Analysis of the Characteristics and Cytotoxicity of Titanium Dioxide Nanomaterials Following Simulated In Vitro Digestion
Abstract
1. Introduction
2. Materials and Methods
2.1. Nanomaterials Properties and Dispersion for Biological Assays
2.2. Simulation of In Vitro Digestion
2.2.1. Chemicals and Reagents
2.2.2. In Vitro Digestion Procedure
2.3. Effect of the In Vitro Simulated Digestion on the NMs Properties
2.4. Cell Culture and Cytotoxicity Assays
2.5. Statistical Analysis
3. Results and Discussion
3.1. Hydrodynamic Size Distribution and Surface Charge
3.2. TEM-EDS Analysis
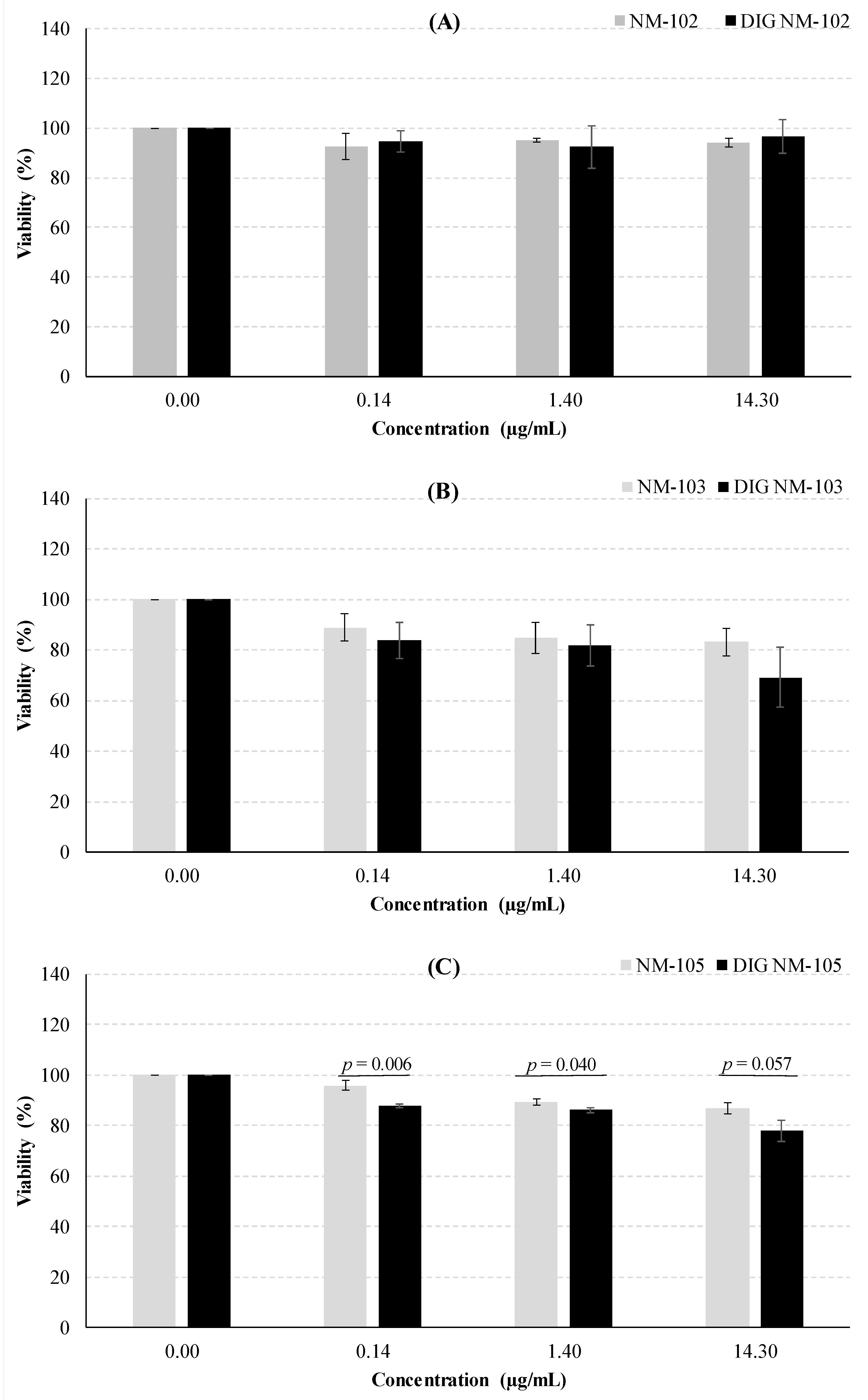
3.3. Cytotoxicity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Peters, R.; Weigel, S.; Marvin, H.; Bouwmeester, H.; Aschberger, K.; Rauscher, H.; Amenta, V.; Arena, M.; Botelho Moniz, F.; Gottardo, S.; et al. Inventory of Nanotechnology Applications in the Agricultural, Feed and Food Sector; EFSA Supporting Publication: Parma, Italy, 2014; pp. 1–125. Available online: www.efsa.europa.eu/publications (accessed on 10 December 2019).
