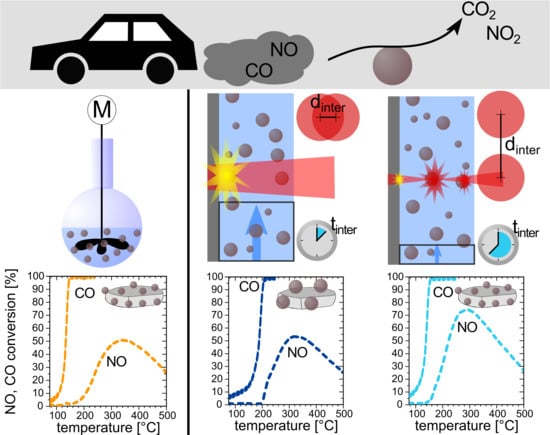
Increasing the Size-Selectivity in Laser-Based g/h Liquid Flow Synthesis of Pt and PtPd Nanoparticles for CO and NO Oxidation in Industrial Automotive Exhaust Gas Treatment Benchmarking
Abstract
:1. Introduction
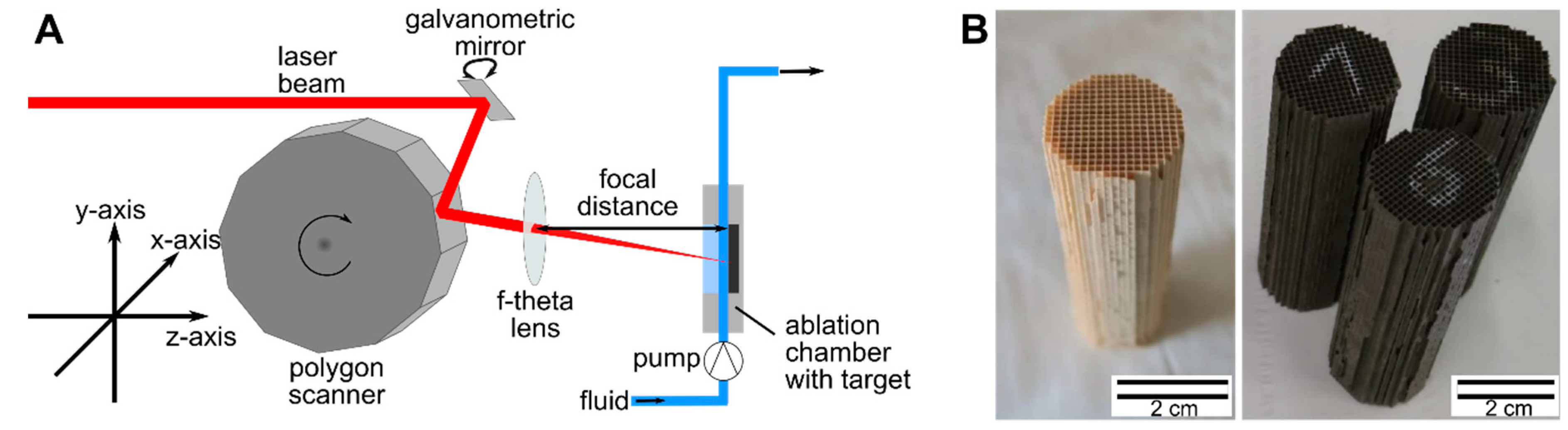
2. Methods and Materials
2.1. Pulsed Laser Ablation in Liquid
2.2. Preparation of PtPd Catalysts and Catalytic Testing
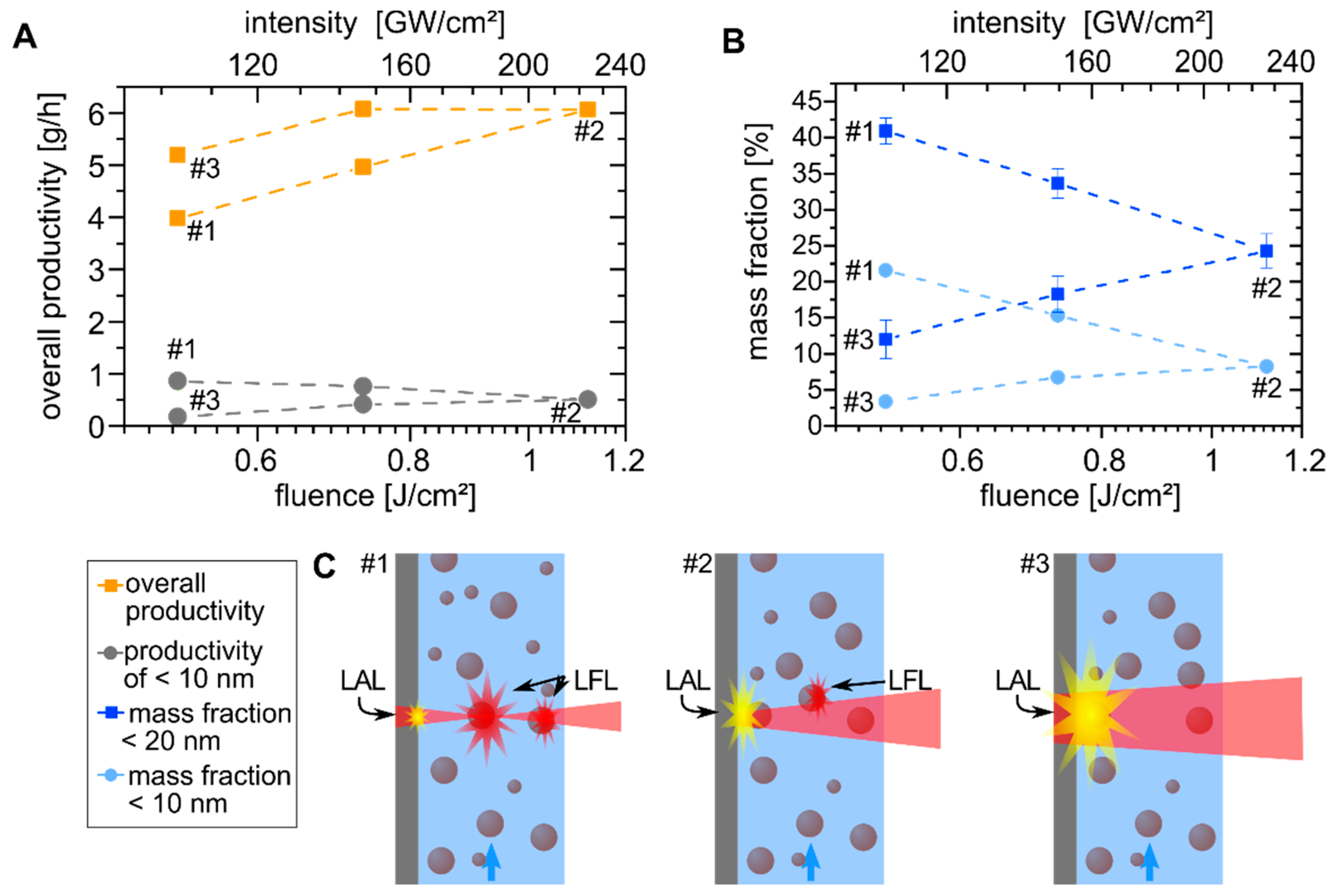
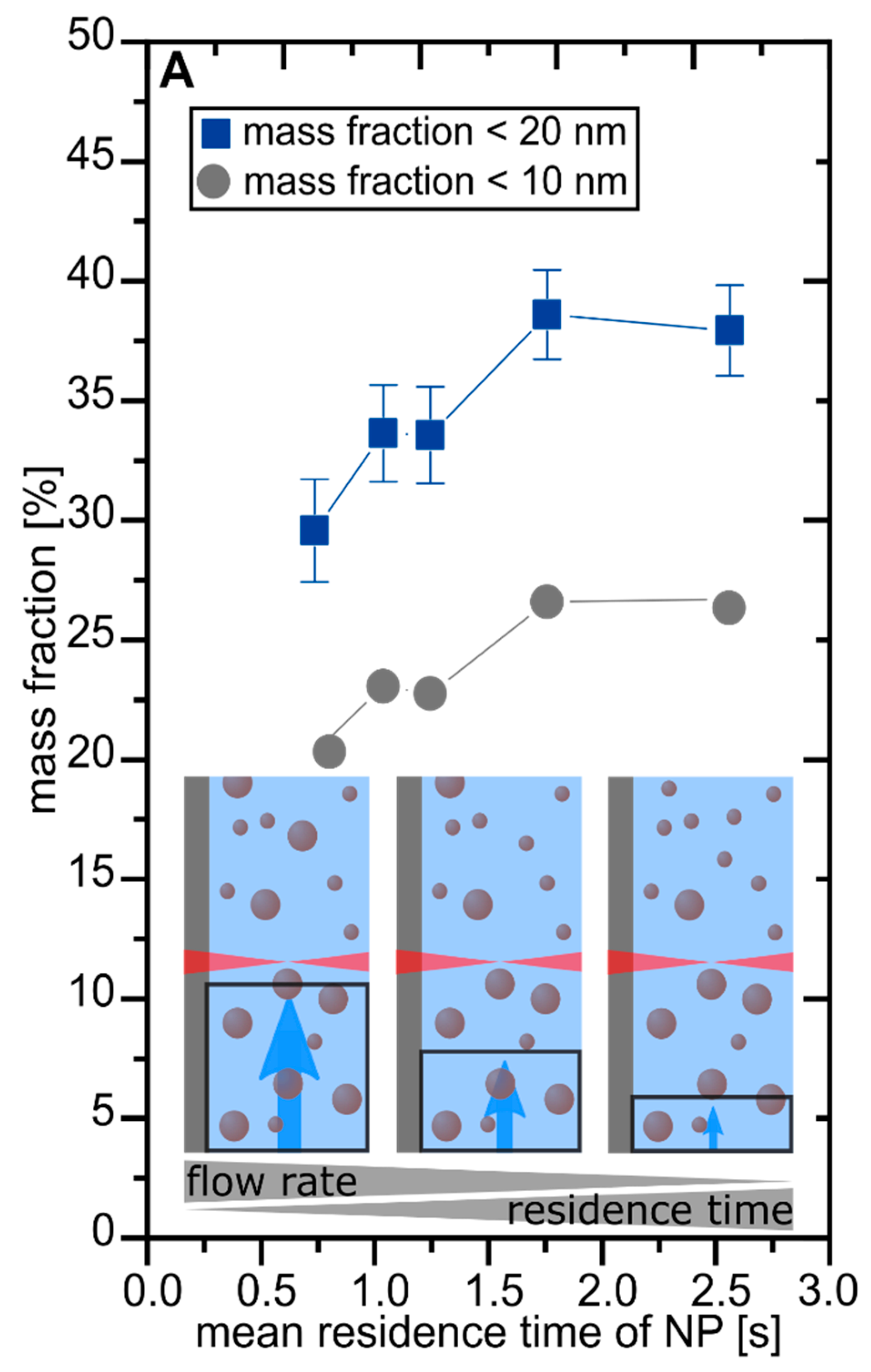
3. Results and Discussion
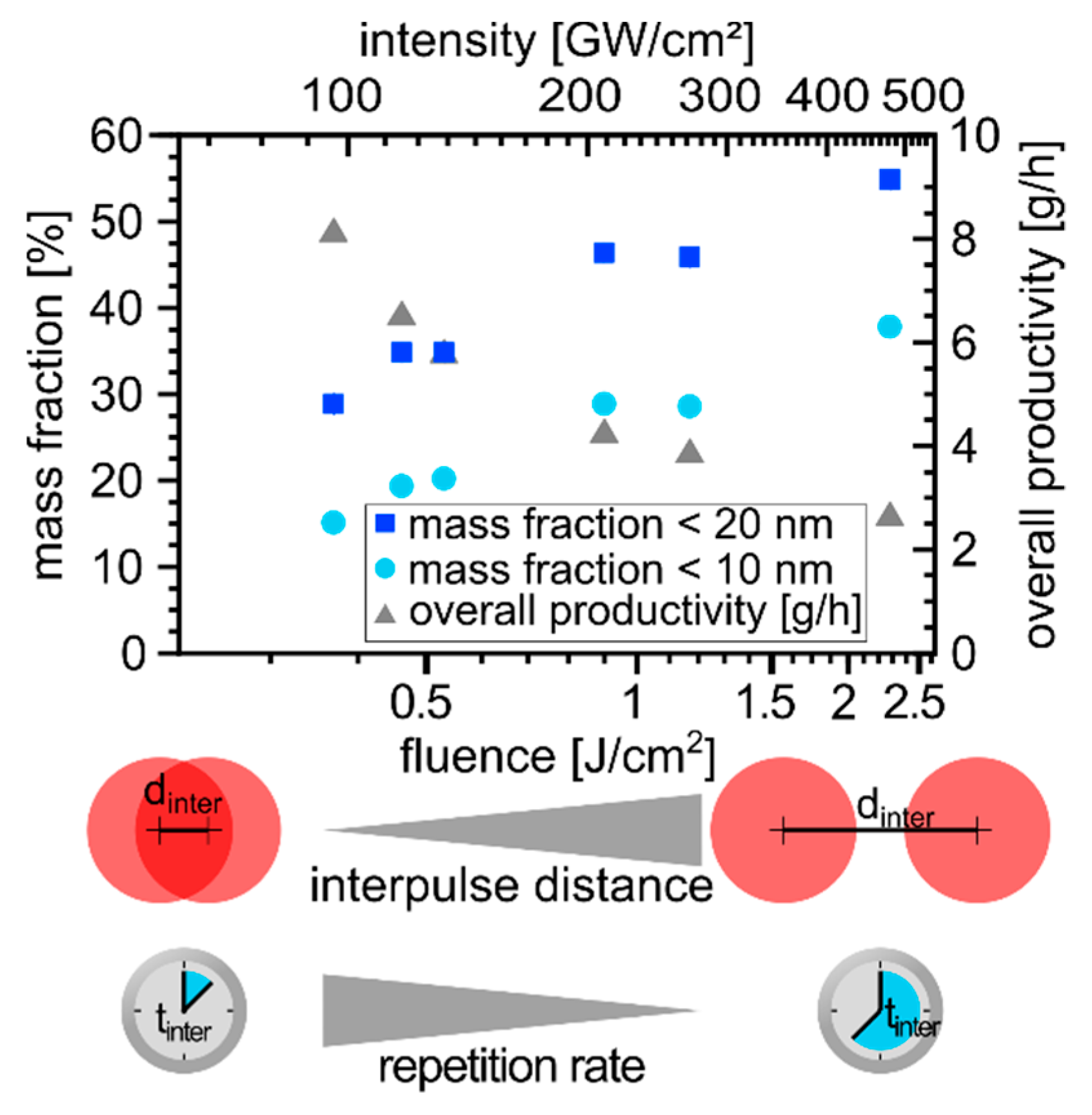
3.1. Determination of Laser Parameter Influence on the Nanoparticle Size Fraction <10 nm
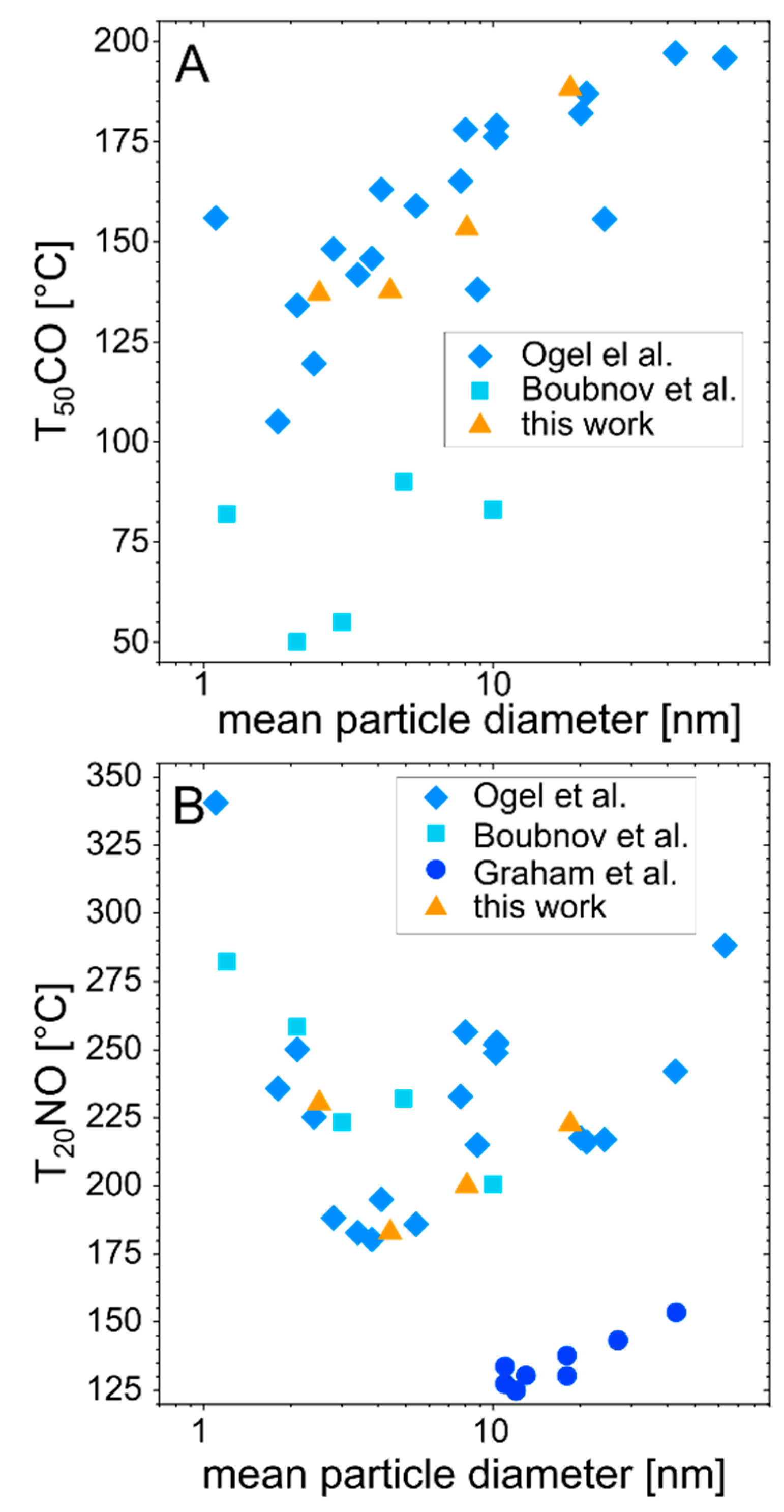
3.2. Examination of the Catalytic Activity of Laser-Generated PtPd Nanoparticles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gremminger, A.; Pihl, J.; Casapu, M.; Grunwaldt, J.-D.; Toops, T.J.; Deutschmann, O. PGM based catalysts for exhaust-gas after-treatment under typical diesel, gasoline and gas engine conditions with focus on methane and formaldehyde oxidation. Appl. Catal. B Environ. 2020, 265, 118571. [Google Scholar] [CrossRef]
- Kunwar, D.; Zhou, S.; DeLaRiva, A.; Peterson, E.J.; Xiong, H.; Pereira-Hernández, X.I.; Purdy, S.C.; ter Veen, R.; Brongersma, H.H.; Miller, J.T.; et al. Stabilizing High Metal Loadings of Thermally Stable Platinum Single Atoms on an Industrial Catalyst Support. ACS Catal. 2019, 9, 3978–3990. [Google Scholar] [CrossRef]
- Ogel, E.; Casapu, M.; Doronkin, D.E.; Popescu, R.; Störmer, H.; Mechler, C.; Marzun, G.; Barcikowski, S.; Türk, M.; Grunwaldt, J.-D. Impact of Preparation Method and Hydrothermal Aging on Particle Size Distribution of Pt/γ-Al2O3 and Its Performance in CO and NO Oxidation. J. Phys. Chem. C 2019, 123, 5433–5446. [Google Scholar] [CrossRef]
- Persson, K.; Ersson, A.; Colussi, S.; Trovarelli, A.; Järås, S.G. Catalytic combustion of methane over bimetallic Pd–Pt catalysts: The influence of support materials. Appl. Catal. B Environ. 2006, 66, 175–185. [Google Scholar] [CrossRef]
- Boubnov, A.; Dahl, S.; Johnson, E.; Molina, A.P.; Simonsen, S.B.; Cano, F.M.; Helveg, S.; Lemus-Yegres, L.J.; Grunwaldt, J.-D. Structure–activity relationships of Pt/Al2O3 catalysts for CO and NO oxidation at diesel exhaust conditions. Appl. Catal. B Environ. 2012, 126, 315–325. [Google Scholar] [CrossRef]
