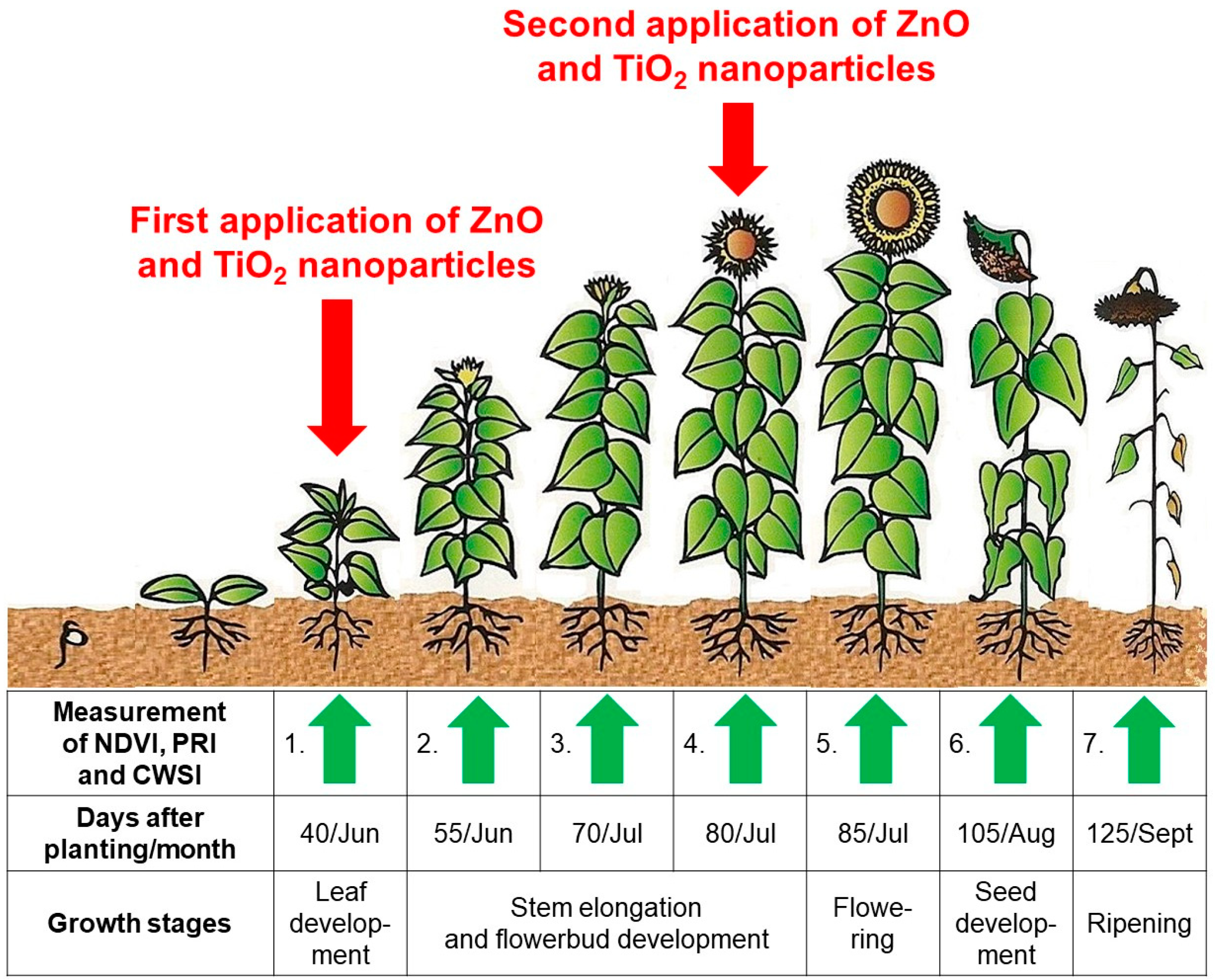
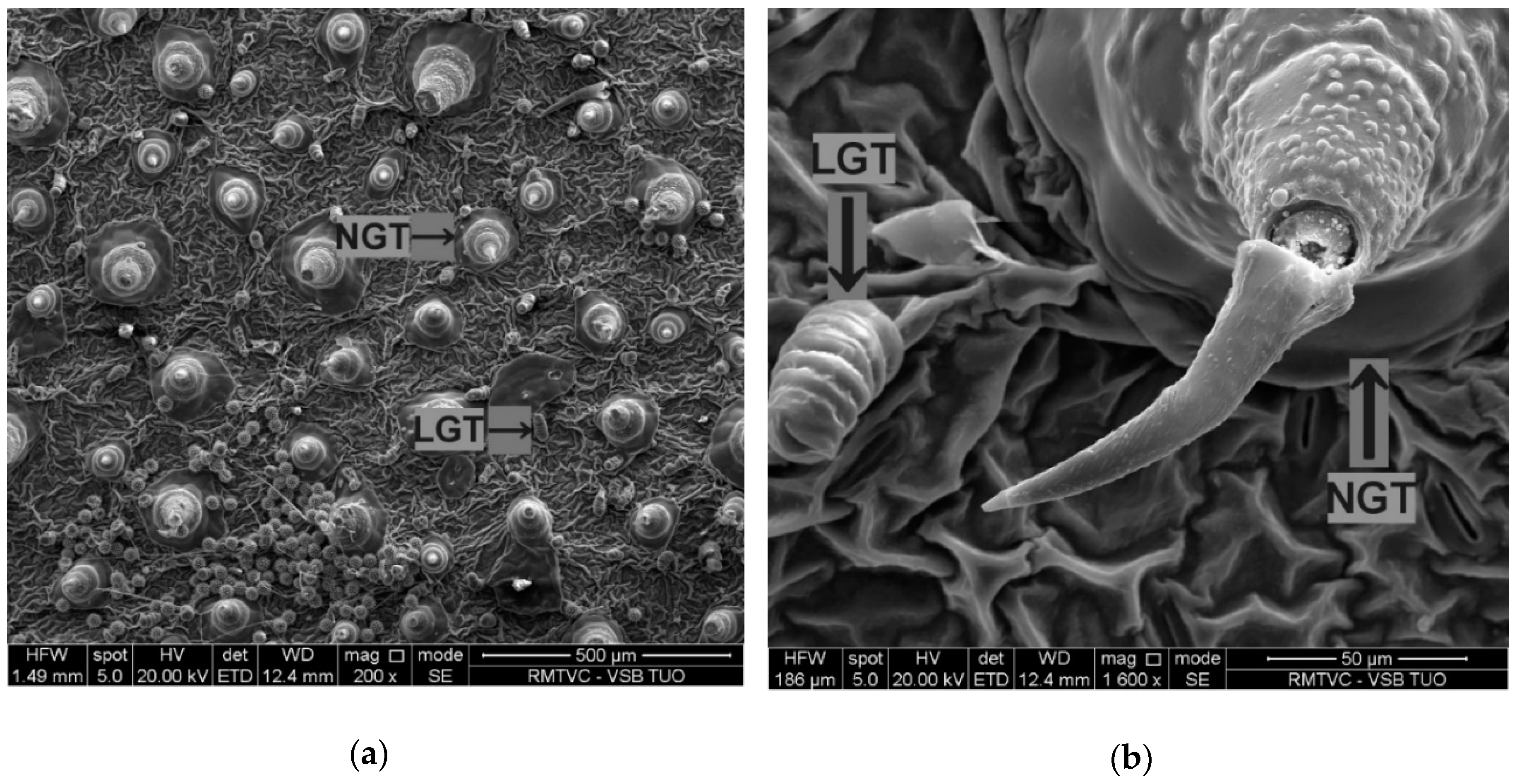
3.1. Characterisation of Titanium Dioxide and Zinc Oxide Nanoparticles and Foliar Application Effects on Surface Leaf Trichomes
Scanning electron microscopy shows that the TiO
2-NPs are predominantly spherical and prismatic crystals with bi-pyramidal terminations and rarely occur as tabular crystals. This morphology corresponds to the rutile and anatase TiO
2 polymorphic modifications identified by XRD analysis (
Figure 3a,b). The mean size of the anatase and rutile crystals is approximately 19.6 ± 0.2 and 30.0 ± 2.0 nm, respectively (
Table 3). The X-ray diffraction analysis in
Table 3 also revealed both rutile and anatase have tetragonal symmetry and different unit cell parameters and relative content. The ZnO-NPs predominantly have spherical shape with 17.3 nm mean crystal size and wurtzite-type structure [
39].