- Kumar, S.; Bhanjana, G.; Sharma, A.; Dilbaghi, N.; Sidhu, M.C.; Kim, K.-H. Development of nanoformulation approaches for the control of weeds. Sci. Total Environ. 2017, 586, 1272–1278. [Google Scholar] [CrossRef] [PubMed]
- Gómez, H.C.; Serpa, A.; Velásquez-Cock, J.; Gañán, P.; Castro, C.; Vélez, L.; Zuluaga, R. Vegetable nanocellulose in food science: A review. Food Hydrocoll. 2016, 57, 178–186. [Google Scholar] [CrossRef]
- Mercier-Bonin, M.; Despax, B.; Raynaud, P.; Houdeau, E.; Thomas, M. Mucus and microbiota as emerging players in gut nanotoxicology: The example of dietary silver and titanium dioxide nanoparticles. Crit. Rev. Food Sci. Nutr. 2018, 58, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Magaye, R.; Castranova, V.; Zhao, J. Titanium dioxide nanoparticles: A review of current toxicological data. Part. Fibre Toxicol. 2013, 10–15. [Google Scholar] [CrossRef]
- Bettini, S.; Boutet-Robinet, E.; Cartier, C.; Coméra, C.; Gaultier, E.; Dupuy, J.; Naud, N.; Taché, S.; Grysan, P.; Reguer, S.; et al. Food-grade TiO2 impairs intestinal and systemic immune homeostasis, initiates preneoplastic lesions and promotes aberrant crypt development in the rat colon. Sci. Rep. 2017, 7, 40373. [Google Scholar] [CrossRef]
- Pele, L.C.; Thoree, V.; Bruggraber, S.F.A.; Koller, D.; Thompson, R.P.H.; Lomer, M.C.; Powell, J.J. Pharmaceutical/food grade titanium dioxide particles are absorbed into the bloodstream of human volunteers. Part. Fibre Toxicol. 2015, 12. [Google Scholar] [CrossRef]
- Böckmann, J.; Lahl, H.; Eckert, T.; Unterhalt, B. Titan-Blutspiegel vor und nach Belastungsversuchen mit Titandioxid\r[Blood titanium levels before and after oral administration titanium dioxide]\r. Pharmazie 2000, 55, 140–143. Available online: http://www.umweltbundesamt.de (accessed on 10 December 2019).
- Heringa, M.B.; Peters, R.J.B.; Bleys, R.L.A.W.; Van der Lee, M.K.; Tromp, P.C.; Van Kesteren, P.C.E.; Van Eijkeren, J.C.H.; Undas, A.K.; Oomen, A.G.; Bouwmeester, H. Detection of titanium particles in human liver and spleen and possible health implications. Part. Fibre Toxicol. 2018, 15, 15. [Google Scholar] [CrossRef]
- EFSA. Re-evaluation of titanium dioxide (E 171) as a food additive. EFSA J. 2016. [Google Scholar] [CrossRef]
- EFSA. EFSA statement on the review of the risks related to the exposure to the food additive titanium dioxide (E 171) performed by the French Agency for Food, Environmental and Occupational Health and Safety (ANSES). EFSA J. 2019, 17. [Google Scholar] [CrossRef]
- Anses. AVIS de l’Agence Nationale de Sécurité Sanitaire de L’alimentation, de L’environnement et du Travail Relatif Aux Risques Liés à L’ingestion de L’additif Alimentaire E171; ANSES: Maisons-Alfort, France, 2019.
