Aerobic Oxidation of 5-Hydroxymethylfurfural over Ag Nanoparticle Catalysts Stabilized by Polyvinylpyrrolidone with Different Molecular Weights
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Catalysts
2.2. Characterization of Catalysts
2.3. Catalytic Reactions and Product Analysis
2.4. Kinetic Study
3. Results and Discussion
3.1. The Nature of the Catalyst
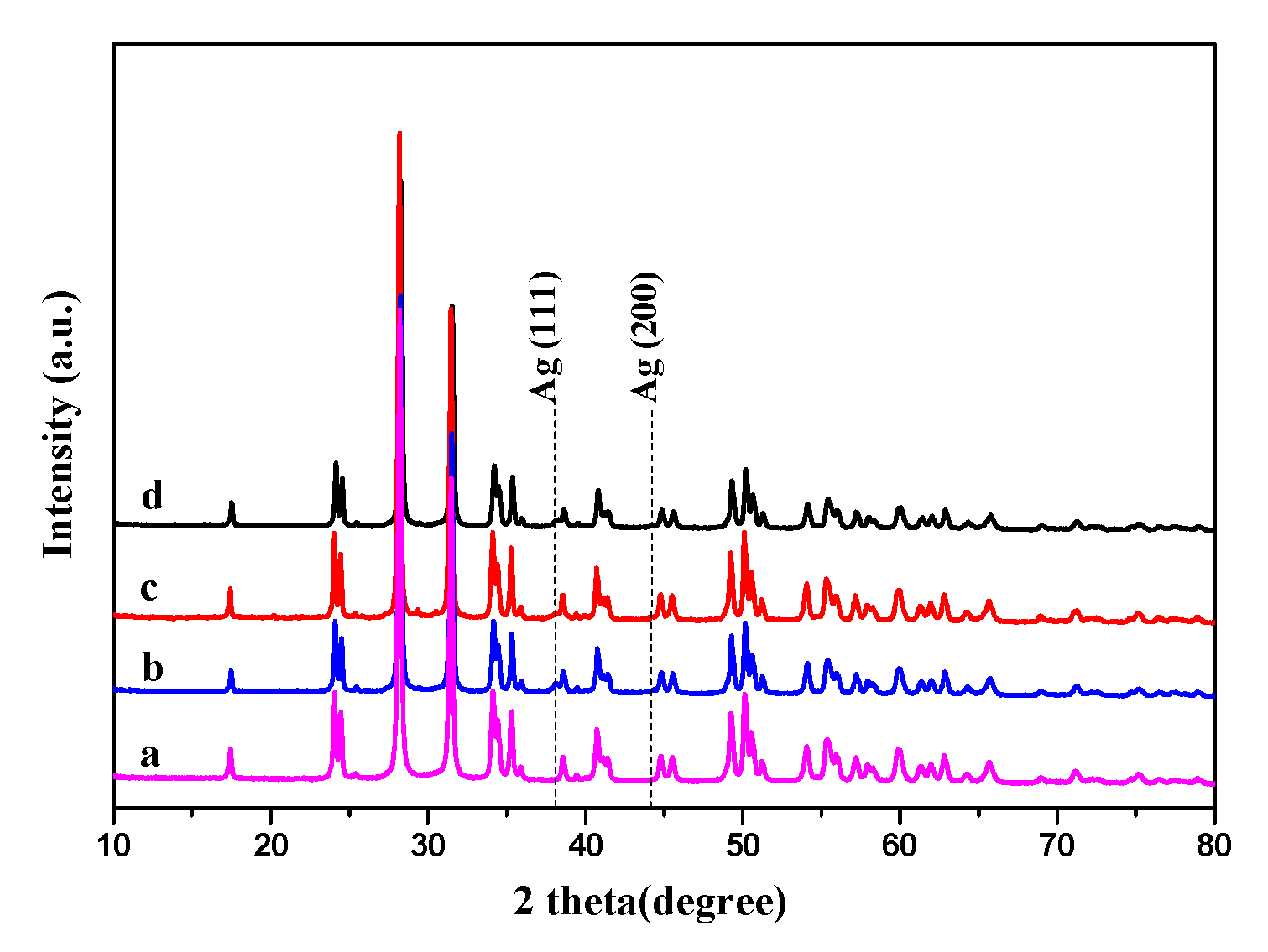
3.1.1. XRD Results
3.1.2. TEM Results
3.1.3. IR Spectra of the Catalysts
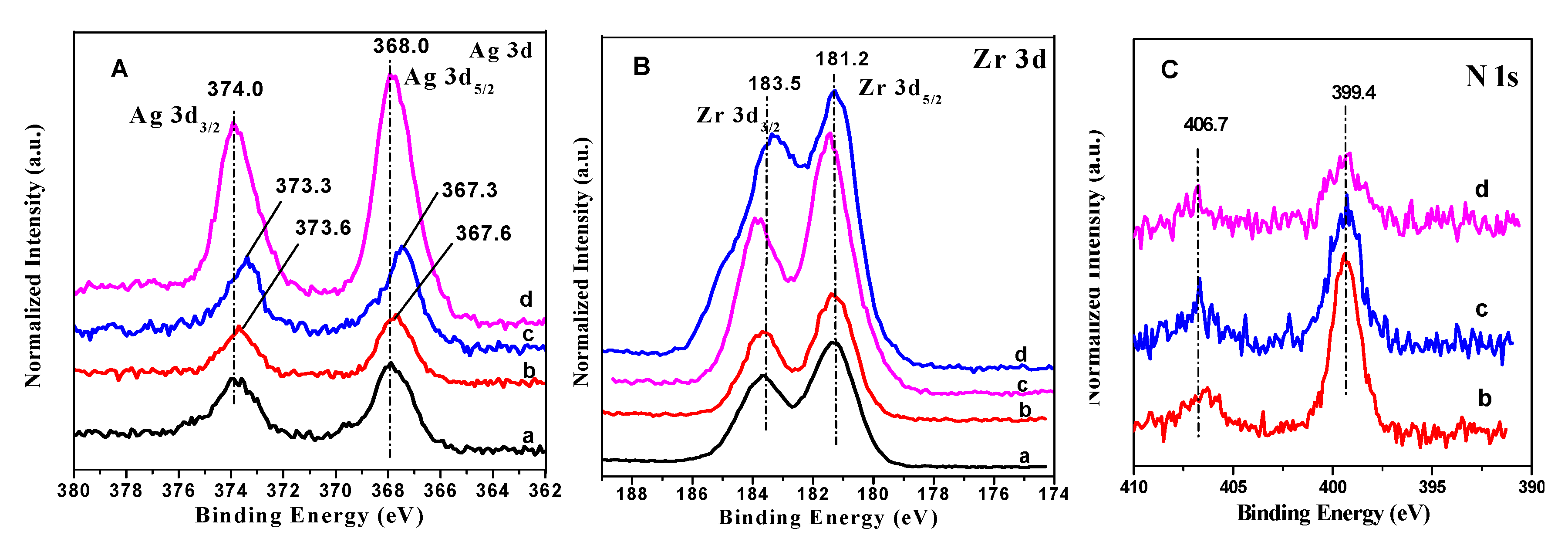
3.1.4. XPS Results
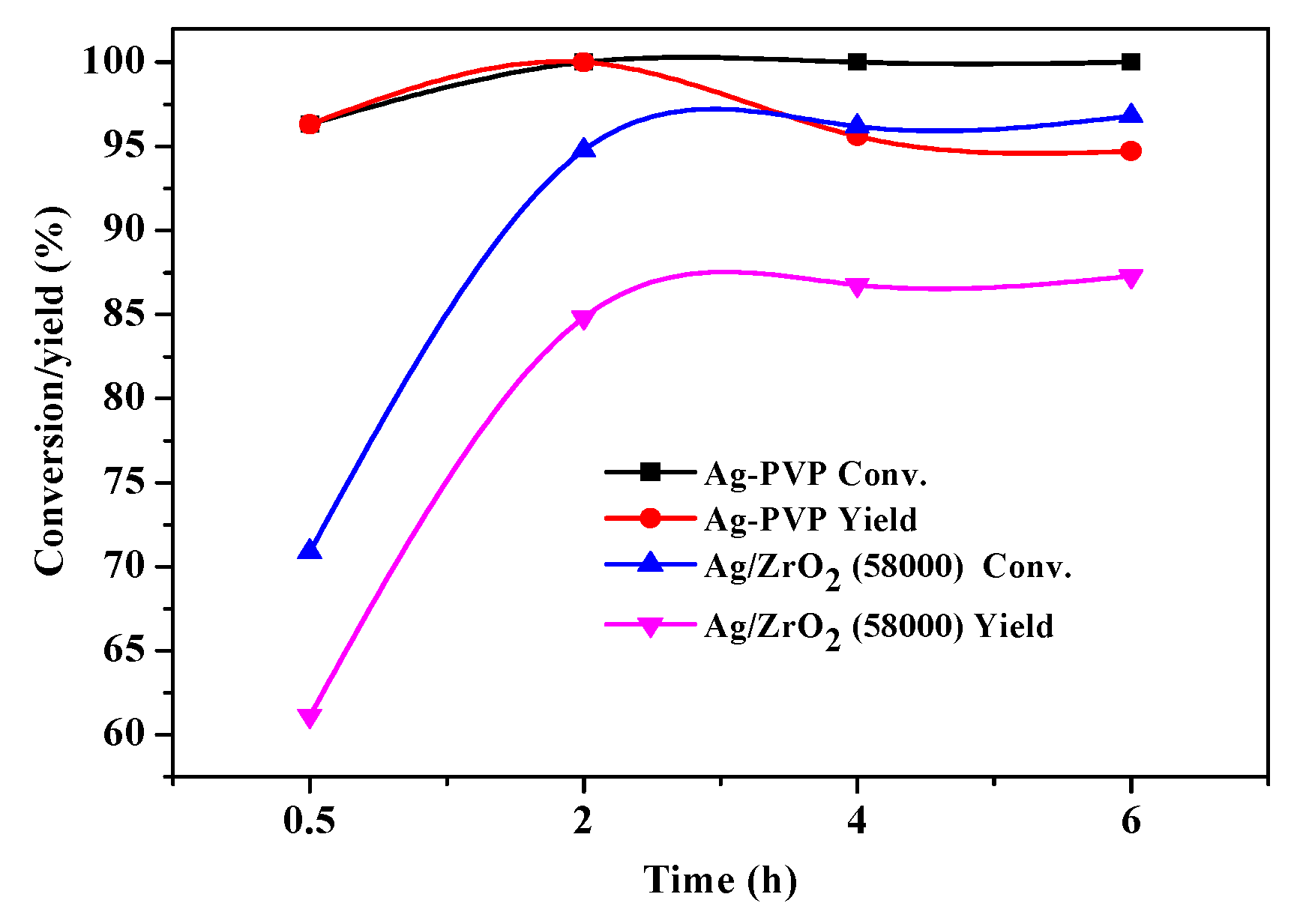
3.2. Oxidation of HMF to HMFCA
3.3. Reaction Kinetics
3.4. The Relationship Between Metal–Support Interaction and PVP Molecular Weight
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jackson, C.; Smith, G.T.; Inwood, D.W.; Leach, A.; Whalley, P.S.; Callisti, M.; Polcar, T.; Russell, A.E.; Levecque, P.B.J.; Kramer, D. Electronic metal–support interaction enhanced oxygen reduction activity and stability of boron carbide supported platinum. Nat. Commun. 2017, 8, 15802. [Google Scholar] [CrossRef]
- Rossi, L.M.; Fiorio, J.L.; Garcia, M.A.S.; Ferraz, C.P. The role and fate of capping ligands in colloidally prepared metal nanoparticle catalysts. Dalton Trans. 2018, 47, 5889–5915. [Google Scholar] [CrossRef]
- Matsubu, J.C.; Zhang, S.; Derita, L.; Marinković, N.; Chen, J.G.; Graham, G.W.; Pan, X.; Christopher, P. Adsorbate-mediated strong metal–support interactions in oxide-supported Rh catalysts. Nat. Chem. 2016, 9, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.-S.; Zhao, Y.-H.; Huang, C.-F.; Song, Y.-H.; Li, D.-P.; Liu, Z.-T.; Liu, Z.-W. Metal–support interactions regulated via carbon coating—A case study of Co/SiO2 for Fischer-Tropsch synthesis. Fuel 2018, 226, 213–220. [Google Scholar] [CrossRef]
- Oh, S.; Kim, Y.K.; Jung, C.H.; Doh, W.H.; Park, J.Y. Effect of the metal–support interaction on the activity and selectivity of methanol oxidation over Au supported on mesoporous oxides. Chem. Commun. 2018, 54, 8174–8177. [Google Scholar] [CrossRef]