The sunflower’s effective nutrient uptake, development, growth and metabolic functions depend on a combination of factors. This is common to all plants undergoing improvement by NPs foliar application, and the most important factors include the plant species and cultivar, ambient light and water conditions and the NPs particle size, stoichiometry, crystallinity and concentration [
68,
69]. The two most usual nanoparticle penetration sites are cuticular and stomatal [
70], but only the stomatal pathway was available to our research Larue et al. [
22] because the cuticular pathway requires less than 5 nm NP-size and we were limited to 10 nm–1μm NP-size with relatively high transport velocity [
70]. More precisely, both our nanoparticle types are less than 30 nm, and they have good stoichiometry and high crystallinity with typical morphology (
Table 3,
Figure 3). However, because of their different metal bases, we expected significantly different photo-chemical behaviour under the plant’s sunlight radiation, specialised leaf anatomy and over-all plant-response.
Exposure to sunlight can induce ZnO-NPs photo-corrosion, and this enables gradual zinc transport into the plant leaves [
2]. Therefore, subsequent ZnO-NPs physiological impact is distinctively different to that of ionic Zn
2+ and micro-sized zinc species [
4]. While TiO
2-NPs have relatively good photo-stability [
7,
45] and high mechanical and chemical resistance, O
• and OH
• radical generation is enhanced when plants are exposed to sunlight, and this can affect plant surface chemistry [
45,
46]. Although better anatase TiO
2-NPs light-utilisation and increased associated photosynthesis was reported in spinach in the 400–800 nm visible and ultraviolet spectra [
44], other researchers observed cuticle and cell wall damage accompanying higher NPs exposure [
22].
Leaf surface analysis showed the presence of non-glandular trichomes (NGTs) and linear glandular trichomes (LGTs) in all variants (
Figure 4), but capitate glandular trichomes (CGT) were found only in the ZnO-NPs-treated variant (
Table 4).
In addition to the differences in trichomes diversity for each treatment, there was also significant individual leaf trichomes variation in the treatments (
Table 4) and a distinct characteristic trend in NGT width and LGT length for the variants in the following order: TiO
2-NPs > control > ZnO-NPs (
Table 5 and
Table 6).
Li et al. [
2] recorded a significant NGT function in sunflower foliar zinc absorption. Although this is the first report of such trichome association with TiO
2-NPs, the wider NGTs and longer LGTs than those in leaves collected from ZnO-NPs treated plants indicate the specific sunflower response to TiO
2-NPs exposure.
3.2. Effects of Titanium Dioxide and Zinc Oxide Nanoparticles on the Sunflower’s Quantitative and Nutritional Parameters
Metal oxides such as ZnO, TiO
2, CuO, and Al
2O
3 are used in nano-fertilisers to boost crop growth [
36], and ZnO nano-fertilisers particularly provide an alternative to conventional chemical fertilisers by introducing the micro-nutrients required for efficient plant growth and development [
71,
72,
73]. General zinc uses include its benefits in catalytic activity; such as its function in dehydrogenases, aldolases, isomerases, transphosphorylases and RNA and DNA polymerases. Zinc is also important in tryptophan synthesis, cell division, and maintaining membrane structure and potential, and it is beneficial in both photosynthesis and as a regulatory cofactor in protein synthesis [
74]. Moreover, zinc has previously been recorded herein as an essential micronutrient for plant growth and development [
75], and our research into its application to the Neostar hybrid sunflower cultivar recorded greater resistance to both drought and water-logging. Zinc therefore has a most important function in our hybrid’s production process because the ZnO nanoparticles are quickly transported into the sunflower and participate in its metabolic processes [
76].
TiO
2, SiO
2, and carbon nanotubes are also part of the new generation of nanoparticle fertilisers. While these have promoted plant growth, controversy surrounds their use because of their potential toxicity. However, beneficial results have been reported by Lu et al. [
77] who recorded increased nitrogen fixation in
Glycine maximum and improved seed germination and growth using a TiO
2 and SiO
2 mixture and Gao et al. [
78] demonstrated that TiO
2 alone increased total nitrogen, protein, and chlorophyll content in the
Spinacia oleracea species.
Our results revealed no statistically significant difference in the number of plants and heads between the plants sprayed with ZnO-NPs and the NP-free controls. This was expected because no significant negative effects of visible leaf damage, growth inhibition, or decreased yield have ever been detected with NPs foliar application [
79]. The TiO
2-NPs also had no negative effect on the number of plants or seed heads, and harmful effects with foliar TiO
2-NP application only occur at concentrations over 1000 mg·kg
−1 [
19].