- Louro, H.; Saruga, A.; Santos, J.; Pinhão, M.; Silva, M.J. Biological impact of metal nanomaterials in relation to their physicochemical characteristics. Toxicol. Vitr. 2019, 56, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Tavares, A.M.; Louro, H.; Antunes, S.; Quarré, S.; Simar, S.; De Temmerman, P.J.; Verleysen, E.; Mast, J.; Jensen, K.A.; Norppa, H.; et al. Genotoxicity evaluation of nanosized titanium dioxide, synthetic amorphous silica and multi-walled carbon nanotubes in human lymphocytes. Toxicol. Vitr. 2014, 28, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Louro, H. Relevance of physicochemical characterization of nanomaterials for understanding nano-cellular interactions. Adv. Exp. Med. Biol. 2018, 1048, 123–142. [Google Scholar] [CrossRef]
- Sohal, I.S.; O’Fallon, K.S.; Gaines, P.; Demokritou, P.; Bello, D. Ingested engineered nanomaterials: State of science in nanotoxicity testing and future research needs. Part. Fibre Toxicol. 2018, 15, 29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, R.; Xiao, H.; Bhattacharya, K.; Bitounis, D.; Demokritou, P.; McClements, D.J. Development of a standardized food model for studying the impact of food matrix effects on the gastrointestinal fate and toxicity of ingested nanomaterials. NanoImpact 2019, 13, 13–25. [Google Scholar] [CrossRef] [PubMed]
- DeLoid, G.M.; Wang, Y.; Kapronezai, K.; Lorente, L.R.; Zhang, R.; Pyrgiotakis, G.; Konduru, N.V.; Ericsson, M.; White, J.C.; De La Torre-Roche, R.; et al. An integrated methodology for assessing the impact of food matrix and gastrointestinal effects on the biokinetics and cellular toxicity of ingested engineered nanomaterials. Part. Fibre Toxicol. 2017, 14, 40. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food-an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Egger, L.; Ménard, O.; Delgado-Andrade, C.; Alvito, P.; Assunção, R.; Balance, S.; Barberá, R.; Brodkorb, A.; Cattenoz, T.; Clemente, A.; et al. The harmonized INFOGEST in vitro digestion method: From knowledge to action. Food Res. Int. 2016, 88, 217–225. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019. [Google Scholar] [CrossRef]
- Improving Health Properties of Food by Sharing our Knowledge on the Digestive Process - Home Page. Available online: https://www.cost-infogest.eu/ (accessed on 10 December 2019).
- Tan, Y.; Li, R.; Zhou, H.; Liu, J.; Muriel Mundo, J.; Zhang, R.; McClements, D.J. Impact of calcium levels on lipid digestion and nutraceutical bioaccessibility in nanoemulsion delivery systems studied using standardized INFOGEST digestion protocol. Food Funct. 2020, 11, 174–186. [Google Scholar] [CrossRef]
- Dorier, M.; Tisseyre, C.; Dussert, F.; Béal, D.; Arnal, M.E.; Douki, T.; Valdiglesias, V.; Laffon, B.; Fraga, S.; Brandão, F.; et al. Toxicological impact of acute exposure to E171 food additive and TiO2 nanoparticles on a co-culture of Caco-2 and HT29-MTX intestinal cells. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2019, 845. [Google Scholar] [CrossRef] [PubMed]
- Jalili, P.; Gueniche, N.; Lanceleur, R.; Burel, A.; Lavault, M.-T.; Sieg, H.; Böhmert, L.; Meyer, T.; Krause, B.-C.; Lampen, A.; et al. Investigation of the In Vitro genotoxicity of two rutile TiO2 nanomaterials in human intestinal and hepatic cells and evaluation of their interference with toxicity assays. NanoImpact 2018, 11, 69–81. [Google Scholar] [CrossRef]
- Sohal, I.S.; Cho, Y.K.; O’Fallon, K.S.; Gaines, P.; Demokritou, P.; Bello, D. Dissolution Behavior and Biodurability of Ingested Engineered Nanomaterials in the Gastrointestinal Environment. ACS Nano 2018, 12, 8115–8128. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Zhang, T.; De Loid, G.M.; Gaffrey, M.J.; Weitz, K.K.; Thrall, B.D.; Qian, W.J.; Demokritou, P. Evaluation of the cytotoxic and cellular proteome impacts of food-grade TiO2 (E171) using simulated gastrointestinal digestions and a tri-culture small intestinal epithelial model. NanoImpact 2020, 17. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, K.; Mast, J.; De Temmerman, P.; Verleysen, E.; Waegeneers, N.; Van Steen, F.; Pizzolon, J.C.; De Temmerman, L.; Jensen, K.A.; Birkedal, R.; et al. Titanium Dioxide, NM-100, NM-101, NM-102, NM-103, NM-104, NM-105: Characterisation and Physico—Chemical Properties. In Science and Policy Report by the Joint Research Centre of the European Commission; Publications Office of the European Union: Luxembourg, 2014. [Google Scholar] [CrossRef]
- Jensen, K.A.; Kembouche, Y.; Christiansen, E.; Jacobsen, N.R.; Wallin, H.; Guiot, C.; Spalla, O.; Witschger, O. Final Protocol for Producing Suitable Manufactured Nanomaterial Exposure Media. 2011. Available online: anses.fr/en/system/files/nanogenotox_deliverable_5.pdf (accessed on 15 January 2019).
