Metal-comparable Surface-Enhanced Raman Scattering. ACS Appl. Mater. Interfaces 2020, 12, 19153–19160. [Google Scholar] [CrossRef]
- Lombardi, J.R.; Birke, R.L. A unified approach to surface-enhanced Raman spectroscopy. J. Phys. Chem. C 2008, 112, 5605–5617. [Google Scholar] [CrossRef]
- Wang, X.; Li, P.; Han, X.X.; Kitahama, Y.; Zhao, B.; Ozaki, Y. An enhanced degree of charge transfer in dye-sensitized solar cells with a ZnO-TiO2/N3/Ag structure as revealed by surface-enhanced Raman scattering. Nanoscale 2017, 9, 15303–15313. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yu, Z.; Ji, W.; Sui, H.; Cong, Q.; Wang, X.; Zhao, B. Charge-Transfer Effect on Surface-Enhanced Raman Scattering (SERS) in an Ordered Ag NPs/4-Mercaptobenzoic Acid/TiO2 System. J. Phys. Chem. C 2015, 119, 22439–22444. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Han, D.; Ma, N.; Gao, R.; Zhu, A.; Guo, S.; Zhang, Y.; Wang, Y.; Yang, J.; Chen, L. Carrier Density-Dependent Localized Surface Plasmon Resonance and Charge Transfer Observed by Controllable Semiconductor Content. J. Phys. Chem. Lett. 2018, 9, 6047–6051. [Google Scholar] [CrossRef]


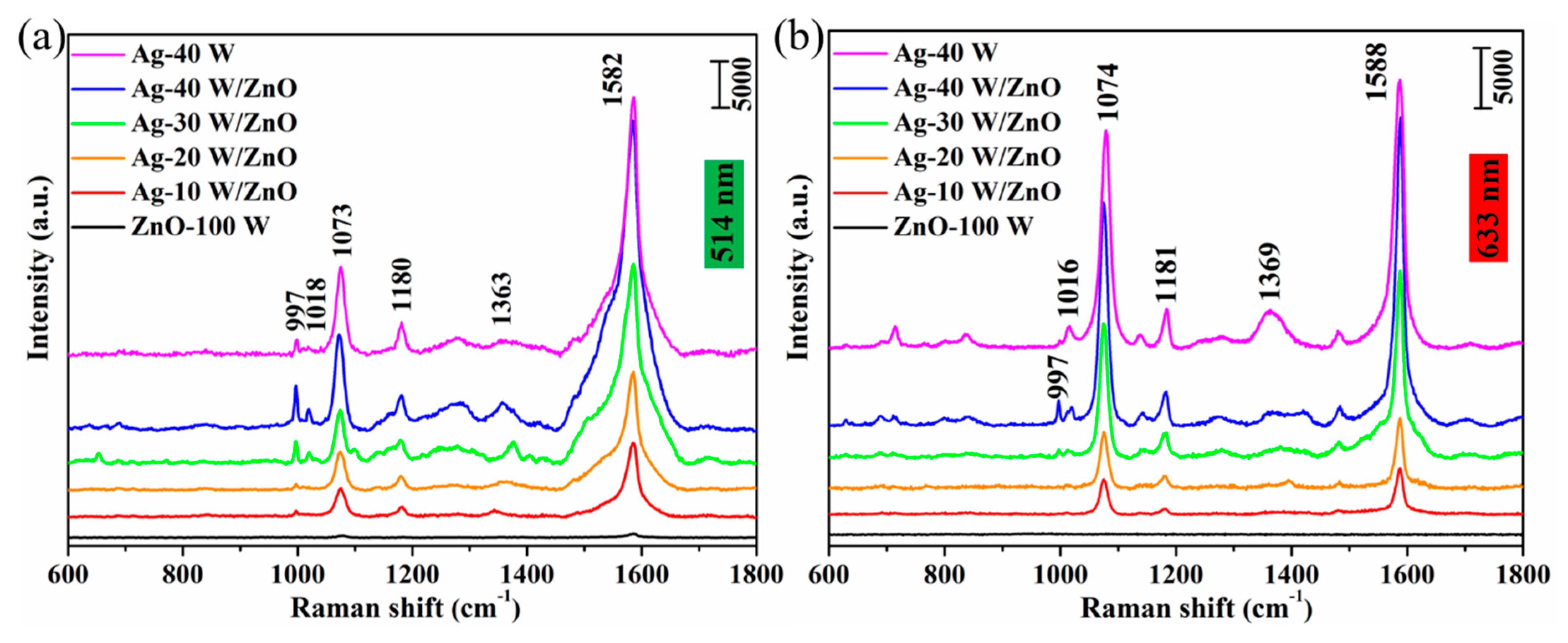

| Wavenumbers/cm−1 | Band Assignments | |
|---|---|---|
| 514 nm | 633 nm | |
| 997 | 997 | in-plane ring breathing, b2 |
| 1018 | 1016 | in-plane ring breathing, b2 |
| 1073 | 1074 | in-plane ring breathing + υ(CS), a1 |
| 1180 | 1181 | C-H deformation modes, a1 |
| 1363 | 1369 | β(OH) + υ(C-ph) + in-plane υ(CC) + asymmetric υ(CO2), b2 |
| 1582 | 1588 | totally symmetric υ(CC), a1 |
| Ag/ZnO | Carrier Density (cm−3) | Measurements Average | Hall Mobility (cm2V−1S−1) |
