Devices with Tuneable Resistance Switching Characteristics Based on a Multilayer Structure of Graphene Oxide and Egg Albumen
Abstract
:1. Introduction
2. Materials and Methods
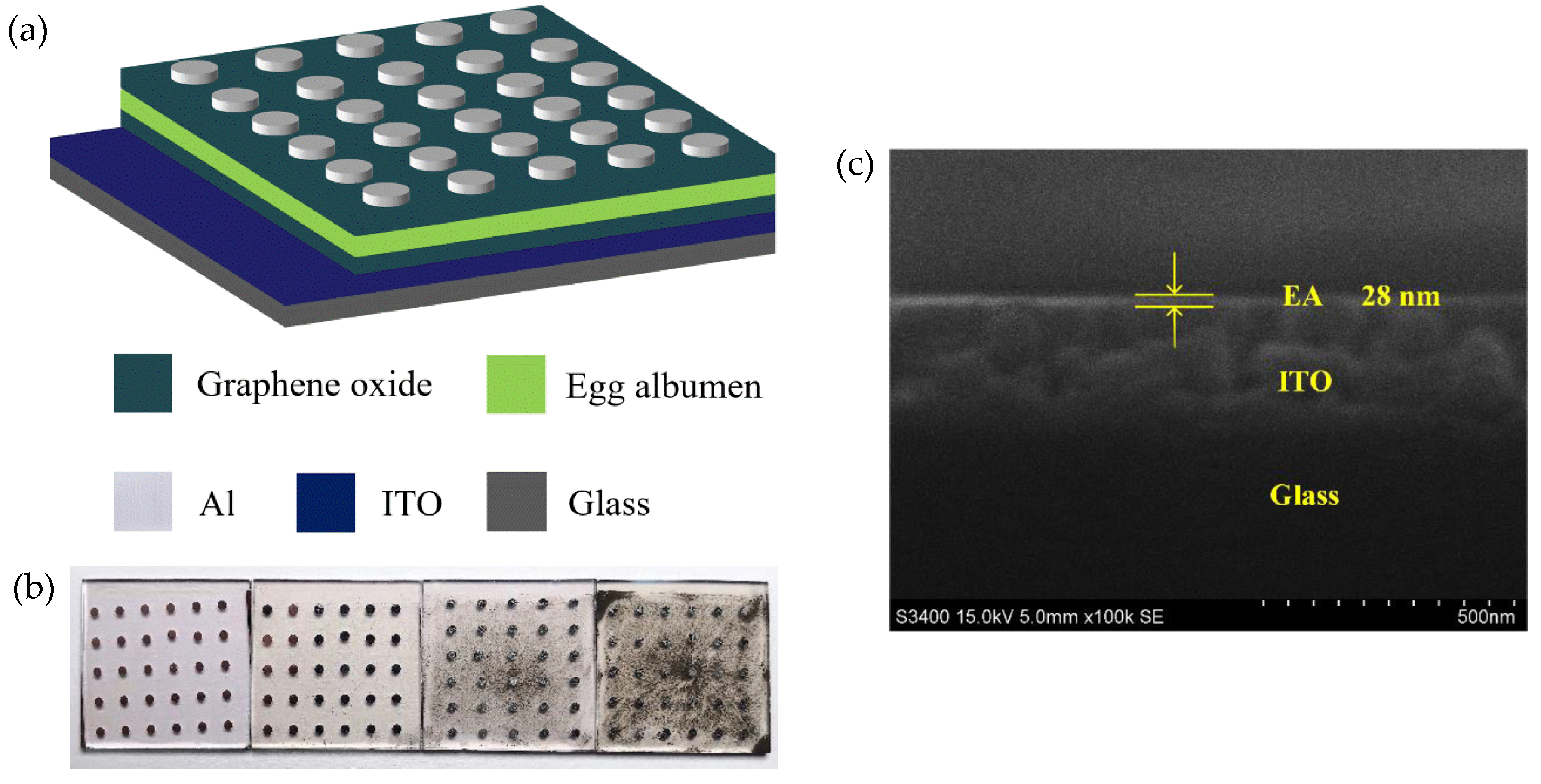
2.1. Materials and Device Fabrication
2.2. Characterization
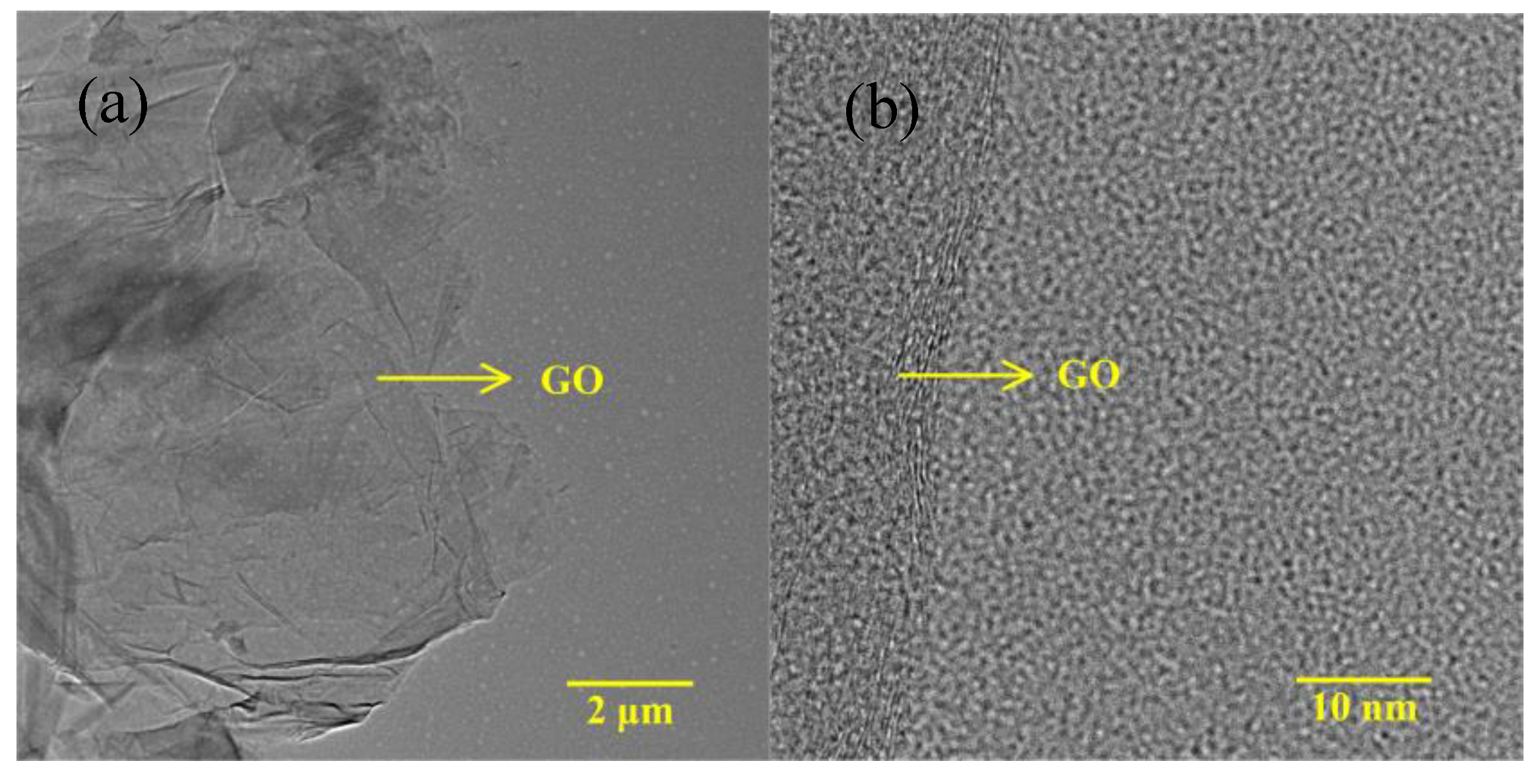
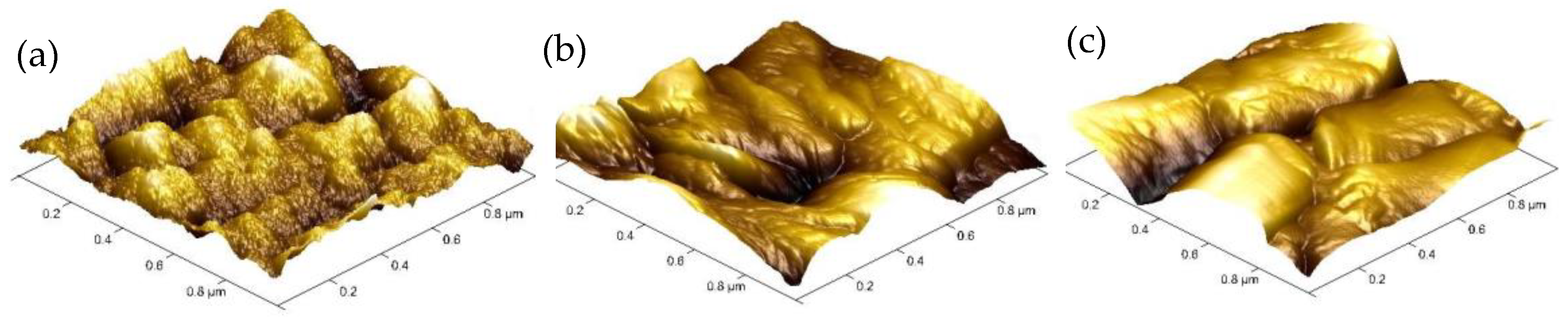
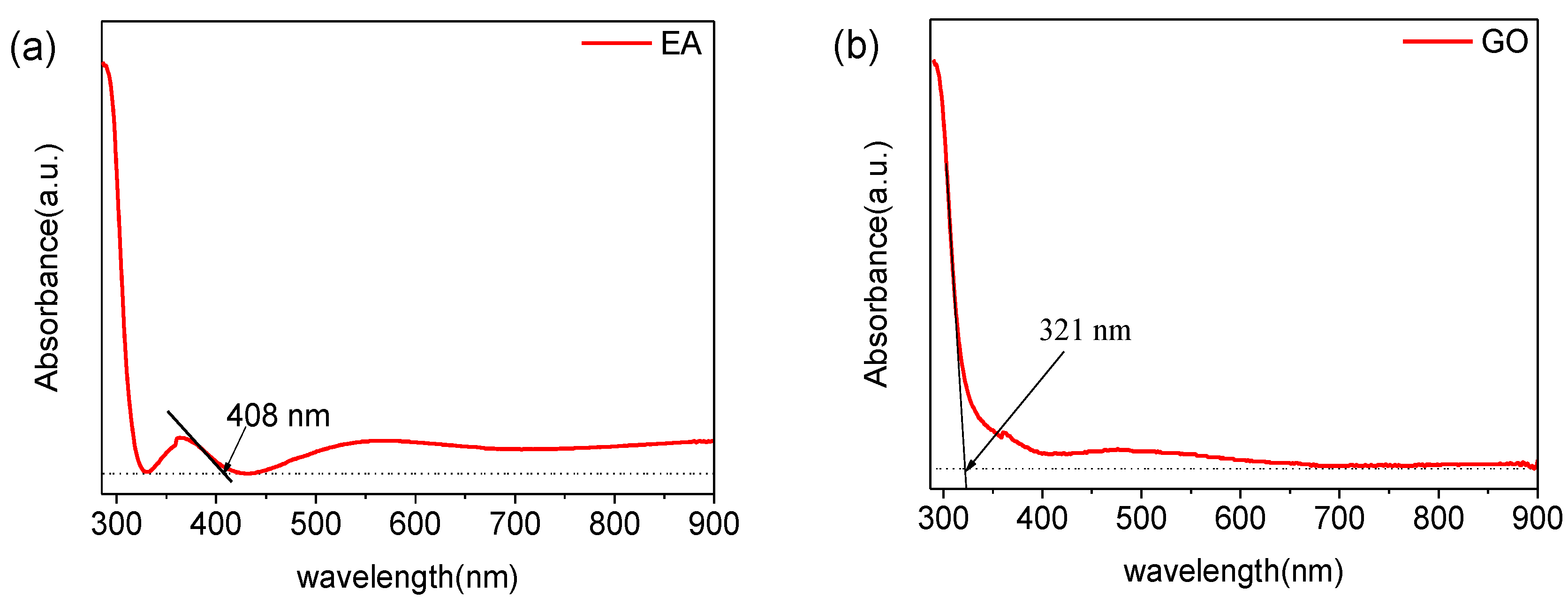
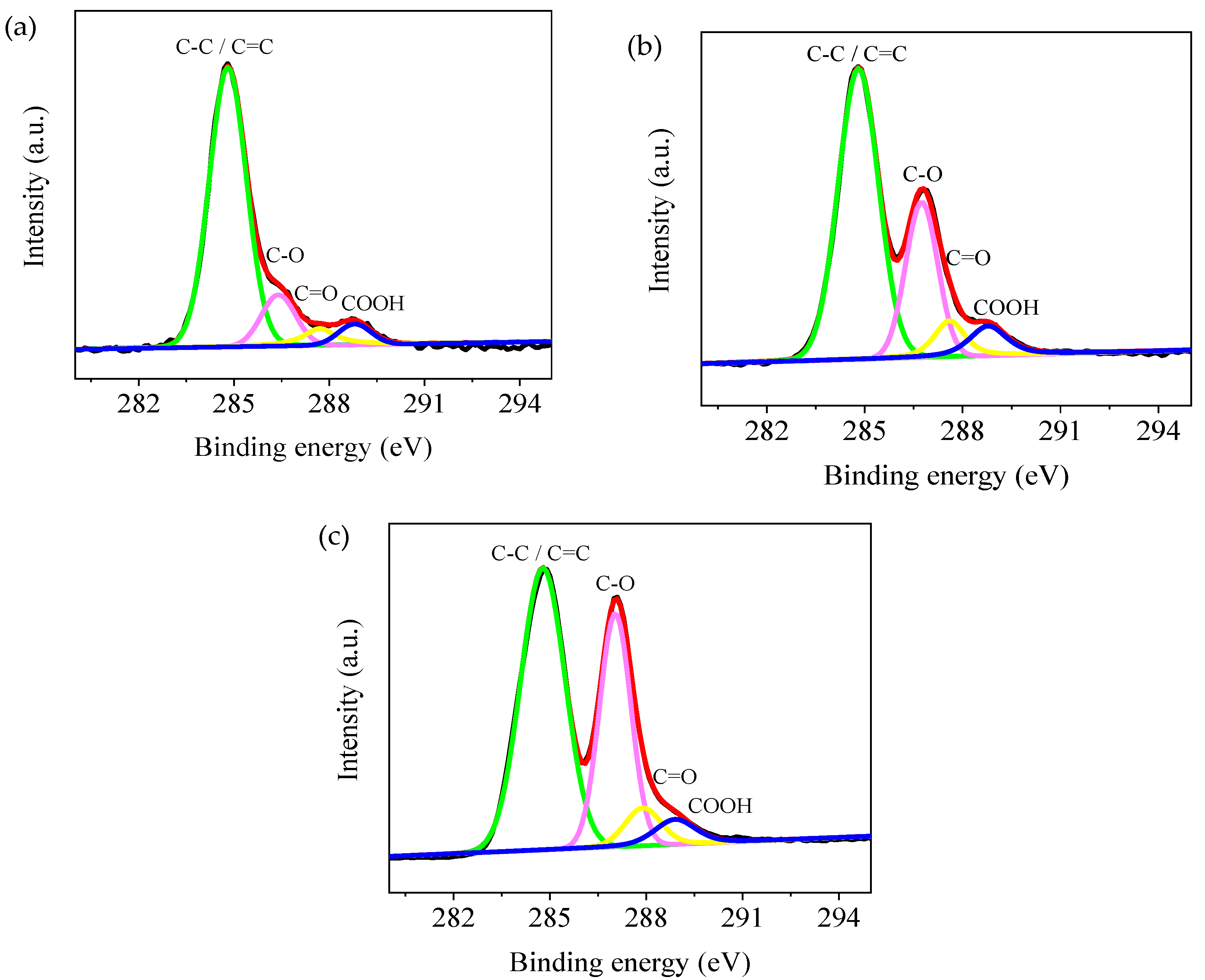
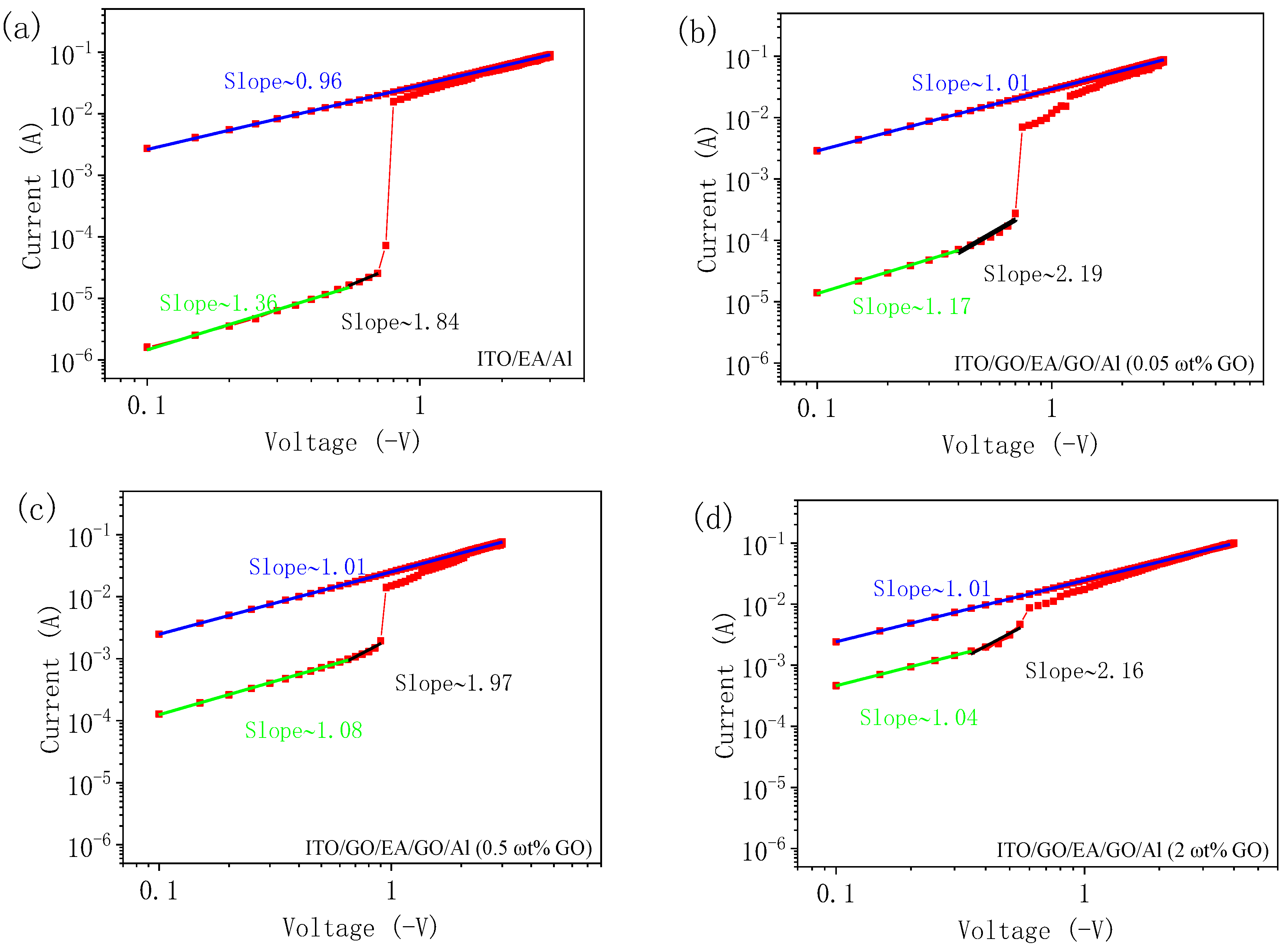
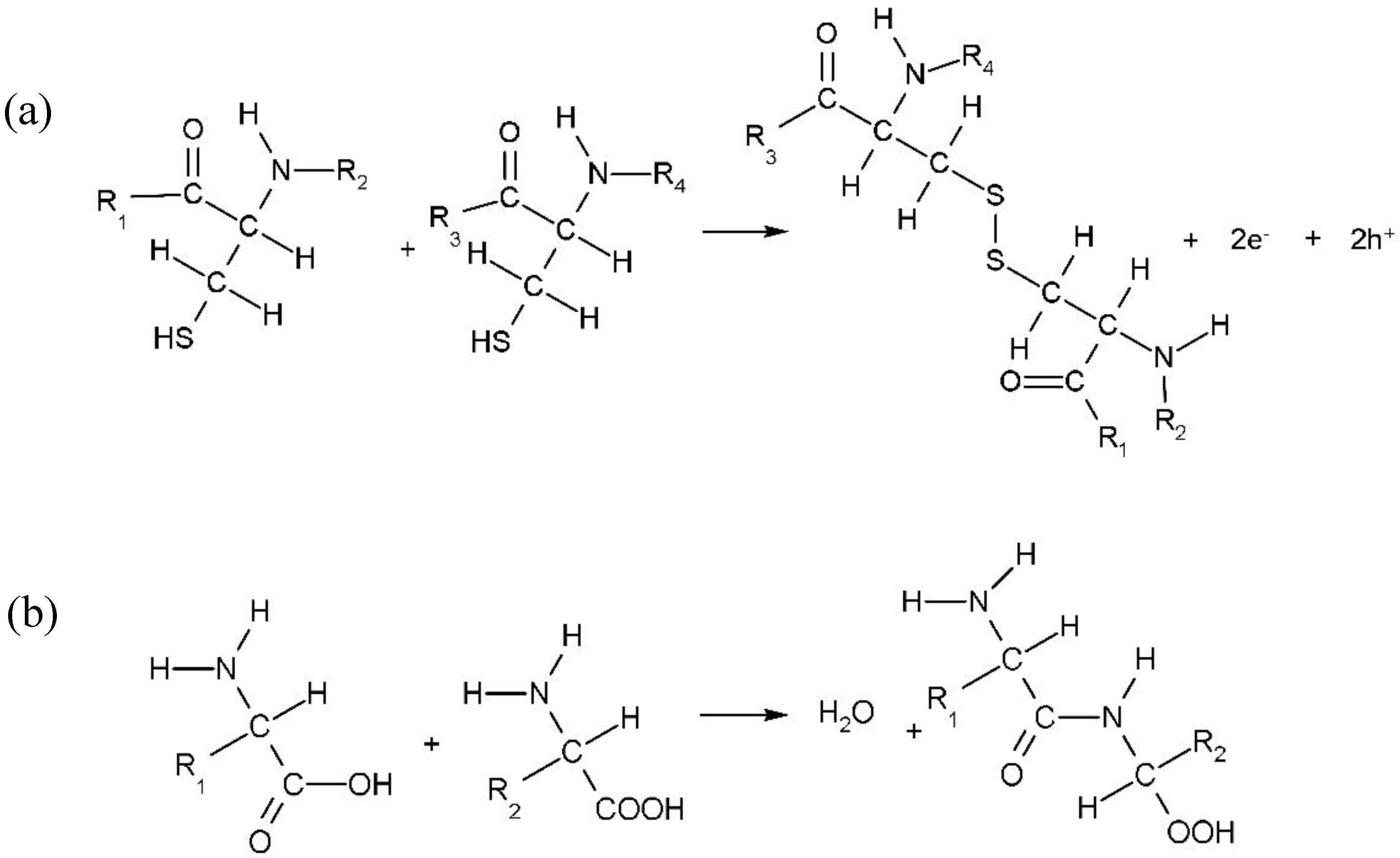
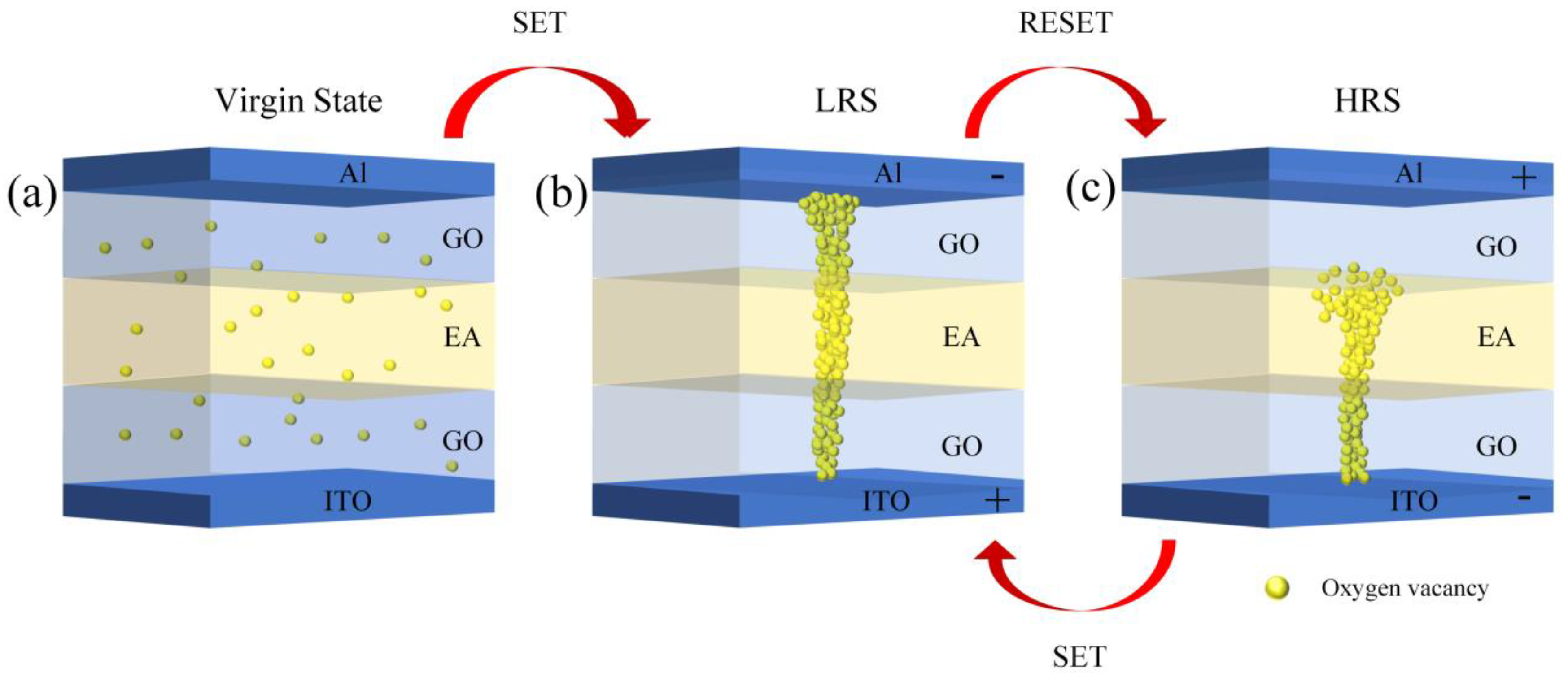
3. Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Raeis-Hosseini, N.; Lee, J.-S. Controlling the Resistive Switching Behavior in Starch-Based Flexible Biomemristors. ACS Appl. Mater. Interfaces 2016, 8, 7326–7332. [Google Scholar] [CrossRef] [PubMed]
- Mirkin, C.A.; Letsinger, R.L.; Mucic, R.C.; Storhoff, J.J. A DNA-based method for rationally assembling nanoparticles into macroscopic materials. Nature 1996, 382, 607–609. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Sun, B.; Fu, G.; Li, T.; Zhu, S.; Zheng, L. A nonvolatile organic resistive switching memory based on lotus leaves. Chem. Phys. 2019, 516, 168–174. [Google Scholar] [CrossRef]
