Green Preparation of Fluorescent Nitrogen-Doped Carbon Quantum Dots for Sensitive Detection of Oxytetracycline in Environmental Samples
Abstract
:1. Introduction
2. Experimental
2.1. Reagents and Chemicals
2.2. Preparation of N-CQDs
2.3. Characterization of N-CQDs
2.4. Pretreatment and Preparation of Environmental Samples
3. Results and Discussion
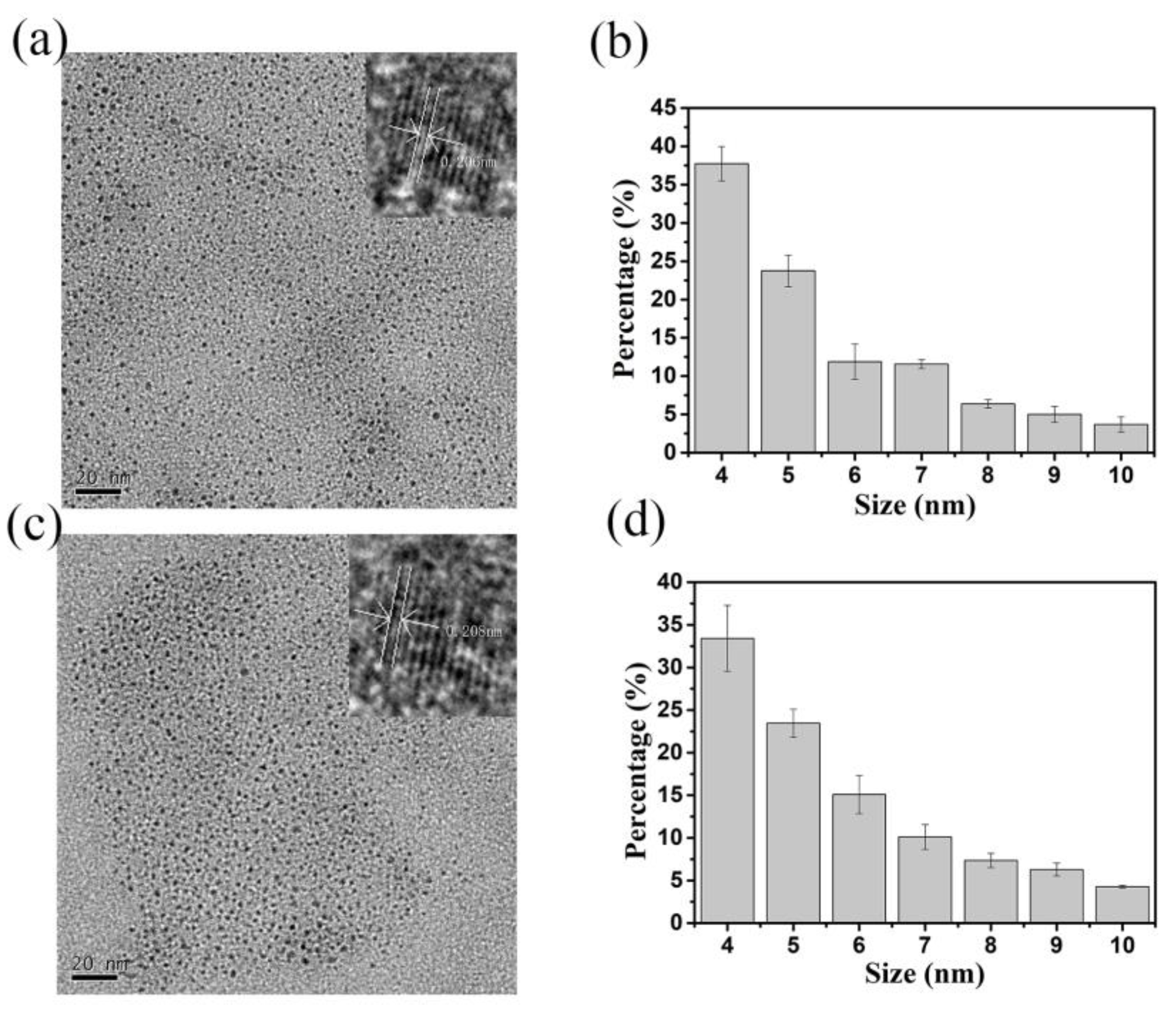
3.1. Morphologies of N-CQDs
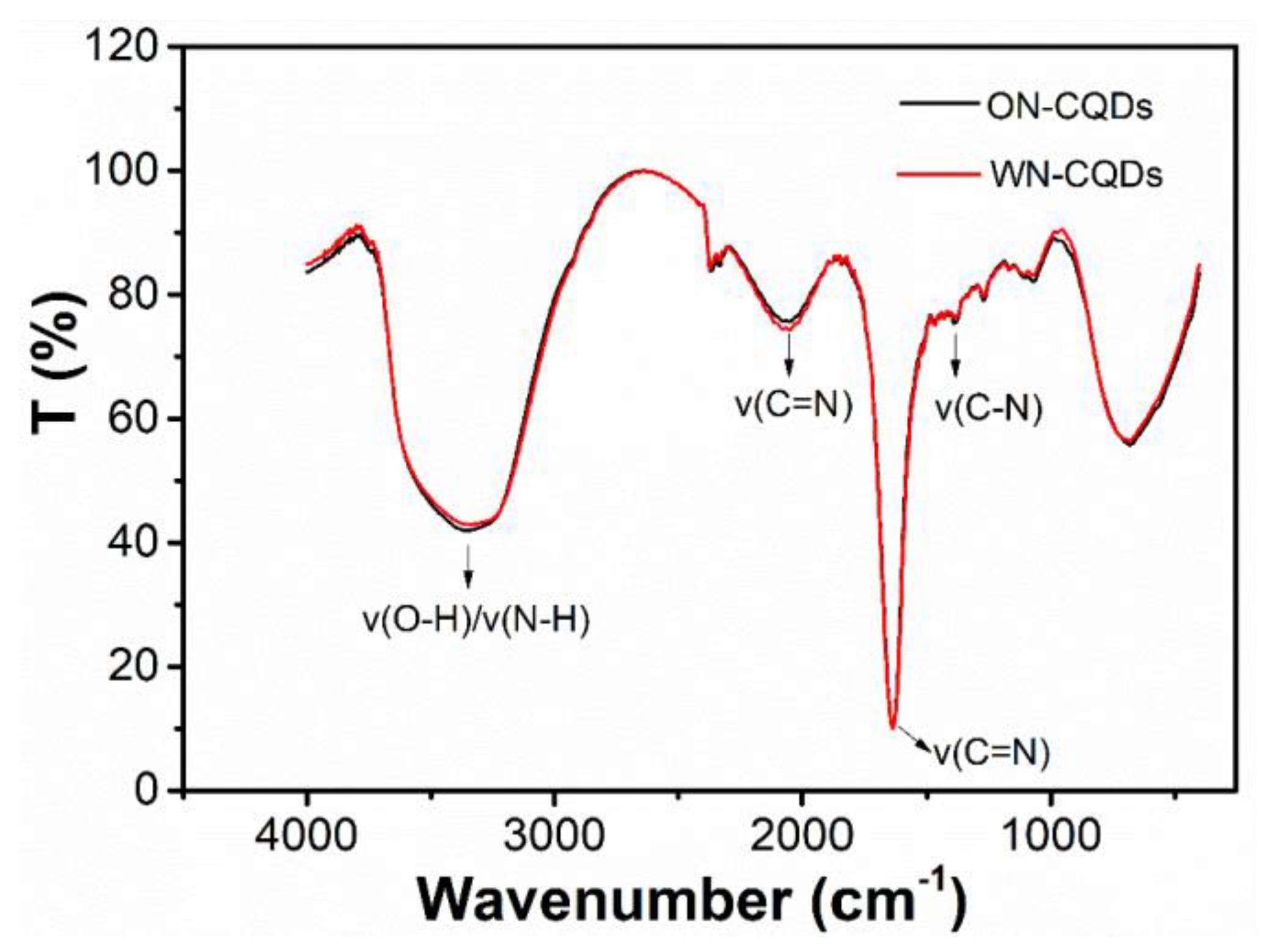
3.2. Composition of N-CQDs
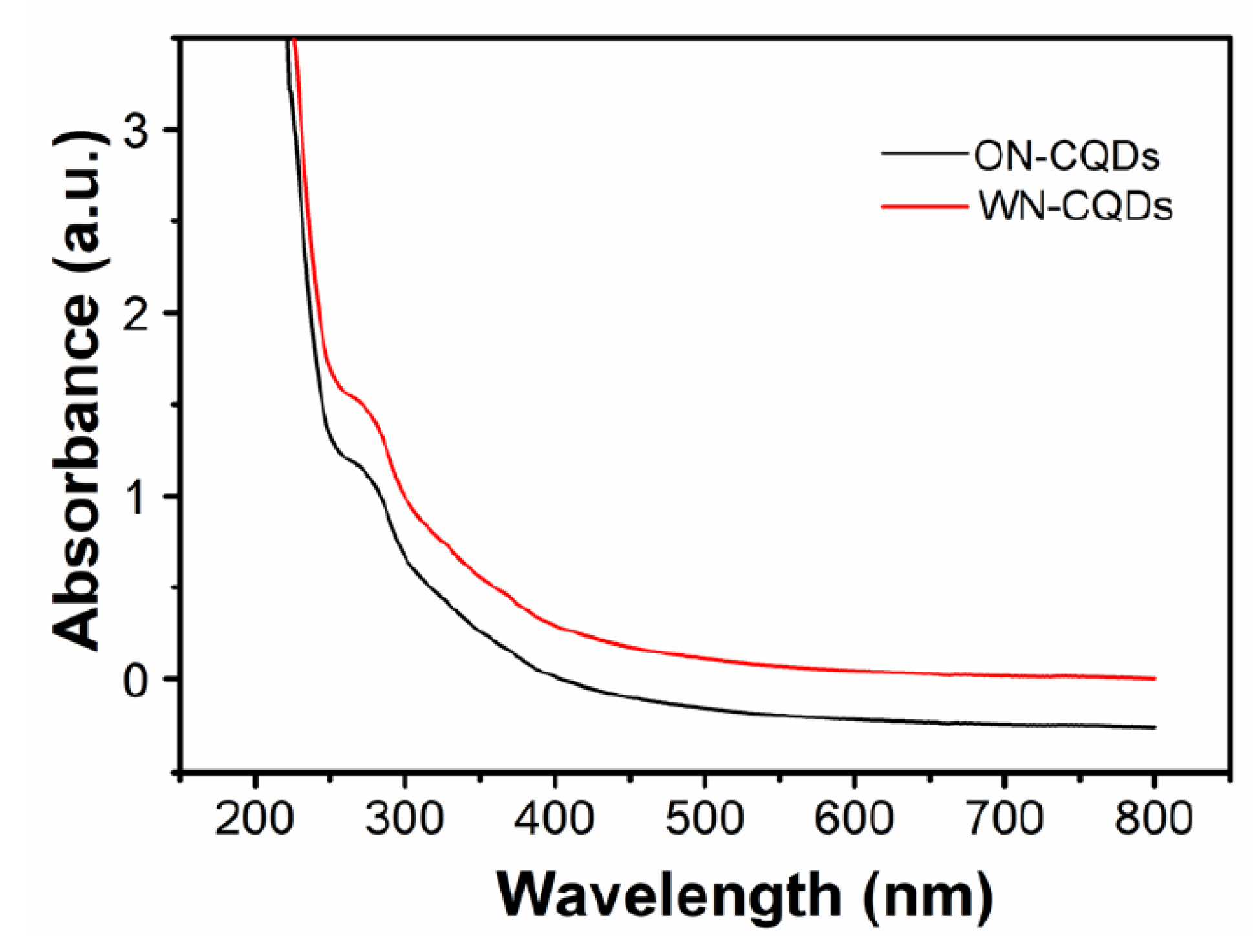
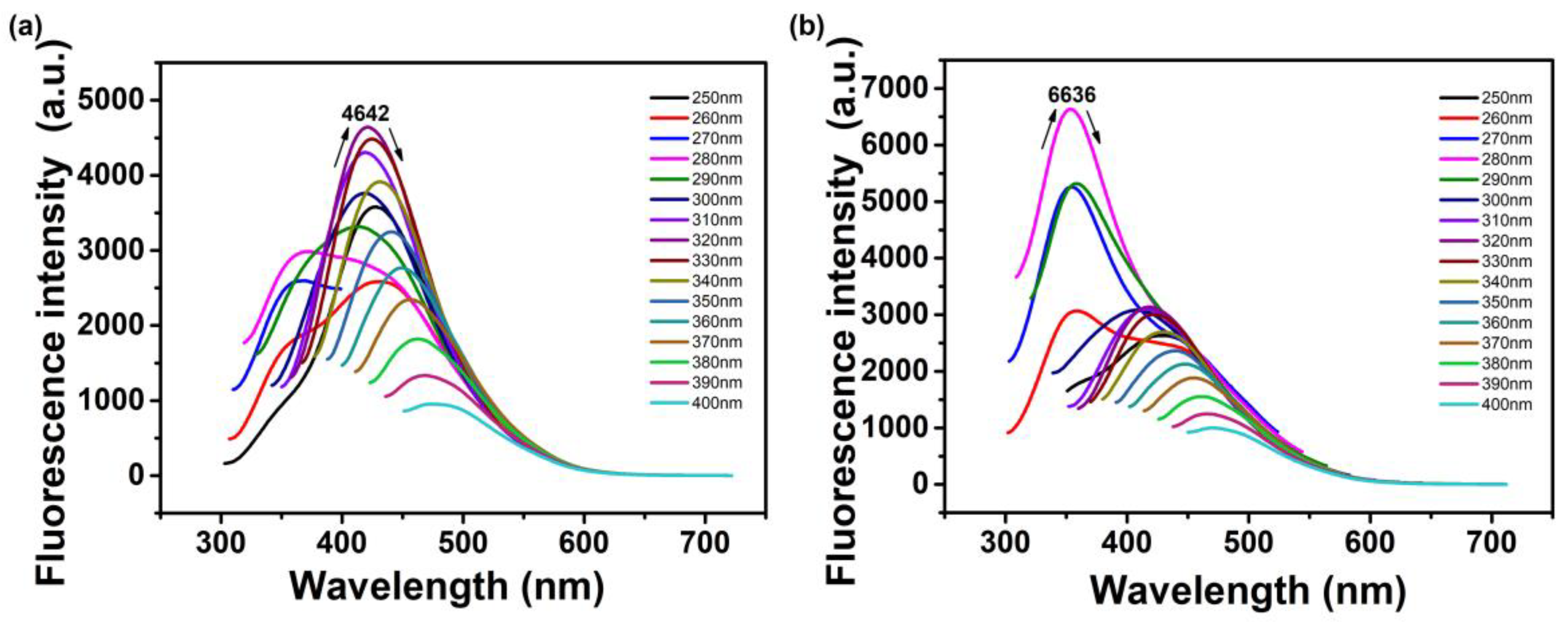
3.3. Optical Properties of N-CQDs
3.4. Basis of OTC Detection
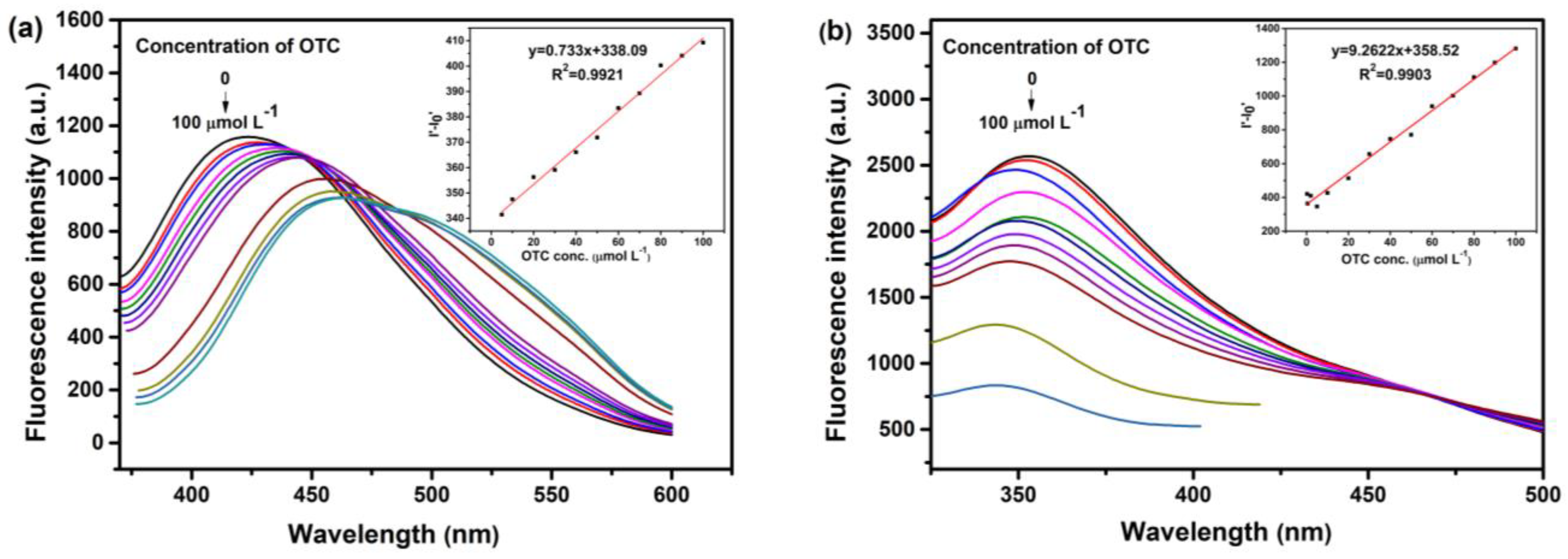
3.5. Linear Range and Detection Limit of OTC
3.6. Determination of OTC in Environmental Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kovalakova, P.; Cizmas, L.; McDonald, T.J.; Marsalek, B.; Feng, M.; Sharma, V.K. Occurrence and toxicity of antibiotics in the aquatic environment: A review. Chemosphere 2020, 251, 126351. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.X.; Feng, J.; Liu, J.; Fu, W.J.; Li, X.Y.; Li, B. Deciphering of microbial community and antibiotic resistance genes in activated sludge reactors under high selective pressure of different antibiotics. Water Res. 2019, 151, 388–402. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.C.; Ding, L.; Chen, L.G.; Chen, Y.H.; Zhou, T.Y.; Li, H.Y.; Xu, Y.; Zhao, L.; Huang, N. A facile, green synthesis of biomass carbon dots coupled with molecularly imprinted polymers for highly selective detection of oxytetracycline. J. Ind. Eng. Chem. 2019, 69, 455–463. [Google Scholar] [CrossRef]
- Xu, N.; Yuan, Y.Q.; Yin, J.H.; Wang, X.; Meng, L. One-pot hydrothermal synthesis of luminescent silicon-based nanoparticles for highly specific detection of oxytetracycline via ratiometric fluorescent strategy. RSC Adv. 2017, 7, 48429–48436. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.L.; Gu, Y.C.; Wu, X.Y.; Zhou, X.Y.; Zhou, H.; Amanze, C.; Shen, L.; Zeng, W.M. Construction of a tetracycline degrading bacterial consortium and its application evaluation in laboratory-scale soil remediation. Microorganisms 2020, 8, 292. [Google Scholar] [CrossRef] [Green Version]
- Ezzariai, A.; Hafidi, M.; Khadra, A.; Aemig, Q.; Loubna, E.F.; Barret, M.; Merlina, G.; Patureau, D.; Pinelli, E. Human and veterinary antibiotics during composting of sludge or manure: Global perspectives on persistence, degradation, and resistance genes. J. Hazard. Mater. 2018, 359, 465–481. [Google Scholar] [CrossRef]
- Conde-Cid, M.; Fernandez-Calvino, D.; Novoa-Munoz, J.C.; Nunez-Delgado, A.; Fernandez-Sanjurjo, M.J.; Arias-Estevez, M.; Alvarez-Rodriguez, E. Experimental data and model prediction of tetracycline adsorption and desorption in agricultural soils. Environ. Res. 2019, 177, 108607. [Google Scholar] [CrossRef]
