Abstract
An in situ high-pressure X-ray diffraction study was performed on Ag2S nanosheets, with an average lateral size of 29 nm and a relatively thin thickness. Based on the experimental data, we demonstrated that under high pressure, the samples experienced two different high-pressure structural phase transitions up to 29.4 GPa: from monoclinic P21/n structure (phase I, α-Ag2S) to orthorhombic P212121 structure (phase II) at 8.9 GPa and then to monoclinic P21/n structure (phase III) at 12.4 GPa. The critical phase transition pressures for phase II and phase III are approximately 2–3 GPa higher than that of 30 nm Ag2S nanoparticles and bulk materials. Additionally, phase III was stable up to the highest pressure of 29.4 GPa. Bulk moduli of Ag2S nanosheets were obtained as 73(6) GPa for phase I and 141(4) GPa for phase III, which indicate that the samples are more difficult to compress than their bulk counterparts and some other reported Ag2S nanoparticles. Further analysis suggested that the nanosize effect arising from the smaller thickness of Ag2S nanosheets restricts the relative position slip of the interlayer atoms during the compression, which leads to the enhancing of phase stabilities and the elevating of bulk moduli.
1. Introduction
As a well-known metal sulfide, Ag2S is a direct narrow band gap semiconductor (~1.5 eV), with high absorption coefficient (104 m−1), good chemical stability and optical properties [1,2,3]. So far, Ag2S materials have been extensively synthesized and studied due to their important applications including semiconductors, photovoltaic cells, infrared detectors, photoelectric switches and oxygen sensors [4,5,6]. Recently, the phase transitions of Ag2S have attracted much attention and a lot of research has been conducted around this topic. Under ambient conditions, Ag2S is stable in a monoclinic structure with P21/n space group (α-Ag2S) [7]. While heating above 450 K, Ag2S undergoes a thermo-induced phase transition and reforms into a body-centered cubic (bcc) structure (β-Ag2S, space group Imm) [8,9]. In this high-temperature structure, silver ions are randomly distributed over the interstitial sites of a bcc sulfur lattice [10,11], leading to a favorable ionic conductivity as high as 5 Ω−1 cm−1 [12]. Thereby, β-Ag2S is considered as a fast ionic conductor (FIC), which has potential applications including in energy, analytical chemistry, biomedicine, solid-state ionic devices and so forth [13,14,15]. At about 860 K, Ag2S further converse into a face-centered cubic (fcc) phase (γ-Ag2S), then keeping stable up to melting temperature [9].
Instead of temperature, high pressure is an effective approach for tuning both structural phase transitions and physical properties [16,17]. Under high pressure, the atomic arrangement of materials would be dramatically changed, and thus, the electronic structures and physical properties could be improved. For bulk Ag2S, structural and optical behaviors under high pressure have been systematically studied by synchrotron X-ray diffraction and infrared spectroscopy measurements [15,18,19]. It is demonstrated that Ag2S bulk material experiences a series of phase transitions up to 40 GPa. At 5.1 GPa, bulk Ag2S transforms from the P21/n structure (α-Ag2S, phase I) to an orthorhombic P212121 structure (phase II). Elevating the pressure up to 8.8 GPa, Ag2S undergoes the second phase transition, from phase II to a monoclinic P21/n structure (phase III), which is isosymmetric to phase I. At 28.4 GPa, phase III further transforms into a Pnma structure (phase IV). Accompanied by structural transformations, the band gap of Ag2S is narrowed gradually by increasing the pressure. Beyond 22 GPa, the pressure effectively tunes the semiconducting Ag2S into a metal [19].
Up to now, with the rapid development of synthesis technology, many kinds of nanostructured Ag2S have been prepared, such as quantum dots [20], nanoparticles [21,22], nanotubes [23], nanowires [24], nanorods [25], sheet-like and cube-like nanocrystals [26] and so forth. This progress inspired researchers to begin to focus on the pressure-induced phase transitions of Ag2S nanomaterials, where they expected to obtain novel properties under pressure. Thus far, there has been one report on the high-pressure behaviors of pure and Y-doped Ag2S nanoparticles [27]. The particle sizes of the two samples were estimated to be about 30 nm. Indeed, Wang et al. have found that both of the samples exhibit slightly higher transition pressure values and obviously increased bulk moduli than that of the corresponding bulk materials. We note that previous studies have been focused on Ag2S nanocrystals with random geometric shapes. It is still unclear what behaviors of Ag2S nanocrystals with special morphologies will actually be induced by high pressure. In particular, it is still unknown whether or not they will choose the known structural evolution laws as well as the nanoparticles. These require further studies.
In this work, we investigate the high-pressure behaviors of Ag2S nanomaterials with sheet-like morphologies using the in situ high-pressure X-ray diffraction up to about 30 GPa. A set of phase transitions of Ag2S nanosheets were observed. Interestingly, the Ag2S nanosheets exhibit even higher structural stability and lower compressibility under high pressure, remarkably different from the Ag2S bulk materials and nanoparticles. Further analysis suggested that the stronger nanosize effect arising from the smaller thickness of Ag2S nanosheets effectively restricts the relative position slip of the interlayer atoms during the compression, which leads to the enhancement of phase stabilities and the elevation of bulk moduli. Our findings give a further insight into high-pressure behaviors of Ag2S nanomaterials.
2. Materials and Methods
In this study, Ag2S nanosheet was provided by Tongshun Wu, Ph. D. of State Key Laboratory of Inorganic Synthesis and Preparative Chemistry, Jilin University (Changchun, China), which was prepared by solvothermal method. The initial sample was dispersed in ethanol, with no substrate or coating. A HITACHI H-8100 (Hitachi, Ltd., Tokyo, Japan) transmission electron microscope (TEM) with accelerating voltage of 200 kV was employed to analyze the typical product. X-ray powder diffraction (XRD) was used to characterize the product and the XRD patterns at ambient condition were collected by a Rigaku D/max-rA XRD (Rigaku Co., Tokyo, Japan) spectrometer (Cu, Kα, λ = 1.5406 Å).
A symmetric diamond anvil cell (DAC) with flat diamond culets of 400 μm in diameter was used to generate high pressure. The DAC allowed access to the X-ray diffraction angular range 4θ = 50°. A sheet of T301 stainless steel was selected as the gasket and pre-indented in the DAC to an initial thickness of about 40 μm. Then, a hole of 150 μm in diameter was drilled manually by a tungsten carbide needle in the middle of the dent, which served as a sample chamber. The Ag2S nanosheet samples along with the dispersant were loaded simultaneously into the sample chamber to keep the dispersity. A mixture of methanol and ethanol at a ratio of 4:1 was used as the pressure transmitting medium. A tiny ruby ball ~5 μm in diameter was loaded in the sample chamber, and pressure was determined by the frequency shift of the ruby R1 fluorescence line. High-pressure X-ray diffraction experiments were carried out up to 30 GPa using a synchrotron X-ray source (λ = 0.386 Å) of the beamline X17C of National Synchrotron Light Source (NSLS), Brookhaven National Lab (BNL). The monochromatic X-ray beam was focused down to 25 μm × 25 μm using Kirkpatrick-Baez mirrors. For filtering out the tail of the X-ray beam, a pinhole was placed before the sample position as a cleanup aperture. The X-ray diffraction images were collected by a MAR345 image plate, located ~350 mm behind the sample. Exposure times were typically 300~600 s. Geometric correction and radial integration was done using the Fit2D software (v12.077, GRENOBLE, France). The observed intensities were integrated as a function of 2θ in order to give conventional, one-dimensional diffraction profiles. Rietveld refinement was performed by GSAS-EXPGUI package (v1.80, Gaithersburg, Maryland, USA). A Birch-Murnaghan (BM) equation of state (EOS) was employed to fit the experimental P-V relationship, using Origin Pro software (v8.0, Northampton, MA, USA).
