Effects of Carbon Content and Current Density on the Li+ Storage Performance for MnO@C Nanocomposite Derived from Mn-Based Complexes
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of the [MnCl2(2-meim)3] Complexes and MnO@C Nanoparticles
2.3. Materials Characterization
2.4. Electrochemical Measurement
3. Results and Discussions
3.1. Composition and Microstructures of MnO@C Nanocomposites
3.2. Electrochemical Property in Half-Cells
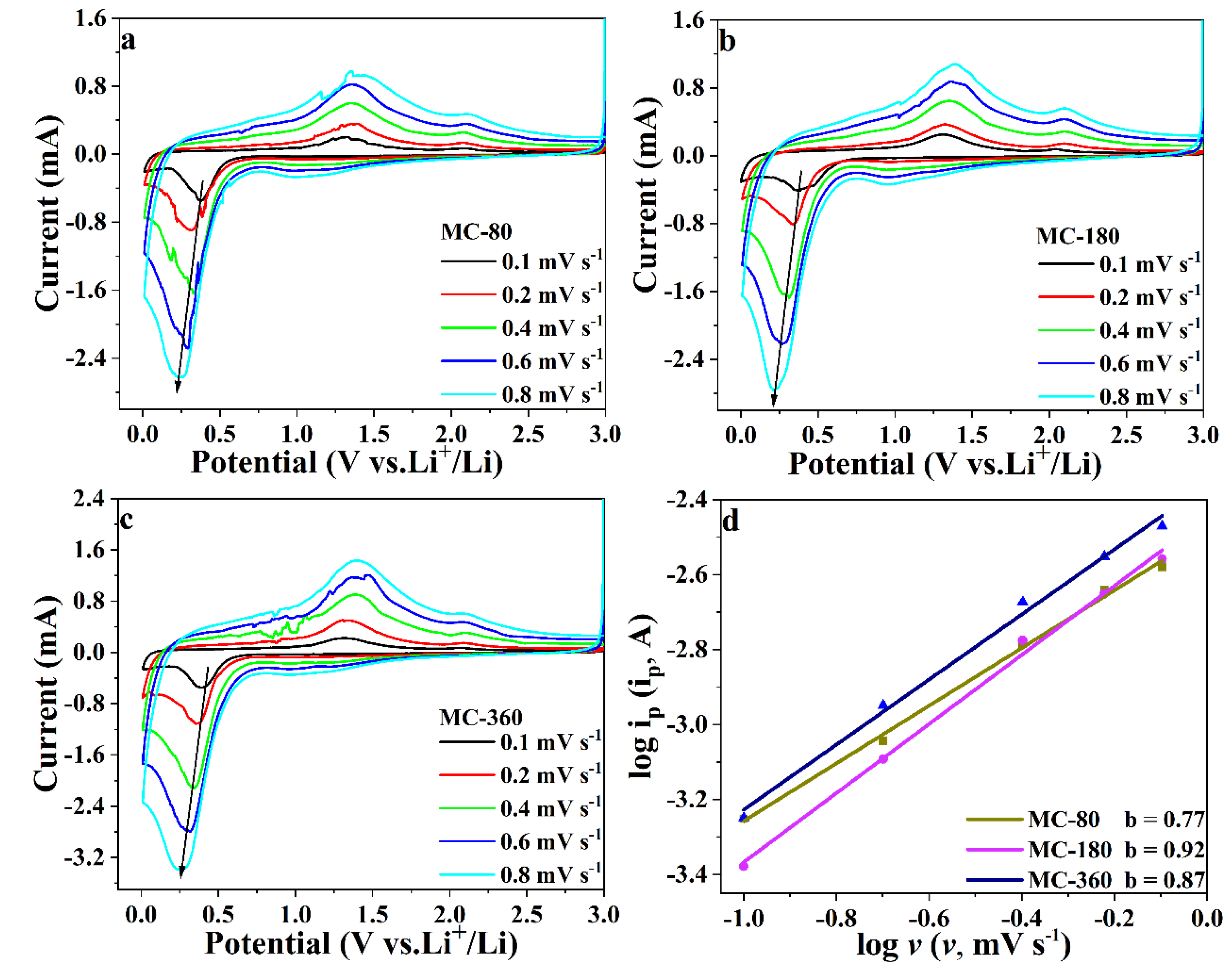
3.3. Electrochemical Mechanism of the MnO@C Nanocomposites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Li, Q.; Li, L.; Wu, P.; Xu, N.; Wang, L.; Li, M.; Dai, A.; Amine, K.; Mai, L.; Lu, J. Silica Restricting the Sulfur Volatilization of Nickel Sulfide for High-Performance Lithium-Ion Batteries. Adv. Energy Mater. 2019, 9, 1901153. [Google Scholar] [CrossRef]
- Xu, K.; Ma, L.; Shen, X.; Ji, Z.; Yuan, A.; Kong, L.; Zhu, G.; Zhu, J. Bimetallic metal-organic framework derived Sn-based nanocomposites for high-performance lithium storage. Electrochim. Acta 2019, 323, 134855. [Google Scholar] [CrossRef]
- An, Q.; Lv, F.; Liu, Q.; Han, C.; Zhao, K.; Sheng, J.; Wei, Q.; Yan, M.; Mai, L. Amorphous vanadium oxide matrixes supporting hierarchical porous Fe3O4/graphene nanowires as a high-rate lithium storage anode. Nano Lett. 2014, 14, 6250–6256. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Y.; Zhou, P.; Lan, Z.; Lu, Y.; Wu, C.; Yan, M. Ultrafast, Highly Reversible, and Cycle-Stable Lithium Storage Boosted by Pseudocapacitance in Sn-Based Alloying Anodes. Adv. Mater. 2017, 29, 1606499. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Jiao, R.; Zuo, X.; Tang, R.; Su, H.; Xu, D.; Sun, D.; Zeng, S.; Zhang, X. Novel Bake-in-Salt Method for the Synthesis of Mesoporous Mn3O4@C Networks with Superior Cycling Stability and Rate Performance. ACS Appl. Mater. Interfaces 2016, 8, 35163–35171. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhang, Y.; Liu, H.; Zhang, X.; Wang, J.-G. Constructing hierarchical MoO2/N-doped carbon hydrangea-like spheres with superior lithium storage properties. J. Alloys Compd. 2019, 787, 45–52. [Google Scholar] [CrossRef]
- Fang, S.; Bresser, D.; Passerini, S. Transition Metal Oxide Anodes for Electrochemical Energy Storage in Lithium- and Sodium-Ion Batteries. Adv. Energy Mater. 2020, 10, 1902485. [Google Scholar] [CrossRef]