- Conde-Cid, M.; Álvarez-Esmorís, C.; Paradelo-Núñez, R.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M.; Álvarez-Rodríguez, E.; Fernández-Sanjurjo, M.J.; Núñez-Delgado, A. Occurrence of tetracyclines and sulfonamides in manures, agricultural soils and crops from different areas in Galicia (NW Spain). J. Clean. Prod. 2018, 197, 491–500. [Google Scholar] [CrossRef]
- Wang, Z.; Du, Y.; Yang, C.; Liu, X.; Zhang, J.Q.; Li, E.H.; Zhang, Q.; Wang, X.L. Occurrence and ecological hazard assessment of selected antibiotics in the surface waters in and around Lake Honghu, China. Sci. Total Environ. 2017, 609, 1423–1432. [Google Scholar] [CrossRef]
- Ghirardini, A.; Grillini, V.; Verlicchi, P. A review of the occurrence of selected micropollutants and microorganisms in different raw and treated manure—Environmental risk due to antibiotics after application to soil. Sci. Total Environ. 2020, 707, 136118. [Google Scholar] [CrossRef]
- Tan, B.; Wang, D.H.; Cai, Z.J.; Quan, X.; Zhao, H.M. Extending suitability of physisorption strategy in fluorescent platforms design: Surface passivation and covalent linkage on MOF nanosheets with enhanced OTC detection sensitivity. Sens. Actuators B Chem. 2020, 303, 127230. [Google Scholar] [CrossRef]
- Zhu, D.; Chen, Q.L.; Ding, J.; Wang, Y.F.; Cui, H.L.; Zhu, Y.G. Antibiotic resistance genes in the soil ecosystem and planetary health: Progress and prospect. Sci. Sin. Vitae 2019, 49, 1652–1663. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Zhao, Y.; Zhu, D.; Gillings, M.; Penuelas, J.; Ok, Y.S.; Capon, A.; Banwart, S. Soil biota, antimicrobial resistance and planetary health. Environ. Int. 2019, 131, 105059. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.Y.; Yan, Z.; Zhang, Y.; Xu, W.L.; Kong, D.Y.; Shan, Z.J.; Wang, N. Behavior of antibiotic resistance genes under extremely high-level antibiotic selection pressures in pharmaceutical wastewater treatment plants. Sci. Total Environ. 2018, 612, 119–128. [Google Scholar] [CrossRef]
- Liang, N.; Huang, P.T.; Hou, X.H.; Li, Z.; Tao, L.; Zhao, L.S. Solid-phase extraction in combination with dispersive liquid-liquid microextraction and ultra-high performance liquid chromatography-tandem mass spectrometry analysis: The ultra-trace determination of 10 antibiotics in water samples. Anal. Bioanal. Chem. 2016, 408, 1701–1713. [Google Scholar] [CrossRef]
- Naik, L.; Sharma, R.; Mann, B.; Lata, K.; Rajput, Y.S.; Surendra Nath, B. Rapid screening test for detection of oxytetracycline residues in milk using lateral flow assay. Food Chem. 2017, 219, 85–92. [Google Scholar] [CrossRef]
- Wang, Y.F.; Xie, T.S.; Yang, J.; Lei, M.; Fan, J.; Meng, Z.H.; Xue, M.; Qiu, L.L.; Qi, F.L.; Wang, Z. Fast screening of antibiotics in milk using a molecularly imprinted two-dimensional photonic crystal hydrogel sensor. Anal. Chim. Acta 2019, 1070, 97–103. [Google Scholar] [CrossRef]