- Richter, J.W.; Shull, G.M.; Fountain, J.H.; Guo, Z.; Musselman, L.P.; Fiumera, A.C.; Mahler, G.J. Titanium dioxide nanoparticle exposure alters metabolic homeostasis in a cell culture model of the intestinal epithelium and Drosophila melanogaster. Nanotoxicology 2018. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Martucci, N.J.; Moreno-Olivas, F.; Tako, E.; Mahler, G.J. Titanium dioxide nanoparticle ingestion alters nutrient absorption in an in vitro model of the small intestine. NanoImpact 2017, 5, 70–82. [Google Scholar] [CrossRef] [PubMed]
- DeLoid, G.M.; Cohen, J.M.; Pyrgiotakis, G.; Demokritou, P. An integrated dispersion preparation, characterization and in vitro dosimetry methodology for engineered nanomaterials. Nat. Protoc. 2017, 12, 335–371. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential - What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Li, Q.; Li, T.; Liu, C.; De Loid, G.; Pyrgiotakis, G.; Demokritou, P.; Zhang, R.; Xiao, H.; McClements, D.J. Potential impact of inorganic nanoparticles on macronutrient digestion: Titanium dioxide nanoparticles slightly reduce lipid digestion under simulated gastrointestinal conditions. Nanotoxicology 2017. [Google Scholar] [CrossRef]
- Saruga, A.S.F. Cyto-and Genotoxicity Assessment of Manufactured Nanomaterials in the A549 Cell Line. Master’s Thesis, NOVA University of Lisbon, Lisbon, Portugal, 2016. Available online: http://hdl.handle.net/10362/19347 (accessed on 10 December 2019).
- Gerloff, K.; Fenoglio, I.; Carella, E.; Kolling, J.; Albrecht, C.; Boots, A.W.; Förster, I.; Schins, R.P.F. Distinctive toxicity of TiO2 rutile/anatase mixed phase nanoparticles on Caco-2 cells. Chem. Res. Toxicol. 2012. [Google Scholar] [CrossRef]