- Murata, K.; Mahara, Y.; Ohyama, J.; Yamamoto, Y.; Arai, S.; Satsuma, A. The Metal–support Interaction Concerning the Particle Size Effect of Pd/Al2O3 on Methane Combustion. Angew. Chem. Int. Ed. 2017, 56, 15993–15997. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Zhu, Y.; Xu, S.; Wang, C.; Bian, C.; Meng, X.; Xiao, F.-S. Strong Metal–Support Interactions Achieved by Hydroxide-to-Oxide Support Transformation for Preparation of Sinter-Resistant Gold Nanoparticle Catalysts. ACS Catal. 2017, 7, 7461–7465. [Google Scholar] [CrossRef]
- An, J.; Sun, G.; Xia, H. Aerobic Oxidation of 5-Hydroxymethylfurfural to High-Yield 5-Hydroxymethyl-2-furancarboxylic Acid by Poly(vinylpyrrolidone)-Capped Ag Nanoparticle Catalysts. ACS Sustain. Chem. Eng. 2019, 7, 6696–6706. [Google Scholar] [CrossRef]
- Sun, G.; An, J.; Hu, H.; Li, C.; Zuo, S.; Xia, H. Green catalytic synthesis of 5-methylfurfural by selective hydrogenolysis of 5-hydroxymethylfurfural over size-controlled Pd nanoparticle catalysts. Catal. Sci. Technol. 2019, 9, 1238–1244. [Google Scholar] [CrossRef]
- Tsuji, M.; Nishizawa, Y.; Matsumoto, K.; Kubokawa, M.; Miyamae, N.; Tsuji, T. Effects of chain length of polyvinylpyrrolidone for the synthesis of silver nanostructures by a microwave-polyol method. Mater. Lett. 2006, 60, 834–838. [Google Scholar] [CrossRef]
- Haesuwannakij, S.; Kimura, T.; Furutani, Y.; Okumura, K.; Kokubo, K.; Sakata, T.; Yasuda, H.; Yakiyama, Y.; Sakurai, H. The Impact of the Polymer Chain Length on the Catalytic Activity of Poly(N-vinyl-2-pyrrolidone)-supported Gold Nanoclusters. Sci. Rep. 2017, 7, 9579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorbanev, Y.Y.; Kegnæs, S.; Riisager, A. Effect of Support in Heterogeneous Ruthenium Catalysts Used for the Selective Aerobic Oxidation of HMF in Water. Top. Catal. 2011, 54, 1318–1324. [Google Scholar] [CrossRef]
