Targeted Assembly of Ultrathin NiO/MoS2 Electrodes for Electrocatalytic Hydrogen Evolution in Alkaline Electrolyte
Abstract
1. Introduction
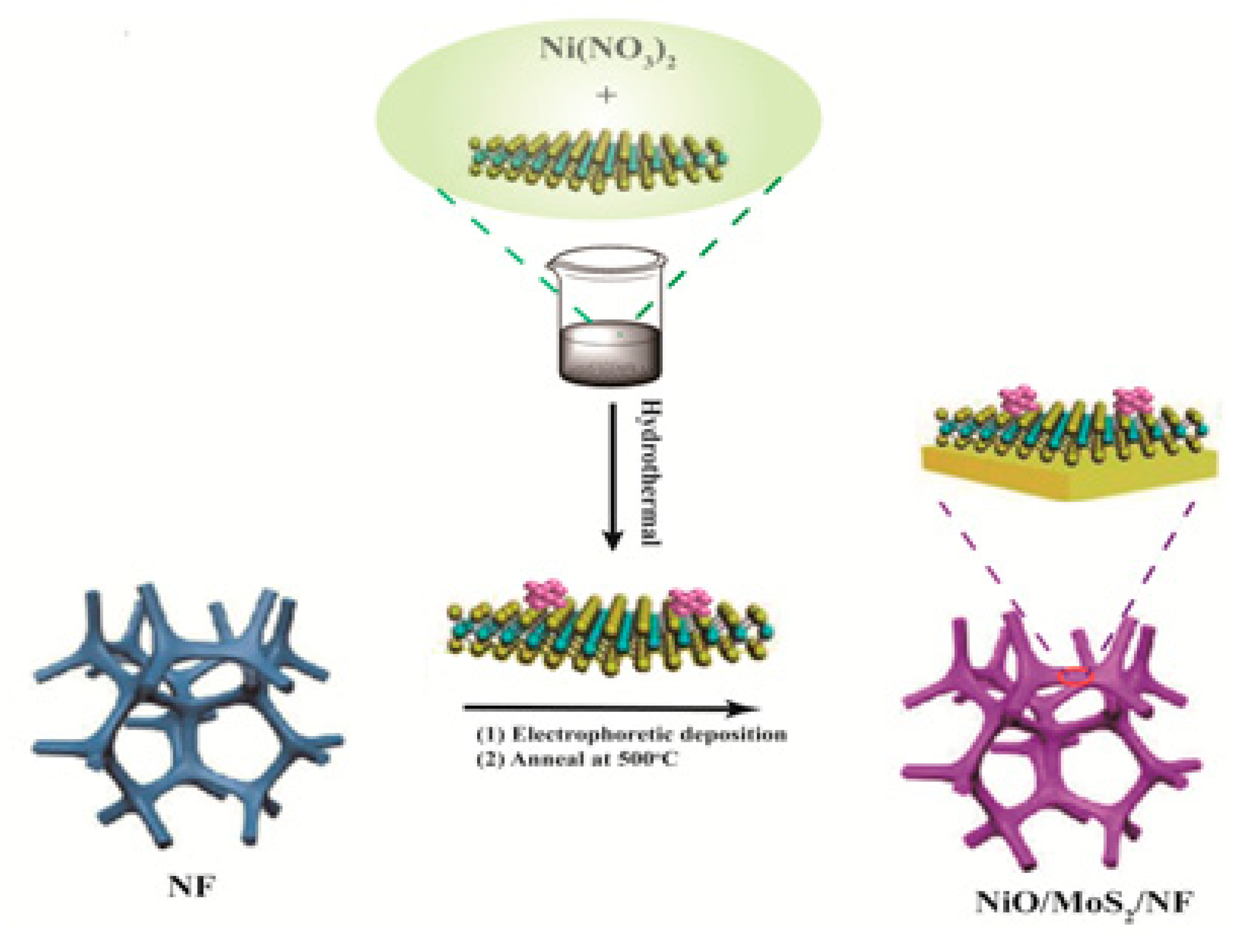
2. Materials and Methods
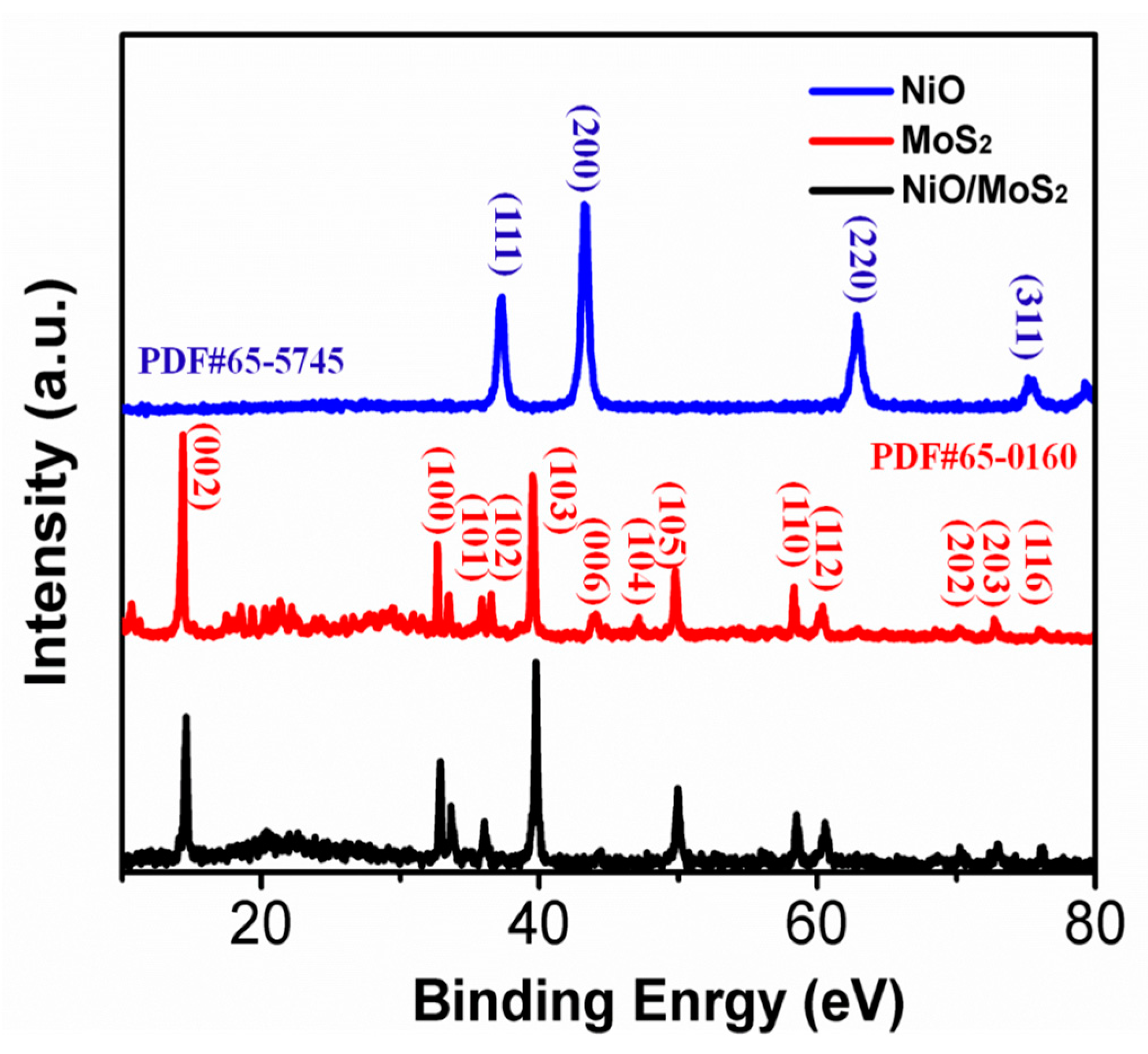
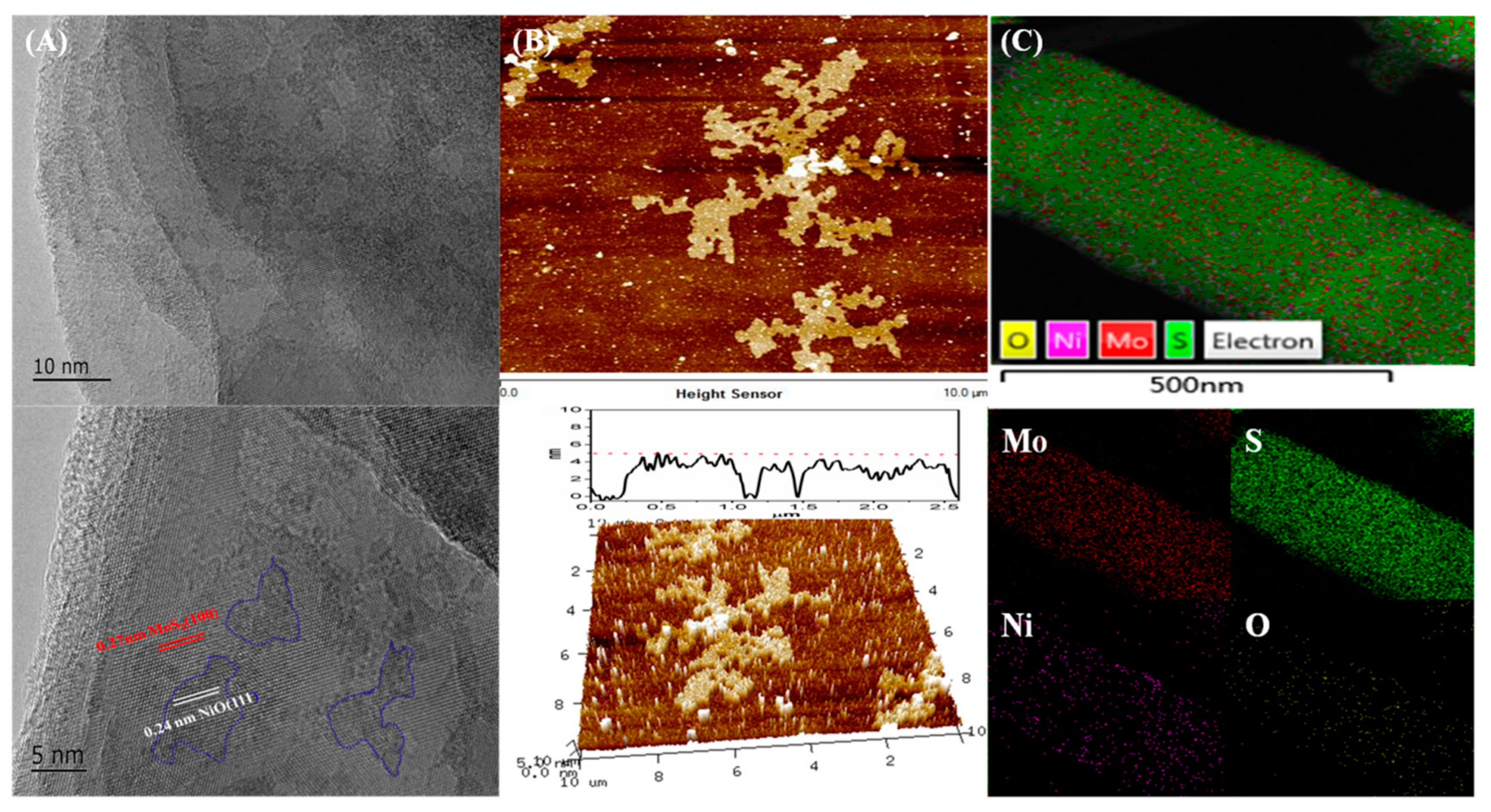
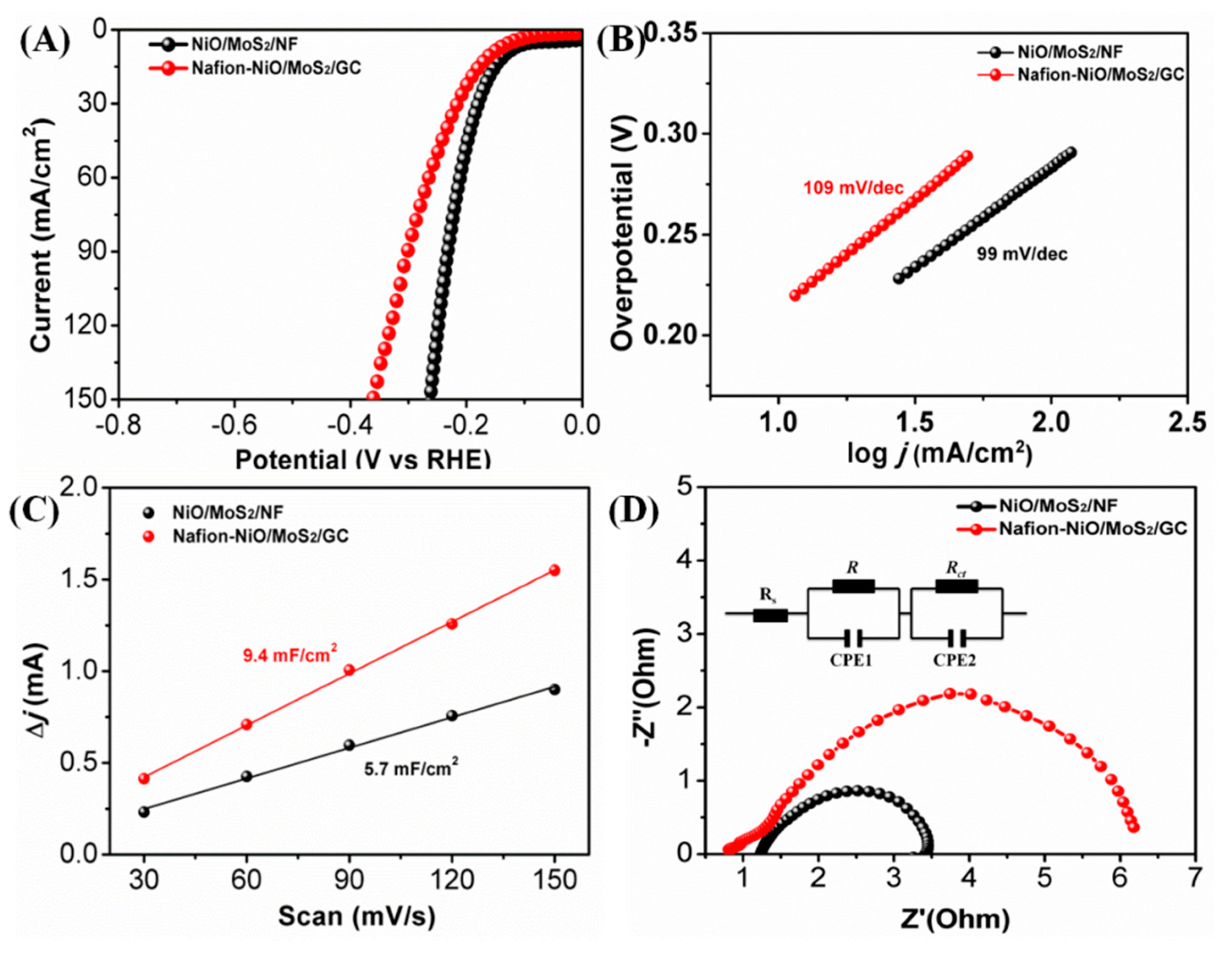
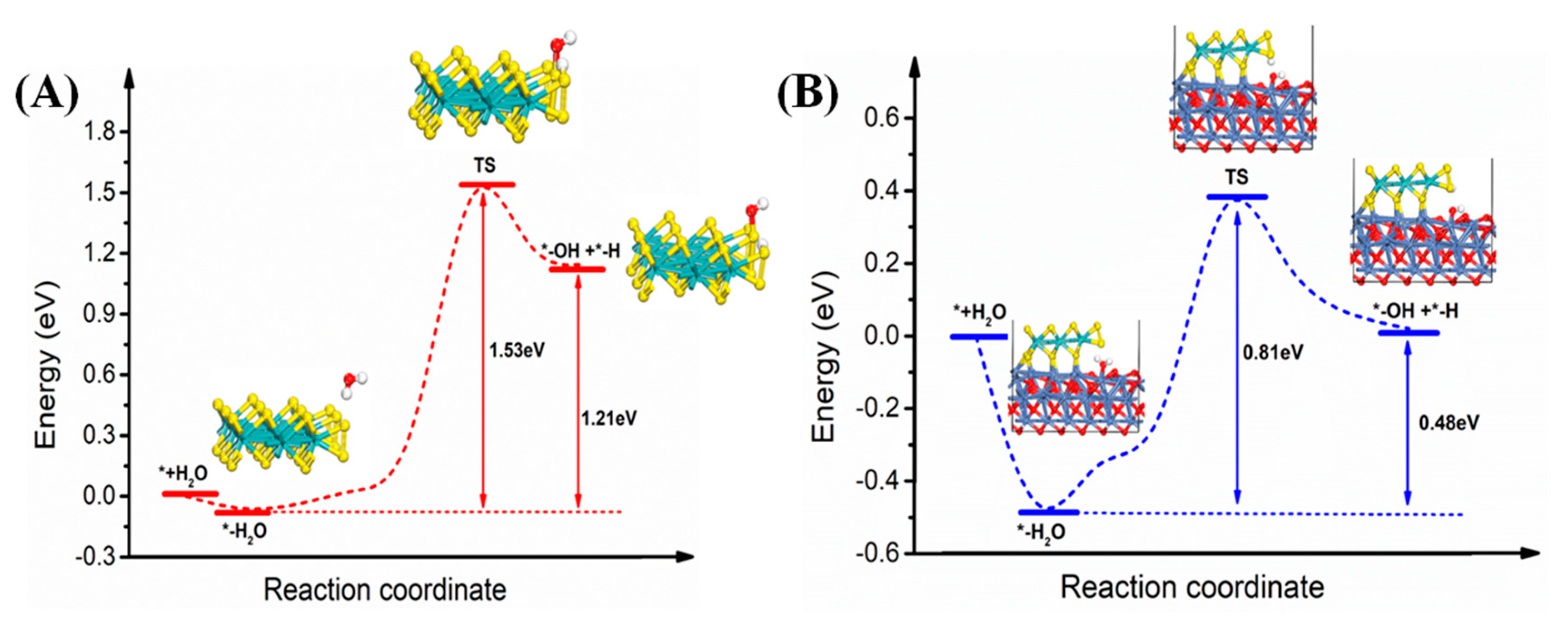
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dresselhaus, M.S.; Thomas, I.L. Alternative energy technologies. Nature 2001, 414, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.J.; Hu, H.; Tan, T.; Li, J. China’s renewable energy goals by 2050. Environ. Dev. 2016, 20, 83–90. [Google Scholar] [CrossRef]
- Li, P.; Chen, W. Recent advances in one-dimensional nanostructures for energy electrocatalysis. Chin. J. Catal. 2019, 40, 4–22. [Google Scholar] [CrossRef]
- Zhou, J.; Dou, Y.B.; Zhou, A.; Guo, R.M.; Zhao, M.J.; Li, J.R. MOF Template-Directed Fabrication of Hierarchically Structured Electrocatalysts for Efficient Oxygen Evolution Reaction. Adv. Energy Mater. 2017, 7, 1602643. [Google Scholar] [CrossRef]
- Hu, H.; Guan, B.; Xia, B.; Lou, X.W. Designed Formation of Co3O4/NiCo2O4 Double-Shelled Nanocages with Enhanced Pseudocapacitive and Electrocatalytic Properties. J. Am. Chem. Soc. 2015, 137, 5590–5595. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Wang, Y.; Dong, J.; He, C.T.; Yin, H.; An, P.; Zhao, K.; Zhang, X.; Gao, C.; Zhang, L.; et al. Ultrathin metal–organic framework nanosheets for electrocatalytic oxygen evolution. Nat. Energy 2016, 1, 16184. [Google Scholar] [CrossRef]