- Berkó, A.; Szökő, J.; Solymosi, F. Effect of CO on the morphology of Pt nanoparticles supported on TiO2(110)-(1 × n). Surf. Sci. 2004, 566–568, 337–342. [Google Scholar] [CrossRef]
- Xiong, H.; Peterson, E.; Qi, G.; Datye, A.K. Trapping mobile Pt species by PdO in diesel oxidation catalysts: Smaller is better. Catal. Today 2016, 272, 80–86. [Google Scholar] [CrossRef] [Green Version]
- Olsson, L.; Fridell, E. The Influence of Pt Oxide Formation and Pt Dispersion on the Reactions NO2⇔NO+1/2 O2 over Pt/Al2O3 and Pt/BaO/Al2O3. J. Catal. 2002, 210, 340–353. [Google Scholar] [CrossRef]
- Graham, G.W.; Jen, H.-W.; Ezekoye, O.; Kudla, R.J.; Chun, W.; Pan, X.Q.; McCabe, R.W. Effect of alloy composition on dispersion stability and catalytic activity for NO oxidation over alumina-supported Pt–Pd catalysts. Catal. Lett. 2007, 116, 1–8. [Google Scholar] [CrossRef]
- Xue, E.; Seshan, K.; Ross, J. Roles of supports, Pt loading and Pt dispersion in the oxidation of NO to NO2 and of SO2 to SO3. Appl. Catal. B Environ. 1996, 11, 65–79. [Google Scholar] [CrossRef] [Green Version]
- Lira, E.; Merte, L.R.; Behafarid, F.; Ono, L.K.; Zhang, L.; Roldan Cuenya, B. Role and Evolution of Nanoparticle Structure and Chemical State during the Oxidation of NO over Size- and Shape-Controlled Pt/γ-Al2O3 Catalysts under Operando Conditions. ACS Catal. 2014, 4, 1875–1884. [Google Scholar] [CrossRef]
- Simonsen, S.B.; Chorkendorff, I.; Dahl, S.; Skoglundh, M.; Sehested, J.; Helveg, S. Direct observations of oxygen-induced platinum nanoparticle ripening studied by in situ TEM. J. Am. Chem. Soc. 2010, 132, 7968–7975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, T.W.; Delariva, A.T.; Challa, S.R.; Datye, A.K. Sintering of catalytic nanoparticles: Particle migration or Ostwald ripening? Acc. Chem. Res. 2013, 46, 1720–1730. [Google Scholar] [CrossRef] [PubMed]
- Pazmiño, J.H.; Miller, J.T.; Mulla, S.S.; Nicholas Delgass, W.; Ribeiro, F.H. Kinetic studies of the stability of Pt for NO oxidation: Effect of sulfur and long-term aging. J. Catal. 2011, 282, 13–24. [Google Scholar] [CrossRef]
- Fridell, E.; Amberntsson, A.; Olsson, L.; Grant, A.W.; Skoglundh, M. Platinum Oxidation and Sulphur Deactivation in NOx Storage Catalysts. Top. Catal. 2004, 30/31, 143–146. [Google Scholar] [CrossRef]