- Li, C.; Zhao, Z.K.; Cai, H.; Wang, A.; Zhang, T. Microwave-promoted conversion of concentrated fructose into 5-hydroxymethylfurfural in ionic liquids in the absence of catalysts. Biomass Bioenergy 2011, 35, 2013–2017. [Google Scholar] [CrossRef]
- Tong, X.; Ma, Y.; Li, Y. Biomass into chemicals: Conversion of sugars to furan derivatives by catalytic processes. Appl. Catal. A Gen. 2010, 385, 1–13. [Google Scholar] [CrossRef]
- Xia, H.; Xu, S.; Yan, X.; Zuo, S. High yield synthesis of 5-hydroxymethylfurfural from cellulose using FePO4 as the catalyst. Fuel Process. Technol. 2016, 152, 140–146. [Google Scholar] [CrossRef]
- Xia, H.; Xu, S.; Yang, L. Efficient conversion of wheat straw into furan compounds, bio-oils, and phosphate fertilizers by a combination of hydrolysis and catalytic pyrolysis. RSC Adv. 2017, 7, 1200–1205. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Yan, X.; Wang, Q.; Wang, Q.; Xia, H. One-pot catalytic conversion of cellulose into polyols with Pt/CNTs catalysts. Carbohydr. Res. 2015, 404, 87–92. [Google Scholar] [CrossRef]
- Xu, S.; Pan, D.; Hu, F.; Wu, Y.; Wang, H.; Chen, Y.; Yuan, H.; Gao, L.; Xiao, G. Highly efficient Cr/beta zeolite catalyst for conversion of carbohydrates into 5-hydroxymethylfurfural: Characterization and performance. Fuel Process. Technol. 2019, 190, 38–46. [Google Scholar] [CrossRef]
- Xu, S.; Yin, C.; Pan, D.; Hu, F.; Wu, Y.; Miao, Y.; Gao, L.; Xiao, G. Efficient conversion of glucose into 5-hydroxymethylfurfural using a bifunctional Fe3+ modified Amberlyst-15 catalyst. Sustain. Energy Fuels 2019, 3, 390–395. [Google Scholar] [CrossRef]
- Biswas, S.; Dutta, B.; Mannodi-Kanakkithodi, A.; Clarke, R.; Song, W.; Ramprasad, R.; Suib, S.L. Heterogeneous mesoporous manganese/cobalt oxide catalysts for selective oxidation of 5-hydroxymethylfurfural to 2,5-diformylfuran. Chem. Commun. 2017, 53, 11751–11754. [Google Scholar] [CrossRef]
- Mishra, D.K.; Cho, J.K.; Kim, Y.J. Facile production of 2,5-diformylfuran from base-free oxidation of 5-hydroxymethyl furfural over manganese–cobalt spinels supported ruthenium nanoparticles. J. Ind. Eng. Chem. 2018, 60, 513–519. [Google Scholar] [CrossRef]