- He, P.; Yu, X.Y.; Lou, X.W. Carbon-Incorporated Nickel-Cobalt Mixed Metal Phosphide Nanoboxes with Enhanced Electrocatalytic Activity for Oxygen Evolution. Angew. Chem. Int. Ed. 2017, 56, 3897–3900. [Google Scholar] [CrossRef]
- Yuan, C.Z.; Sun, Z.T.; Jiang, Y.F.; Yang, Z.K.; Jiang, N.; Zhao, Z.W.; Qazi, U.Y.; Zhang, W.H.; Xu, A.W. One-Step In Situ Growth of Iron-Nickel Sulfide Nanosheets on FeNi Alloy Foils: High-Performance and Self-Supported Electrodes for Water Oxidation. Small 2017, 13, 1604161. [Google Scholar] [CrossRef]
- Wu, H.; Chen, Z.; Zhang, J.; Wu, F.; Xiao, F.; Du, S.; He, C.; Wu, Y.; Ren, Z. Generalized Synthesis of Ultrathin Cobalt-Based Nanosheets from Metallophthalocyanine-Modulated Self-Assemblies for Complementary Water Electrolysis. Small 2018, 14, 1702896. [Google Scholar] [CrossRef]
- Wu, D.; Wei, Y.; Ren, X.; Ji, X.; Liu, Y.; Guo, X.; Liu, Z.; Asiri, A.M.; Wei, Q.; Sun, X. Co(OH)2 Nanoparticle-Encapsulating Conductive Nanowires Array: Room-Temperature Electrochemical Preparation for High-Performance Water Oxidation Electrocatalysis. Adv. Mater. 2018, 30, 1705366. [Google Scholar] [CrossRef]
- Wu, J.; Ge, X.B.; Li, Z.H.; Cao, D.; Xiao, J.Y. Highly dispersed NiCoP nanoparticles on carbon nanotubes modified nickel foam for efficient electrocatalytic hydrogen production. Electrochim. Acta 2017, 252, 101–108. [Google Scholar] [CrossRef]
- Xiao, C.H.; Zhang, B.; Li, D. Partial-sacrificial-template Synthesis of Fe/Ni Phosphides on Ni Foam: A Strongly Stabilized and Efficient Catalyst for Electrochemical Water Splitting. Electrochim. Acta 2017, 242, 260–267. [Google Scholar] [CrossRef]