3. Results and Discussion
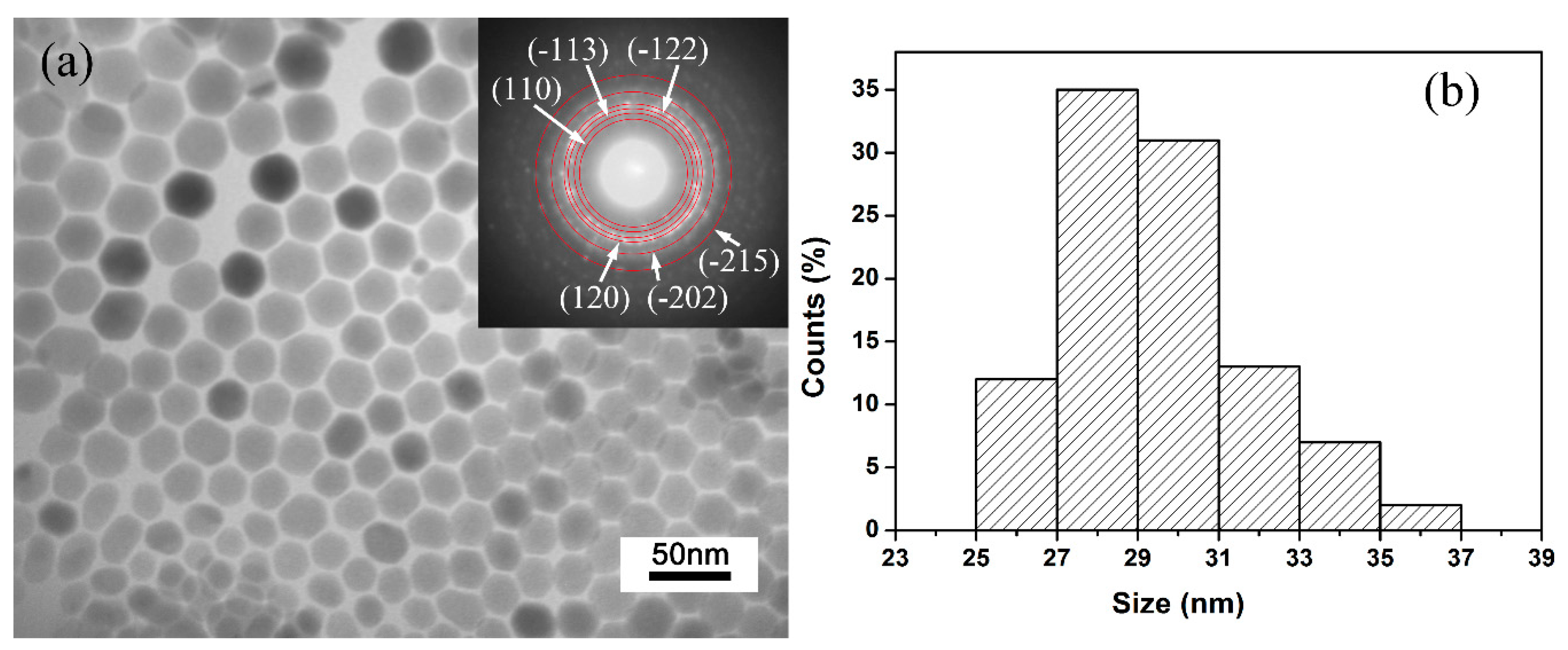
To investigate the morphology and structure of the Ag2S samples, TEM and selected area electron diffraction (SAED) analysis was performed. Figure 1a shows the TEM image and SAED pattern of the Ag2S samples. It can be clearly seen that all Ag2S nanocrystals are well dispersed and possess sheet-like morphology; no other shapes can be observed. SAED pattern (inset in Figure 1a) determines the samples are well crystalized in a monoclinic P21/n structure. Figure 1b shows the lateral size distribution of Ag2S nanosheets. The distribution is relatively narrow, and the average lateral size is 29 ± 2 nm. In principle, for the TEM image, the contrast of the image normally represents the thickness of samples. The smooth contrast of each Ag2S nanosheet grain proves that the samples have uniform thickness. Interestingly, the Ag2S nanosheets are approximately semitransparent (see the sample overlaps in TEM image), which demonstrates that the thickness of samples is relatively thin, far below 29 nm. All the analyses show that Ag2S samples are well crystallized and have an almost-uniform morphology as nanosheets, providing ideal samples for high-pressure research.
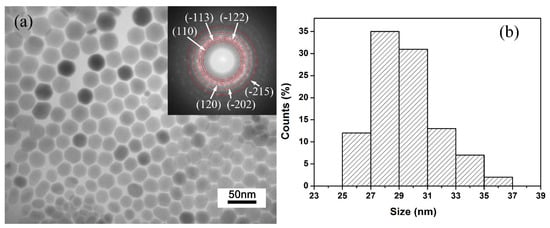
Figure 1.
Typical transmission electron microscope (TEM) images and particle size distribution: (a) TEM images and selected area electron diffraction (SAED) image (inset) of the samples. (b) Size distribution of Ag2S nanosheets.