- Ning, L.; Liao, S.; Sun, Y.; Yu, L.; Tong, X. The Efficient Oxidation of Biomass-Derived 5-Hydroxymethyl Furfural to Produce 2,5-Diformylfuran Over Supported Cobalt Catalysts. Waste Biomass-Valorization 2016, 9, 95–101. [Google Scholar] [CrossRef]
- Hong, M.; Min, J.; Wu, S.; Cui, H.; Zhao, Y.; Li, J.; Wang, S. Metal Nitrate Catalysis for Selective Oxidation of 5-Hydroxymethylfurfural into 2,5-Diformylfuran under Oxygen Atmosphere. ACS Omega 2019, 4, 7054–7060. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, B.; Lv, K.; Sun, J.; Deng, K. Aerobic oxidation of biomass derived 5-hydroxymethylfurfural into 5-hydroxymethyl-2-furancarboxylic acid catalyzed by a montmorillonite K-10 clay immobilized molybdenum acetylacetonate complex. Green Chem. 2014, 16, 2762. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Z. Cs-substituted tungstophosphate-supported ruthenium nanoparticles: An effective catalyst for the aerobic oxidation of 5-hydroxymethylfurfural into 5-hydroxymethyl-2-furancarboxylic acid. J. Taiwan Inst. Chem. Eng. 2017, 70, 1–6. [Google Scholar] [CrossRef]
- Schade, O.R.; Kalz, K.F.; Neukum, D.; Kleist, W.; Grunwaldt, J.-D. Supported gold- and silver-based catalysts for the selective aerobic oxidation of 5-(hydroxymethyl)furfural to 2,5-furandicarboxylic acid and 5-hydroxymethyl-2-furancarboxylic acid. Green Chem. 2018, 20, 3530–3541. [Google Scholar] [CrossRef] [Green Version]
- Ardemani, L.; Cibin, G.; Dent, A.J.; Isaacs, M.; Kyriakou, G.; Lee, A.F.; Parlett, C.M.A.; Parry, S.A.; Wilson, K. Solid base catalysed 5-HMF oxidation to 2,5-FDCA over Au/hydrotalcites: Fact or fiction? Chem. Sci. 2015, 6, 4940–4945. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Su, K.; Li, Z.; Cheng, B. Selective oxidation of 5-hydroxymethylfurfural with H2O2 catalyzed by a molybdenum complex. Green Chem. 2016, 18, 2122–2128. [Google Scholar] [CrossRef]
