Nanoengineering in Cardiac Regeneration: Looking Back and Going Forward
Abstract
:1. Introduction: Clinical Need
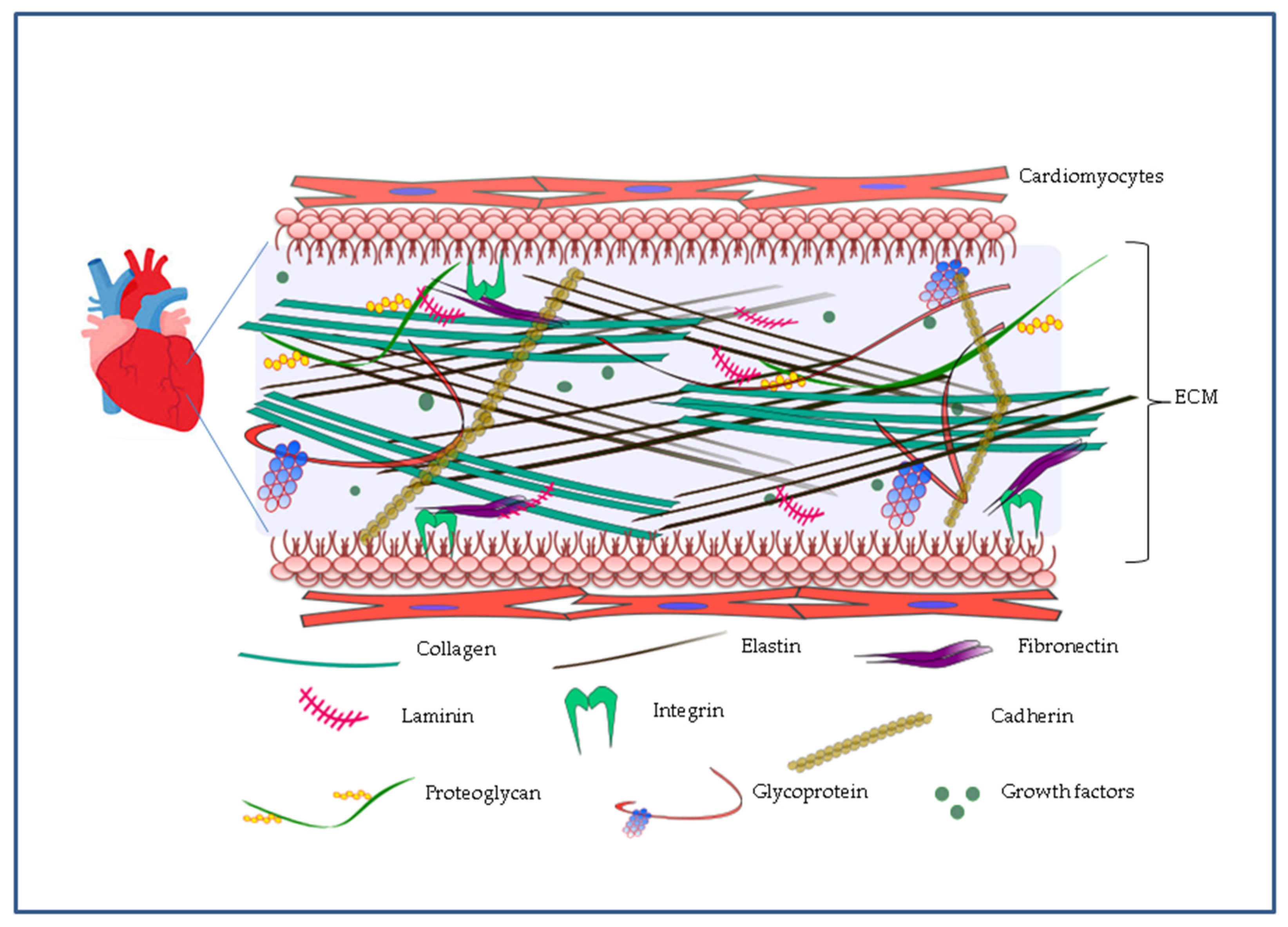
2. Cardiac ECM as a Guide to Nanoengineering
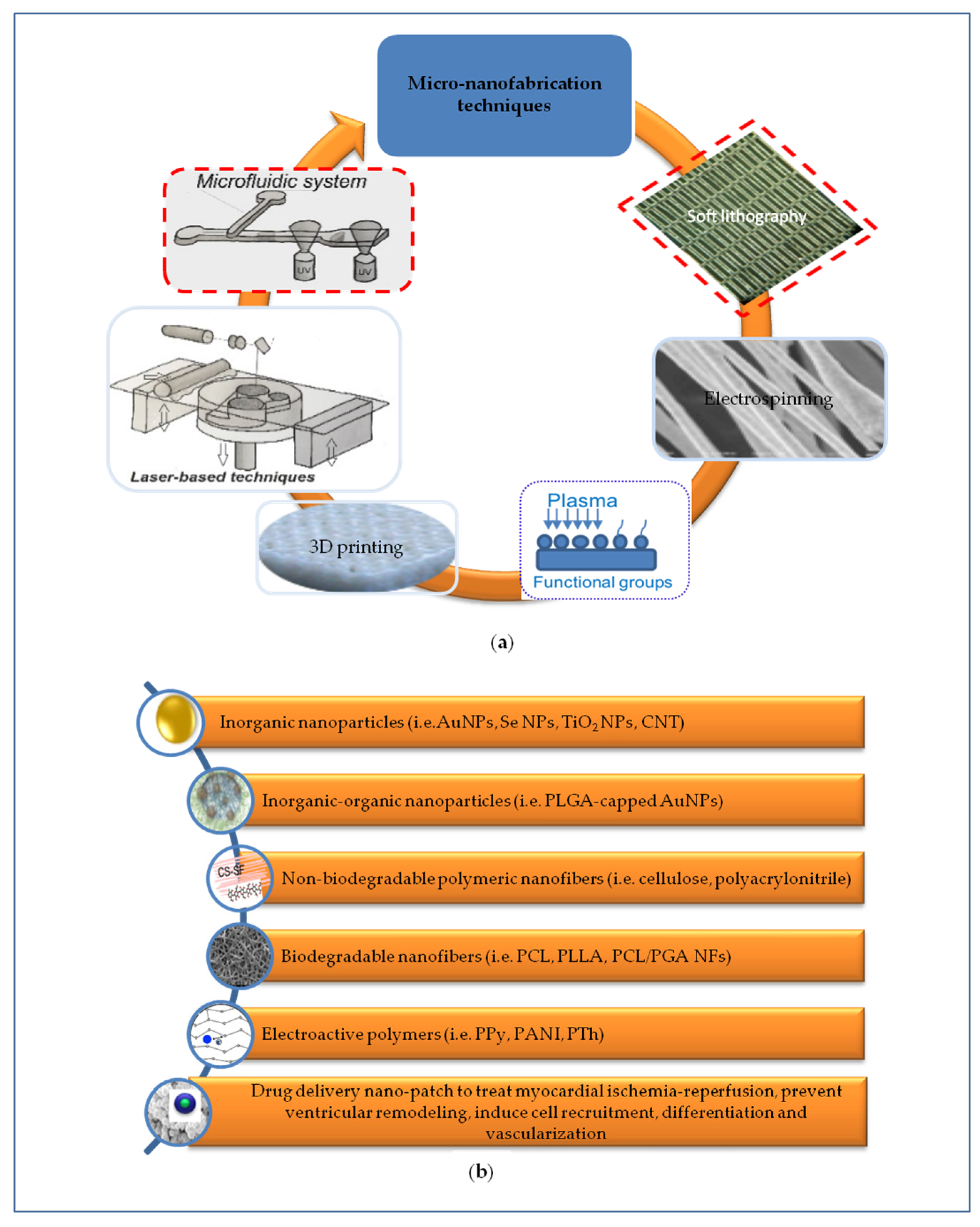
3. Nanopatterning Technologies for Cardiac Tissue Regeneration
3.1. Micro-Nanotopography
3.2. Nanomaterials for Cardiac Patches
3.2.1. Inorganic Nanoparticles
3.2.2. Inorganic-Organic Nanoparticles
3.2.3. Polymeric Nanofibers
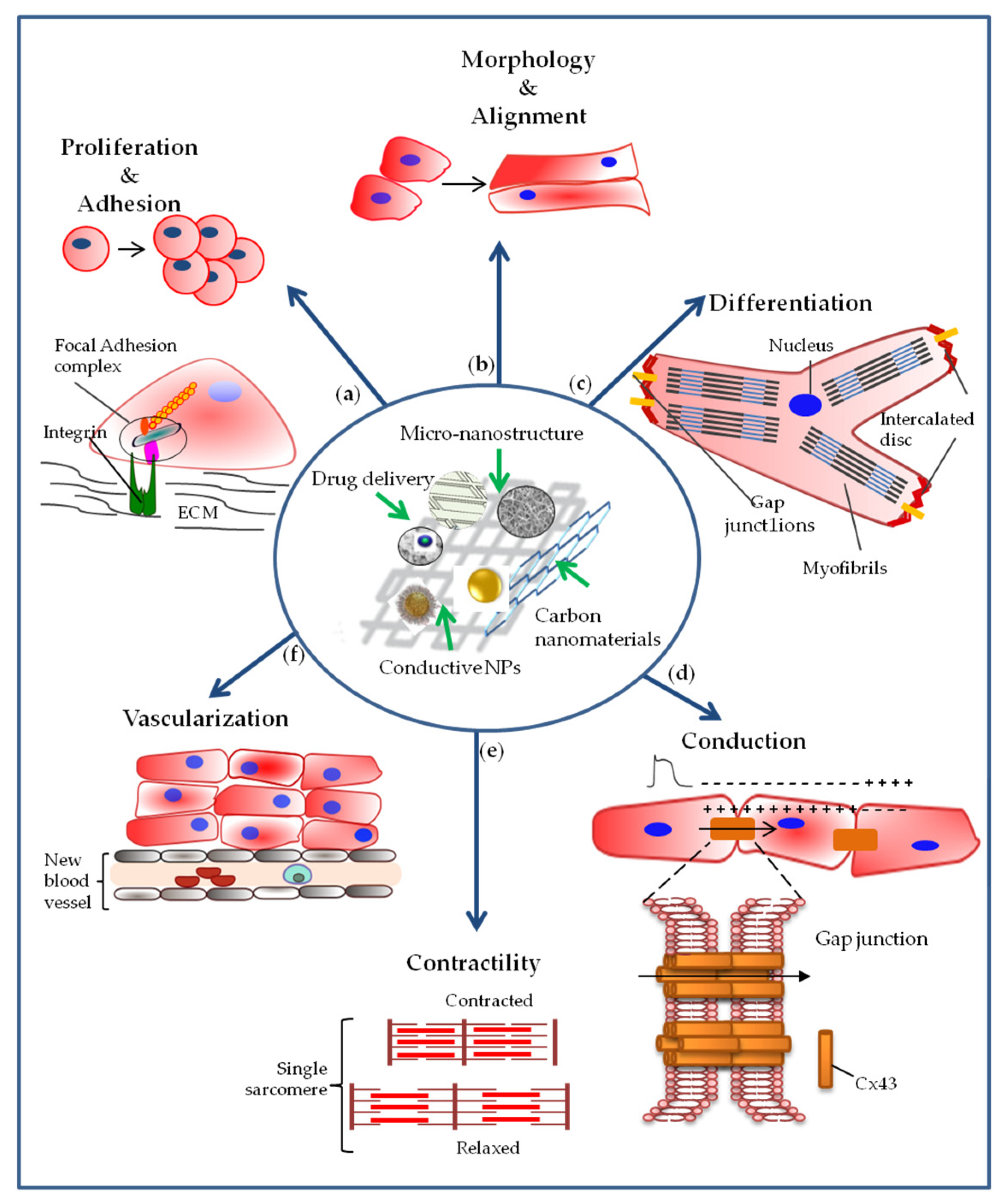
4. Cardiac Stem, Precursor and Differentiated Cell Interactions with Nanoengineered Materials
4.1. Effect of Nanoengineered Materials on Cell Adhesion and Proliferation
4.2. Effect of Nanoengineered Materials on Cell Morphology
4.3. Effect of Nanoengineered Materials on Cell Differentiation
4.4. Effect of Nanoengineered Materials on Cellular Electrical Coupling and Conduction of the Impulse
4.5. Effect of Nanoengineered Materials on Cell Contractility
4.6. Effect of Nanoengineered Materials on Vascularization
5. Challenges and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 3D | Tridimensional |
| AMI | Acute myocardial infarction |
| BADSC | Brown adipose-derived stem cell |
| CM | Cardiomyocyte |
| CNT | Carbon nanotube |
| CPCs | Cardiac progenitor cells |
| CS/FS | Chitosan/silk fibroin |
| cTn | Cardiac troponin |
| Cx43 | Connexin 43 |
| EC | Endothelial cell |
