Titanium Dioxide-Coated Zinc Oxide Nanorods as an Efficient Photoelectrode in Dye-Sensitized Solar Cells
Abstract
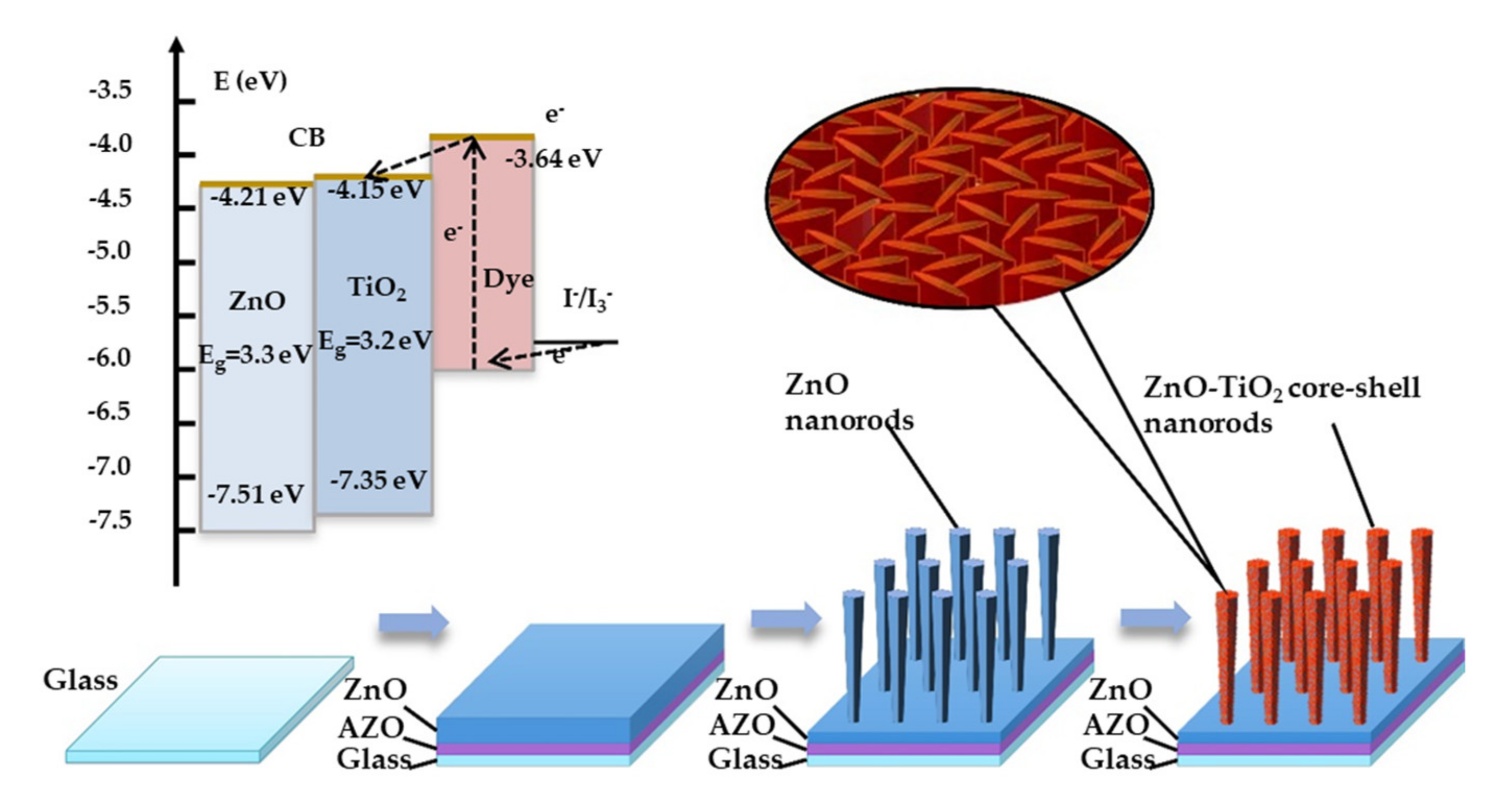
:1. Introduction
2. Materials and Methods
2.1. Deposition of Thin Films
2.2. Fabrication of ZnO Nanorods
2.3. Fabrication of ZnO–TiO2 Core–Shell Nanorods
2.4. Fabrication of DSSC
2.5. Characterization
3. Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Prabavathy, N.; Shalini, S.; Balasundaraprabhu, R.; Velauthapillai, D.; Prasanna, S.; Muthukumarasamy, N. Enhancement in the photostability of natural dyes for dye-sensitized solar cell (DSSC) applications: A review. Int. J. Energy Res. 2017, 41, 1372–1396. [Google Scholar] [CrossRef]
- Ahmad, M.S.; Pandey, A.K.; Rahim, N.A. Advancements in the development of TiO2 photoanodes and its fabrication methods for dye sensitized solar cell (DSSC) applications. A review. Renew. Sustain. Energy Rev. 2017, 77, 89–108. [Google Scholar] [CrossRef]
- Das, T.K.; Ilaiyaraja, P.; Sudakar, C. Template assisted nanoporous TiO2 nanoparticles: The effect of oxygen vacancy defects on photovoltaic performance of DSSC and QDSSC. Sol. Energy 2018, 159, 920–929. [Google Scholar] [CrossRef]
- Vaghasiya, J.V.; Sonigara, K.K.; Soni, S.S.; Tan, S.C. Dual functional hetero-anthracene based single component organic ionic conductors as redox mediator cum light harvester for solid state photoelectrochemical cells. J. Mater. Chem. A 2018, 6, 4868–4877. [Google Scholar] [CrossRef]
- Kakiage, K.; Aoyama, Y.; Yano, T.; Oya, K.; Fujisawa, J.-I.; Hanaya, M. Highly-efficient dye-sensitized solar cells with collaborative sensitization by silyl-anchor and carboxy-anchor dyes. Chem. Commun. 2015, 51, 15894–15897. [Google Scholar] [CrossRef]
- Law, M.; Greene, L.E.; Johnson, J.C.; Saykally, R.; Yang, P. Nanowire dye-sensitized solar cells. Nat. Mater. 2005, 4, 455–459. [Google Scholar] [CrossRef]
- Zhang, Q.; Dandeneau, C.S.; Zhou, X.; Cao, G. ZnO nanostructures for dye-sensitized solar cells. Adv. Mater. 2009, 21, 4087–4108. [Google Scholar] [CrossRef]
- Gopalan, S.-A.; Gopalan, A.-I.; Vinu, A.; Lee, K.-P.; Kang, S.-W. Solar Energy Materials and Solar Cells A new optical-electrical integrated bu ff er layer design based on gold nanoparticles tethered thiol containing sulfonated polyaniline towards enhancement of solar cell performance. Sol. Energy Mater. Sol. Cells 2018, 174, 112–123. [Google Scholar] [CrossRef]