Khater [
41] reported that 6 mg·L
−1 TiO
2-NPs spray-application to
Coriandrum sativum L. increased the plant height, number of branches and fruit yield, and Moaveni et al. [
48] added the positive effect on
Hordem vulgare L. yield. These results support our statistically significantly increase in head diameter, dry-seed head weight, grain yield, and TSW. The positive effect of 0.02% TiO
2-NPs on
Triticum aestivum L., cv. ‘Pishtaz’ spring wheat TSW weight was also confirmed by Jaberzadeh et al. [
3], in their highlights of TiO
2 benefits. Although our previous research in Kolenčík et al. [
39] indicated little to no ZnO-NPs effect on foxtail millet quantitative parameters, the positive effects of ZnO-NPs treatment in this research was statistically significant compared to the control. While this was noted in head diameter, dry-seed head weight, yield and TSW, there was less growth improvement than in the TiO
2-NPs-treated variant. Hussain et al. [
33] also reported that ZnO-NPs increased yield, wheat growth, and dry weights after NPs foliar application under Cd stress condition. The ZnO-NPs there most likely increased plant zinc distribution, with consequent Cd stress reduction. Additional authors have also confirmed ZnO-NPs beneficial impact on plant growth parameters [
11,
24,
50].
Statistically significant differences were also found in nutritional parameters. This was especially apparent in terms of the increased oil content, which was 63.6% in the TiO
2-NPs variant compared to 59.2% in the control. Although the 60.5% sunflower oil content in the ZnO-NPs-treated variant was only slightly higher than the control, this increase was valuable (
Table 7). These results are important for agriculture science and quite impressive when compared to 44% USDA seed oil content [
80], and the recent FAO report of up to 50% in new Russian sunflower variety seeds [
81].
The superior TiO
2-NPs variant performance was unexpected because these are usually considered only plant-growth enhancers and do not have the overall ZnO-NPs nanofertiliser effects [
15]. Raliya et al. [
19] also implied this phenomenon in their report of higher tomato lycopene production by TiO
2-NPs use compared to ZnO-NPs. Herein, we established that foliar application of both ZnO-NPs and TiO
2-NPs have positive effects on the common sunflower physiological parameters, plant growth and seed quality. Especially in the case of ZnO-NPs, the full assimilation by plant and transformation to chelated Zn species, including zinc-citrate, -oxalate and -phytate [
2] and their subsequent transport into seeds would be beneficial for food industry.
In contrast,