| ECM | Extracellular matrix |
| ESC | embryonic stem cell |
| FGF | Fibroblast growth factor |
| IGF | Insulin growth factor |
| GelMA | Gelatin-Methacryloyl |
| GO | Graphen oxide |
| H9c2 | Cardiomyoblasts |
| HF | Heart failure |
| IHD | Ischaemic heart disease |
| iPSCs | Induced pluripotent stem cells |
| MeTro | Methacrylated tropoelastin |
| MHC | Myosin heavy chain |
| MLC | Myosin light chain |
| MSC | Mesenchymal stem cell |
| NF | Nanofibrous |
| NP | Nanoparticle |
| NR | Nanorod |
| NW | Nanowire |
| PANI | Polyaniline |
| PCL | Poly(ε-caprolactone) |
| PEG | Polyethylene glycol |
| PEGDA | Poly(ethylene glycol) diacrylate |
| PEOz | Poly (2-ethyl-2-oxazoline) |
| PGA | Poly glycolic acid |
| PGS | Poly(Glycerol Sebacate) |
| PHB | Poly(3-hydroxybutyrate) |
| PLCL | Poly (L-lactide-co-caprolactone) |
| PLGA | Poly(lactic-co-glycolic acid) |
| PLL | Poly L-lysine |
| PLLA | Poly(L-lactic acid) |
| PMGI | Polydimethylglutarimide |
| PPS | Poly(propylene sulfide) |
| PS | Polystyrene |
| PPy | Polypyrrole |
| PTh | Polythiophene |
| PVA | Poly(vinyl alcohol) |
| SC | Stem cell |
| SF | Silk Fibroin |
| TGF-β1 | Transforming growth factor-β1 |
| TiO2 | Titanium dioxide |
| TMC | N,N,N-trimethylchitosan chloride |
| VEGF | Vascular endothelial growth factor |
| α-SA | α-sarcomeric actinin |
References
- The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 15 May 2020).