- Sivkov, A.A.; Xing, Y.; Cheong, K.Y. Investigation of honey thin film as a resistive switching material for nonvolatile memories. Mater. Lett. 2020, 271, 127796. [Google Scholar] [CrossRef]
- Güzel, R.; Ocak, Y.S.; Karuk, S.N. Light harvesting and photo-induced electrochemical devices based on bionanocage proteins. J. Power Sour. 2019, 440, 227119. [Google Scholar]
- Yukimoto, T.; Uemura, S.; Kamata, T. Non-volatile transistor memory fabricated using DNA and eliminating influence of mobile ions on electric properties. J. Mater. Chem. 2011, 21, 15575–15579. [Google Scholar] [CrossRef]
- Chang, J.W.; Wang, C.G.; Huang, C.Y. Chicken Albumen Dielectrics in Organic Field-Effect Transistors. Adv. Mater. 2011, 23, 4077–4081. [Google Scholar] [CrossRef]
- Zhu, Z.; Tam, M.T.; Sun, F. A high-energy-density sugar biobattery based on a synthetic enzymatic pathway. Nat. Commun. 2014, 5, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Gomez, E.F.; Venkatraman, V.; Grote, J.G. DNA Bases Thymine and Adenine in Bio-Organic Light Emitting Diodes. Sci. Rep. 2014, 4, 7105. [Google Scholar]
- Zheng, L.; Sun, B.; Chen, Y. The redox of hydroxyl-assisted metallic filament induced resistive switching memory based on a biomaterial-constructed sustainable and environment-friendly device. Mater. Today Chem. 2018, 10, 167–174. [Google Scholar] [CrossRef]