- Hu, Z.T.; Sun, P.D.; Hu, Z.R.; Han, J.Y.; Wang, R.Y.; Jiao, L.; Yang, P.F. Short-term performance of enhanced biological phosphorus removal (EBPR) system exposed to erythromycin (ERY) and oxytetracycline (OTC). Bioresour. Technol. 2016, 221, 15–25. [Google Scholar] [CrossRef]
- Fan, H.Z.; Zhang, M.; Bhandari, B.; Yang, C.H. Food waste as a carbon source in carbon quantum dots technology and their applications in food safety detection. Trends Food Sci. Technol. 2020, 95, 86–96. [Google Scholar] [CrossRef]
- Li, M.; Chen, T.; Gooding, J.J.; Liu, J. Review of carbon and graphene quantum dots for sensing. ACS Sens. 2019, 4, 1732–1748. [Google Scholar] [CrossRef]
- Zhou, J.W.; Zou, X.M.; Song, S.H.; Chen, G.H. Quantum dots applied to methodology on detection of pesticide and veterinary drug residues. J. Agric. Food Chem. 2018, 66, 1307–1319. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S.; Varma, R.S. Green synthesis, biomedical and biotechnological applications of carbon and graphene quantum dots. A review. Environ. Chem. Lett. 2020, 18, 703–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, P.; Zhu, Z.Q.; Chen, M.Z.; Chen, W.M.; Zhou, X.Y. Microwave-assisted synthesis of xylan-derived carbon quantum dots for tetracycline sensing. Opt. Mater. 2018, 85, 329–336. [Google Scholar] [CrossRef]
- Qi, H.J.; Teng, M.; Liu, M.; Liu, S.X.; Li, J.; Yu, H.P.; Teng, C.B.; Huang, Z.H.; Liu, H.; Shao, Q.; et al. Biomass-derived nitrogen-doped carbon quantum dots: Highly selective fluorescent probe for detecting Fe3+ ions and tetracyclines. J. Colloid Interface Sci. 2019, 539, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.J.; Zhang, L.Z.; Wang, Z.W.; Sun, Y.T.; Liu, Q.S.; Dong, W.; Hao, A.J. Fluorescent carbon dots based sensing system for detection of enrofloxacin in water solutions. Spectrochim. Acta Part A 2019, 219, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Cheng, Z.H.; Zhou, L. Nitrogen-doping enhanced fluorescent carbon dots: Green synthesis and their applications for bioimaging and label-free detection of Au3+ ions. ACS Sustain. Chem. Eng. 2016, 4, 3053–3061. [Google Scholar] [CrossRef]
- Guo, Y.M.; Zhang, L.F.; Cao, F.P.; Leng, Y.M. Thermal treatment of hair for the synthesis of sustainable carbon quantum dots and the applications for sensing Hg2+. Sci. Rep. 2016, 6, 35795. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.L.; Zhang, Y.; Niu, Q.Q.; Mou, M.Y.; Wu, Y.; Liu, X.X.; Yan, Z.Y.; Liao, S.H. A fluorescence probe based on the nitrogen-doped carbon dots prepared from orange juice for detecting Hg2+ in water. J. Lumin. 2017, 187, 274–280. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, Y.; Zhao, X.; Deng, Y.; Xia, Y. Facile synthesis of nitrogen-doped carbon quantum dots with chitosan for fluorescent detection of Fe3+. Polymers 2019, 11, 1731. [Google Scholar] [CrossRef] [Green Version]