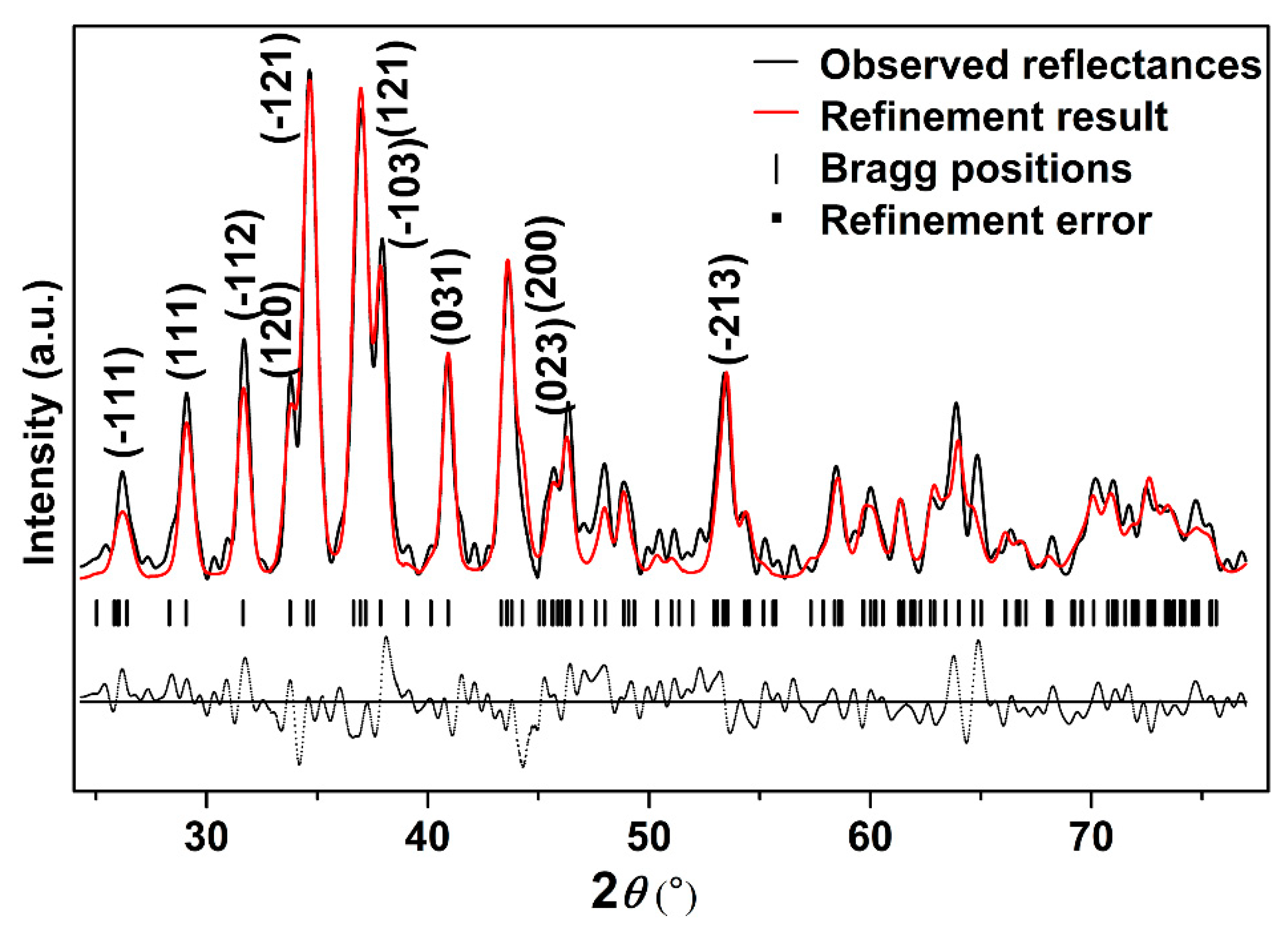
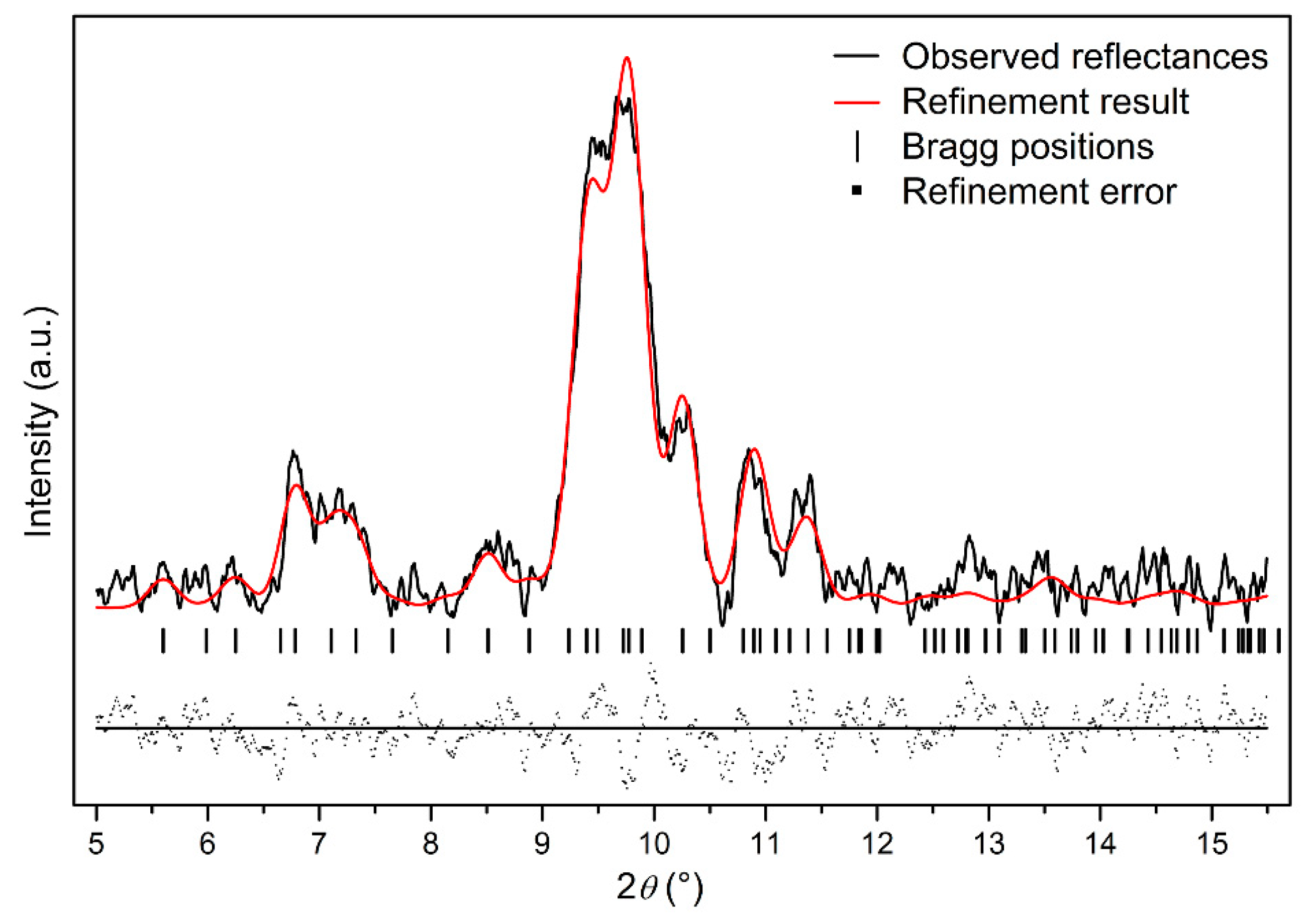
In order to further study the crystal structure of Ag2S nanosheets, XRD analysis was carried out. Figure 2 shows the typical XRD pattern of the samples and its Rietveld refinement under ambient conditions. It can be seen that the diffraction pattern can be indexed into α-Ag2S phase (Joint Committee on Powder Diffraction Standards (JCPDS) card no. 14-0072) (monoclinic structure, space group P21/n). Vertical markers indicate the Bragg reflections of the P21/n monoclinic structure. It can be seen that no other diffraction peaks exist, which means that no other phases or other impurities are mixing in the sample. The Rietveld refinement of the XRD pattern at ambient pressure yields a = 4.214 ± 0.001 Å, b = 6.912 ± 0.001 Å, c = 7.853 ± 0.001 Å, β = 99.580° ± 0.010°, V0 = 225.55 ± 0.034 Å3 for Ag2S nanosheets, slightly smaller than 30 nm Ag2S nanoparticles and bulk materials [27,28]. The existence of a lattice shrinking phenomenon of Ag2S nanosheets could be attributed to the stronger nanosize effect as a result of their smaller thickness.
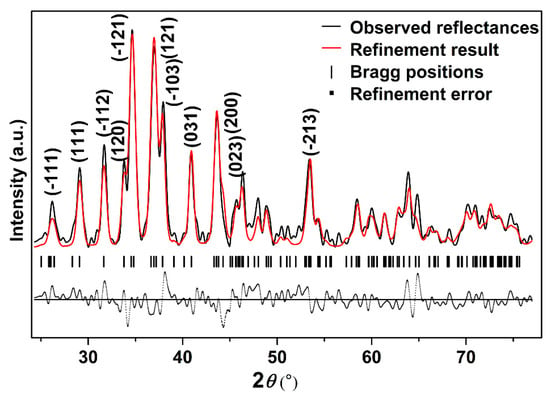
Figure 2.