- Zhou, G.; Sun, B.; Zhou, A. A larger nonvolatile bipolar resistive switching memory behaviour fabricated using eggshells. Curr. Appl. Phys. 2017, 17, 235–239. [Google Scholar] [CrossRef]
- Abbas, Y.; Dugasani, S.R.; Raza, M.T. The observation of resistive switching characteristics using transparent and biocompatible Cu2+-doped salmon DNA composite thin film. Nanotechnology 2019, 30, 335203. [Google Scholar] [CrossRef] [PubMed]
- Celano, U.; Nagashima, K.; Koga, H.; Nogi, M.; Zhuge, F.; Meng, G.; He, Y.; Boeck, J.D.; Jurczak, M.; Vandervorst, W.; et al. All-nanocellulose nonvolatile resistive memory. Npg Asia Mater. 2016, 8, e310. [Google Scholar] [CrossRef]
- Wang, H.; Meng, F.; Ca, Y.; Zheng, L.; Li, Y.; Liu, Y.; Jiang, Y.; Wang, X.; Chen, X. Sericin for resistance switching device with multilevel nonvolatile memory. Adv. Mater. 2013, 25, 5498–5503. [Google Scholar] [CrossRef]
- Mukherjee, C.; Hota, M.K.; Naskar, D. Resistive switching in natural silk fibroin protein-based bio-memristors. Physica Status Solidi (a) 2013, 210, 1797–1805. [Google Scholar] [CrossRef]
- Bok, C.H.; Woo, S.J.; Wu, C. Flexible bio-memristive devices based on chicken egg albumen:Au@SiO2 core-shell nanoparticle nanocomposites. Sci. Rep. 2017, 7, 12033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, Y.; Lee, J.-S. Artificial Synapses with Short- and Long-Term Memory for Spiking Neural Networks Based on Renewable Materials. Acs Nano 2017, 11, 8962–8969. [Google Scholar] [CrossRef] [PubMed]