- Nandakumar, D.K.; Vaghasiya, J.V.; Yang, L.; Zhang, Y.; Tan, S.C. A solar cell that breathes in moisture for energy generation. Nano Energy 2020, 68, 104263. [Google Scholar] [CrossRef]
- Nandakumar, D.K.; Ravi, S.K.; Zhang, Y.; Guo, N.; Zhang, C.; Tan, S.C. A super hygroscopic hydrogel for harnessing ambient humidity for energy conservation and harvesting. Energy Environ. Sci. 2018, 11, 2179–2187. [Google Scholar] [CrossRef]
- Kolodziejczak-Radzimska, A.; Jesionowski, T. Zinc oxide-from synthesis to application: A review. Materials (Basel) 2014, 7, 2833–2881. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-C.; Gopalan, A.-I.; Saianand, G.; Lee, K.-P.; Kim, W.-J. Manganese and graphene included titanium dioxide composite nanowires: Fabrication, characterization and enhanced photocatalytic activities. Nanomaterials 2020, 10, 456. [Google Scholar] [CrossRef] [Green Version]
- Tiwana, P.; Docampo, P.; Johnston, M.B.; Snaith, H.J.; Herz, L.M. Electron mobility and injection dynamics in mesoporous ZnO, SnO2, and TiO2 films used in dye-sensitized solar cells. ACS Nano. 2011, 5, 5158–5166. [Google Scholar] [CrossRef]
- Gonzalez-Valls, I.; Lira-Cantu, M. Vertically-aligned nanostructures of ZnO for excitonic solar cells: A review. Energy Environ. Sci. 2009, 2, 19–34. [Google Scholar] [CrossRef]
- Vittal, R.; Ho, K.-C. Zinc oxide based dye-sensitized solar cells: A review. Renew. Sustain. Energy Rev. 2017, 70, 920–935. [Google Scholar] [CrossRef]
- Lu, L.; Li, R.; Fan, K.; Peng, T. Effects of annealing conditions on the photoelectrochemical properties of dye-sensitized solar cells made with ZnO nanoparticles. Sol. Energy. 2010, 84, 844–853. [Google Scholar] [CrossRef]
- Ambade, S.B.; Mane, R.S.; Ghule, A.V.; Takwale, M.G.; Abhyankar, A.; Cho, B.W.; Han, S.H. Contact angle measurement: A preliminary diagnostic method for evaluating the performance of ZnO platelet-based dye-sensitized solar cells. Scr. Mater. 2009, 61, 12–15. [Google Scholar] [CrossRef]
- Yan, F.; Huang, L.; Zheng, J.; Huang, J.; Lin, Z.; Huang, F.; Wei, M. Effect of surface etching on the efficiency of ZnO-based dye-sensitized solar cells. Langmuir. 2010, 26, 7153–7156. [Google Scholar] [CrossRef]
- Horiuchi, H.; Katoh, R.; Hara, K.; Yanagida, M.; Murata, S.; Arakawa, H.; Tachiya, M. Electron injection efficiency from excited N3 into nanocrystalline ZnO films: Effect of (N3-Zn2+) aggregate formation. J. Phys. Chem. B. 2003, 107, 2570–2574. [Google Scholar] [CrossRef]