- Dorier, M.; Béal, D.; Marie-Desvergne, C.; Dubosson, M.; Barreau, F.; Houdeau, E.; Herlin-Boime, N.; Carriere, M. Continuous in vitro exposure of intestinal epithelial cells to E171 food additive causes oxidative stress, inducing oxidation of DNA bases but no endoplasmic reticulum stress. Nanotoxicology 2017. [Google Scholar] [CrossRef] [PubMed]
- McCracken, C.; Zane, A.; Knight, D.A.; Dutta, P.K.; Waldman, W.J. Minimal intestinal epithelial cell toxicity in response to short- and long-term food-relevant inorganic nanoparticle exposure. Chem. Res. Toxicol. 2013, 26, 1514–1525. [Google Scholar] [CrossRef] [PubMed]
- Ammendolia, M.G.; Iosi, F.; Maranghi, F.; Tassinari, R.; Cubadda, F.; Aureli, F.; Raggi, A.; Superti, F.; Mantovani, A.; De Berardis, B. Short-term oral exposure to low doses of nano-sized TiO2 and potential modulatory effects on intestinal cells. Food Chem. Toxicol. 2017, 102, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Kukia, N.R.; Rasmi, Y.; Abbasi, A.; Koshoridze, N.; Shirpoor, A.; Burjanadze, G.; Saboory, E. Bio-effects of TiO2 nanoparticles on human colorectal cancer and umbilical vein endothelial cell lines. Asian Pac. J. Cancer Prev. 2018, 19, 2821–2829. [Google Scholar] [CrossRef]
- Schneider, T.; Westermann, M.; Glei, M. In Vitro uptake and toxicity studies of metal nanoparticles and metal oxide nanoparticles in human HT29 cells. Arch. Toxicol. 2017, 91, 3517–3527. [Google Scholar] [CrossRef]
- Huet, G.; Kim, I.; De Bolos, C.; Lo-Guidice, J.M.; Moreau, O.; Hemon, B.; Richet, C.; Delannoy, P.; Real, F.X.; Degand, P. Characterization of mucins and proteoglycans synthesized by a mucin-secreting HT-29 cell subpopulation. J. Cell Sci. 1995, 108, 1275–1285. [Google Scholar]