- Lu, M.C.; Duan, Y.X.; Song, Y.H.; Tan, J.S.; Zhou, L. Green preparation of versatile nitrogen-doped carbon quantum dots from watermelon juice for cell imaging, detection of Fe3+ ions and cysteine, and optical thermometry. J. Mol. Liq. 2018, 269, 766–774. [Google Scholar] [CrossRef]
- Wu, M.; Fan, Y.J.; Li, J.W.; Lu, D.Q.; Guo, Y.P.; Xie, L.W.; Wu, Y.Q. Vinyl phosphate-functionalized, magnetic, molecularly-imprinted polymeric microspheres’ enrichment and carbon dots’ fluorescence-detection of organophosphorus pesticide residues. Polymers 2019, 11, 1770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.L.; Liu, M.W.; Wu, Z.P.; He, Y.; Ge, Y.L.; Song, G.W.; Zhou, J.G. Carbon dots co-doped with nitrogen and chlorine for “off-on” fluorometric determination of the activity of acetylcholinesterase and for quantification of organophosphate pesticides. Mikrochim. Acta 2019, 186, 585. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.Q.; Guo, J.Q.; Zheng, N.; Nie, Y.J.; Ye, S.; Tang, D.P. Selective determination of 2, 4, 6-trinitrophenol by using a novel carbon nanoparticles as a fluorescent probe in real sample. Anal. Bioanal. Chem. 2020, 412, 3083–3090. [Google Scholar] [CrossRef]
- Wang, M.; Gao, M.J.; Deng, L.L.; Kang, X.; Zhang, K.L.; Fu, Q.F.; Xia, Z.N.; Gao, D. A sensitive and selective fluorescent sensor for 2, 4, 6-trinitrophenol detection based on the composite material of magnetic covalent organic frameworks, molecularly imprinted polymers and carbon dots. Microchem. J. 2020, 154, 104590. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, A.Y.; Huang, Y.Y.; He, X.; Xie, X.F.; He, B.; Yang, J.H.; Wang, X.Y. Off-on fluorescent switching of boron-doped carbon quantum dots for ultrasensitive sensing of catechol and glutathione. Carbon 2020, 162, 234–244. [Google Scholar] [CrossRef]
- Zhu, P.D.; Gan, Y.; Lin, K.P.; Lin, C.; Li, S.S.; Yu, S.L.; Shi, J.H. Dual-response detection of oxidized glutathione, ascorbic acid, and cell imaging based on pH/redox dual-sensitive fluorescent carbon dots. ACS Omega 2020, 5, 4482–4489. [Google Scholar] [CrossRef]
- Pawar, S.; Togiti, U.K.; Bhattacharya, A.; Nag, A. Functionalized chitosan-carbon dots: A fluorescent probe for detecting trace amount of water in organic solvents. ACS Omega 2019, 4, 11301–11311. [Google Scholar] [CrossRef]
- Yu, D.; Wang, L.; Zhou, H.Y.; Zhang, X.J.; Wang, L.; Qiao, N. Fluorimetric detection of candida albicans using cornstalk N-carbon quantum dots modified with amphotericin B. Bioconjug. Chem. 2019, 30, 966–973. [Google Scholar] [CrossRef]