- Salomons, S.; Hayes, R.E.; Votsmeier, M.; Drochner, A.; Vogel, H.; Malmberg, S.; Gieshoff, J. On the use of mechanistic CO oxidation models with a platinum monolith catalyst. Appl. Catal. B Environ. 2007, 70, 305–313. [Google Scholar] [CrossRef]
- Carrillo, C.; Johns, T.R.; Xiong, H.; DeLaRiva, A.; Challa, S.R.; Goeke, R.S.; Artyushkova, K.; Li, W.; Kim, C.H.; Datye, A.K. Trapping of Mobile Pt Species by PdO Nanoparticles under Oxidizing Conditions. J. Phys. Chem. Lett. 2014, 5, 2089–2093. [Google Scholar] [CrossRef]
- Mulla, S.; Chen, N.; Cumaranatunge, L.; Blau, G.; Zemlyanov, D.; Delgass, W.; Epling, W.; Ribeiro, F. Reaction of NO and O2 to NO2 on Pt: Kinetics and catalyst deactivation. J. Catal. 2006, 241, 389–399. [Google Scholar] [CrossRef]
- Duan, Z.; Henkelman, G. CO Oxidation on the Pd(111) Surface. ACS Catal. 2014, 4, 3435–3443. [Google Scholar] [CrossRef]
- Xu, Y.; Getman, R.B.; Shelton, W.A.; Schneider, W.F. A first-principles investigation of the effect of Pt cluster size on CO and NO oxidation intermediates and energetics. Phys. Chem. Chem. Phys. 2008, 10, 6009–6018. [Google Scholar] [CrossRef]
- Auvray, X.; Olsson, L. Stability and activity of Pd-, Pt- and Pd–Pt catalysts supported on alumina for NO oxidation. Appl. Catal. B Environ. 2015, 168–169, 342–352. [Google Scholar] [CrossRef]
- Russell, A.; Epling, W.S. Diesel Oxidation Catalysts. Catal. Rev. 2011, 53, 337–423. [Google Scholar] [CrossRef]
- Blommaerts, N.; Vanrompay, H.; Nuti, S.; Lenaerts, S.; Bals, S.; Verbruggen, S.W. Unraveling Structural Information of Turkevich Synthesized Plasmonic Gold-Silver Bimetallic Nanoparticles. Small 2019, 15, e1902791. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Kusada, K.; Wu, D.; Ogiwara, N.; Yamamoto, T.; Toriyama, T.; Matsumura, S.; Kawaguchi, S.; Kubota, Y.; Honma, T.; et al. Solid-solution alloy nanoparticles of a combination of immiscible Au and Ru with a large gap of reduction potential and their enhanced oxygen evolution reaction performance. Chem. Sci. 2019, 10, 5133–5137. [Google Scholar] [CrossRef] [Green Version]
- Marzun, G.; Levish, A.; Mackert, V.; Kallio, T.; Barcikowski, S.; Wagener, P. Laser synthesis, structure and chemical properties of colloidal nickel-molybdenum nanoparticles for the substitution of noble metals in heterogeneous catalysis. J. Colloid Interface Sci. 2017, 489, 57–67. [Google Scholar] [CrossRef]