Figure 5 shows no significant difference in zinc and titanium concentrations in treated fully-ripe seeds compared to the control. However, we consider that this is most likely due to the low-level NPs concentrations applied.
3.3. Sunflower Physiological Response to Titanium Dioxide and Zinc Oxide Nanoparticle Foliar Application
Authors recorded that nano-fertilisers positively affect plant growth, flowering and fruit maturation [
19,
32] and they also provide protection in negative seasonal conditions [
82,
83], such as salt stress and drought [
24,
84].
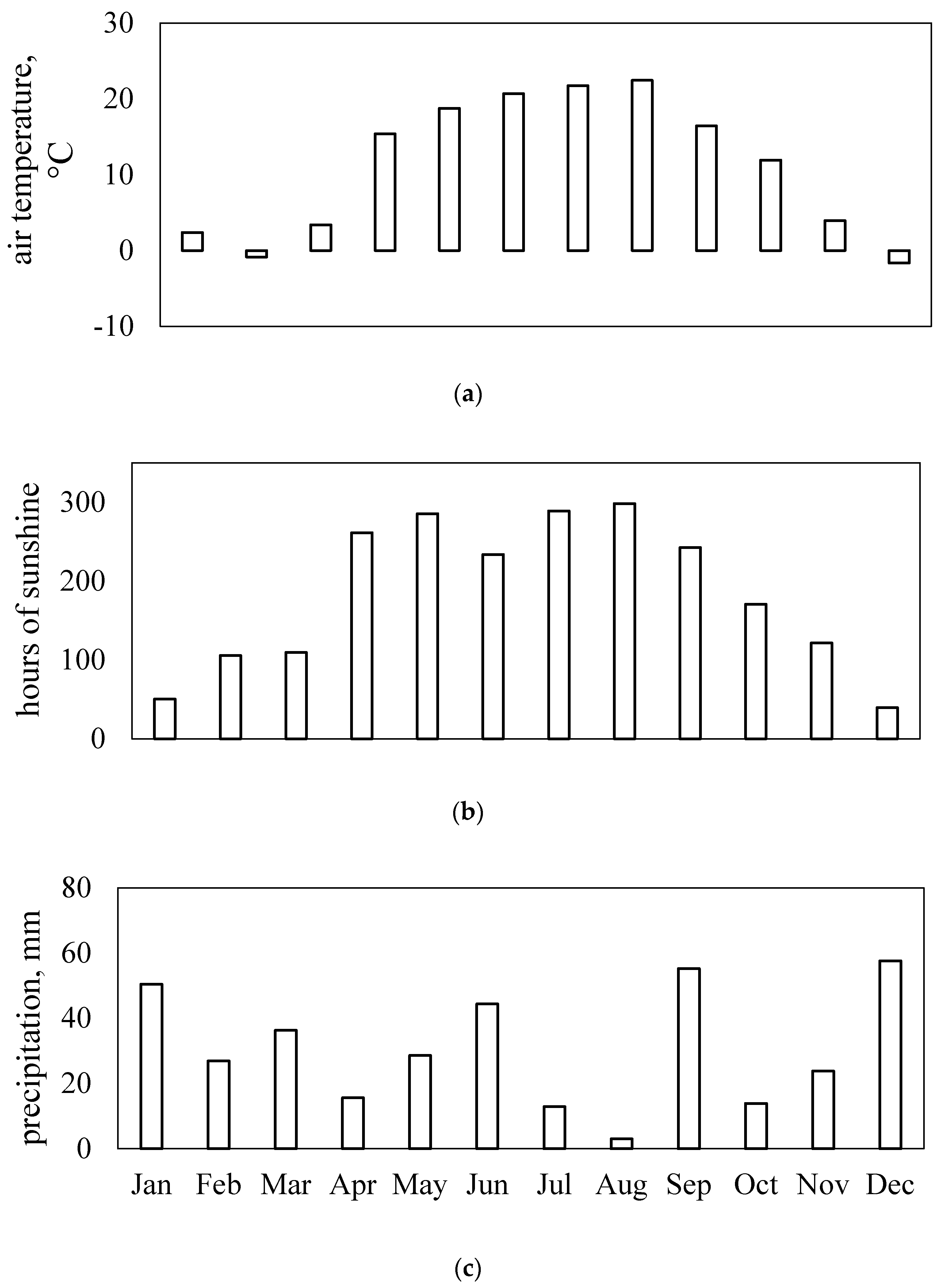
Figure 6 shows that local weather dynamics in the 2018 growing season indicated greater than normal drought.
However, chlorophyll content increases when the sunflower is subject to the foliar application of suitable concentrations of crystalline anastase TiO
2-NPs in adequate environmental conditions. These conditions include sunlight, higher temperature and appropriate precipitation, and the enhanced chlorophyll content then enables plants to synthesise more light-harvesting pigment-protein complexes (LHCII) which absorb greater light energy. Modification was employed because nano-size anatase encourages oxidation-reduction under specific light wavelengths, and this enhances charge transfer between the nanoparticles and LHCII with resultant photosynthesis increase [
85]. In support of this, Lei et al. [
44] identified that TiO
2-NPs under visible and ultra-violet radiation significantly enhances the entire chlorophyll chain electron transport, photosystem II reduction, oxygen production and photo-phosphorylation.
The average normalized difference vegetation index value (NDVI) generally reflects leaf chlorophyll content, photosynthesis-activity, stomatal volume and transpiration [
86,
87]. While
Table 7 shows that this had the following trend: ZnO-NPs variant > TiO
2-NPs variant > control,
Figure 7a highlights that it was statistically significant only for ZnO-NPs at plant ripening on the 125th experimental day. This indicates a vital and prolonged vegetation period from ZnO-NPs application, and early plant maturation with lower chlorophyll content for plants exposed to TiO
2-NPs. There were also statistically significant differences at stem elongation and flower-bud formation on the 70th day for both TiO
2-NPs and ZnO-NPs compared to the control, and statistically significant difference between the TiO
2-NPs variant and control at seed development on the 105th day (
Figure 7a).
The photo-chemical reflectance index (PRI) indicates increased photo-chemical activity and photosynthesis [
87]. It also shows the assimilation of photosynthetic light-use-efficiency normally linked to the de-exposition stage of xanthophyll cycle pigments which protect the photosynthetic apparatus against photo-damage [
88].