- Cahill, T.J.; Kharbanda, R.K. Heart failure after myocardial infarction in the era of primary percutaneous coronary intervention: Mechanisms, incidence and identification of patients at risk. World J. Cardiol. 2017, 9, 407. [Google Scholar] [CrossRef] [PubMed]
- Tzahor, E.; Poss, K.D. Cardiac regeneration strategies: Staying young at heart. Science 2017, 1039, 1035–1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broughton, K.M.; Sussman, M.A. Enhancement Strategies for Cardiac Regenerative Cell Therapy: Focus on Adult Stem Cells. Circ. Res. 2018, 132, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Eschenhagen, T.; Bolli, R.; Braun, T.; Field, L.J.; Fleischmann, B.K.; Frisén, J.; Giacca, M.; Hare, J.M.; Houser, S.; Lee, R.T.; et al. Cardiomyocyte regeneration: A consensus statement. Circulation 2017, 136, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Madonna, R.; Van Laake, L.W.; Davidson, S.M.; Engel, F.B.; Hausenloy, D.J.; Lecour, S.; Leor, J.; Perrino, C.; Schulz, R.; Ytrehus, K.; et al. Position Paper of the European Society of Cardiology Working Group Cellular Biology of the Heart: Cell-based therapies for myocardial repair and regeneration in ischemic heart disease and heart failure. Eur. Heart J. 2016, 37, 1789–1798. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, L.; Zeng, C.; Wang, W.E. Functionally Improved Mesenchymal Stem Cells to Better Treat Myocardial Infarction. Stem Cells Int. 2018, 2018, 7045245. [Google Scholar] [CrossRef]
- Skalak, R.; Fox, C.F. Tissue Engineering: Proceedings of a Workshop Held at Granlibakken, Lake Tahoe, California, 26–29 February 1988; (UCLA Symposia on Molecular and Cellular Biology, New Ser., V. 107); Liss: New York, NY, USA, 1988. [Google Scholar]
- Cristallini, C.; Cibrario, E.; Accomasso, L.; Folino, A.; Gallina, C.; Muratori, L.; Pagliaro, P.; Rastaldo, R.; Raimondo, S.; Saviozzi, S.; et al. The effect of bioarti fi cial constructs that mimic myocardial structure and biomechanical properties on stem cell commitment towards cardiac lineage. Biomaterials 2014, 35, 92–104. [Google Scholar] [CrossRef]
- Davis, M.E.; Hsieh, P.C.H.; Grodzinsky, A.J.; Lee, R.T. Custom Design of the Cardiac Microenvironment with Biomaterials. Circ. Res. 2005, 97, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Chun, Y.W.; Crowder, S.W.; Mehl, S.C.; Wang, X.; Bae, H.; Sung, H. Therapeutic application of nanotechnology in cardiovascular and pulmonary regeneration. Comput. Struct. Biotechnol. J. 2013, 7, e201304005. [Google Scholar] [CrossRef] [Green Version]
- Radisic, M.; Park, H.; Shing, H.; Consi, T.; Schoen, F.J.; Langer, R.; Freed, L.E.; Vunjak-Novakovic, G. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proc. Natl. Acad. Sci. USA 2004, 101, 18129–18134. [Google Scholar] [CrossRef] [Green Version]
- Gupta, M.K.; Walthall, J.M.; Venkataraman, R.; Crowder, S.W.; Kwang, D.; Yu, S.S.; Feaster, T.K.; Wang, X.; Giorgio, T.D.; Charles, C.; et al. Combinatorial Polymer Electrospun Matrices Promote Physiologically-Relevant Cardiomyogenic Stem Cell Differentiation. PLoS ONE 2011, 6, e28935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tandon, N.; Cannizzaro, C.; Chao, P.H.G.; Maidhof, R.; Marsano, A.; Au, H.T.H.; Radisic, M.; Vunjak-Novakovic, G. Electrical stimulation systems for cardiac tissue engineering. Nat. Protoc. 2009, 4, 155–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, J.; Wang, F.; Li, Z.; Chen, J.; Guo, X.; Liao, J.; Moldovan, N.I. The stimulation of the cardiac differentiation of mesenchymal stem cells in tissue constructs that mimic myocardium structure and biomechanics. Biomaterials 2011, 32, 5568–5580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mooney, E.; Mackle, J.N.; Blond, D.J.; Cearbhaill, E.O.; Shaw, G.; Blau, W.J.; Barry, F.P.; Barron, V.; Murphy, J.M. The electrical stimulation of carbon nanotubes to provide a cardiomimetic cue to MSCs. Biomaterials 2012, 33, 6132–6139. [Google Scholar] [CrossRef] [Green Version]
- JiangYan, C.; Zeng, D.; Ding, L.; Li, X.; Liu, X.; Li, W.; Wei, T.; Yan, S.; Xie, J.; Wei, L.; et al. Three-dimensional poly-(ε-caprolactone) nanofibrous scaffolds directly promote the cardiomyocyte differentiation of murine-induced pluripotent stem cells through Wnt/β-catenin signaling. BMC Cell Biol. 2015, 16, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Gaetani, R.; Doevendans, P.A.; Metz, C.H.G.; Alblas, J.; Messina, E.; Giacomello, A.; Sluijter, J.P.G. Cardiac tissue engineering using tissue printing technology and human cardiac progenitor cells. Biomaterials 2012, 33, 1782–1790. [Google Scholar] [CrossRef]
- Cristallini, C.; Rocchietti, E.C.; Gagliardi, M.; Mortati, L.; Saviozzi, S.; Bellotti, E.; Turinetto, V.; Sassi, M.P.; Barbani, N.; Giachino, C. Micro- and Macrostructured PLGA/Gelatin Scaffolds Promote Early Cardiogenic Commitment of Human Mesenchymal Stem Cells In Vitro. Stem Cells Int. 2016, 2016, 7176154. [Google Scholar] [CrossRef] [Green Version]
- Cassidy, J.W. Nanotechnology in the Regeneration of Complex Tissues. Bone Tissue Regen. Insights 2015, 25–35. [Google Scholar] [CrossRef]
- Andreu, I.; Luque, T.; Sancho, A.; Pelacho, B.; Iglesias-garcía, O.; Melo, E.; Farré, R.; Prósper, F.; Elizalde, M.R.; Navajas, D. Heterogeneous micromechanical properties of the extracellular matrix in healthy and infarcted hearts. Acta Biomater. 2014, 10, 3235–3242. [Google Scholar] [CrossRef]
- Nielsen, S.H.; Mouton, A.J.; DeLeon-Pennell, K.Y.; Genovese, F.; Karsdal, M.; Lindsey, M.L. Understanding cardiac extracellular matrix remodeling to develop biomarkers of myocardial infarction outcomes. Matrix Biol. 2019, 75–76, 43–57. [Google Scholar] [CrossRef]
- Ott, H.C.; Matthiesen, T.S.; Goh, S.; Black, L.D.; Kren, S.M.; Netoff, T.I.; Taylor, D.A. Perfusion-decellularized matrix: Using nature’s platform to engineer a bioartificial heart. Nat. Med. 2008, 14, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Oberwallner, B.; Brodarac, A.; Anić, P.; Šarić, T.; Wassilew, K.; Neef, K.; Choi, Y.H.; Stamm, C. Human cardiac extracellular matrix supports myocardial lineage commitment of pluripotent stem cells. Eur. J. Cardio-Thorac. Surg. 2015, 47, 416–425. [Google Scholar] [CrossRef] [Green Version]