- Oko, D.N.; Zhang, J.; Garbarino, S.; Chaker, M.; Ma, D.; Tavares, A.C.; Guay, D. Formic acid electro-oxidation at PtAu alloyed nanoparticles synthesized by pulsed laser ablation in liquids. J. Power Sources 2014, 248, 273–282. [Google Scholar] [CrossRef]
- Zhang, J.; Oko, D.N.; Garbarino, S.; Imbeault, R.; Chaker, M.; Tavares, A.C.; Guay, D.; Ma, D. Preparation of PtAu Alloy Colloids by Laser Ablation in Solution and Their Characterization. J. Phys. Chem. C 2012, 116, 13413–13420. [Google Scholar] [CrossRef]
- Hu, S.; Tian, M.; Ribeiro, E.L.; Duscher, G.; Mukherjee, D. Tandem laser ablation synthesis in solution-galvanic replacement reaction (LASiS-GRR) for the production of PtCo nanoalloys as oxygen reduction electrocatalysts. J. Power Sources 2016, 306, 413–423. [Google Scholar] [CrossRef]
- Prymak, O.; Jakobi, J.; Rehbock, C.; Epple, M.; Barcikowski, S. Crystallographic characterization of laser-generated, polymer-stabilized 4 nm silver-gold alloyed nanoparticles. Mater. Chem. Phys. 2018, 207, 442–450. [Google Scholar] [CrossRef]
- Neumeister, A.; Jakobi, J.; Rehbock, C.; Moysig, J.; Barcikowski, S. Monophasic ligand-free alloy nanoparticle synthesis determinants during pulsed laser ablation of bulk alloy and consolidated microparticles in water. Phys. Chem. Chem. Phys. 2014, 16, 23671–23678. [Google Scholar] [CrossRef] [Green Version]
- Censabella, M.; Torrisi, V.; Boninelli, S.; Bongiorno, C.; Grimaldi, M.G.; Ruffino, F. Laser ablation synthesis of mono- and bimetallic Pt and Pd nanoparticles and fabrication of Pt-Pd/Graphene nanocomposites. Appl. Surf. Sci. 2019, 475, 494–503. [Google Scholar] [CrossRef]
- Reichenberger, S.; Marzun, G.; Barcikowski, S.; Muhler, M. Perspective of surfactant-free colloidal nanoparticles in heterogeneous catalysis. ChemCatChem 2019, 11, 4489–4518. [Google Scholar] [CrossRef]
- Marzun, G.; Nakamura, J.; Zhang, X.; Barcikowski, S.; Wagener, P. Size control and supporting of palladium nanoparticles made by laser ablation in saline solution as a facile route to heterogeneous catalysts. Appl. Surf. Sci. 2015, 348, 75–84. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, G.; Chaker, M.; Rosei, F.; Ma, D. Gold nanoparticle decorated ceria nanotubes with significantly high catalytic activity for the reduction of nitrophenol and mechanism study. Appl. Catal. B Environ. 2013, 132–133, 107–115. [Google Scholar] [CrossRef]