|---|---|---|---|
| ZnO-100 W | 4.0912 × 1015 4.5542 × 1015 2.1728 × 1015 9.8474 × 1015 | 5.1664 × 1015 | 1.6243 × 102 1.4591 × 102 3.0583 × 102 6.7481 × 101 |
| Ag-10 W/ZnO-100 W | 3.6921 × 1019 2.7065 × 1019 3.7385 × 1019 4.5268 × 1019 | 3.6659 × 1019 | 8.9640 × 100 1.2229 × 100 8.8529 × 100 7.3113 × 100 |
| Ag-20 W/ZnO-100 W | 2.2082 × 1020 4.4512 × 1020 9.4287 × 1020 4.7943 × 1020 | 3.4691 × 1020 | 9.3925 × 100 6.4190 × 100 2.7538 × 100 1.2458 × 101 |
| Ag-30 W/ZnO-100 W | 4.2259 × 1021 1.2989 × 1021 1.2541 × 1021 2.2139 × 1021 | 2.2482 × 1021 | 1.3310 × 100 4.3302 × 100 4.4847 × 100 2.5406 × 100 |
| Ag-40 W/ZnO-100 W | 4.1634 × 1021 2.2812 × 1021 5.7905 × 1021 6.4167 × 1021 | 4.6629 × 1021 | 4.6887 × 10−1 8.5573 × 10−1 3.3711 × 10−1 3.0422 × 10−1 |
| Ag-40 W | 6.1689 × 1022 4.7839 × 1022 3.0875 × 1022 1.1936 × 1022 | 3.8084 × 1022 | 3.8932 × 10−1 5.0203 × 10−2 7.7787 × 10−2 2.0122 × 10−1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, B.; Guo, S.; Jin, S.; Park, E.; Xue, X.; Chen, L.; Jung, Y.M. Improved Charge Transfer Contribution by Cosputtering Ag and ZnO. Nanomaterials 2020, 10, 1455. https://doi.org/10.3390/nano10081455
Han B, Guo S, Jin S, Park E, Xue X, Chen L, Jung YM. Improved Charge Transfer Contribution by Cosputtering Ag and ZnO. Nanomaterials. 2020; 10(8):1455. https://doi.org/10.3390/nano10081455
Chicago/Turabian StyleHan, Bingbing, Shuang Guo, Sila Jin, Eungyeong Park, Xiangxin Xue, Lei Chen, and Young Mee Jung. 2020. "Improved Charge Transfer Contribution by Cosputtering Ag and ZnO" Nanomaterials 10, no. 8: 1455. https://doi.org/10.3390/nano10081455