- Liu, R.; Chen, X.; Zhou, C.; Li, A.; Gong, Y.; Muhammad, N.; Song, H. Controlled synthesis of porous 3D interconnected MnO /C composite aerogel and their excellent lithium-storage properties. Electrochim. Acta 2019, 306, 143–150. [Google Scholar] [CrossRef]
- Wang, S.; Ren, Y.; Liu, G.; Xing, Y.; Zhang, S. Peanut-like MnO@C core-shell composites as anode electrodes for high-performance lithium ion batteries. Nanoscale 2014, 6, 3508–3512. [Google Scholar] [CrossRef]
- Sun, B.; Chen, Z.; Kim, H.-S.; Ahn, H.; Wang, G. MnO/C core–shell nanorods as high capacity anode materials for lithium-ion batteries. J. Power Sources 2011, 196, 3346–3349. [Google Scholar] [CrossRef]
- Xu, G.L.; Xu, Y.F.; Sun, H.; Fu, F.; Zheng, X.M.; Huang, L.; Li, J.T.; Yang, S.H.; Sun, S.G. Facile synthesis of porous MnO/C nanotubes as a high capacity anode material for lithium ion batteries. Chem. Commun. (Camb.) 2012, 48, 8502–8504. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.-C.; Xu, C.-Y.; Sun, X.-Y.; Pei, Y.; Wang, P.-P.; Ma, F.-X.; Zhen, L. Constructing yolk-shell MnO@C nanodiscs through a carbothermal reduction process for highly stable lithium storage. Chem. Eng. J. 2018, 336, 427–435. [Google Scholar] [CrossRef]
- Tao, S.; Li, B.; Zhang, J.; Cui, P.; Wu, D.; Chu, W.; Qian, B.; Song, L. In situ synthesis of ultrasmall MnO nanoparticles encapsulated by a nitrogen-doped carbon matrix for high-performance lithium-ion batteries. Chem. Commun. (Camb.) 2019, 55, 9184–9187. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Zhou, X.; Yang, J.; Sun, A.; Wang, H.; Tang, J. MnO Nanoparticles Sandwiched within 3D Graphene-Based Hierarchical Architecture for Efficient Lithium Storage. Inorg. Chem. 2019, 58, 3329–3337. [Google Scholar] [CrossRef]
- Guo, J.; Liang, J.; Cui, C.; Ma, J. Oleic acid-treated synthesis of MnO@C with superior electrochemical properties. J. Energy Chem. 2017, 26, 340–345. [Google Scholar] [CrossRef][Green Version]
- Phillips, F.L.; Shreeve, F.M.; Skapski, A.C. Crystal and Molecular Structure of Dichlorotris-(2-methylimidazole)manganese(II); a High-Spin Pentacoordinate Complex of Manganese.pdf. Acta Cryst. 1976, 32, 687–692. [Google Scholar] [CrossRef]
- Zhou, X.; Cheng, F.; Yang, J.; Jia, M.; Sun, A.; Tang, J. N-doped carbon encapsulated MnO nanoparticles for enhanced lithium storage. Mater. Lett. 2019, 234, 335–338. [Google Scholar] [CrossRef]
- Dubey, R.J.; Sasikumar, P.V.W.; Cerboni, N.; Aebli, M.; Krumeich, F.; Blugan, G.; Kravchyk, K.V.; Graule, T.; Kovalenko, M.V. Silicon oxycarbide-antimony nanocomposites for high-performance Li-ion battery anodes. Nanoscale 2020, 12, 13540–13547. [Google Scholar] [CrossRef]