- Kohsakowski, S.; Streubel, R.; Radev, I.; Peinecke, V.; Barcikowski, S.; Marzun, G.; Reichenberger, S. First PEM fuel cell based on ligand-free, laser-generated platinum nanoparticles. Appl. Surf. Sci. 2019, 467–468, 486–492. [Google Scholar] [CrossRef]
- Shokouhimehr, M. Magnetically Separable and Sustainable Nanostructured Catalysts for Heterogeneous Reduction of Nitroaromatics. Catalysts 2015, 5, 534–560. [Google Scholar] [CrossRef]
- Feng, Y.; Li, Z.; Liu, H.; Dong, C.; Wang, J.; Kulinich, S.A.; Du, X. Laser-Prepared CuZn Alloy Catalyst for Selective Electrochemical Reduction of CO2 to Ethylene. Langmuir 2018, 34, 13544–13549. [Google Scholar] [CrossRef]
- Shih, C.-Y.; Shugaev, M.V.; Wu, C.; Zhigilei, L.V. The effect of pulse duration on nanoparticle generation in pulsed laser ablation in liquids: Insights from large-scale atomistic simulations. Phys. Chem. Chem. Phys. 2020, 13, 7077–7099. [Google Scholar] [CrossRef]
- Jendrzej, S.; Goekce, B.; Epple, M.; Barcikowski, S. How Size Determines the Value of Gold: Economic Aspects of Wet Chemical and Laser-Based Metal Colloid Synthesis. Chem. Phys. Phys. Chem. 2017, 18, 1012–1019. [Google Scholar] [CrossRef]
- Streubel, R.; Barcikowski, S.; Goekce, B. Continuous multigram nanoparticle synthesis by high-power, high-repetition-rate ultrafast laser ablation in liquids. Opt. Lett. 2016, 41, 1486–1489. [Google Scholar] [CrossRef]
- Kohsakowski, S.; Seiser, F.; Wiederrecht, J.-P.; Reichenberger, S.; Vinnay, T.; Barcikowski, S.; Marzun, G. Effective size separation of laser-generated, surfactant-free nanoparticles by continuous centrifugation. Nanotechnology 2019, 31, 95603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dittrich, S.; Streubel, R.; McDonnell, C.; Huber, H.P.; Barcikowski, S.; Gökce, B. Comparison of the productivity and ablation efficiency of different laser classes for laser ablation of gold in water and air. Appl. Phys. A 2019, 125, 432. [Google Scholar] [CrossRef]
- Shih, C.-Y.; Streubel, R.; Heberle, J.; Letzel, A.; Shugaev, M.V.; Wu, C.; Schmidt, M.; Gökce, B.; Barcikowski, S.; Zhigilei, L.V. Two mechanisms of nanoparticle generation in picosecond laser ablation in liquids: The origin of the bimodal size distribution. Nanoscale 2018, 10, 6900–6910. [Google Scholar] [CrossRef] [Green Version]