- Wang, Y.F.; Hu, A.G. Carbon quantum dots: Synthesis, properties and applications. J. Mater. Chem. C 2014, 2, 6921. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.F.; Zhang, L.L.; Lin, L.Y.; Li, X.F.; Zhao, S.L.; Liang, H. Preparation of carbon quantum dots from lycium chinensis and as a fluorescent probe for high sensitive detection of D-penicillamine. Sci. Sin. Chim. 2017, 47, 258–266. [Google Scholar] [CrossRef]
- Thambiraja, S.; Shankaran, D.R. Green synthesis of highly fluorescent carbon quantum dots from sugarcane bagasse pulp. Appl. Surf. Sci. 2016, 390, 435–443. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Kasouni, A.; Sygellou, L.; Avgeropoulos, A.; Troganis, A.; Stalikas, C. Two of a kind but different: Luminescent carbon quantum dots from Citrus peels for iron and tartrazine sensing and cell imaging. Talanta 2017, 175, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.; Wang, Y.Y.; Yang, X.M. Carbon dots derived from tobacco for visually distinguishing and detecting three kinds of tetracyclines. Nanoscale 2018, 10, 8139–8145. [Google Scholar] [CrossRef] [PubMed]
- Sangubotla, R.; Kim, J. A facile enzymatic approach for selective detection of γ-aminobutyric acid using corn-derived fluorescent carbon dots. Appl. Surf. Sci. 2019, 490, 61–69. [Google Scholar] [CrossRef]
- Kasibabu, B.S.; D’Souza, S.L.; Jha, S.; Kailasa, S.K. Imaging of bacterial and fungal cells using fluorescent carbon dots prepared from carica papaya juice. J. Fluoresc. 2015, 25, 803–810. [Google Scholar] [CrossRef]
- Prasannan, A.; Imae, T. One-pot synthesis of fluorescent carbon dots from orange waste peels. Ind. Eng. Chem. Res. 2013, 52, 15673–15678. [Google Scholar] [CrossRef]
- Wang, R.X.; Wang, X.F.; Sun, Y.M. One-step synthesis of self-doped carbon dots with highly photoluminescence as multifunctional biosensors for detection of iron ions and pH. Sens. Actuators B Chem. 2017, 241, 73–79. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.A.; Park, S.J.; Zhang, Y.F.; Kim, T.; Chae, S.; Park, M.; Kim, H.Y. One-step synthesis of robust nitrogen-doped carbon dots: Acid-evoked fluorescence enhancement and their application in Fe3+ detection. J. Mater. Chem. A 2015, 3, 17747–17754. [Google Scholar] [CrossRef]
- Wang, X.W.; Sun, G.; Routh, P.; Kim, D.-H.; Huang, W.; Chen, P. Heteroatom-doped graphene materials: Syntheses, properties and applications. Chem. Soc. Rev. 2014, 43, 7067–7098. [Google Scholar] [CrossRef] [Green Version]
- Hung, Y.H.; Dutta, D.; Tseng, C.J.; Chang, J.K.; Bhattacharyya, A.J.; Su, C.Y. Manipulation of heteroatom substitution on nitrogen and phosphorus co-doped graphene as a high active catalyst for hydrogen evolution reaction. J. Phys. Chem. C 2019, 123, 22202–22211. [Google Scholar] [CrossRef] [Green Version]
- Telychko, M.; Mutombo, P.; Ondracek, M.; Hapala, P.; Bocquet, F.; Kolorenc, J.; Vondracek, M.; Jelinek, P.; Svec, M. Achieving high-quality single-atom nitrogen doping of graphene/SiC(0001) by ion implantation and subsequent thermal stabilizatio. ACS Nano 2014, 8, 7318–7324. [Google Scholar] [CrossRef] [PubMed]