- Gagnon, M.; Zihler Berner, A.; Chervet, N.; Chassard, C.; Lacroix, C. Comparison of the Caco-2, HT-29 and the mucus-secreting HT29-MTX intestinal cell models to investigate Salmonella adhesion and invasion. J. Microbiol. Methods 2013, 94, 274–279. [Google Scholar] [CrossRef]
- Song, B.; Zhou, T.; Yang, W.L.; Liu, J.; Shao, L.Q. Contribution of oxidative stress to TiO2 nanoparticle-induced toxicity. Environ. Toxicol. Pharmacol. 2016, 48, 130–140. [Google Scholar] [CrossRef]
- Li, W.; Jia, M.X.; Deng, J.; Wang, J.H.; Zuberi, Z.; Yang, S.; Ba, J.; Chen, Z. MicroRNA response and toxicity of potential pathways in human colon cancer cells exposed to titanium dioxide nanoparticles. Cancers 2020, 12, 1236. [Google Scholar] [CrossRef]
- Song, Z.M.; Chen, N.; Liu, J.H.; Tang, H.; Deng, X.; Xi, W.S.; Han, K.; Cao, A.; Liu, Y.; Wang, H. Biological effect of food additive titanium dioxide nanoparticles on intestine: An In Vitro study. J. Appl. Toxicol. 2015, 35, 1169–1178. [Google Scholar] [CrossRef]
- Gerloff, K.; Pereira, D.I.A.; Faria, N.; Boots, A.W.; Kolling, J.; Förster, I.; Albrecht, C.; Powell, J.J.; Schins, R.P.F. Influence of simulated gastrointestinal conditions on particle-induced cytotoxicity and interleukin-8 regulation in differentiated and undifferentiated Caco-2 cells. Nanotoxicology 2013, 7, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Brun, E.; Barreau, F.; Veronesi, G.; Fayard, B.; Sorieul, S.; Chanéac, C.; Carapito, C.; Rabilloud, T.; Mabondzo, A.; Herlin-Boime, N.; et al. Titanium dioxide nanoparticle impact and translocation through ex vivo, in vivo and in vitro gut epithelia. Part. Fibre Toxicol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Hansen, G.H.; Rasmussen, K.; Niels-Christiansen, L.L.; Danielsen, E.M. Endocytic trafficking from the small intestinal brush border probed with FM dye. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297. [Google Scholar] [CrossRef] [PubMed]