- Haxhiaj, I.; Tigges, S.; Firla, D.; Zhang, X.; Hagemann, U.; Kondo, T.; Nakamura, J.; Marzun, G.; Barcikowski, S. Platinum nanoparticles supported on reduced graphene oxide prepared in situ by a continuous one-step laser process. Appl. Surf. Sci. 2019, 469, 811–820. [Google Scholar] [CrossRef]
- Mafuné, F.; Okamoto, T.; Ito, M. Surfactant-free small Ni nanoparticles trapped on silica nanoparticles prepared by pulsed laser ablation in liquid. Chem. Phys. Lett. 2014, 591, 193–196. [Google Scholar] [CrossRef]
- Bonaccorso, F.; Zerbetto, M.; Ferrari, A.C.; Amendola, V. Sorting Nanoparticles by Centrifugal Fields in Clean Media. J. Phys. Chem. C 2013, 117, 13217–13229. [Google Scholar] [CrossRef]
- Mafuné, F.; Kohno, J.-Y.; Takeda, Y.; Kondow, T. Growth of Gold Clusters into Nanoparticles in a Solution Following Laser-Induced Fragmentation. J. Phys. Chem. B 2002, 106, 8555–8561. [Google Scholar] [CrossRef]
- Hashimoto, S.; Werner, D.; Uwada, T. Studies on the interaction of pulsed lasers with plasmonic gold nanoparticles toward light manipulation, heat management, and nanofabrication. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 28–54. [Google Scholar] [CrossRef]
- Amendola, V.; Fortunati, I.; Marega, C.; Abdelhady, A.L.; Saidaminov, M.I.; Bakr, O.M. High-Purity Hybrid Organolead Halide Perovskite Nanoparticles Obtained by Pulsed-Laser Irradiation in Liquid. Chemphyschem 2017, 18, 1047–1054. [Google Scholar] [CrossRef]
- Ziefuß, A.R.; Reichenberger, S.; Rehbock, C.; Chakraborty, I.; Gharib, M.; Parak, W.J.; Barcikowski, S. Laser Fragmentation of Colloidal Gold Nanoparticles with High-Intensity Nanosecond Pulses is Driven by a Single-Step Fragmentation Mechanism with a Defined Educt Particle-Size Threshold. J. Phys. Chem. C 2018, 122, 22125–22136. [Google Scholar] [CrossRef]
- Lin, J.-Y.; Xi, C.; Li, Z.; Feng, Y.; Wu, D.-Y.; Dong, C.-K.; Yao, P.; Liu, H.; Du, X.-W. Lattice-strained palladium nanoparticles as active catalysts for the oxygen reduction reaction. Chem. Commun. (Camb.) 2019, 55, 3121–3123. [Google Scholar] [CrossRef] [PubMed]