- Velez-Fort, E.; Mathieu, C.; Pallecchi, E.; Pigneur, M.; Silly, M.G.; Belkhou, R.; Marangolo, M.; Shukla, A.; Sirotti, F.; Ouerghi, A. Epitaxial graphene on 4H-SiC(0001) grown under nitrogen flux: Evidence of low nitrogen-doping and high charge transfer. ACS Nano 2012, 6, 10893–10900. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Pang, J.H.; Xu, F.; Zhang, X.M. Simple approach to synthesize amino-functionalized carbon dots by carbonization of chitosan. Sci. Rep. 2016, 6, 31100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.Y.; Mo, Z.L.; Niu, X.H.; Yang, X.; Jiang, Y.Y.; Zhao, P.; Liu, N.J.; Guo, R.B. Highly sensitive fluorescence sensor for mercury(II) based on boron- and nitrogen-co-doped graphene quantum dots. J. Colloid Interface Sci. 2020, 566, 357–368. [Google Scholar] [CrossRef]
- Ma, P.P.; Sun, X.B.; Pan, W.; Yu, G.F.; Wang, J.P. Green and orange emissive carbon dots with high quantum yields dispersed in matrices for phosphor-based white LEDs. ACS Sustain. Chem. Eng. 2020, 8, 3151–3161. [Google Scholar] [CrossRef]
- Lu, J.; Yang, J.X.; Wang, J.Z.; Lim, A.; Wang, S.; Loh, K.P. One-pot synthesis of fluorescent carbon nanoribbons, nanoparticles, and graphene by the exfoliation of graphite in ionic liquids. ACS Nano 2009, 3, 2367–2375. [Google Scholar] [CrossRef]
- Yang, X.M.; Luo, Y.W.; Zhu, S.S.; Feng, Y.J.; Zhuo, Y.; Dou, Y. One-pot synthesis of high fluorescent carbon nanoparticles and their applications as probes for detection of tetracyclines. Biosens. Bioelectron. 2014, 56, 6–11. [Google Scholar] [CrossRef]
- Martindale, B.C.M.; Hutton, G.A.M.; Caputo, C.A.; Reisner, E. Solar hydrogen production using carbon quantum dots and a molecular nickel catalyst. J. Am. Chem. Soc. 2015, 137, 6018–6025. [Google Scholar] [CrossRef]
- Carneiro Cruz, A.A.; Freire, R.M.; Froelich, D.B.; Alves de Lima, A.C.; Muniz, A.R.; Ferreira, O.P.; Fechine, P.B.A. Fluorescence based platform to discriminate protein using carbon quantum dots. ChemistrySelect 2019, 4, 5619–5627. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Aseer, K.R.; Perumal, S.; Karthik, N.; Lee, Y.R. Highly fluorescent nitrogen-doped carbon dots derived from Phyllanthus acidus utilized as a fluorescent probe for label-free selective detection of Fe3+ ions, live cell imaging and fluorescent ink. Biosens. Bioelectron. 2018, 99, 303–311. [Google Scholar] [CrossRef]
- Shi, B.F.; Su, Y.B.; Zhang, L.L.; Huang, M.J.; Liu, R.J.; Zhao, S.L. Nitrogen and phosphorus co-doped carbon nanodots as a novel fluorescent probe for highly sensitive detection of Fe3+ in human serum and living cells. ACS Appl. Mater. Interfaces 2016, 8, 10717–10725. [Google Scholar] [CrossRef] [PubMed]