The typical X-ray powder diffraction (XRD) pattern of Ag2S nanosheets under ambient conditions; Rwp = 0.3133, Rp = 0.2028, and chi^2 = 84.89.
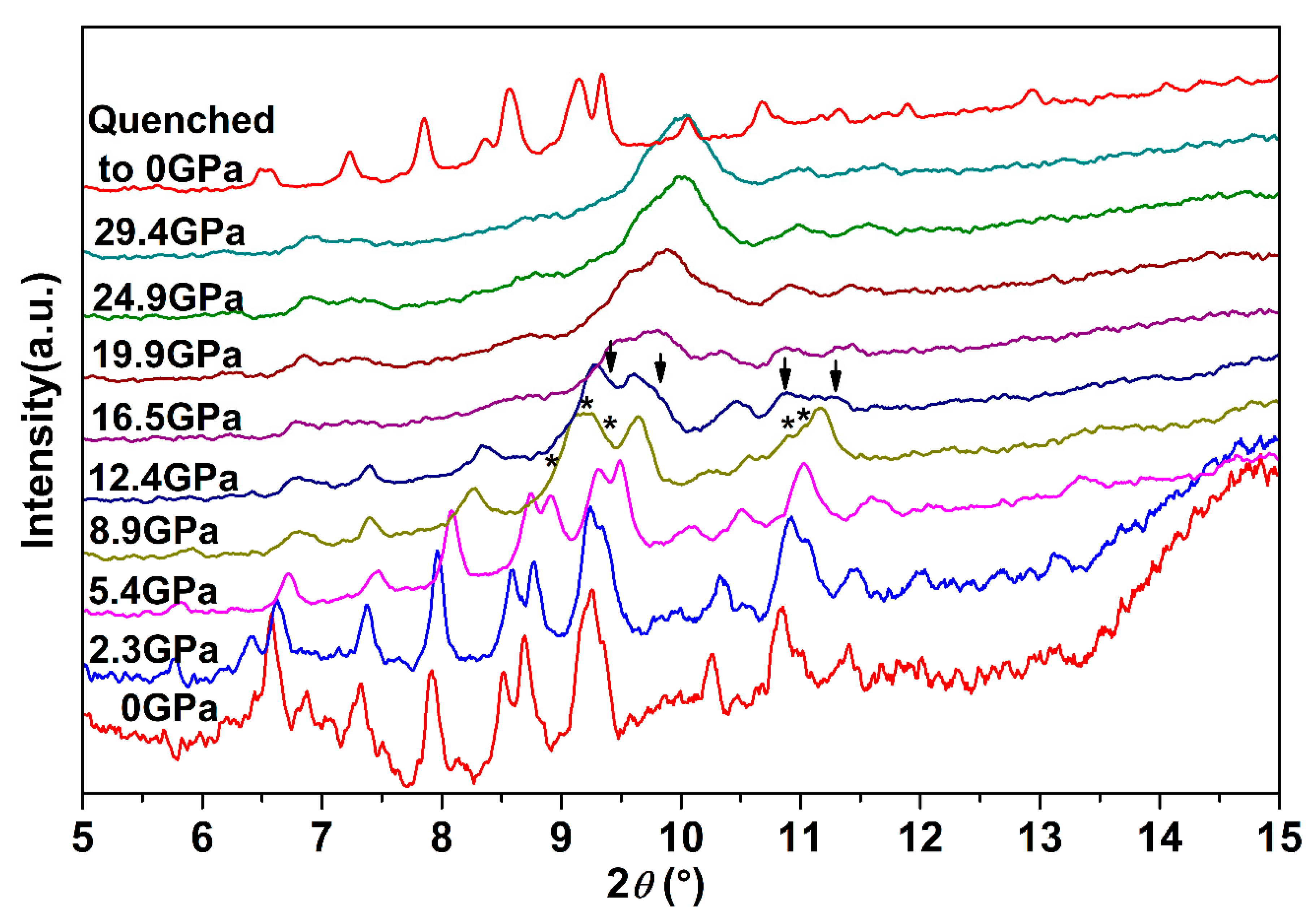
To systematically explore the high-pressure behavior of Ag2S nanosheets, we carried out an in situ high-pressure X-ray diffraction study. Figure 3 shows synchrotron X-ray diffraction patterns of Ag2S nanosheets under different pressures. It is clear that under high pressures, two phase transitions for the samples are observed up to 29.4 GPa. With pressure increasing, all the peaks of α-Ag2S phase shift to smaller d-spacing, indicating a pressure-induced reduction of d-spacing and shrinkage of the unit cell. The initial phase can be maintained up to 8.9 GPa, much more stable than that in Ag2S nanoparticles and bulk materials [19,27]. At 5.4 GPa (the first phase transition point of bulk Ag2S), the measured XRD pattern is consistent with the starting monoclinic P21/n structure (phase I). The mutual agreements encourage us to perform subsequent fine Rietveld analysis on our experimental data by using the P21/n structure. As shown in Figure 4, the fitting gives a successful result to the experimental data. The lattice constants of Ag2S nanosheets at 5.38 GPa are as follows: a = 4.107 ± 0.002 Å, b = 6.659 ± 0.003 Å, c = 7.616 ± 0.004 Å, β = 99.62° ± 0.03, V0 = 205.3 ± 0.1 Å3. According to the fitting data, all of the diffraction peaks can be indexed to the initial monoclinic P21/n structure. No new phases were observed at this pressure.
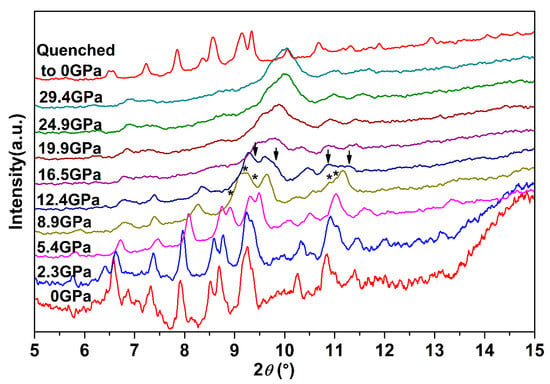
Figure 3.
Synchrotron X-ray diffraction patterns of Ag2S nanosheets under different pressures. The asterisks and arrows denote the occurrences of new peaks for phase II and phase III, respectively.
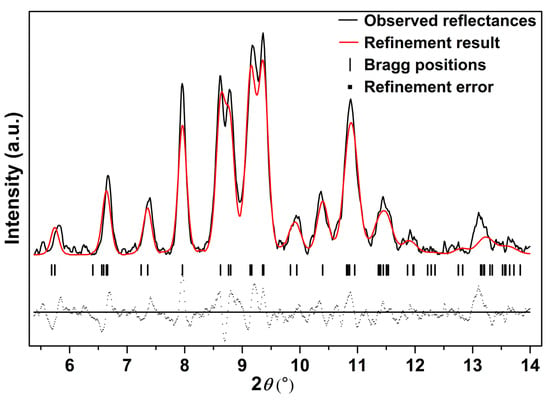
Figure 4.