- Law, M.; Greene, L.E.; Radenovic, A.; Kuykendall, T.; Liphardt, J.; Yang, P. ZnO-Al2O3 and ZnO-TiO2 core-shell nanowire dye-sensitized solar cells. J. Phys. Chem. B. 2006, 110, 22652–22663. [Google Scholar] [CrossRef] [PubMed]
- Chandiran, A.K.; Abdi-Jalebi, M.; Nazeeruddin, M.K.; Grätzel, M. Analysis of electron transfer properties of ZnO and TiO2 photoanodes for dye-sensitized solar cells. ACS Nano. 2014, 8, 2261–2268. [Google Scholar] [CrossRef] [PubMed]
- Atienzar, P.; Ishwara, T.; Illy, B.N.; Ryan, M.P.; O’Regan, B.C.; Durrant, J.R.; Nelson, J. Control of photocurrent generation in polymer/ZnO nanorod solar cells by using a solution-processed TiO2 overlayer. J. Phys. Chem. Lett. 2010, 1, 708–713. [Google Scholar] [CrossRef]
- Feng, Y.; Ji, X.; Duan, J.; Zhu, J.; Jiang, J.; Ding, H.; Meng, G.; Ding, R.; Liu, J.; Hu, A.; et al. Synthesis of ZnO@TiO2 core-shell long nanowire arrays and their application on dye-sensitized solar cells. J. Solid State Chem. 2012, 190, 303–308. [Google Scholar] [CrossRef]
- Prabakar, K.; Son, M.; Kim, W.-Y.; Kim, H. TiO2 thin film encapsulated ZnO nanorod and nanoflower dye sensitized solar cells. Mater. Chem. Phys. 2011, 125, 12–14. [Google Scholar] [CrossRef]
- Zhao, R.; Zhu, L.; Cai, F.; Yang, Z.; Gu, X.; Huang, J.; Cao, L. ZnO/TiO2 core-shell nanowire arrays for enhanced dye-sensitized solar cell efficiency. Appl. Phys. A Mater. Sci. Process. 2013, 113, 67–73. [Google Scholar] [CrossRef]
- Goh, G.K.L.; Le, H.Q.; Huang, T.J.; Hui, B.T.T. Low temperature grown ZnO@TiO2 core shell nanorod arrays for dye sensitized solar cell application. J. Solid State Chem. 2014, 214, 17–23. [Google Scholar] [CrossRef]
- Greene, L.E.; Law, M.; Yuhas, B.D.; Yang, P. ZnO-TiO2 core-shell nanorod/P3HT solar cells. J. Phys. Chem. C. 2007, 111, 18451–18456. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Kawaharamura, T.; Wang, D.; Nitta, N.; Furuta, M.; Furuta, H.; Hatta, A. Influence of substrates on formation of zinc oxide nanostructures by a novel reducing annealing method. Nanosci. Nanotechnol. Lett. 2014, 6, 174–180. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Hou, S.; Hatta, A.; Yu, J.; Jiang, N. Thickness of ITO thin film influences on fabricating ZnO nanorods applying for dye-sensitized solar cell. Compos. Part B Eng. 2015, 74, 147–152. [Google Scholar] [CrossRef]