- Raeis-Hosseini, N.; Park, Y.; Lee, J.-S. Flexible Artificial Synaptic Devices Based on Collagen from Fish Protein with Spike-Timing-Dependent Plasticity. Adv. Funct. Mater. 2018, 28, 1800553. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, W. Nanoscale resistive switching devices: Mechanisms and modeling. Nanoscale 2013, 5, 10076. [Google Scholar] [CrossRef]
- Celano, U.; Goux, L.; Degraeve, R.; Fantini, A.; Richard, O.; Bender, H.; Jurcza, M.; Vandervorst, W. Imaging the three-dimensional conductive channel in filamentary-based oxide resistive switching memory. Nano Lett. 2015, 15, 7970–7975. [Google Scholar] [CrossRef]
- Chandrakishore, S.; Pandurangan, A. Facile synthesis of carbon nanotubes and their use in the fabrication of resistive switching memory devices. Rsc Adv. 2014, 4, 9905–9911. [Google Scholar] [CrossRef]
- Avila-Nino, J.A.; Machado, W.S.; Sustaita, A.O. Organic low voltage rewritable memory device based on PEDOT:PSS/f-MWCNTs thin film. Org. Electron. 2012, 13, 2582–2588. [Google Scholar] [CrossRef]
- Chaoxing, W.; Li, F.; Zhang, Y. Highly reproducible memory effect of organic multilevel resistive-switch device utilizing graphene oxide sheets/polyimide hybrid nanocomposite. Appl. Phys. Lett. 2011, 99, 183. [Google Scholar]