- Gao, X.; Qi, J.; Wan, S.; Zhang, W.; Wang, Q.; Cao, R. Conductive Molybdenum Sulfide for Efficient Electrocatalytic Hydrogen Evolution. Small 2018, 14, e1803361. [Google Scholar] [CrossRef] [PubMed]
- Cong, M.; Sun, D.; Zhang, L.; Ding, X. In situ assembly of metal-organic framework-derived N-doped carbon/Co/CoP catalysts on carbon paper for water splitting in alkaline electrolytes. Chin. J. Catal. 2020, 41, 242–248. [Google Scholar] [CrossRef]
- Ding, X.; Zhang, L.L.; Wang, Y.; Liu, A.H.; Gao, Y. Design of photoanode-based dye-sensitized photoelectrochemical cells assembling with transition metal complexes for visible light-induced water splitting. Coord. Chem. Rev. 2018, 357, 130–143. [Google Scholar] [CrossRef]
- Ding, X.; Gao, Y.; Zhang, L.L.; Yu, Z.; Liu, J.H.; Sun, L.C. Visible Light-Driven Water Splitting in Photoelectrochemical Cells with Supramolecular Catalysts on Photoanodes. ACS Catal. 2014, 4, 2347–2350. [Google Scholar] [CrossRef]
- Gao, Y.; Ding, X.; Liu, J.; Wang, L.; Lu, Z.; Li, L.; Sun, L. Visible light driven water splitting in a molecular device with unprecedentedly high photocurrent density. J. Am. Chem. Soc. 2013, 135, 4219–4222. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, H.; Ge, R.; Ren, X.; Ren, J.; Yang, D.; Zhang, L.; Sun, X. Phosphorus-Doped Co3O4 Nanowire Array: A Highly Efficient Bifunctional Electrocatalyst for Overall Water Splitting. ACS Catal. 2018, 8, 2236–2241. [Google Scholar] [CrossRef]