- Ahadian, S.; Yamada, S.; Ramón-Azcón, J.; Estili, M.; Liang, X.; Nakajima, K.; Shiku, H.; Khademhosseini, A.; Matsue, T. Hybrid hydrogel-aligned carbon nanotube scaffolds to enhance cardiac differentiation of embryoid bodies. Acta Biomater. 2016, 31, 134–143. [Google Scholar] [CrossRef]
- Kitsara, M.; Kontziampasis, D.; Agbulut, O.; Chen, Y. Heart on a chip: Micro-nanofabrication and microfluidics steering the future of cardiac tissue engineering. Microelectron. Eng. 2019, 203, 44–62. [Google Scholar] [CrossRef]
- Trantidou, T.; Humphrey, E.J.; Poulet, C.; Gorelik, J.; Prodromakis, T.; Terracciano, C.M. Surface Chemistry and Microtopography of Parylene C Films Control the Morphology and Microtubule Density of Cardiac Myocytes. Tissue Eng. Part. C Methods 2016, 22, 464–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.; Lipke, E.A.; Kim, P.; Cheong, R.; Thompson, S.; Delannoy, M. Nanoscale cues regulate the structure and function of macroscopic cardiac tissue constructs. Proc. Natl. Acad. Sci. USA 2009, 107, 565–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, P.; Ding, S.; Sumer, H.; Wong, R.C.B.; Kingshott, P. Heterogeneity of mesenchymal and pluripotent stem cell populations grown on nanogrooves and nanopillars. J. Mater. Chem. B 2017, 5, 7927–7938. [Google Scholar] [CrossRef]
- Patz, T.M.; Doraiswamy, A.; Narayan, R.J.; Modi, R.; Chrisey, D.B. Two-dimensional differential adherence and alignment of C2C12 myoblasts. Mater. Sci. Eng. B 2005, 123, 242–247. [Google Scholar] [CrossRef]
- Engelmayr, G.C.; Cheng, M.; Bettinger, C.J.; Borenstein, J.T.; Langer, R.; Freed, L.E. Accordion-like honeycombs for tissue engineering of cardiac anisotropy. Nat. Mater. 2008, 7, 1003–1010. [Google Scholar] [CrossRef]
- Masoumi, N.; Zugates, J.T.; Johnson, K.L.; Engelmayr, G.C. Laser microfabricated poly (glycerol sebacate) scaffolds for heart valve tissue engineering. J. Biomed. Mater. Res. A 2012, 2, 104–114. [Google Scholar] [CrossRef]
- Castellano, D.; Blanes, M.; Marco, B.; Cerrada, I.; Ruiz-Saurí, A.; Pelacho, B.; Araña, M.; Montero, J.A.; Cambra, V.; Prosper, F.; et al. A comparison of electrospun polymers reveals poly(3-hydroxybutyrate) fiber as a superior scaffold for cardiac repair. Stem Cells Dev. 2014, 23, 1479–1490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, D.A.; Beygui, R.E.; MacLellan, W.R.; Laks, H.; Dunn, J.C.Y.; Wu, B.M. Modulation of gene expression in neonatal rat cardiomyocytes by surface modification of polylactide-co-glycolide substrates. J. Biomed. Mater. Res. Part. A 2005, 74, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, R.; Sridhar, R.; Venugopal, J.R.; Sundarrajan, S.; Mukherjee, S.; Ramakrishna, S. Gold nanoparticle loaded hybrid nanofibers for cardiogenic differentiation of stem cells for infarcted myocardium regeneration. Macromol. Biosci. 2014, 14, 515–525. [Google Scholar] [CrossRef]
- Jung, D.; Minami, I.; Patel, S.; Lee, J.; Jiang, B.; Yuan, Q.; Li, L.; Kobayashi, S.; Chen, Y.; Lee, K.-B.; et al. Incorporation of functionalized gold nanoparticles into nanofibers for enhanced attachment and differentiation of mammalian cells. J. Nanobiotechnol. 2012, 10, 23. [Google Scholar] [CrossRef] [Green Version]
- Kharaziha, M.; Shin, S.R.; Nikkhah, M.; Topkaya, S.N.; Masoumi, N.; Annabi, N.; Dokmeci, M.R.; Khademhosseini, A. Tough and flexible CNT-polymeric hybrid scaffolds for engineering cardiac constructs. Biomaterials 2014, 35, 7346–7354. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Lee, A.R.; Lin, W.H.; Lin, C.W.; Wu, Y.K.; Tsai, W.B. Electrospun PLGA fibers incorporated with functionalized biomolecules for cardiac tissue engineering. Tissue Eng. Part. A 2014, 20, 1896–1907. [Google Scholar] [CrossRef] [Green Version]
- Bhaarathy, V.; Venugopal, J.; Gandhimathi, C.; Ponpandian, N.; Mangalaraj, D.; Ramakrishna, S. Biologically improved nanofibrous scaffolds for cardiac tissue engineering. Mater. Sci. Eng. C 2014, 44, 268–277. [Google Scholar] [CrossRef]
- Ho, C.M.B.; Mishra, A.; Lin, P.T.P.; Ng, S.H.; Yeong, W.Y.; Kim, Y.J.; Yoon, Y.J. 3D Printed Polycaprolactone Carbon Nanotube Composite Scaffolds for Cardiac Tissue Engineering. Macromol. Biosci. 2017, 17, 1600250. [Google Scholar] [CrossRef]
- Gao, L.; Kupfer, M.E.; Jung, J.P.; Yang, L.; Zhang, P.; Da Sie, Y.; Tran, Q.; Ajeti, V.; Freeman, B.T.; Fast, V.G.; et al. Myocardial Tissue Engineering with Cells Derived from Human-Induced Pluripotent Stem Cells and a Native-Like, High-Resolution, 3-Dimensionally Printed Scaffold. Circ. Res. 2017, 120, 1318–1325. [Google Scholar] [CrossRef] [Green Version]
- Ciocci, M.; Mochi, F.; Carotenuto, F.; Di Giovanni, E.; Prosposito, P.; Francini, R.; De Matteis, F.; Reshetov, I.; Casalboni, M.; Melino, S.; et al. Scaffold-in-scaffold potential to induce growth and differentiation of cardiac progenitor cells. Stem Cells Dev. 2017, 26, 1438–1447. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Hong, M.; Dai, R.; Wu, H.; Zhu, P. Engineered bioactive nanoparticles incorporated biofunctionalized ECM/silk proteins based cardiac patches combined with MSCs for the repair of myocardial infarction: In vitro and in vivo evaluations. Sci. Total Environ. 2020, 707, 135976. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.S.; Ameer, J.M.; Alison, M.R.; Anilkumar, T.V. A gold nanoparticle coated porcine cholecyst-derived bioscaffold for cardiac tissue engineering. Colloids Surf. B Biointerfaces 2017, 157, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Dvir, T.; Timko, B.P.; Brigham, M.D.; Naik, S.R.; Sandeep, S.; Levy, O.; Jin, H.; Parker, K.K.; Langer, R.; Daniel, S. Nanowired three dimensional cardiac patches. Nat. Nanotechnol. 2012, 6, 720–725. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Fan, W.; Wang, K.; Wei, H.; Zhang, R.; Wu, Y. Novel preparation of Au nanoparticles loaded Laponite nanoparticles/ECM injectable hydrogel on cardiac differentiation of resident cardiac stem cells to cardiomyocytes. J. Photochem. Photobiol. B Biol. 2019, 192, 49–54. [Google Scholar] [CrossRef]