The refinement result for the monoclinic P21/n structure (phase I) of Ag2S nanosheets at 5.38 GPa, Rwp = 0.3011, Rp = 0.1836 and chi^2 = 109.9.
While compressing up to 8.9 GPa, several new peaks (marked as asterisks) start to emerge obviously and their intensity grows with pressure increasing (see Figure 3), indicating that α-Ag2S undergoes a phase transition. According to previous reports, these new peaks located at 8.96°, 9.18°, 9.39°, 10.90° and 11.05° can be indexed into the (120), (112), (121), (113) and (201) diffractions of orthorhombic P212121 structure (phase II). Both phase I and phase II of Ag2S coexist under this pressure. No pure phase II structure could be obtained in our work. With continuous compression, all the peaks of ambient phase I became weak gradually.
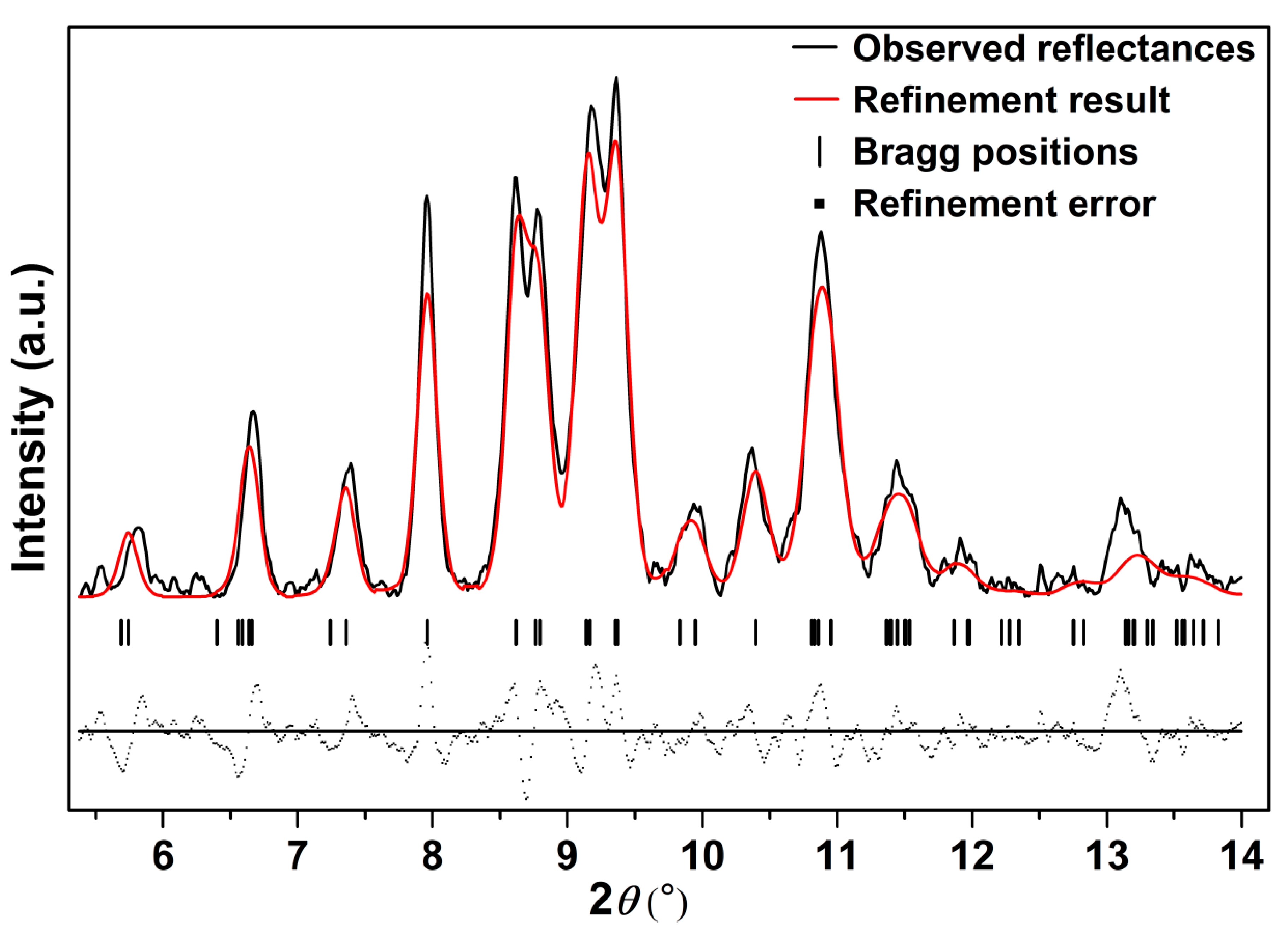
Further compressed to 12.4 GPa, it can be clearly seen that a set of new diffraction peaks (2θ = 9.39°, 9.78°, 10.87°, 11.30°) emerged (marked as arrows in Figure 3), indicating that Ag2S nanosheets undergo the second phase transition. These new diffraction peaks can be indexed into (022), (12-1), (200) and (014) diffractions of monoclinic P21/n structure (phase III), isosymmetric to the ambient phase. Both phase II and phase III coexist under this pressure. However, the peaks corresponding to phase II lost a lot of intensity and then completely disappeared at the pressure of 16.5 GPa. Under this pressure, all of the remaining peaks can be indexed to phase III, indicating Ag2S nanosheets crystallized in monoclinic P21/n structure completely. Typical Rietveld refinement for phase III is plotted in Figure 5, yielding a = 4.079 ± 0.003 Å, b = 5.800 ± 0.006 Å, c = 7.948 ± 0.008 Å and β = 93.15° ± 0.07 (V = 187.7 ± 0.2 Å3). Compressing the samples up to 29.4 GPa, the highest pressure in our study, no new diffraction peaks were observed, Ag2S nanosheets are mainly still maintaining in phase III.
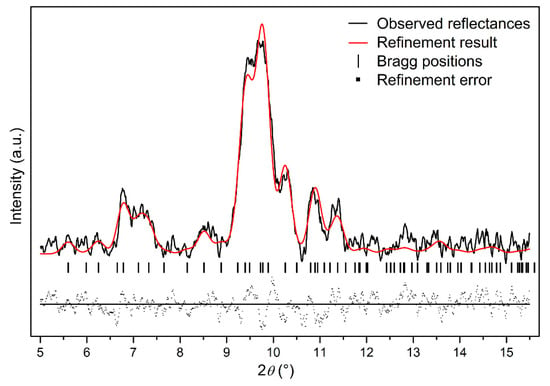
Figure 5.
The refinement result for phase III of Ag2S nanosheets at 16.5 GPa, Rwp = 0.1921, Rp = 0.1402 and chi^2 = 67.78.
In previous studies, we noted that bulk Ag2S undergoes the phase transition sequence P21/n (phase I) → P212121 (phase II) → P21/n (phase III) → Pnma (phase IV) at pressures of 5.1GPa, 8.8 GPa and 28.4 GPa, respectively [19]. For pure 30 nm Ag2S nanoparticles, the transition pressures elevate slightly compared to the bulk: from phase I to phase II at about 6.83 GPa and then to phase III at 9.3 GPa [27]. Compared to these reports, we noted that phase transition pressure in Ag2S nanosheets was about 2–3 GPa higher than that in 30 nm Ag2S nanoparticles and bulk crystals. Moreover, phase IV [19], which formed at 28.4 GPa in bulk counterparts, was not observed in our samples. Thus, our work reveals that Ag2S nanosheets exhibit a different high-pressure property compared with previous reports. According to the analysis in the TEM image before, it was shown that the Ag2S nanosheets have a relatively thin thickness, far below 29 nm (the average size of the sample). Therefore, we suggest that the stronger nanosize effect arising from the smaller thickness of Ag2S nanosheets effectively restricts the relative position slip of the interlayer atoms during compression, which leads to the enhancing of phase stabilities.