- Huang, T.; Shen, T.; Gong, M.; Deng, S.; Lai, C.; Liu, X.; Zhao, T.; Teng, L.; Wang, D. Ultrafine Ni-B nanoparticles for efficient hydrogen evolution reaction. Chin. J. Catal. 2019, 40, 1867–1873. [Google Scholar] [CrossRef]
- Indra, A.; Paik, U.; Song, T. Boosting Electrochemical Water Oxidation with Metal Hydroxide Carbonate Templated Prussian Blue Analogues. Angew. Chem. Int. Ed. 2018, 57, 1241–1245. [Google Scholar] [CrossRef]
- Zhou, D.; Cai, Z.; Lei, X.; Tian, W.; Bi, Y.; Jia, Y.; Han, N.; Gao, T.; Zhang, Q.; Kuang, Y.; et al. NiCoFe-Layered Double Hydroxides/N-Doped Graphene Oxide Array Colloid Composite as an Efficient Bifunctional Catalyst for Oxygen Electrocatalytic Reactions. Adv. Energy Mater. 2018, 8, 1701905. [Google Scholar] [CrossRef]
- Gu, Y.; Chen, S.; Ren, J.; Jia, Y.A.; Chen, C.; Komarneni, S.; Yang, D.; Yao, X. Electronic Structure Tuning in Ni3FeN/r-GO Aerogel toward Bifunctional Electrocatalyst for Overall Water Splitting. ACS Nano 2018, 12, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.S.; Wang, J.W.; Luo, J.; Liu, R.R.; Zhang, Z.M.; He, C.T.; Lu, T.B. Extraction of nickel from NiFe-LDH into Ni2P@NiFe hydroxide as a bifunctional electrocatalyst for efficient overall water splitting. Chem. Sci. 2018, 9, 1375–1384. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Chen, H.; Ji, Y.; Huang, H.; Claesson, P.M.; Daniel, Q.; Philippe, B.; Rensmo, H.; Li, F.; Luo, Y.; et al. Nickel-vanadium monolayer double hydroxide for efficient electrochemical water oxidation. Nat. Commun. 2016, 7, 11981. [Google Scholar] [CrossRef] [PubMed]