- Kalishwaralal, K.; Jeyabharathi, S.; Sundar, K.; Selvamani, S.; Prasanna, M.; Muthukumaran, A. A novel biocompatible chitosan–Selenium nanoparticles (SeNPs) film with electrical conductivity for cardiac tissue engineering application. Mater. Sci. Eng. C 2018, 92, 151–160. [Google Scholar] [CrossRef]
- Liu, N.; Chen, J.; Zhuang, J.; Zhu, P. Fabrication of engineered nanoparticles on biological macromolecular (PEGylated chitosan) composite for bio-active hydrogel system in cardiac repair applications. Int. J. Biol. Macromol. 2018, 117, 553–558. [Google Scholar] [CrossRef]
- Crowder, S.W.; Liang, Y.; Rath, R.; Park, A.M.; Maltais, S.; Pintauro, P.N.; Hofmeister, W.; Lim, C.C.; Wang, X.; Sung, H.J. Poly(e-caprolactone)-carbon nanotube composite scaffolds for enhanced cardiac differentiation of human mesenchymal stem cells. Nanomedicine 2013, 8, 1763–1776. [Google Scholar] [CrossRef] [Green Version]
- Stout, D.A.; Yoo, J.; Noemi Santiago-Miranda, A.N.; Webster, T.J. Mechanisms of greater cardiomyocytes functions on conductive nanoengineered composites for cardiovascular applications. Int. J. Nanomed. 2012, 7, 5356–5369. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Liu, Q.; Liu, Y.; Ju, F.; Ma, Q.; He, Q. In vivo evaluation of enhanced drug carrier efficiency and cardiac anti-hypertrophy therapeutic potential of nano-curcumin encapsulated photo-plasmonic nanoparticles combined polymerized nano-vesicles: A novel strategy. J. Photochem. Photobiol. B Biol. 2019, 199, 111619. [Google Scholar] [CrossRef]
- Chen, J.; Zhan, Y.; Wang, Y.; Han, D.; Tao, B.; Luo, Z.; Ma, S.; Wang, Q.; Li, X.; Fan, L.; et al. Chitosan/silk fibroin modified nanofibrous patches with mesenchymal stem cells prevent heart remodeling post-myocardial infarction in rats. Acta Biomater. 2018, 80, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Tian, S.; Zhao, C.; Chen, X.; Lei, I.; Wang, Z.; Ma, P.X. Porous nanofibrous poly(l-lactic acid) scaffolds supporting cardiovascular progenitor cells for cardiac tissue engineering. Acta Biomater. 2015, 26, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Aghdam, R.M.; Shakhesi, S.; Najarian, S.; Mohammadi, M.; Hossein, S.; Tafti, A.; Mirzadeh, H. Fabrication of a Nanofibrous Scaffold for the In Vitro Culture of Cardiac Progenitor Cells for Myocardial Regeneration. Int. J. Polym. Mater. Polym. Biomater. 2014, 63, 229–239. [Google Scholar] [CrossRef]
- Shin, S.R.; Jung, S.M.; Zalabany, M.; Kim, K.; Zorlutuna, P.; Kim, S.B.; Nikkhah, M.; Khabiry, M.; Azize, M.; Kong, J.; et al. Carbon-nanotube-embedded hydrogel sheets for engineering cardiac constructs and bioactuators. ACS Nano 2013, 7, 2369–2380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.; Zhou, J.; Huang, Z.; Qu, L.; Lin, N.; Liang, C.; Dai, R.; Tang, L.; Tian, F. Carbon nanotube-incorporated collagen hydrogels improve cell alignment and the performance of cardiac constructs. Int. J. Nanomed. 2017, 12, 3109–3120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, S.R.; Shin, C.; Memic, A.; Shadmehr, S.; Miscuglio, M.; Jung, H.Y.; Jung, S.M.; Bae, H.; Khademhosseini, A.; Tang, X.; et al. Aligned Carbon Nanotube-Based Flexible Gel Substrates for Engineering Biohybrid Tissue Actuators. Adv. Funct. Mater. 2015, 25, 4486–4495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trantidou, T.; Rao, C.; Barrett, H.; Camelliti, P.; Pinto, K.; Yacoub, M.H.; Athanasiou, T.; Toumazou, C.; Terracciano, C.M.; Prodromakis, T. Selective hydrophilic modification of Parylene C films: A new approach to cell micro-patterning for synthetic biology applications. Biofabrication 2014, 6, 025004. [Google Scholar] [CrossRef] [PubMed]
- Radisic, M.; Marsano, A.; Maidhof, R.; Wang, Y.; Vunjak-Novakovic, G. Cardiac tissue engineering using perfusion bioreactor systems. Nat. Protoc. 2008, 3, 719–738. [Google Scholar] [CrossRef] [Green Version]
- Horvath, P.; Aulner, N.; Bickle, M.; Davies, A.M.; Del Nery, E.; Ebner, D.; Montoya, M.C.; Östling, P.; Pietiäinen, V.; Price, L.S.; et al. Screening out irrelevant cell-based models of disease. Nat. Rev. Drug Discov. 2016, 15, 751–769. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.D.; Ko, M.C.; Wu, S.T.; Li, S.F.; Hu, J.F.; Lai, Y.J.; Harn, H.I.C.; Laio, I.C.; Yeh, M.L.; Yeh, H.I.; et al. A nanopatterned cell-seeded cardiac patch prevents electro-uncoupling and improves the therapeutic efficacy of cardiac repair. Biomater. Sci. 2014, 2, 567–580. [Google Scholar] [CrossRef]
- Amezcua, R.; Shirolkar, A.; Fraze, C.; Stout, D.A. Nanomaterials for cardiac myocyte tissue engineering. Nanomaterials 2016, 6, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashtari, K.; Nazari, H.; Ko, H.; Tebon, P.; Akhshik, M.; Akbari, M.; Alhosseini, S.N.; Mozafari, M.; Mehravi, B.; Soleimani, M.; et al. Electrically conductive nanomaterials for cardiac tissue engineering. Adv. Drug Deliv. Rev. 2019, 144, 162–179. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, Y.; Guo, R.; Li, S.; Ni, J.; Gao, S.; Gao, X.; Mao, J.; Zhu, Y.; Wu, P.; et al. Ginsenoside Rg3-loaded, reactive oxygen species-responsive polymeric nanoparticles for alleviating myocardial ischemia-reperfusion injury. J. Control. Release 2020, 317, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Cristallini, C.; Vaccari, G.; Barbani, N.; Cibrario Rocchietti, E.; Barberis, R.; Falzone, M.; Cabiale, K.; Perona, G.; Bellotti, E.; Rastaldo, R.; et al. Cardioprotection of PLGA/gelatine cardiac patches functionalised with adenosine in a large animal model of ischaemia and reperfusion injury: A feasibility study. J. Tissue Eng. Regen. Med. 2019, 13, 1253–1264. [Google Scholar] [CrossRef]
- A Polymeric Scaffold for Cardiac Regeneration and Protection from Reperfusion Injury. PCT/IB2014/058025. Available online: http://hdl.handle.net/2318/142361 (accessed on 28 May 2020).