- Murgunde, B.K.; Rabinal, M.K. Solution processed bilayer junction of silk fibroin and semiconductor quantum dots as multilevel memristor devices. Org. Electron. 2017, 48, 276–284. [Google Scholar] [CrossRef]
- Thakre, A.; Borkar, H.; Singh, B.P.; Kumar, A. Electroforming free high resistance resistive switching of graphene oxide modified polar-PVDF. Rsc Adv. 2015, 5, 57406–57413. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Chen, Y.; Ren, Y. In Situ Synthesis and Nonvolatile Rewritable-Memory Effect of Polyaniline-Functionalized Graphene Oxide. Chemistry 2013, 19, 6265–6273. [Google Scholar] [CrossRef]
- Yang, P.; Ma, X.; Ni, X. Nonvolatile resistance switching memory devices fabricated from the photopolymerized poly(N-vinylcarbazole)-graphene oxide composites. J. Mater. Sci. Mater. Electron. 2016, 28, 3695–3702. [Google Scholar] [CrossRef]
- Choi, J.-Y.; Yu, H.-C.; Lee, J. Preparation of Polyimide/Graphene Oxide Nanocomposite and Its Application to Nonvolatile Resistive Memory Device. Polymers 2018, 10, 901. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.; Kim, D.-K.; Kim, J. Resistive switching behaviour of multi- stacked PVA/graphene oxide + PVA composite/PVA insulating layer-based RRAM devices. Semicond. Sci. Technol. 