- Sun, X. Ni foam-supported NiCoP nanosheets as bifunctional electrocatalysts for efficient overall water splitting. Chin. J. Catal. 2019, 40, 1405–1407. [Google Scholar] [CrossRef]
- Tian, J.; Liu, Q.; Asiri, A.M.; Sun, X. Self-supported nanoporous cobalt phosphide nanowire arrays: An efficient 3D hydrogen-evolving cathode over the wide range of pH 0-14. J. Am. Chem. Soc. 2014, 136, 7587–7590. [Google Scholar] [CrossRef]
- Shi, H.; Liang, H.; Ming, F.; Wang, Z. Efficient Overall Water-Splitting Electrocatalysis Using Lepidocrocite VOOH Hollow Nanospheres. Angew. Chem. Int. Ed. Engl. 2017, 56, 573–577. [Google Scholar] [CrossRef]
- Xue, Z.H.; Su, H.; Yu, Q.Y.; Zhang, B.; Wang, H.H.; Li, X.H.; Chen, J.S. Janus Co/CoP Nanoparticles as Efficient Mott-Schottky Electrocatalysts for Overall Water Splitting in Wide pH Range. Adv. Energy Mater. 2017, 7, 1602355. [Google Scholar] [CrossRef]
- Wu, Z.X.; Wang, J.; Zhu, J.; Guo, J.P.; Xiao, W.P.; Xuan, C.J.; Lei, W.; Wang, D.L. Highly efficient and stable MoP-RGO nanoparticles as electrocatalysts for hydrogen evolution. Electrochim. Acta 2017, 232, 254–261. [Google Scholar] [CrossRef]
- Gupta, S.; Patel, N.; Fernandes, R.; Hanchate, S.; Miotello, A.; Kothari, D.C. Co-Mo-B Nanoparticles as a non-precious and efficient Bifunctional Electrocatalyst for Hydrogen and Oxygen Evolution. Electrochim. Acta 2017, 232, 64–71. [Google Scholar] [CrossRef]
- Liu, Y.R.; Hu, W.H.; Han, G.Q.; Dong, B.; Li, X.; Shang, X.; Chai, Y.M.; Liu, Y.Q.; Liu, C.G. Novel CoP Hollow Prisms as Bifunctional Electrocatalysts for Hydrogen Evolution Reaction in Acid media and Overall Water-splitting in Basic media. Electrochim. Acta 2016, 220, 98–106. [Google Scholar] [CrossRef]