- Amendola, V.; Amans, D.; Ishikawa, Y.; Koshizaki, N.; Scirè, S.; Compagnini, G.; Reichenberger, S.; Barcikowski, S. Room-temperature laser synthesis in liquid of oxide, metal-oxide core-shells and doped oxide nanoparticles. Chemistry 2020. [Google Scholar] [CrossRef] [PubMed]
- Wagener, P.; Schwenke, A.; Chichkov, B.N.; Barcikowski, S. Pulsed Laser Ablation of Zinc in Tetrahydrofuran: Bypassing the Cavitation Bubble. J. Phys. Chem. C 2010, 114, 7618–7625. [Google Scholar] [CrossRef]
- Jendrzej, S.; Gökce, B.; Amendola, V.; Barcikowski, S. Barrierless growth of precursor-free, ultrafast laser-fragmented noble metal nanoparticles by colloidal atom clusters—A kinetic in situ study. J. Colloid Interface Sci. 2016, 463, 299–307. [Google Scholar] [CrossRef]
- Marzun, G.; Streich, C.; Jendrzej, S.; Barcikowski, S.; Wagener, P. Adsorption of colloidal platinum nanoparticles to supports: Charge transfer and effects of electrostatic and steric interactions. Langmuir 2014, 30, 11928–11936. [Google Scholar] [CrossRef]
- Zhang, D.; Gökce, B.; Barcikowski, S. Laser Synthesis and Processing of Colloids: Fundamentals and Applications. Chem. Rev. 2017, 117, 3990–4103. [Google Scholar] [CrossRef]
- Feng, Q.; Moloney, J.V.; Newell, A.C.; Wright, E.M.; Cook, K.; Kennedy, P.K.; Hammer, D.X.; Rockwell, B.A.; Thompson, C.R. Theory and simulation on the threshold of water breakdown induced by focused ultrashort laser pulses. IEEE J. Quantum Electron. 1997, 33, 127–137. [Google Scholar] [CrossRef]
- Noack, J.; Vogel, A. Laser-induced plasma formation in water at nanosecond to femtosecond time scales: Calculation of thresholds, absorption coefficients, and energy density. IEEE J. Quantum Electron. 1999, 35, 1156–1167. [Google Scholar] [CrossRef] [Green Version]
- Neuenschwander, B.; Jaeggi, B.; Schmid, M.; Hennig, G. Surface Structuring with Ultra-short Laser Pulses: Basics, Limitations and Needs for High Throughput. Phys. Procedia 2014, 56, 1047–1058. [Google Scholar] [CrossRef] [Green Version]
- Waag, F.; Gökce, B.; Barcikowski, S. Ablation target cooling by maximizing the nanoparticle productivity in laser synthesis of colloids. Appl. Surf. Sci. 2019, 466, 647–656. [Google Scholar] [CrossRef]
- Kalus, M.-R.; Lanyumba, R.; Lorenzo-Parodi, N.; Jochmann, M.A.; Kerpen, K.; Hagemann, U.; Schmidt, T.C.; Barcikowski, S.; Gökce, B. Determining the role of redox-active materials during laser-induced water decomposition. Phys. Chem. Chem. Phys. 2019, 21, 18636–18651. [Google Scholar] [CrossRef] [PubMed]