- Murugana, N.; Prakash, M.; Jayakumar, M.; Sundaramurthy, A.; Sundramoorthy, A.K. Green synthesis of fluorescent carbon quantum dots from Eleusine coracana and their application as a fluorescence ‘turn-off’ sensor probe for selective detection of Cu2+. Appl. Surf. Sci. 2019, 476, 468–480. [Google Scholar] [CrossRef]
- Vaz, R.; Bettini, J.; Júnior, J.G.F.; Lima, E.D.S.; Botero, W.G.; Santos, J.C.C.; Schiavon, M.A. High luminescent carbon dots as an eco-friendly fluorescence sensor for Cr(VI) determination in water and soil samples. J. Photochem. Photobiol. A 2017, 346, 502–511. [Google Scholar] [CrossRef]
- Song, Z.Q.; Quan, F.Y.; Xu, Y.H.; Liu, M.L.; Cui, L.; Liu, J.Q. Multifunctional N,S co-doped carbon quantum dots with pH and thermo-dependent switchable fluorescent properties and highly selective detection of glutathione. Carbon 2016, 104, 169–178. [Google Scholar] [CrossRef]
- Sharma, A.; Gadly, T.; Gupta, A.; Ballal, A.; Ghosh, S.K.; Kumbhakar, M. Origin of excitation dependent fluorescence in carbon nanodots. J. Phys. Chem. Lett. 2016, 7, 3695–3702. [Google Scholar] [CrossRef]
- Goreham, R.V.; Schroeder, K.L.; Holmes, A.; Bradley, S.J.; Nann, T. Demonstration of the lack of cytotoxicity of unmodified and folic acid modified graphene oxide quantum dots, and their application to fluorescence lifetime imaging of HaCaT cells. Mikrochim. Acta 2018, 185, 128. [Google Scholar] [CrossRef]
- Becker, W. Fluorescence lifetime imaging-techniques and applications. J. Microsc. 2012, 247, 119–136. [Google Scholar] [CrossRef]
- Li, Q.; Luo, T.Y.; Zhou, M.; Abroshan, H.; Huang, J.; Kim, H.J.; Rosi, N.L.; Shao, Z.; Jin, R. Silicon nanoparticles with surface nitrogen: 90% quantum yield with narrow luminescence bandwidth and the ligand structure based energy law. ACS Nano 2016, 10, 8385–8393. [Google Scholar] [CrossRef]
- Chandra, S.; Ghosh, B.; Beaune, G.; Nagarajan, U.; Yasui, T.; Nakamura, J.; Tsuruoka, T.; Baba, Y.; Shirahata, N.; Winnik, F.M. Functional double-shelled silicon nanocrystals for two-photon fluorescence cell imaging: Spectral evolution and tuning. Nanoscale 2016, 8, 9009–9019. [Google Scholar] [CrossRef]
- Fang, Y.X.; Guo, S.J.; Li, D.; Zhu, C.Z.; Ren, W.; Dong, S.J.; Wang, E.K. Easy synthesis and imaging applications of cross-linked green fluorescent hollow carbon nanoparticles. ACS Nano 2012, 6, 400–409. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, T.; Cai, Y.; Chen, X.Y.; Shang, S.M.; Li, J. Highly fluorescent N, S-co-doped carbon dots: Synthesis and multiple applications. New J. Chem. 2017, 41, 11125–11137. [Google Scholar] [CrossRef]
- Meng, A.; Xu, Q.H.; Zhao, K.; Li, Z.J.; Liang, J.; Li, Q.D. A highly selective and sensitive “on-off-on” fluorescent probe for detecting Hg(II) based on Au/N-doped carbon quantum dots. Sens. Actuators B Chem. 2018, 255, 657–665. [Google Scholar] [CrossRef]