- Wang, J.; Luo, C.; Mao, J.; Zhu, Y.; Fan, X.; Gao, T.; Mignerey, A.C.; Wang, C. Solid-State Fabrication of SnS2/C Nanospheres for High-Performance Sodium Ion Battery Anode. ACS Appl. Mater. Interfaces 2015, 7, 11476–11481. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Wan, W.; Li, L.; Dong, Y.; Zhao, Z.; Qiu, J. Multifunctional nitrogen-doped graphene nanoribbon aerogels for superior lithium storage and cell culture. Nanoscale 2016, 8, 2159–2167. [Google Scholar] [CrossRef]
- Zhu, S.; Li, J.; He, C.; Zhao, N.; Liu, E.; Shi, C.; Zhang, M. Soluble salt self-assembly-assisted synthesis of three-dimensional hierarchical porous carbon networks for supercapacitors. J. Mater. Chem. A 2015, 3, 22266–22273. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, X.; Luo, W.; Huang, Y. Porous carbon-modified MnO disks prepared by a microwave-polyol process and their superior lithium-ion storage properties. J. Mater. Chem. 2012, 22, 19190. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, H.; Liu, Z.; Zhao, H.; Huang, L.; Wang, Q.; Liu, H.; Zhang, Y. Tailoring sandwich-like CNT@MnO@N-doped carbon hetero-nanotubes as advanced anodes for boosting lithium storage. Electrochim. Acta 2019, 304, 158–167. [Google Scholar] [CrossRef]
- Xiao, Y.; Cao, M. Carbon-Anchored MnO Nanosheets as an Anode for High-Rate and Long-Life Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2015, 7, 12840–12849. [Google Scholar] [CrossRef]
- Guo, S.; Lu, G.; Qiu, S.; Liu, J.; Wang, X.; He, C.; Wei, H.; Yan, X.; Guo, Z. Carbon-coated MnO microparticulate porous nanocomposites serving as anode materials with enhanced electrochemical performances. Nano Energy 2014, 9, 41–49. [Google Scholar] [CrossRef]
- Li, Q.; Li, L.; Owusu, K.A.; Luo, W.; An, Q.; Wei, Q.; Zhang, Q.; Mai, L. Self-adaptive mesoporous CoS@alveolus-like carbon yolk-shell microsphere for alkali cations storage. Nano Energy 2017, 41, 109–116. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, X.; Wang, W.; Zhao, D.; Cao, M. Engineering hybrid between MnO and N-doped carbon to achieve exceptionally high capacity for lithium-ion battery anode. ACS Appl. Mater. Interfaces 2014, 6, 2051–2058. [Google Scholar] [CrossRef]
- Chu, Y.; Guo, L.; Xi, B.; Feng, Z.; Wu, F.; Lin, Y.; Liu, J.; Sun, D.; Feng, J.; Qian, Y.; et al. Embedding MnO@Mn3O4 Nanoparticles in an N-Doped-Carbon Framework Derived from Mn-Organic Clusters for Efficient Lithium Storage. Adv. Mater. 2017, 30, 1704244. [Google Scholar] [CrossRef]