2019, 34, 065006. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, L.; Wang, L. Covalent Modification of Graphene Oxide with Poly(N-vinylcarbazole) Containing Pendant Azobenzene Chromophores for Nonvolatile Ternary memories. Carbon 2018, 134, 500–506. [Google Scholar] [CrossRef]
- Jesuraj, P.J.; Parameshwari, R.; Jeganathan, K. Improved performance of graphene oxide based resistive memory devices through hydrogen plasma. Mater. Lett. 2018, 232, 62–65. [Google Scholar] [CrossRef]
- Khurana, G.; Misra, P.; Katiyar, R.S. Forming free resistive switching in graphene oxide thin film for thermally stable nonvolatile memory applications. J. Appl. Phys. 2013, 114, 124508. [Google Scholar] [CrossRef]
- Jeong, H.Y.; Kim, J.Y.; Kim, J.W. Graphene Oxide Thin Films for Flexible Nonvolatile Memory Applications. Nano Lett. 2010, 10, 4381–4386. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Siddik, M.; Shin, J. Low temperature solution-processed graphene oxide/Pr0.7Ca0.3MnO3 based resistive-memory device. Appl. Phys. Lett. 2011, 99, 042101. [Google Scholar] [CrossRef]
- Khurana, G.; Misra, P.; Kumar, N. Tunable Power Switching in Nonvolatile Flexible Memory Devices Based on Graphene Oxide Embedded with ZnO Nanorods. J. Phys. Chem. C 2014, 118, 21357–21364. [Google Scholar] [CrossRef]