- Van Nguyen, C.; Liao, Y.-T.; Kang, T.-C.; Chen, J.E.; Yoshikawa, T.; Nakasaka, Y.; Masuda, T.; Wu, K.C.-W. A metal-free, high nitrogen-doped nanoporous graphitic carbon catalyst for an effective aerobic HMF-to-FDCA conversion. Green Chem. 2016, 18, 5957–5961. [Google Scholar] [CrossRef]
- Zhang, Z.; Deng, K. Recent Advances in the Catalytic Synthesis of 2,5-Furandicarboxylic Acid and Its Derivatives. ACS Catal. 2015, 5, 6529–6544. [Google Scholar] [CrossRef]
- Xia, H.; An, J.; Hong, M.; Xu, S.; Zhang, L.; Zuo, S. Aerobic oxidation of 5-hydroxymethylfurfural to 2,5-difurancarboxylic acid over Pd-Au nanoparticles supported on Mg-Al hydrotalcite. Catal. Today 2018, 319, 113–120. [Google Scholar] [CrossRef]
- Liu, K.-J.; Zeng, T.-Y.; Zeng, J.-L.; Gong, S.-F.; He, J.-Y.; Lin, Y.-W.; Tan, J.-X.; Cao, Z.; He, W.-M. Solvent-dependent selective oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid under neat conditions. Chin. Chem. Lett. 2019, 30, 2304–2308. [Google Scholar] [CrossRef]
- Casanova, O.; Iborra, S.; Corma, A. Biomass into Chemicals: Aerobic Oxidation of 5-Hydroxymethyl-2-furfural into 2,5-Furandicarboxylic Acid with Gold Nanoparticle Catalysts. ChemSusChem 2009, 2, 1138–1144. [Google Scholar] [CrossRef] [PubMed]
- Nossova, L.; Caravaggio, G.; Couillard, M.; Ntais, S. Effect of preparation method on the performance of silver-zirconia catalysts for soot oxidation in diesel engine exhaust. Appl. Catal. B Environ. 2018, 225, 538–549. [Google Scholar] [CrossRef]
- García-Aguilar, J.; Navlani-García, M.; Berenguer-Murcia, A.; Mori, K.; Kuwahara, Y.; Yamashita, H.; Cazorla-Amoros, D. Evolution of the PVP–Pd Surface Interaction in Nanoparticles through the Case Study of Formic Acid Decomposition. Langmuir 2016, 32, 12110–12118. [Google Scholar] [CrossRef]
- Evangelisti, C.; Panziera, N.; D’Alessio, A.; Bertinetti, L.; Botavina, M.; Vitulli, G. New monodispersed palladium nanoparticles stabilized by poly-(N-vinyl-2-pyrrolidone): Preparation, structural study and catalytic properties. J. Catal. 2010, 272, 246–252. [Google Scholar] [CrossRef]