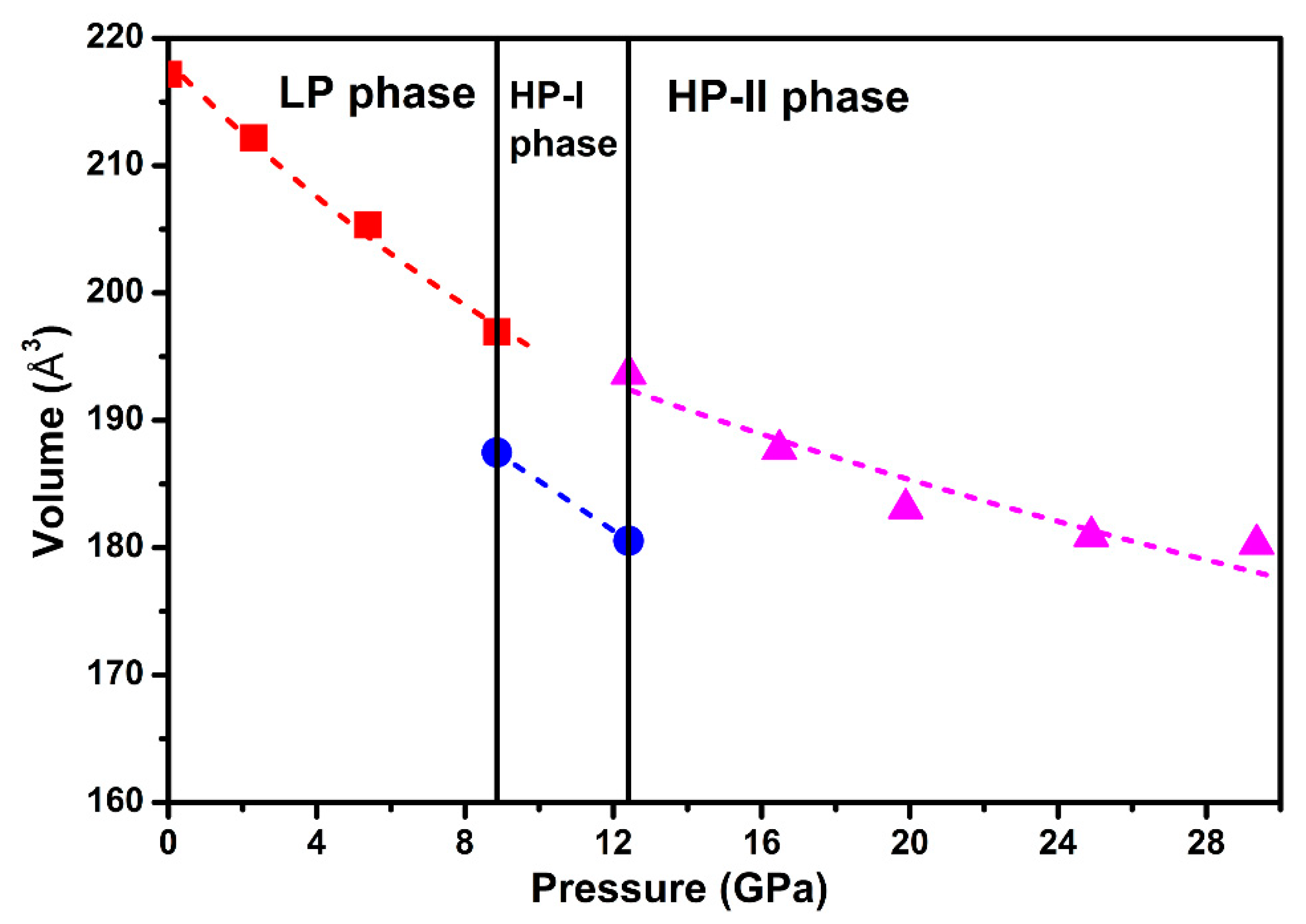
Figure 6 shows the compressive behavior of Ag2S nanosheets. A third-order BM EOS was introduced to fit our pressure-volume data. For monoclinic P21/n structure (phase I), by fixing the first pressure derivative of isothermal bulk modulus (B0′) to 4, the bulk modulus (B0) was obtained as 73(6) GPa. The obtained bulk modulus B0 for phase I, the ambient phase, was much higher than that of the bulk Ag2S (~34–38(2) GPa), even higher than that of the 30 nm Ag2S nanoparticles (B0 = 57.3(6) GPa) [19,27]. For phase III, during the fitness, the values of the bulk modulus (B0) and zero pressure cell volume (V0) were left to vary freely, and the first derivative of B0 with pressure (B0′) was fixed to 4. As shown in Figure 6, the fitting to the P-V curve yields a bulk modulus B0 of 141(4) GPa and unit cell volume V0 of 207(6) Å3. Compared to previous studies, the obtained bulk modulus B0 for phase III of Ag2S nanosheets was also larger than the bulk counterparts (102(4) GPa) and 30 nm nanoparticles (123.1(0) GPa) [19,27]. According to the above analysis, we consider that Ag2S nanosheets exhibit significant difference in compression properties under high pressure, a much lower volume compressibility than previous reports. A similar phenomenon was observed in some other nanomaterials, e.g., C60 nanosheets and ZnO nanocrystal [29,30], and has potential benefits in keeping the stability of materials. It is well known that nanosize effect can strongly influence the bulk modulus; thereby, the 30 nm nanoparticles exhibit higher bulk modulus than the bulk materials. In our experiment, the Ag2S nanosheets have a very small size in thickness, much lower than their grain size of 29 nm. Thus, the stronger nanosize effect from our samples may cause the bulk modulus to largely elevate.
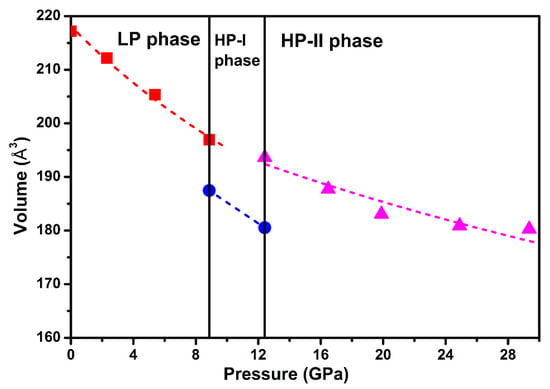
Figure 6.
Room-temperature equation of state data for Ag2S nanosheets. A third-order Birch-Murnaghan (BM) equation of state (EOS) is introduced to fit our pressure-volume data.