- Lei, H.; Chen, M.; Liang, Z.; Liu, C.; Zhang, W.; Cao, R. Ni2P hollow microspheres for electrocatalytic oxygen evolution and reduction reactions. Catal. Sci. Technol. 2018, 8, 2289–2293. [Google Scholar] [CrossRef]
- Chen, M.; Qi, J.; Zhang, W.; Cao, R. Electrosynthesis of NiPx nanospheres for electrocatalytic hydrogen evolution from a neutral aqueous solution. Chem Commun. 2017, 53, 5507–5510. [Google Scholar] [CrossRef]
- Wang, Y.; Kong, B.; Zhao, D.Y.; Wang, H.T.; Selomulya, C. Strategies for developing transition metal phosphides as heterogeneous electrocatalysts for water splitting. Nano Today 2017, 15, 26–55. [Google Scholar] [CrossRef]
- Zhu, Z.; Yin, H.; He, C.T.; Mamun, M.A.; Liu, P.; Jiang, L.; Zhao, Y.; Wang, Y.; Yang, H.G.; Tang, Z.; et al. Ultrathin Transition Metal Dichalcogenide/3d Metal Hydroxide Hybridized Nanosheets to Enhance Hydrogen Evolution Activity. Adv Mater. 2018, 30, e1801171. [Google Scholar] [CrossRef]
- Shang, B.; Ma, P.; Fan, J.; Jiao, L.; Liu, Z.; Zhang, Z.; Chen, N.; Cheng, Z.; Cui, X.; Zheng, W. Stabilized monolayer 1T MoS2 embedded in CoOOH for highly efficient overall water splitting. Nanoscale 2018, 10, 12330–12336. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zheng, Y.; Zhu, D.; Vasileff, A.; Ling, T.; Qiao, S.Z. Identification of pH-dependent synergy on Ru/MoS2 interface: A comparison of alkaline and acidic hydrogen evolution. Nanoscale 2017, 9, 16616–16621. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Zhang, B.; Ruan, Y.; Wan, H.; Ji, X.; Xu, K.; Zha, D.; Miao, L.; Jiang, J. Mutually beneficial Co3O4@MoS2 heterostructures as a highly efficient bifunctional catalyst for electrochemical overall water splitting. J. Mater. Chem. A. 2018, 6, 2067–2072. [Google Scholar] [CrossRef]