- Cristallini, C.; Gagliardi, M.; Barbani, N.; Giannessi, D.; Guerra, G.D. Novel biodegradable, biomimetic and functionalised polymer scaffolds to prevent expansion of post-infarct left ventricular remodelling. J. Mater. Sci. Mater. Med. 2012, 23, 205–216. [Google Scholar] [CrossRef]
- Bauer, S.; Park, J.; Faltenbacher, J.; Berger, S.; Von Der Mark, K.; Schmuki, P. Size selective behavior of mesenchymal stem cells on ZrO2 and TiO2 nanotube arrays. Integr. Biol. 2009, 1, 525–532. [Google Scholar] [CrossRef]
- Park, J.; Bauer, S.; Schmuki, P.; von der Mark, K. Narrow window in nanoscale dependent activation of endothelial cell growth and differentiation on TiO2 nanotube surfaces. Nano Lett. 2009, 9, 3157–3164. [Google Scholar] [CrossRef]
- Kim, D.H.; Smith, R.R.; Kim, P.; Ahn, E.H.; Kim, H.N.; Marbán, E.; Suh, K.Y.; Levchenko, A. Nanopatterned cardiac cell patches promote stem cell niche formation and myocardial regeneration. Integr. Biol. 2012, 4, 1019–1033. [Google Scholar] [CrossRef]
- Discher, D.E.; Janmey, P.; Wang, Y.L. Tissue cells feel and respond to the stiffness of their substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Kim, P.; Song, I.; Cha, J.M.; Lee, S.H.; Kim, B.; Suh, K.Y. Guided Three-Dimensional Growth of Functional Cardiomyocytes on Polyethylene Glycol Nanostructures. Langmuir 2006, 22, 5419–5426. [Google Scholar] [CrossRef]
- Stout, D.A.; Basu, B.; Webster, T.J. Poly (lactic-co-glycolic acid): Carbon nanofiber composites for myocardial tissue engineering applications. Acta Biomater. 2011, 7, 3101–3112. [Google Scholar] [CrossRef]
- Wickham, A.M.; Islam, M.M.; Mondal, D.; Phopase, J.; Sadhu, V.; Polisetti, N.; Richter-dahlfors, A.; Liedberg, B.; Griffith, M. Polycaprolactone-thiophene-conjugated carbon nanotube meshes as scaffolds for cardiac progenitor cells. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.R.; Aghaei-Ghareh-Bolagh, B.; Dang, T.T.; Topkaya, S.N.; Tang, X.S.; Khademhosseini, A. Cell-laden Microengineered and Mechanically Tunable Hybrid Hydrogels of Gelatin and Graphene Oxide. Adv. Mater. 2013, 25, 6385–6391. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.R.; Aghaei-Ghareh-Bolagh, B.; Gao, X.; Nikkhah, M.; Jung, S.M.; Dolatshahi-Pirouz, A.; Kim, S.B.; Kim, S.M.; Dokmeci, M.R.; Tang, X.; et al. Layer-by-layer assembly of 3D tissue constructs with functionalized graphene. Adv. Funct. Mater. 2014, 24, 6136–6144. [Google Scholar] [CrossRef] [PubMed]
- Navaei, A.; Saini, H.; Christenson, W.; Tanner, R.; Ros, R.; Nikkhah, M. Gold nanorod-incorporated gelatin-based conductive hydrogels for engineering cardiac tissue constructs. Acta Biomater. 2016, 41, 133–146. [Google Scholar] [CrossRef]
- Sun, H.; Tang, J.; Mou, Y.; Zhou, J.; Qu, L.; Duval, K.; Huang, Z.; Lin, N.; Dai, R.; Liang, C.; et al. Carbon nanotube-composite hydrogels promote intercalated disc assembly in engineered cardiac tissues through β1-integrin mediated FAK and RhoA pathway. Acta Biomater. 2017, 48, 88–99. [Google Scholar] [CrossRef]
- Black, L.D.; Meyers, J.D.; Weinbaum, J.S.; Shvelidze, Y.A.; Tranquillo, R.T. Cell-Induced Alignment Augments Twitch Force in Fibrin Gel-Based Engineered Myocardium via Gap Junction Modification. Tissue Eng. Part A 2009, 15, 3099–3108. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.Y.; Teo, E.Y.; Chong, M.S.K.; Zhang, Q.Y.; Lim, J.; Zhang, Z.Y.; Hong, M.H.; Thian, E.S.; Chan, J.K.Y.; Teoh, S.H. Biomimetic three-dimensional anisotropic geometries by uniaxial stretch of poly(ε-Caprolactone) films for mesenchymal stem cell proliferation, alignment, and myogenic differentiation. Tissue Eng. Part C Methods 2013, 19, 538–549. [Google Scholar] [CrossRef] [Green Version]
- Kai, D.; Prabhakaran, M.P.; Jin, G.; Ramakrishna, S. Guided orientation of cardiomyocytes on electrospun aligned nanofibers for cardiac tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 98, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Xu, Y.; Hua, S.; Johnson, J.; Belevych, A.; Janssen, P.M.L.; Gyorke, S.; Guan, J.; Angelos, M.G. Evaluation of changes in morphology and function of human induced pluripotent stem cell derived cardiomyocytes (HiPSC-CMs) cultured on an aligned-nanofiber cardiac patch. PLoS ONE 2015, 10, e0126338. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, L.; Guo, B.; Ma, P.X. Interwoven Aligned Conductive Nanofiber Yarn/Hydrogel Composite Scaffolds for Engineered 3D Cardiac Anisotropy. ACS Nano 2017, 11, 5646–5659. [Google Scholar] [CrossRef]
- Sun, H.; Mou, Y.; Li, Y.; Li, X.; Chen, Z.; Duval, K.; Huang, Z.; Dai, R.; Tang, L.; Tian, F. Carbon nanotube-based substrates promote cardiogenesis in brown adipose-derived stem cells via β1-integrin-dependent TGF-β1 signaling pathway. Int. J. Nanomed. 2016, 11, 4381–4395. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Manoharan, V.; Cheung, L.; Lee, S.; Cha, B.H.; Newman, P.; Farzad, R.; Mehrotra, S.; Zhang, K.; Khan, F.; et al. Nanoparticle-Based Hybrid Scaffolds for Deciphering the Role of Multimodal Cues in Cardiac Tissue Engineering. ACS Nano 2019, 13, 12525–12539. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Park, S.; Ryu, S.; Bhang, S.H.; Kim, J.; Yoon, J.K.; Park, Y.H.; Cho, S.P.; Lee, S.; Hong, B.H.; et al. Graphene-regulated cardiomyogenic differentiation process of mesenchymal stem cells by enhancing the expression of extracellular matrix proteins and cell signaling molecules. Adv. Healthc. Mater. 2014, 3, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Shevach, M.; Fleischer, S.; Shapira, A.; Dvir, T. Gold nanoparticle-decellularized matrix hybrids for cardiac tissue engineering. Nano Lett. 2014, 14, 5792–5796. [Google Scholar] [CrossRef] [PubMed]
- You, J.O.; Rafat, M.; Ye, G.J.C.; Auguste, D.T. Nanoengineering the heart: Conductive scaffolds enhance connexin 43 expression. Nano Lett. 2011, 11, 3643–3648. [Google Scholar] [CrossRef] [PubMed]