- Hou, S.; Li, C. Aluminum-doped zinc oxide thin film as seeds layer effects on the alignment of zinc oxide nanorods synthesized in the chemical bath deposition. Thin Solid Films 2016, 605, 37–43. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Kawaharamura, T.; Wang, D.; Nitta, N.; Furuta, M.; Furuta, H.; Hatta, A. Fabrication of zinc oxide nanostructures by mist chemical vapor deposition. Trans. Mater. Res. Soc. Jpn. 2014, 164, 161–164. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Li, C. TiO2 coated ZnO nanorods by mist chemical vapor deposition for application as photoanodes for dye-sensitized solar cells. Nanomaterials 2019, 9, 1339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, X.; Du, Y.; Meng, X. Cupric oxide film with a record hole mobility of 48.44 cm2/Vs via direct-current reactive magnetron sputtering for perovskite solar cell application. Sol. Energy 2019, 191, 205–209. [Google Scholar] [CrossRef]
- Fang, X.S.; Bando, Y.; Shen, G.Z.; Ye, C.H.; Gautam, U.K.; Costa, P.M.F.J.; Zhi, C.Y.; Tang, C.C.; Golberg, D. Ultrafine ZnS nanobelts as field emitters. Adv. Mater. 2007, 19, 2593–2596. [Google Scholar] [CrossRef]






| Film | AZO | ZnO |
|---|---|---|
| Target | AZO (2 wt.%) | ZnO (5N) |
| Working gas, Flow Rate (sccm) | Argon, 30 | Argon, 30 |
| Working distance (mm) | 60 | 60 |
| Deposition Temperature (°C) | 150 | 150 |
| Pressure (Pa) | 1 | 7 |
| RF Power (W) | 60 | 180 |
| Step | Gas | Temperature (°C) | Time (min) |
|---|---|---|---|
| 1 | H2 in N2 (1.96%) | 300 | 120 |
| 2 | H2 in N2 (1.96%) | 450 | 180 |
| 3 | O2 | 450 | 40 |
| 4 | H2 in N2 (1.96%) | 450 | 120 |
| Solvent | Ethanol |
| Solute | TTIP |
| Concentration (mol/L) | 0.10 |
| Deposition Temperature (°C) | 450 |
| Carrier Gas, Flow Rate (L/min) | Compressed Air (dried), 2.5 |
| Dilution Gas, Flow Rate (L/min) | Compressed Air (dried), 4.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Hou, S.; Li, C. Titanium Dioxide-Coated Zinc Oxide Nanorods as an Efficient Photoelectrode in Dye-Sensitized Solar Cells. Nanomaterials 2020, 10, 1598. https://doi.org/10.3390/nano10081598
Zhang Q, Hou S, Li C. Titanium Dioxide-Coated Zinc Oxide Nanorods as an Efficient Photoelectrode in Dye-Sensitized Solar Cells. Nanomaterials. 2020; 10(8):1598. https://doi.org/10.3390/nano10081598
Chicago/Turabian StyleZhang, Qiang, Shengwen Hou, and Chaoyang Li. 2020. "Titanium Dioxide-Coated Zinc Oxide Nanorods as an Efficient Photoelectrode in Dye-Sensitized Solar Cells" Nanomaterials 10, no. 8: 1598. https://doi.org/10.3390/nano10081598
APA StyleZhang, Q., Hou, S., & Li, C. (2020). Titanium Dioxide-Coated Zinc Oxide Nanorods as an Efficient Photoelectrode in Dye-Sensitized Solar Cells. Nanomaterials, 10(8), 1598. https://doi.org/10.3390/nano10081598