- Subbaraman, R.; Tripkovic, D.; Strmcnik, D.; Chang, K.C.; Uchimura, M.; Paulikas, A.P.; Stamenkovic, V.; Markovic, N.M. Enhancing hydrogen evolution activity in water splitting by tailoring Li(+)-Ni(OH)(2)-Pt interfaces. Science 2011, 334, 1256–1260. [Google Scholar] [CrossRef]
- Ma, Y.Y.; Wu, C.X.; Feng, X.J.; Tan, H.Q.; Yan, L.K.; Liu, Y.; Kang, Z.H.; Wang, E.B.; Li, Y.G. Highly efficient hydrogen evolution from seawater by a low-cost and stable CoMoP@C electrocatalyst superior to Pt/C. Energy Environ. Sci. 2017, 10, 788–798. [Google Scholar] [CrossRef]
- Ye, L.; Chai, G.L.; Wen, Z.H. Zn-MOF-74 Derived N-Doped Mesoporous Carbon as pH-Universal Electrocatalyst for Oxygen Reduction Reaction. Adv. Funct. Mater. 2017, 27, 1606190. [Google Scholar] [CrossRef]
- Tabassum, H.; Guo, W.H.; Meng, W.; Mahmood, A.; Zhao, R.; Wang, Q.F.; Zou, R.Q. Metal-Organic Frameworks Derived Cobalt Phosphide Architecture Encapsulated into B/N Co-Doped Graphene Nanotubes for All pH Value Electrochemical Hydrogen Evolution. Adv. Energy Mater. 2017, 7, 1601671. [Google Scholar] [CrossRef]
- Yang, F.; Zhao, P.; Hua, X.; Luo, W.; Cheng, G.; Xing, W.; Chen, S. A cobalt-based hybrid electrocatalyst derived from a carbon nanotube inserted metal-organic framework for efficient water-splitting. J. Mater. Chem. A. 2016, 4, 16057–16063. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, W.; Shi, Z.; Che, M.; Yu, X.; Tang, Y.; Gao, Q. Electrospinning Hetero-Nanofibers of Fe3C-Mo2C/Nitrogen-Doped-Carbon as Efficient Electrocatalysts for Hydrogen Evolution. ChemSusChem 2017, 10, 2597–2604. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Xia, X.H.; Cao, X.; Zhang, B.W.; Tiep, N.H.; He, H.Y.; Chen, S.; Huang, Y.Z.; Fan, H.J. Ultrafine Metal Nanoparticles/N-Doped Porous Carbon Hybrids Coated on Carbon Fibers as Flexible and Binder-Free Water Splitting Catalysts. Adv. Energy Mater. 2017, 7, 1700220. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, K.; Cong, M.; Xu, F.; Ding, X.; Zhang, X. Targeted Assembly of Ultrathin NiO/MoS2 Electrodes for Electrocatalytic Hydrogen Evolution in Alkaline Electrolyte. Nanomaterials 2020, 10, 1547. https://doi.org/10.3390/nano10081547
Xia K, Cong M, Xu F, Ding X, Zhang X. Targeted Assembly of Ultrathin NiO/MoS2 Electrodes for Electrocatalytic Hydrogen Evolution in Alkaline Electrolyte. Nanomaterials. 2020; 10(8):1547. https://doi.org/10.3390/nano10081547
Chicago/Turabian StyleXia, Kai, Meiyu Cong, Fanfan Xu, Xin Ding, and Xiaodong Zhang. 2020. "Targeted Assembly of Ultrathin NiO/MoS2 Electrodes for Electrocatalytic Hydrogen Evolution in Alkaline Electrolyte" Nanomaterials 10, no. 8: 1547. https://doi.org/10.3390/nano10081547
APA StyleXia, K., Cong, M., Xu, F., Ding, X., & Zhang, X. (2020). Targeted Assembly of Ultrathin NiO/MoS2 Electrodes for Electrocatalytic Hydrogen Evolution in Alkaline Electrolyte. Nanomaterials, 10(8), 1547. https://doi.org/10.3390/nano10081547