- Xu, S.H.; Li, X.L.; Mao, Y.N.; Gao, T.; Feng, X.Y.; Luo, X.L. Novel dual ligand co-functionalized fluorescent gold nanoclusters as a versatile probe for sensitive analysis of Hg2+ and oxytetracycline. Anal. Bioanal. Chem. 2016, 408, 2955–2962. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.Q.; Yi, X.F.; Wu, Q.; Zhu, Y.C.; Ou, M.R.; Xu, X.P. First report on a Bodipy-based fluorescent probe for sensitive detection of oxytetracycline: Application for the rapid determination of oxytetracycline in milk, honey and pork. RSC Adv. 2016, 6, 89288–89297. [Google Scholar] [CrossRef]




| Method | Linear Range (µmol L−1) | Detection Limit (µmol L−1) | Citations |
|---|---|---|---|
| N-CQDs fluorescent probe | 3.32–32.26 | 0.3739 | [24] |
| Fluorescent sensor for OTC based on SiNPs | 0.2–20 | 0.18 | [4] |
| Gold nanoclusters fluorescent probe | 0.375–12.5 | 0.15 | [73] |
| BODIPY fluorescent probe | 0–42 | 0.72 | [74] |
| ON-CQDs fluorescent probe | 2–100 | 0.973 | This work |
| WN-CQDs fluorescent probe | 0.25–100 | 0.077 | This work |
| Samples | Spiked | Measured Concentration | Recovery Rate (%) | RSD (%, n = 5) |
|---|---|---|---|---|
| Lake water 1 | 5 µmol L−1 | 4.870 µmol L−1 | 97.408 | 0.62% |
| Tap water 1 | 10 µmol L−1 | 9.754 µmol L−1 | 97.544 | 0.94% |
| Soil 1 | 12.240 µmol Kg−1 | 12.632 µmol Kg−1 | 103.206 | 0.82% |
| Tap water 1 | 30 µmol L−1 | 27.517 µmol L−1 | 91.724 | 1.06% |
| Lake water 1 | 40 µmol L−1 | 40.341 µmol L−1 | 100.853 | 0.62% |
| Soil 1 | 30.600 µmol Kg−1 | 31.468 µmol Kg−1 | 102.837 | 0.88% |
| Tap water 2 | 2 µmol L−1 | 1.844µmol L−1 | 92.203 | 0.84% |
| Soil 2 | 9.180 µmol Kg−1 | 9.255 µmol Kg−1 | 100.826 | 0.81% |
| Lake water 2 | 20 µmol L−1 | 19.399µmol L−1 | 96.996 | 1.05% |
| Tap water 2 | 35 µmol L−1 | 34.514µmol L−1 | 98.613 | 0.94% |
| Lake water 2 | 45 µmol L−1 | 45.311µmol L−1 | 100.691 | 0.88% |
| Soil 2 | 30.600 µmol Kg−1 | 31.469 µmol Kg−1 | 102.844 | 5.35% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, R.; Wu, Z.; Wang, L.; Liu, J.; Deng, Y.; Xiao, Z.; Fang, J.; Liang, Y. Green Preparation of Fluorescent Nitrogen-Doped Carbon Quantum Dots for Sensitive Detection of Oxytetracycline in Environmental Samples. Nanomaterials 2020, 10, 1561. https://doi.org/10.3390/nano10081561
Gao R, Wu Z, Wang L, Liu J, Deng Y, Xiao Z, Fang J, Liang Y. Green Preparation of Fluorescent Nitrogen-Doped Carbon Quantum Dots for Sensitive Detection of Oxytetracycline in Environmental Samples. Nanomaterials. 2020; 10(8):1561. https://doi.org/10.3390/nano10081561
Chicago/Turabian StyleGao, Rong, Zhibin Wu, Li Wang, Jiao Liu, Yijun Deng, Zhihua Xiao, Jun Fang, and Yunshan Liang. 2020. "Green Preparation of Fluorescent Nitrogen-Doped Carbon Quantum Dots for Sensitive Detection of Oxytetracycline in Environmental Samples" Nanomaterials 10, no. 8: 1561. https://doi.org/10.3390/nano10081561