- Xian, J.; Hua, Q.; Jiang, Z.; Ma, Y.; Huang, W. Size-Dependent Interaction of the Poly(N-vinyl-2-pyrrolidone) Capping Ligand with Pd Nanocrystals. Langmuir 2012, 28, 6736–6741. [Google Scholar] [CrossRef]
- Boukhvalov, D.W.; Zhidkov, I.S.; Kurmaev, E.Z.; Fazio, E.; Cholakh, S.O.; D’Urso, L. Atomic and electronic structures of stable linear carbon chains on Ag-nanoparticles. Carbon 2018, 128, 296–301. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Wei, C.; Song, Y.; Song, X.; Sun, Z. Nanoporous Ag–ZrO2 composites prepared by chemical dealloying for borohydride electro-oxidation. Int. J. Hydrog. Energy 2014, 39, 15646–15655. [Google Scholar] [CrossRef]
- Collins, G.; Schmidt, M.; McGlacken, G.P.; O’Dwyer, C.; Holmes, J.D. Stability, Oxidation, and Shape Evolution of PVP-Capped Pd Nanocrystals. J. Phys. Chem. C 2014, 118, 6522–6530. [Google Scholar] [CrossRef] [Green Version]
- Jenness, G.R.; Schmidt, J.R. Unraveling the Role of Metal–Support Interactions in Heterogeneous Catalysis: Oxygenate Selectivity in Fischer–Tropsch Synthesis. ACS Catal. 2013, 3, 2881–2890. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, H.; An, J.; Zhang, W. Aerobic Oxidation of 5-Hydroxymethylfurfural over Ag Nanoparticle Catalysts Stabilized by Polyvinylpyrrolidone with Different Molecular Weights. Nanomaterials 2020, 10, 1624. https://doi.org/10.3390/nano10091624
Xia H, An J, Zhang W. Aerobic Oxidation of 5-Hydroxymethylfurfural over Ag Nanoparticle Catalysts Stabilized by Polyvinylpyrrolidone with Different Molecular Weights. Nanomaterials. 2020; 10(9):1624. https://doi.org/10.3390/nano10091624
Chicago/Turabian StyleXia, Haian, Jiahuan An, and Weizi Zhang. 2020. "Aerobic Oxidation of 5-Hydroxymethylfurfural over Ag Nanoparticle Catalysts Stabilized by Polyvinylpyrrolidone with Different Molecular Weights" Nanomaterials 10, no. 9: 1624. https://doi.org/10.3390/nano10091624