- Pyatenko, A.; Wang, H.; Koshizaki, N.; Tsuji, T. Mechanism of pulse laser interaction with colloidal nanoparticles. Laser Photonics Rev. 2013, 7, 596–604. [Google Scholar] [CrossRef]
- Ziefuss, A.R.; Reich, S.; Reichenberger, S.; Levantino, M.; Plech, A. In situ structural kinetics of picosecond laser-induced heating and fragmentation of colloidal gold spheres. Phys. Chem. Chem. Phys. 2020, 22, 4993–5001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casapu, M.; Grunwaldt, J.; Maciejewski, M.; Baiker, A.; Eckhoff, S.; Gobel, U.; Wittrock, M. The fate of platinum in Pt/Ba/CeO2 and Pt/Ba/Al2O3 catalysts during thermal aging. J. Catal. 2007, 251, 28–38. [Google Scholar] [CrossRef]

| T50CO (°C) | T20NO (°C) | Maximal NO Conversion (%) | ||||
|---|---|---|---|---|---|---|
| Fresh | Aged | Fresh | Aged | Fresh | Aged | |
| reference, 2.5 nm | 117 | 137 | 219 | 230 | 55 | 51 |
| supernatant, 4.4 nm | 97 | 138 | 188 | 183 | 80 | 74 |
| raw colloid, 8.1 nm | 168 | 153 | 198 | 200 | 73 | 66 |
| re-dispersed, 18 nm | 163 | 188 | 222 | 223 | 52 | 54 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dittrich, S.; Kohsakowski, S.; Wittek, B.; Hengst, C.; Gökce, B.; Barcikowski, S.; Reichenberger, S. Increasing the Size-Selectivity in Laser-Based g/h Liquid Flow Synthesis of Pt and PtPd Nanoparticles for CO and NO Oxidation in Industrial Automotive Exhaust Gas Treatment Benchmarking. Nanomaterials 2020, 10, 1582. https://doi.org/10.3390/nano10081582
Dittrich S, Kohsakowski S, Wittek B, Hengst C, Gökce B, Barcikowski S, Reichenberger S. Increasing the Size-Selectivity in Laser-Based g/h Liquid Flow Synthesis of Pt and PtPd Nanoparticles for CO and NO Oxidation in Industrial Automotive Exhaust Gas Treatment Benchmarking. Nanomaterials. 2020; 10(8):1582. https://doi.org/10.3390/nano10081582
Chicago/Turabian StyleDittrich, S., S. Kohsakowski, B. Wittek, C. Hengst, B. Gökce, S. Barcikowski, and S. Reichenberger. 2020. "Increasing the Size-Selectivity in Laser-Based g/h Liquid Flow Synthesis of Pt and PtPd Nanoparticles for CO and NO Oxidation in Industrial Automotive Exhaust Gas Treatment Benchmarking" Nanomaterials 10, no. 8: 1582. https://doi.org/10.3390/nano10081582