- Bian, W.; Jackman, C.P.; Bursac, N. Controlling the structural and functional anisotropy of engineered cardiac tissues. Biofabrication 2014, 6, 024109. [Google Scholar] [CrossRef]
- Martinelli, V.; Cellot, G.; Toma, F.M.; Long, C.S.; Caldwell, J.H.; Zentilin, L.; Giacca, M.; Turco, A.; Prato, M.; Ballerini, L.; et al. Carbon nanotubes promote growth and spontaneous electrical activity in cultured cardiac myocytes. Nano Lett. 2012, 12, 1831–1838. [Google Scholar] [CrossRef]
- Ahadian, S.; Davenport Huyer, L.; Estili, M.; Yee, B.; Smith, N.; Xu, Z.; Sun, Y.; Radisic, M. Moldable elastomeric polyester-carbon nanotube scaffolds for cardiac tissue engineering. Acta Biomater. 2017, 52, 81–91. [Google Scholar] [CrossRef]
- Shevach, M.; Maoz, B.M.; Feiner, R.; Shapira, A.; Dvir, T. Nanoengineering gold particle composite fibers for cardiac tissue engineering. J. Mater. Chem. B 2013, 1, 5210–5217. [Google Scholar] [CrossRef]
- Pok, S.; Vitale, F.; Eichmann, S.L.; Benavides, O.M.; Pasquali, M.; Jacot, J.G. Biocompatible carbon nanotube-chitosan scaffold matching the electrical conductivity of the heart. ACS Nano 2014, 8, 9822–9832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sewanan, L.R.; Schwan, J.; Kluger, J.; Park, J.; Jacoby, D.L.; Qyang, Y.; Campbell, S.G. Extracellular matrix from hypertrophic myocardium provokes impaired twitch dynamics in healthy cardiomyocytes. JACC Basic Transl. Sci. 2019, 4, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.W.; Corselli, M.; Péault, B.; Huard, J. Human blood-vessel-derived stem cells for tissue repair and regeneration. J. Biomed. Biotechnol. 2012, 2012, 597439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, I.; Fuseler, J.W.; Price, R.L.; Borg, T.K.; Baudino, T.A. Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. Am. J. Physiol. Hear. Circ. Physiol. 2007, 293, 1883–1891. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Shah, A.M. ROS signalling between endothelial cells and cardiac cells. Cardiovasc. Res. 2014, 102, 249–257. [Google Scholar] [CrossRef] [Green Version]
- Rademakers, T.; Horvath, J.M.; van Blitterswijk, C.A.; LaPointe, V.L.S. Oxygen and nutrient delivery in tissue engineering: Approaches to graft vascularization. J. Tissue Eng. Regen. Med. 2019, 13, 1815–1829. [Google Scholar] [CrossRef]
- Shimizu, T.; Sekine, H.; Yang, J.; Isoi, Y.; Yamato, M.; Kikuchi, A.; Kobayashi, E.; Okano, T. Polysurgery of cell sheet grafts overcomes diffusion limits to produce thick, vascularized myocardial tissues. FASEB J. 2006, 20, 708–710. [Google Scholar] [CrossRef]
- Tulloch, N.L.; Muskheli, V.; Razumova, M.V.; Korte, F.S.; Regnier, M.; Hauch, K.D.; Pabon, L.; Reinecke, H.; Murry, C.E. Growth of engineered human myocardium with mechanical loading and vascular coculture. Circ. Res. 2011, 109, 47–59. [Google Scholar] [CrossRef]
- Zhang, H.; van Olden, C.; Sweeney, D.; Martin-Rendon, E. Blood vessel repair and regeneration in the ischaemic heart. Open Heart 2014, 1, e000016. [Google Scholar] [CrossRef] [Green Version]
- Tan, Q.; Tang, H.; Hu, J.; Hu, Y.; Zhou, X.; Tao, Y.; Wu, Z. Controlled release of chitosan/heparin nanoparticle-delivered VEGF enhances regeneration of decellularized tissue-engineered scaffolds. Int. J. Nanomed. 2011, 6, 929–942. [Google Scholar] [CrossRef] [Green Version]
- Moulisová, V.; Gonzalez-García, C.; Cantini, M.; Rodrigo-Navarro, A.; Weaver, J.; Costell, M.; Sabater, I.; Serra, R.; Dalby, M.J.; García, A.J.; et al. Engineered microenvironments for synergistic VEGF—Integrin signalling during vascularization. Biomaterials 2017, 126, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Izadifar, M.; Kelly, M.E.; Chen, X. Regulation of sequential release of growth factors using bilayer polymeric nanoparticles for cardiac tissue engineering. Nanomedicine 2016, 11, 3237–3259. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanan, R.; Kumaraswamy, P.; Krishnan, U.M.; Sethuraman, S. Engineering a growth factor embedded nanofiber matrix niche to promote vascularization for functional cardiac regeneration. Biomaterials 2016, 97, 176–195. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Ye, G.; Song, C.; Li, C.; Xiong, W.; Yu, L.; Qiu, X.; Wang, L. Mussel-inspired conductive nanofibrous membranes repair myocardial infarction by enhancing cardiac function and revascularization. Theranostics 2018, 8, 5159–5177. [Google Scholar] [CrossRef]
- Hirt, M.N.; Hansen, A.; Eschenhagen, T. Cardiac tissue engineering: State of the art. Circ. Res. 2014, 114, 354–367. [Google Scholar] [CrossRef] [Green Version]
- Sekine, H.; Shimizu, T.; Hobo, K.; Sekiya, S.; Yang, J.; Yamato, M.; Kurosawa, H.; Kobayashi, E.; Okano, T. Endothelial cell coculture within tissue-engineered cardiomyocyte sheets enhances neovascularization and improves cardiac function of ischemic hearts. Circulation 2008, 118, 145–152. [Google Scholar] [CrossRef] [Green Version]
- Wierzbicki, M.; Sawosz, E.; Grodzik, M.; Hotowy, A.; Prasek, M.; Jaworski, S.; Sawosz, F.; Chwalibog, A. Carbon nanoparticles downregulate expression of basic fibroblast growth factor in the heart during embryogenesis. Int. J. Nanomed. 2013, 8, 3427–3435. [Google Scholar] [CrossRef] [Green Version]
- Setyawati, M.I.; Tay, C.Y.; Chia, S.L.; Goh, S.L.; Fang, W.; Neo, M.J.; Chong, H.C.; Tan, S.M.; Loo, S.C.J.; Ng, K.W.; et al. Titanium dioxide nanomaterials cause endothelial cell leakiness by disrupting the homophilic interaction of VE-cadherin. Nat. Commun. 2013, 4, 1673. [Google Scholar] [CrossRef]
- Setyawati, M.I.; Tay, C.Y.; Bay, B.H.; Leong, D.T. Gold Nanoparticles Induced Endothelial Leakiness Depends on Particle Size and Endothelial Cell Origin. ACS Nano 2017, 11, 5020–5030. [Google Scholar] [CrossRef]
- Song, J.; Kim, M.; Lee, H. Recent Advances on Nanofiber Fabrications: Unconventional State-of-the-Art Spinning Techniques. Polymers 2020, 12, 1386. [Google Scholar] [CrossRef]