- Pradhan, S.K.; Xiao, B.; Mishra, S.; Killam, A.; Pradhan, A.K. Resistive switching behavior of reduced graphene oxide memory cells for low power nonvolatile device application. Sci. Rep. 2016, 6, 26763. [Google Scholar] [CrossRef]
- Sharma, S.K.; Prakash, J.; Pujari, P.K. Effects of the molecular level dispersion of graphene oxide on the free volume characteristics of poly (vinyl alcohol) and its impact on the thermal and mechanical properties of their nanocomposites. Phys. Chem. Chem. Phys. 2015, 17, 29201–29209. [Google Scholar] [CrossRef]
- Zhu, C.; Mahmood, Z.; Zhang, W.; Akram, M.W.; Ainur, D.; Ma, H. In situ investigation of acute exposure of graphene oxide on activated sludge: Biofilm characteristics, microbial activity and cytotoxicity. Ecotoxicol. Environ. Saf. 2020, 199, 110639. [Google Scholar] [CrossRef]
- Banerjee, I.; Harris, P.; Salimian, A. Graphene oxide thin films for resistive memory switches. Let Circuits Devices Syst. 2015, 9, 428–433. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Li, G. Multi-Bit biomemory based on chitosan: Graphene oxide nanocomposite with wrinkled surface. Micromachines 2020, 11, 580. [Google Scholar] [CrossRef]
- Alves, A.K.; Frantz, A.C.S.; Berutti, F.A. Microwave-assisted oleothermal synthesis of graphene-TiO2 quantum dots for photoelectrochemical oxygen evolution reaction. Flatchem 2018, 12, 26–34. [Google Scholar] [CrossRef]