- Hou, C.; Tai, Z.; Zhao, L.; Zhai, Y.; Hou, Y.; Fan, Y.; Dang, F.; Wang, J.; Liu, H. High performance MnO@C microcages with a hierarchical structure and tunable carbon shell for efficient and durable lithium storage. J. Mater. Chem. A 2018, 6, 9723–9736. [Google Scholar] [CrossRef]
- Li, W.; An, C.; Guo, H.; Sun, J.; Wang, M.; Li, Y.; Jiao, L.; Wang, Y. In Situ Synthesis of 1D Mesoporous MnO@C Nanorods for High Performance Li-Ion Batteries. ACS Sustain. Chem. Eng. 2018, 7, 139–146. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, L.; Song, H.; Chen, X.; Zhou, J. Enhanced electrochemical performance of MnO nanowire/graphene composite during cycling as the anode material for lithium-ion batteries. Nano Energy 2014, 10, 172–180. [Google Scholar] [CrossRef]
- Jiang, H.; Hu, Y.; Guo, S.; Yan, C.; Lee, P.S.; Li, C. Rational design of MnO/carbon nanopeapods with internal void space for high-rate and long-life Li-ion batteries. ACS Nano 2014, 8, 6038–6046. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Zeng, W.; Lin, H.; Liu, S.; Tian, X.; Yang, J.; Li, J.; Yang, Y. Na2SnO3 as a novel anode for high performance lithium storage and its electrochemical reaction mechanism. Electrochim. Acta 2019, 315, 48–57. [Google Scholar] [CrossRef]
- Cao, G.; Zhu, J.; Li, Y.; Jin, Z.; Xu, B.; Chen, Y.; Deng, S.; Chang, S.; Lei, T.; Guo, J. Towards superior high-rate cyclability of fine LiNi0.88Co0.12O2 cathode materials for lithium-ion battery via a solvothermal routine. Mater. Lett. 2019, 246, 169–173. [Google Scholar] [CrossRef]
- Jiang, Z.; Jiang, Z.-J. Effects of carbon content on the electrochemical performance of LiFePO4/C core/shell nanocomposites fabricated using FePO4/polyaniline as an iron source. J. Alloys Compd. 2012, 537, 308–317. [Google Scholar] [CrossRef]
- Brezesinski, T.; Wang, J.; Tolbert, S.H.; Dunn, B. Ordered mesoporous alpha-MoO3 with iso-oriented nanocrystalline walls for thin-film pseudocapacitors. Nat. Mater. 2010, 9, 146–151. [Google Scholar] [CrossRef]
- Yang, L.C.; Sun, W.; Zhong, Z.W.; Liu, J.W.; Gao, Q.S.; Hu, R.Z.; Zhu, M. Hierarchical MoO2/N-doped carbon heteronanowires with high rate and improved long-term performance for lithium-ion batteries. J. Power Sources 2016, 306, 78–84. [Google Scholar] [CrossRef]
- Zhang, Y.; Tao, H.; Ma, H.; Du, S.; Li, T.; Zhang, Y.; Li, J.; Yang, X. Three-dimensional MoO2@few-layered MoS2 covered by S-doped graphene aerogel for enhanced lithium ion storage. Electrochim. Acta 2018, 283, 619–627. [Google Scholar] [CrossRef]