Table 7 shows that this is followed by analogous NDVI trend with total seasonal-measured values, and
Figure 7b demonstrates the effect at the ripening phase. The results, however, indicate it was only statistically different for the ZnO-NPs variant compared to the control.
Figure 7b also highlights the high assimilation efficiency of the ZnO-NPs variant compared to both TiO
2-NPs and control. However, the apparent decrease in PRI value was also observed at seed development where both ZnO-NPs and TiO
2-NPs-treated plants had statistically significantly higher values than the control.
Finally, the total average of all crop water stress indices (CWSI) measured throughout the whole season demonstrate better values for the ZnO-NPs variant than for both TiO
2-NPs and control (
Table 7).
Figure 7c then illustrates that periodical CWSI ZnO-NPs variant values at ripening were statistically significantly higher than for the control. While this result for ZnO-NPs was unexpected, the TiO
2-NPs variant values most likely reflect early plant maturation.
There was statistically significant improvement in the common sunflower physiological parameters at stem elongation and flower-bud formation stage two weeks after the first foliar TiO
2-NPs application. Similarly, the second application of TiO
2-NPs led to plant positive response at seed development. Hong et al. [
43] also reported positive effects, including enhanced light absorption, light energy transformation and protoplast protection which counteracted spinach aging. In contrast, TiO
2-NPs statistically significantly negatively affected sunflower physiology at the ripening stage. This was most likely related to leaf-yellowing and decreased chlorophyll content and photosynthetic activity. This was not observed in ZnO-NPs treated plants, possibly because of sufficient sunlight radiation or higher air temperature during this stage of the sunflower life cycle (
Figure 6).
The TiO
2-NPs effects are therefore most likely caused by their physical-chemical nature and depend on its photo-corrosion stability and physical-mechanical resistance compared to ZnO-NPs [
37,
44,
45,
46]. Finally, foliar application of both TiO
2-NPs and ZnO-NPs is reported to have beneficial impact on early stem development, flowering and fruit appearance compared to the control; but no earlier fruit maturation was noted [
19].
The statistically significant differences between ZnO-NPs treatment and the control emerged one-month after the first foliar application and was apparent at stem elongation and flower-bud formation. However, it was surprising that although the NDVI and PRI values at the end of the experiment indicated positive ZnO-NPs plant effects, water stress significantly increased (CWSI). It appears that the ZnO-NPs-treated variant resulted in prolonged sunflower vegetative phase compared to the TiO
2-NPs and control variants. The slightly delayed sunflower physiological response to ZnO-NPs may have been caused by the low-level NPs concentration, their rapid uptake and assimilation [
2], or low precipitation and other weather conditions during the vegetation season (
Figure 6).
Soil condition is an important environmental factor that affects plant physiological parameters. The availability of soil zinc for the plant is mainly influenced by lower, or higher pH and higher clay content and carbonates or natural organic matter [
89]. However, our
Table 1 highlights no soil zinc deficit at 0.9 mg·kg
−1, neutral pH (pH = 7), low 1.28% carbon content and approximately 2% humus content. This reflects the Dolná Malanta silt loam haplic Luvisol [
53], and the total soil zinc content corresponds to the global average [
90]. Moreover, foliar application of ZnO-NPs is most easily assimilated by the plant and freely available for the physiological processes required for prolonged plant vegetation. Our results therefore preclude the sunflower sensitivity to zinc deficiency reported in [
91]. Finally, Lyu et al. [
40] consider that low titanium soil concentration is beneficial for plant production because it simulates certain enzyme enhancement of essential nutrient uptake like Fe, increases crop yield, and promotes a decrease in stress tolerance.
To the best of our knowledge, there is no literature on sunflower interaction with ZnO-NPs related to a wide variety of physiological parameters and its impact on oil content. The available literature contains only the positive ZnO-NP effects on the quantitative and physiological parameters of winter wheat and maize with direct impact on chlorophyll content and photosynthesis [
50,
92,
93]. This is normally an obligatory requirement to increase oil content [
94].
In addition, Kirnak et al. [
95] reported that decreased CWSI resulted in higher oil content and Candogan et al. [
96] supported this with lower CWSI increasing soy seed-proteins and oil content. Finally, Ahmad et al. [
97] found statistically significant differences in
Mentha piperita essential oil content and quality after their 150 mg·L
−1 TiO
2-NPs foliar application, and 90 mg·L
−1 TiO
2-NPs concentration improved
Vetiveria zizanioides photosynthesis and essential oil yield [
98].