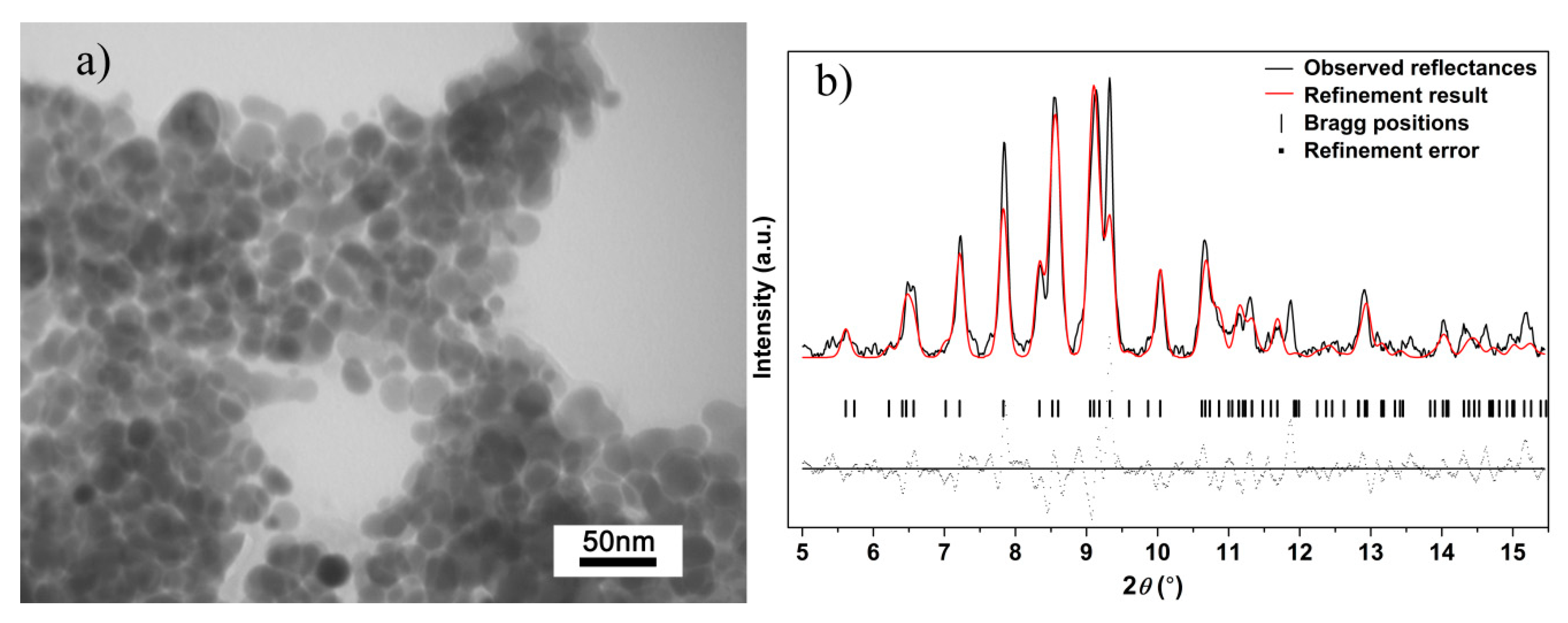
In our high-pressure research, the quench process is implemented. TEM images and XRD pattern of Ag2S nanosheets quenched to the ambient conditions are shown in Figure 7. According to the TEM image of the quenched sample, although Ag2S samples could not maintain their uniform shape because of the high pressure and high shear stresses, it still keeps the sheet-like morphology in nanoscale. Based on this result, we can reasonably draw a conclusion that during the compressing process, Ag2S samples in our experiment remain nanomaterials, and no agglomeration took place. After decompressing to ambient pressure, the shapes of all the diffraction peaks for Ag2S nanosheets return to the initial monoclinic P21/n structure (phase I), indicating the reversibility of the pressure-induced structural transitions. Further analysis showed that the (-112), (-103) peak was enhanced after compression. The effect of non-hydrostatic pressure and high pressure gives nanosheet samples a certain orientation and texture.
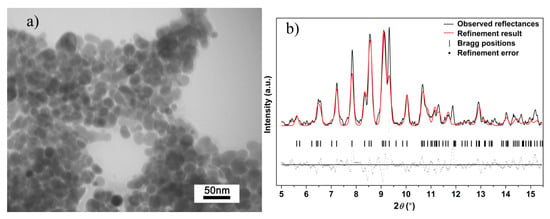
Figure 7.
(a) TEM images and (b) Rietveld refinement result of Ag2S nanosheets quenched to ambient conditions; Rwp = 0.3982, Rp = 0.2929 and chi^2 = 160.3.
4. Conclusions
In summary, we studied the high-pressure behaviors of Ag2S nanomaterials with sheet-like morphologies using in situ high-pressure X-ray diffraction up to about 30 GPa. Under high pressure, Ag2S nanosheets undergo the phase transition sequence P21/n (phase I) → P212121 (phase II) → P21/n (phase III) at pressures of 8.9 GPa and 12.4 GPa. Interestingly, the Ag2S nanosheets exhibit a wide field of structural stability and much lower compressibility under high pressure, remarkably different from the corresponding bulk materials and 30 nm nanoparticles. Further analysis suggests that a stronger nanosize effect arising from smaller thickness in Ag2S nanosheets effectively restricts the relative position slip of the interlayer atoms during the compression, which leads to the enhancing of phase stabilities and the elevating of bulk moduli. Our findings give a further insight into high-pressure behaviors in Ag2S nanomaterials.
Author Contributions
Conceptualization, R.L. and B.-B.L.; investigation, R.L.; writing—original draft preparation, R.L. and B.L.; writing—review and editing, Q.-J.L. and B.-B.L.; project administration, B.-B.L.; funding acquisition, B.-B.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Key R&D Program of China (2018YFA0305900) and the NSFC (11634004, 51320105007, 11604116, 11847094 and 51602124), Program for Changjiang Scholars and Innovative Research Team in University (IRT1132).