- Soliva-Fortuny, R.; Balasa, A.; Knorr, D. Effects of pulsed electric fields on bioactive compounds in foods: A review. Trends Food Sci. Technol. 2009, 20, 544–556. [Google Scholar] [CrossRef]
- He, X.L.; Zhang, J.; Wang, W.B.; Xuan, W.P.; Wang, X.Z.; Zhang, Q.L.; Smith, C.G.; Luo, J.K. Transient resistive switching devices made from egg albumen dielectrics and dissolvable electrodes. Acs Appl. Mater. Interfaces 2016, 8, 10954–10960. [Google Scholar] [CrossRef]








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Wang, J.; Wen, D. Devices with Tuneable Resistance Switching Characteristics Based on a Multilayer Structure of Graphene Oxide and Egg Albumen. Nanomaterials 2020, 10, 1491. https://doi.org/10.3390/nano10081491
Wang L, Wang J, Wen D. Devices with Tuneable Resistance Switching Characteristics Based on a Multilayer Structure of Graphene Oxide and Egg Albumen. Nanomaterials. 2020; 10(8):1491. https://doi.org/10.3390/nano10081491
Chicago/Turabian StyleWang, Lu, Jinyi Wang, and Dianzhong Wen. 2020. "Devices with Tuneable Resistance Switching Characteristics Based on a Multilayer Structure of Graphene Oxide and Egg Albumen" Nanomaterials 10, no. 8: 1491. https://doi.org/10.3390/nano10081491