- Graça, D.; Louro, H.; Santos, J.; Dias, K.; Almeida, A.J.; Gonçalves, L.; Silva, M.J.; Bettencourt, A. Toxicity screening of a novel poly(methylmethacrylate)-Eudragit nanocarrier on L929 fibroblasts. Toxicol. Lett. 2017. [Google Scholar] [CrossRef]
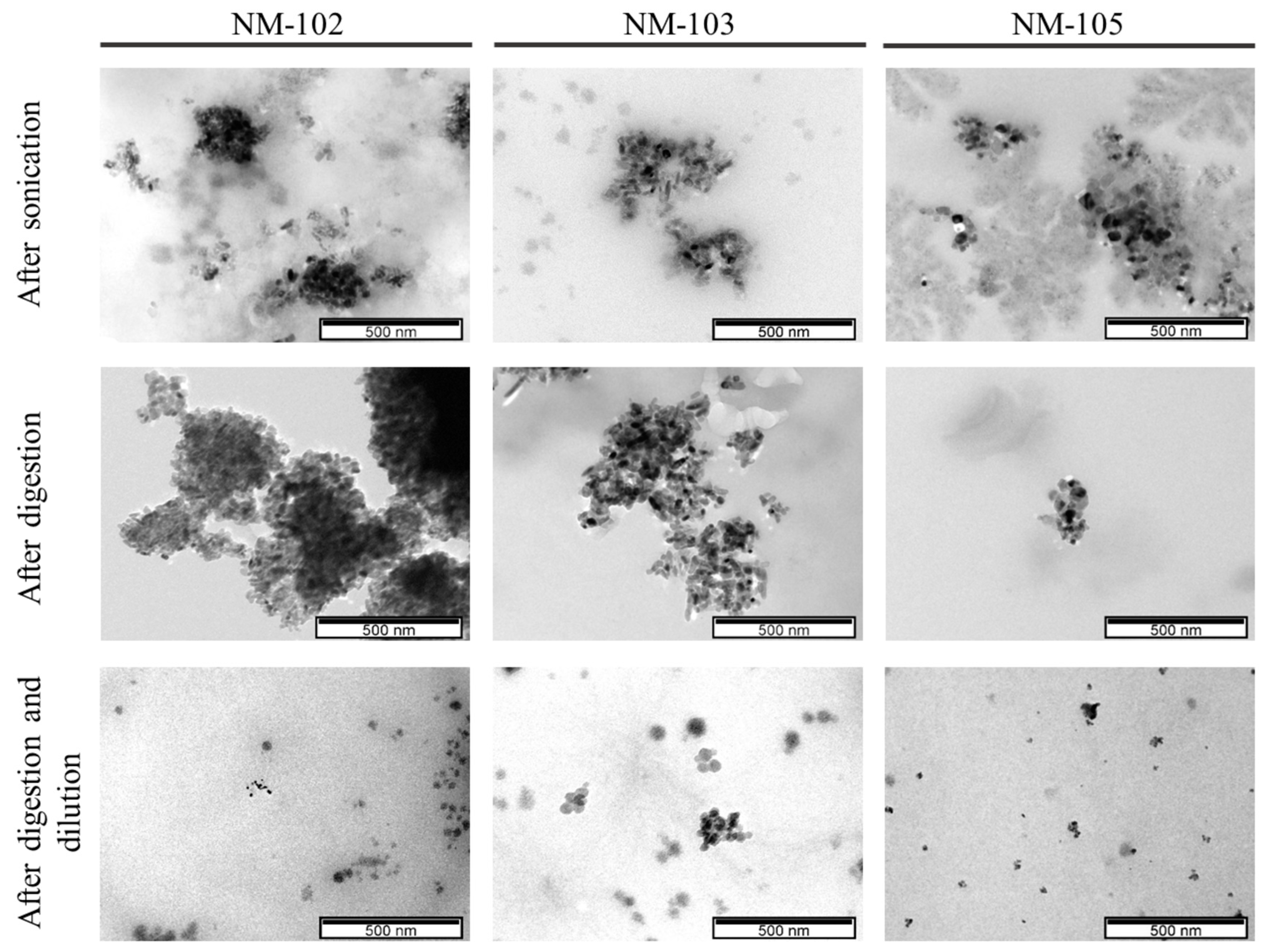
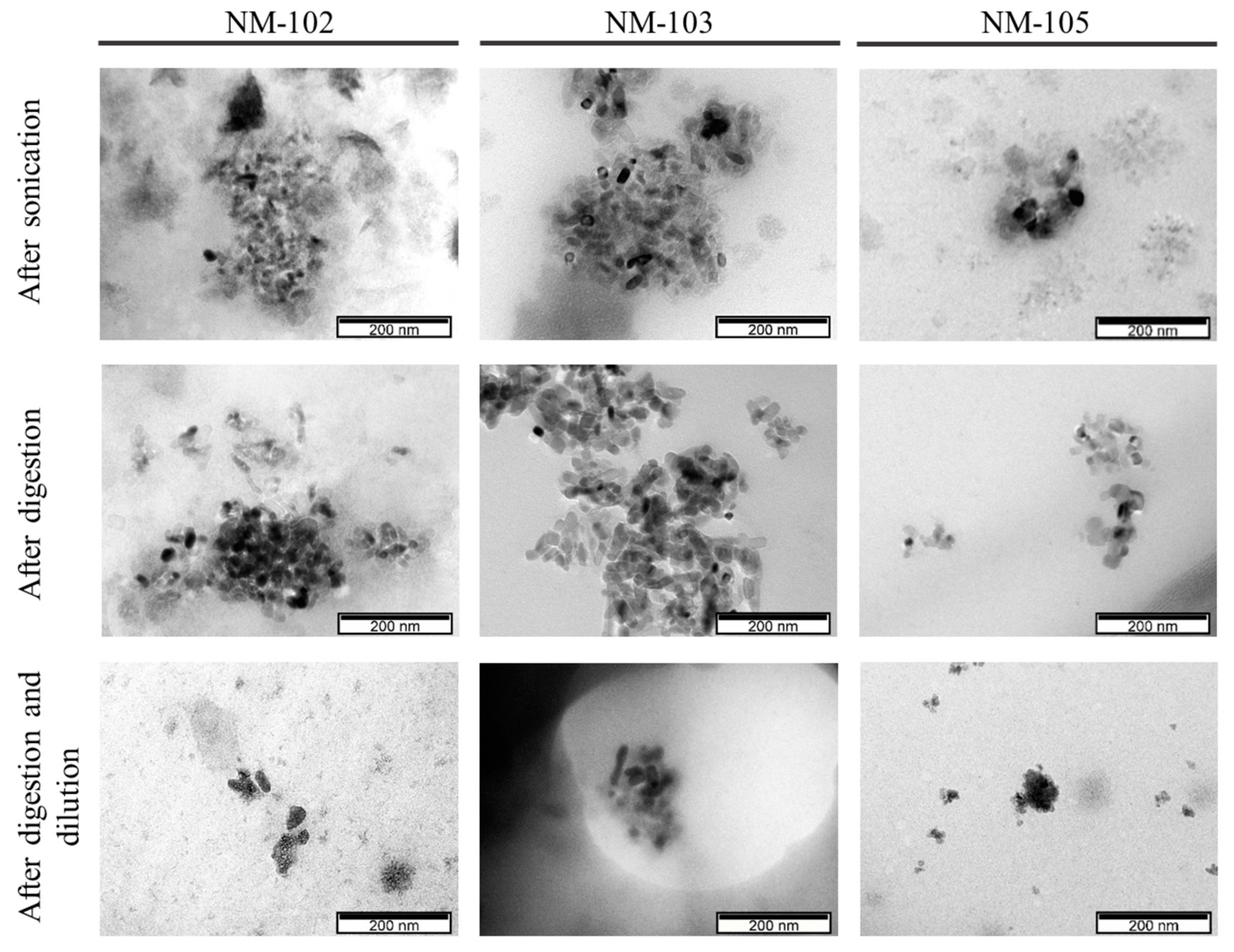
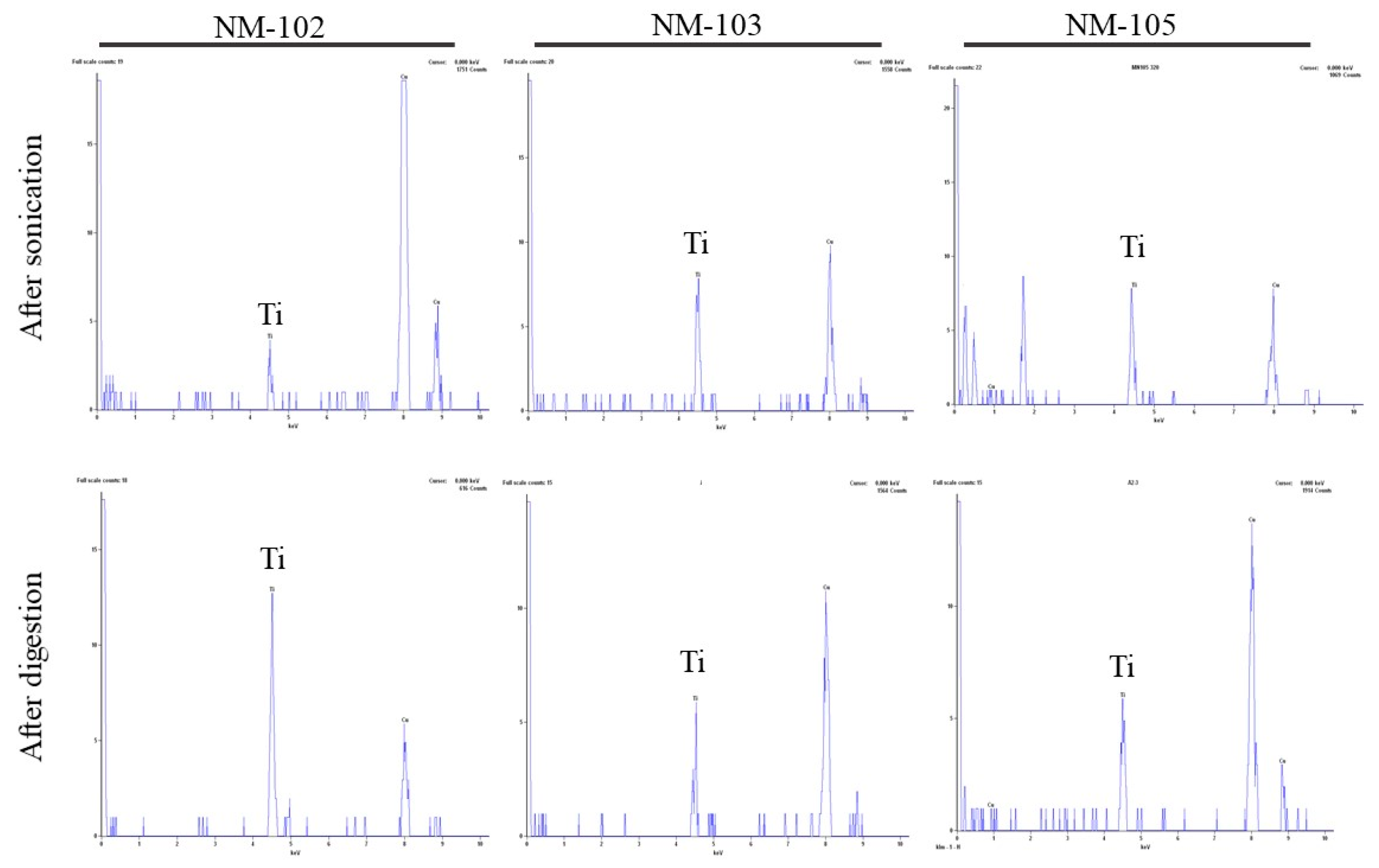

| NM-102 | NM-103 | NM-105 | |
|---|---|---|---|
| Batch# | JRCNM10202a | JRCNM62001a | JRCNM01005a |
| Crystalline phase | Anatase | Rutile | Anatase and rutile (81.5:18.5.) |
| Primary particle size (nm) | 22 | 28 | 30 |
| DLS (nm) | Zav = 442.6 PDI = 0.428 | Zav = 113.8 PDI = 0.252 | Zav = 124.5 PDI = 0.172 |
| Specific surface area (m2/g) | 77.99 | 50.84 | 46.18 |
| Agglomerates/ aggregates size (nm) | 100–500 | NA | 20–500 |
| NM | Undigested | Digested | ||||
|---|---|---|---|---|---|---|
| Zav (nm) | PDI | Zeta Potential (mV) | Zav (nm) | PDI | Zeta Potential (mV) | |
| NM-102 | 551.30 ± 46.53 | 0.49 ± 0.14 | −22.0 ± 2.8 | 560.78 ± 254.75 | 0.70 ± 0.08 | −21.4 ± 1.5 |
| NM-103 | 545.10 ± 490.87 | 0.58 ± 0.43 | −27.0 ± 0.6 | 478.60 ± 199.97 | 0.77 ± 0.15 | −26.8 ± 0.4 |
| NM-105 | 292.40 ± 0.85 | 0.73 ± 0.21 | −22.2 ± 2.0 | 190.40 * ± 9.33 | 0.52 ± 0.36 | −26.7 ± 0.8 |
| Feret Min (nm)± | Feret Max (nm)* | Feret Mean (nm) | % of Particles < 100 nm | Aspect Ratio | Agglomerate/ Aggregate Size Range (nm) | ||
|---|---|---|---|---|---|---|---|
| NM-102 | U | 20.2 ± 1.1 | 29.8 ± 1.3 | 24.6 ± 1.3 | 100 | 1.5 ± 1.3 | 111.8–315.0 |
| D | 21.2 ± 1.1 | 31.2 ± 1.1 | 25.7 ± 1.1 | 100 | 1.5 ± 1.1 | 123.9–334.0 | |