Acknowledgments
We thank Tongshun Wu (Ph.D. of State Key Laboratory of Inorganic Synthesis and Preparative Chemistry, Jilin University, Changchun, China) for providing ideal Ag2S nanosheet samples, thank Dongmei Li (State Key Laboratory of Superhard Materials, Jilin University, Changchun, China) for helping us to get excellent TEM images; we also thank Zhiqiang Chen (X17C, NSLS, BNL, Upton, NY, USA) for helping us to collect perfect HP in-situ synchrotron XDR data.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Yang, W.; Zhang, L.; Hu, Y.; Zhong, Y.; Wu, H.B.; Lou, X.W. Microwave-assisted synthesis of porous Ag2S-Ag hybrid nanotubes with high visible-light photocatalytic activity. Angew. Chem. 2012, 51, 11501–11504. [Google Scholar] [CrossRef]
- Cui, C.; Li, X.; Liu, J.; Hou, Y.; Zhao, Y.; Zhong, G. Synthesis and Functions of Ag2S Nanostructures. Nanoscale Res. Lett. 2015, 10, 431. [Google Scholar] [CrossRef] [PubMed]
- Sadovnikov, S.I.; Gusev, A.I. Universal Approach to the Synthesis of Silver Sulfide in the Forms of Nanopowders, Quantum Dots, Core-Shell Nanoparticles, and Heteronanostructures. Eur. J. Inorg. Chem. 2016, 2016, 4944–4957. [Google Scholar] [CrossRef]
- Xu, Z.; Bando, Y.; Wang, W.; Bai, X.; Dmitri, G. Real-Time in Situ HRTEM-Resolved Resistance Switching of Ag2S Nanoscale Ionic Conductor. ACS Nano 2010, 4, 2515–2522. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Li, X.; Shi, Z.; Zheng, C.; Li, X.; Leng, D.; Wang, Y.; Liu, J.; Zhu, L. 2D Ductile Transition Metal Chalcogenides (TMCs): Novel High—Performance Ag2S Nanosheets for Ultrafast Photonics. Adv. Opt. Mater. 2020, 8, 1901762. [Google Scholar] [CrossRef]
- Robinson, D.A.; White, H.S. Electrochemical Synthesis of Individual Core@Shell and Hollow Ag/Ag2S Nanoparticles. Nano Lett. 2019, 19, 5612–5619. [Google Scholar] [CrossRef]
- Jiang, W.; Wu, Z.; Yue, X.; Yuan, S.; Lu, H.; Liang, B. Photocatalytic performance of Ag2S under irradiation with visible and near-infrared light and its mechanism of degradation. RSC Adv. 2015, 5, 24064–24071. [Google Scholar] [CrossRef]
- Sadanaga, R.S. Sigeho X-Ray Study of the Alpha-beta Transition of Ag2S. Miner. J. 1967, 5, 124–148. [Google Scholar] [CrossRef]
- Sadovnikov, S.I.; Gusev, A.I.; Chukin, A.V.; Rempel, A.A. High-temperature X-ray diffraction and thermal expansion of nanocrystalline and coarse-crystalline acanthite alpha-Ag2S and argentite beta-Ag2S. Phys. Chem. Chem. Phys. 2016, 18, 4617–4626. [Google Scholar] [CrossRef] [PubMed]
- Grier, B.H.; Shapiro, S.M.; Cava, R.J. Inelastic neutron scattering measurements of the diffusion inβ−Ag2S. Phys. Rev. B 1984, 29, 3810–3814. [Google Scholar] [CrossRef]
- Ray, J.R.; Vashishta, P. Low temperature phase transformation in superionic conductors: A molecular dynamics study of silver sulfide. J. Chem. Phys. 1989, 90, 6580–6586. [Google Scholar] [CrossRef]
- Miyatani, S. Ionic Conductivity in Silver Chalcogenides. J. Phys. Soc. Jpn. 1981, 50, 3415–3418. [Google Scholar] [CrossRef]
- Shen, S.; Zhang, Y.; Peng, L.; Xu, B.; Du, Y.; Deng, M.; Xu, H.; Wang, Q. Generalized synthesis of metal sulfide nanocrystals from single-source precursors: Size, shape and chemical composition control and their properties. CrystEngComm 2011, 13, 4572–4579. [Google Scholar] [CrossRef]
- Bourret, G.R.; Lennox, R.B. Electrochemical synthesis of Ag(0)/A2S heterojunctions templated on pre-formed Ag2S nanowires. Nanoscale 2011, 3, 1838–1844. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, C.; Zhang, X.; Ke, F.; Han, Y.; Peng, G.; Ma, Y.; Gao, C. Impurity level evolution and majority carrier-type inversion of Ag2S under extreme compression: Experimental and theoretical approaches. Appl. Phys. Lett. 2013, 103, 082116. [Google Scholar] [CrossRef]
- Martins, L.G.P.; Matos, M.J.S.; Paschoal, A.R.; Freire, P.T.C.; Andrade, N.F.; Aguiar, A.L.; Kong, J.; Neves, B.R.A.; de Oliveira, A.B.; Mazzoni, M.S.C.; et al. Raman evidence for pressure-induced formation of diamondene. Nat. Commun. 2017, 8, 96. [Google Scholar] [CrossRef]
- Dong, X.; Oganov, A.R.; Goncharov, A.F.; Stavrou, E.; Lobanov, S.; Saleh, G.; Qian, G.R.; Zhu, Q.; Gatti, C.; Deringer, V.L.; et al. A stable compound of helium and sodium at high pressure. Nat. Chem. 2017, 9, 440–445. [Google Scholar] [CrossRef]
- Santamaria-Perez, D.; Marques, M.; Chulia-Jordan, R.; Menendez, J.M.; Gomis, O.; Ruiz-Fuertes, J.; Sans, J.A.; Errandonea, D.; Recio, J.M. Compression of silver sulfide: X-ray diffraction measurements and total-energy calculations. Inorg. Chem. 2012, 51, 5289–5298. [Google Scholar] [CrossRef]
- Zhao, Z.; Wei, H.; Mao, W.L. Pressure tuning the lattice and optical response of silver sulfide. Appl. Phys. Lett. 2016, 108, 261902. [Google Scholar] [CrossRef]
- Liu, J.; Raveendran, P.; Shervani, Z.; Ikushima, Y. Synthesis of Ag2S quantum dots in water-in-CO2 microemulsions. Chem. Commun. 2004, 22, 2582–2583. [Google Scholar] [CrossRef]
- Xiao, J.; Xie, Y.; Tang, R.; Luo, W. Template-based synthesis of nanoscale Ag2E (E = S, Se) dendrites. J. Mater. Chem. 2002, 12, 1148–1151. [Google Scholar] [CrossRef]
- Wang, G.; Liu, J.; Zhu, L.; Guo, Y.; Yang, L. Silver sulfide nanoparticles for photodynamic therapy of human lymphoma cells via disruption of energy metabolism. RSC Adv. 2019, 9, 29936–29941. [Google Scholar] [CrossRef]
- Fu, X.; Zou, H.; Zhou, L. A novel synthesis route of Ag2S nanotubes by sulfidizing silver nanowires in ambient atmosphere. J. Nanosci. Nanotechnol. 2010, 10, 5851–5856. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.-M.; Hou, C.; Zhang, H.-Z.; Wang, D.-S.; Yu, D.-P. Evolution of resistive switching over bias duration of single Ag2S nanowires. Appl. Phys. Lett. 2010, 96, 203109. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, D.; Shi, W.; Wang, F. A gamma-ray irradiation reduction route to prepare rod-like Ag2S nanocrystallines at room temperature. Mater. Lett. 2007, 61, 3232–3234. [Google Scholar] [CrossRef]
- Lim, W.P.; Zhang, Z.; Low, H.Y.; Chin, W.S. Preparation of Ag2S nanocrystals of predictable shape and size. Angew. Chem. Int. Ed. Engl. 2004, 43, 5685–5689. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, R.; Wu, L.; Zhang, M. Effect of Y doping on high-pressure behavior of Ag2S nanocrystals. RSC Adv. 2017, 7, 35105–35110. [Google Scholar] [CrossRef]
- Fouddad, F.Z.; Hiadsi, S.; Bouzid, L.; Ghrici, Y.F.; Bekhadda, K. Low temperature study of the structural stability, electronic and optical properties of the acanthite α-Ag2S: Spin-orbit coupling effects and new important ultra-refraction property. Mater. Sci. Semicond. Process. 2020, 107, 104801. [Google Scholar] [CrossRef]
- Wang, L.; Liu, B.-B.; Liu, D.-D.; Yao, M.-G.; Yu, S.-D.; Hou, Y.-Y.; Zou, B.; Cui, T.; Zou, G.-T. Synthesis and high pressure induced amorphization of C60 nanosheets. Appl. Phys. Lett. 2007, 91, 103112. [Google Scholar] [CrossRef]
- Jiang, J.Z.; Olsen, J.S.; Gerward, L.; Frost, D.; Rubie, D.; Peyronneau, J. Structural stability in nanocrystalline ZnO. Europhys. Lett. 2000, 50, 48–53. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).