- Wang, W.; Shi, G.; Cai, H.; Zhao, C.; Wu, J.; Yu, Y.; Hu, J.; Fang, Z.; Yan, J.; Liu, B. Yolk-shell structured Mo/MoO2 composite microspheres function as high-performance anode materials for lithium-ion batteries. J. Alloys Compd. 2019, 792, 191–202. [Google Scholar] [CrossRef]
- Yang, L.; Li, X.; Ouyang, Y.; Gao, Q.; Ouyang, L.; Hu, R.; Liu, J.; Zhu, M. Hierarchical MoO2/Mo2C/C Hybrid Nanowires as High-Rate and Long-Life Anodes for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 19987–19993. [Google Scholar] [CrossRef]
- Yao, X.; Ke, Y.; Ren, W.; Wang, X.; Xiong, F.; Yang, W.; Qin, M.; Li, Q.; Mai, L. Defect-Rich Soft Carbon Porous Nanosheets for Fast and High-Capacity Sodium-Ion Storage. Adv. Energy Mater. 2018, 9, 1803260. [Google Scholar] [CrossRef]
- Lu, L.Q.; Wang, Y. Facile synthesis of graphene-supported shuttle- and urchin-like CuO for high and fast Li-ion storage. Electrochem. Commun. 2012, 14, 82–85. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, Y.; Zhao, Z.; Yuan, C.; Lou, X.W.D. A Ternary Fe1−xS@Porous Carbon Nanowires/Reduced Graphene Oxide Hybrid Film Electrode with Superior Volumetric and Gravimetric Capacities for Flexible Sodium Ion Batteries. Adv. Energy Mater. 2019, 9, 1803052. [Google Scholar] [CrossRef]










© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, R.; Zhao, L.; Zhou, S.; Zhai, Y.; Wei, D.; Zeng, S.; Zhang, X. Effects of Carbon Content and Current Density on the Li+ Storage Performance for MnO@C Nanocomposite Derived from Mn-Based Complexes. Nanomaterials 2020, 10, 1629. https://doi.org/10.3390/nano10091629
Jiao R, Zhao L, Zhou S, Zhai Y, Wei D, Zeng S, Zhang X. Effects of Carbon Content and Current Density on the Li+ Storage Performance for MnO@C Nanocomposite Derived from Mn-Based Complexes. Nanomaterials. 2020; 10(9):1629. https://doi.org/10.3390/nano10091629
Chicago/Turabian StyleJiao, Ranran, Li Zhao, Shuli Zhou, Yanjun Zhai, Denghu Wei, Suyuan Zeng, and Xianxi Zhang. 2020. "Effects of Carbon Content and Current Density on the Li+ Storage Performance for MnO@C Nanocomposite Derived from Mn-Based Complexes" Nanomaterials 10, no. 9: 1629. https://doi.org/10.3390/nano10091629
APA StyleJiao, R., Zhao, L., Zhou, S., Zhai, Y., Wei, D., Zeng, S., & Zhang, X. (2020). Effects of Carbon Content and Current Density on the Li+ Storage Performance for MnO@C Nanocomposite Derived from Mn-Based Complexes. Nanomaterials, 10(9), 1629. https://doi.org/10.3390/nano10091629