| NM-103 | U | 16.6 ± 1.2 | 30.1 ± 1.4 | 22.4 ± 1.4 | 100 | 1.8 ± 1.4 | 102.3–400.4 |
| D | 16.7 ± 1.3 | 29.6 ± 1.4 | 22.2 ± 1.4 | 100 | 1.8 ± 1.4 | 94.3–198.2 | |
| NM-105 | U | 17.6 ± 1.3 | 24.3 ± 1.2 | 20.7 ± 1.2 | 100 | 1.4 ± 1.2 | 66.4–162.2 |
| D | 18.3 ± 1.3 | 25.4 ± 1.3 | 21.6 ± 1.3 | 100 | 1.4 ± 1.3 | 48.7–195.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bettencourt, A.; Gonçalves, L.M.; Gramacho, A.C.; Vieira, A.; Rolo, D.; Martins, C.; Assunção, R.; Alvito, P.; Silva, M.J.; Louro, H. Analysis of the Characteristics and Cytotoxicity of Titanium Dioxide Nanomaterials Following Simulated In Vitro Digestion. Nanomaterials 2020, 10, 1516. https://doi.org/10.3390/nano10081516
Bettencourt A, Gonçalves LM, Gramacho AC, Vieira A, Rolo D, Martins C, Assunção R, Alvito P, Silva MJ, Louro H. Analysis of the Characteristics and Cytotoxicity of Titanium Dioxide Nanomaterials Following Simulated In Vitro Digestion. Nanomaterials. 2020; 10(8):1516. https://doi.org/10.3390/nano10081516
Chicago/Turabian StyleBettencourt, Ana, Lídia M. Gonçalves, Ana C. Gramacho, Adriana Vieira, Dora Rolo, Carla Martins, Ricardo Assunção, Paula Alvito, Maria João Silva, and Henriqueta Louro. 2020. "Analysis of the Characteristics and Cytotoxicity of Titanium Dioxide Nanomaterials Following Simulated In Vitro Digestion" Nanomaterials 10, no. 8: 1516. https://doi.org/10.3390/nano10081516
APA StyleBettencourt, A., Gonçalves, L. M., Gramacho, A. C., Vieira, A., Rolo, D., Martins, C., Assunção, R., Alvito, P., Silva, M. J., & Louro, H. (2020). Analysis of the Characteristics and Cytotoxicity of Titanium Dioxide Nanomaterials Following Simulated In Vitro Digestion. Nanomaterials, 10(8), 1516. https://doi.org/10.3390/nano10081516