- Jang, J.; Park, H.J.; Kim, S.W.; Kim, H.; Park, J.Y.; Na, S.J.; Kim, H.J.; Park, M.N.; Choi, S.H.; Park, S.H.; et al. 3D printed complex tissue construct using stem cell-laden decellularized extracellular matrix bioinks for cardiac repair. Biomaterials 2017, 112, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Heid, S.; Boccaccini, A.R. Advancing bioinks for 3D bioprinting using reactive fillers: A review. Acta Biomater. 2020, 113, 1–22. [Google Scholar] [CrossRef] [PubMed]
| Material | NM | Cells | Approaches/Method of Assembly | Specific Application | Biological Effects | Ref. |
|---|---|---|---|---|---|---|
| GelMA and MeTro | neonatal rat CMs | Micromolding, photolithography | in vitro MTE | Cell attachment and beating | [28] | |
| PEG | CMs | Soft lithography | in vitro MTE | Alignment of the focal adhesions | [29] | |
| PS | Human AM-MSCs + mouse ESCs | Soft lithography | in vitro MTE | Early differentiation of mESCs (in cardiac-like beating cells) and heterogeneous cells | [30] | |
| Agarose | C2C12 | Laser ablation | in vitro MTE | Improved attachment and differentiation of myoblasts | [31] | |
| PGS | neonatal rat CMs | Laser-assisted technique | in vitro MTE | Increased alignment of heart cells and improved mechanical properties | [32,33] | |
| PHB | MSCs, CMs, CFs | Electrospinning | in vitro MTE | Induced angiogenesis, reparative process and remodeling | [34] | |
| PLGA | neonatal rat CMs | Plasma etching, adsorption fibronectin | in vitro MTE | Modulation of gene expression in cells | [35] | |
| BSA/PVA | AuNPs | Human MSCs | Electrospinning | in vitro MTE | Cardiogenic differentiation of MSCs | [36] |
| PMGI + heparin-binding peptide I | AuNPs | HeLa, hPSCs | Co-electrospinning | in vitro MTE | Enhanced HeLa cell attachment and potentiated CM differentiation of hPSCs | [37] |
| PGS/gelatin | CNTs | CMs | Electrospinning | in vitro MTE | Superior mechanical properties, enhanced CM beating properties | [38] |
| PLGA + YIGSR | neonatal rat CMs | Electrospinning | in vitro MTE | Higher expression of a myosin and b-tubulin, faster and latest longer contraction of CMs | [39] | |
| PLGA + SF + Aloe Vera | CMs | Electrospinning | in vitro MTE | Increased cell proliferation and enhanced cardiac expression proteins | [40] | |
| PCL | CNTs | H9c2 | 3D-printing | in vitro MTE | Cell proliferation | [41] |
| GelMA | hciPSC-CMs, -SMCs, -ECs | 3D-MPE | in vitro MTE | Potentiated cell viability, electromechanical coupling in vitro, improvements in cardiac function, infarct size, vascularizing in a murine MI model | [42] | |
| PEGDA-Wp in PEGDA hydrogel | Human CPCs | Microstereolithography | in vitro MTE | hCPCs differentiation and 3D spatial orientation, activation of connexin 43 expression | [43] | |
| ECM/SF | AuNPs | MSCs, CMs | Spray drying machine | in vitro MTE | Higher cell survival and retention of CMs | [44] |
| cholecyst-ECM | AuNPs | H9c2 | EDC-NHS functionalization to cholecyst -ECM | in vitro MTE | Suitability for the growth and proliferation of cardiomyoblasts | [45] |
| Alginate | AuNW | CMs and fibroblasts | Ionic crosslinking | in vitro MTE | Improved electrical connectivity and functionality | [46] |
| ECM | Lap-AuNPs | CMs | Decellularization process | in vitro MTE | Effective cardiac protein expressions and phenotype maturations of cardiac specific proteins on the CMs | [47] |
| Chitosan | Se-NPs | H9c2 | Film preparation | in vitro MTE | Attachment and proliferation of H9c2 cells, functional electrical connectivity between heart cells and films | [48] |
| PEG/Chitosan | TiO2 NPs | neonatal rat CMs | Chemical crosslinking | in vitro MTE | Adhesion, elongation and spreading of cells | [49] |
| PCL + azacytidine | CNTs | Human MSCs | Electrospinning | in vitro MTE | In vitro cardiac differentiation of hMSCs | [50] |
| PLGA | CNF | Human CMs | Film preparation | in vitro MTE | Increased CM growth, increased conductivity of the composites | [51] |
| CAu-PLGA NPs | Double emulsion solvent | in vivo drug delivery for cardiac anti-ipertrophy | Improved survival rate and in vivo cardiac ant-hypertrophy | [52] | ||
| Cellulose + CS/SF | AD-MSCs | Electrospinning | in vitro MTE | Reduced ventricular remodeling post-MI | [53] | |
| PLLA | CPCs | Sugar template, freeze drying | in vitro MTE | Cell extension, growth and differentiation towards desired lineages in vitro, larger number of living cells | [54] | |
| PCL:PGA | CPCs | Electrospinning | in vitro MTE | Cell attachment and differentiation in vitro and support living cells in vivo | [55] | |
| GelMA | CNT | neonatal rat CMs | Hydrogel preparation, UV irradiation | in vitro MTE | Transmission of action potential between cells and synchronous spontaneous beating | [56] |
| Collagen | CNT | neonatal rat CMs | Hydrogel preparation | in vitro MTE | Spontaneous Ca2+ transients and synchronous rhythm | [57] |
| GelMA, PEG | CNT | neonatal rat CMs | Microelectrode array, photolitography | in vitro MTE | Enhanced electrical synapse formation and electrical coupling | [58] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cristallini, C.; Vitale, E.; Giachino, C.; Rastaldo, R. Nanoengineering in Cardiac Regeneration: Looking Back and Going Forward. Nanomaterials 2020, 10, 1587. https://doi.org/10.3390/nano10081587
Cristallini C, Vitale E, Giachino C, Rastaldo R. Nanoengineering in Cardiac Regeneration: Looking Back and Going Forward. Nanomaterials. 2020; 10(8):1587. https://doi.org/10.3390/nano10081587
Chicago/Turabian StyleCristallini, Caterina, Emanuela Vitale, Claudia Giachino, and Raffaella Rastaldo. 2020. "Nanoengineering in Cardiac Regeneration: Looking Back and Going Forward" Nanomaterials 10, no. 8: 1587. https://doi.org/10.3390/nano10081587
APA StyleCristallini, C., Vitale, E., Giachino, C., & Rastaldo, R. (2020). Nanoengineering in Cardiac Regeneration: Looking Back and Going Forward. Nanomaterials, 10(8), 1587. https://doi.org/10.3390/nano10081587








