Preparation of Boron Nitride Nanoplatelets via Amino Acid Assisted Ball Milling: Towards Thermal Conductivity Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
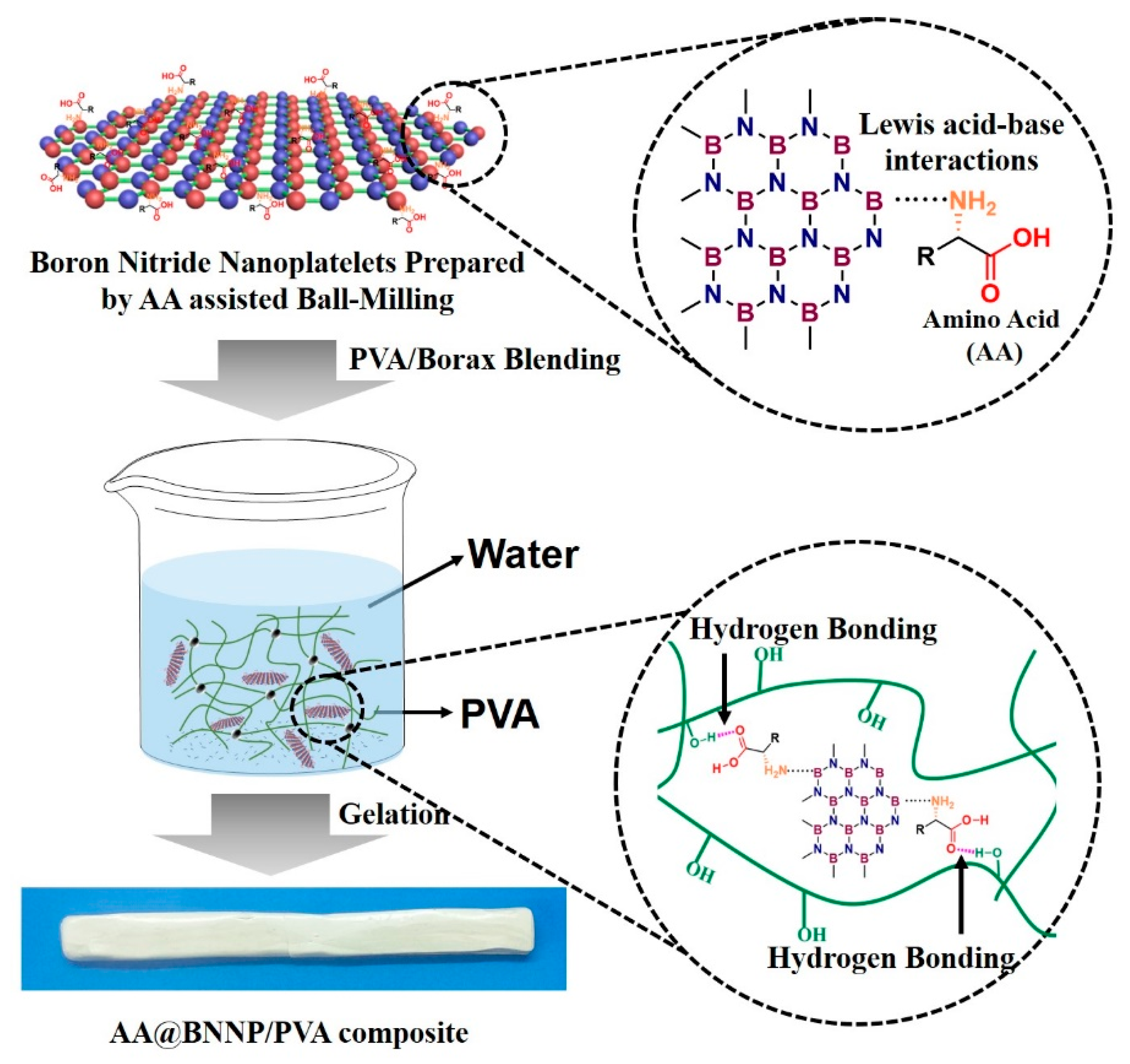
2.2. Experiment
2.2.1. Exfoliation of BNNP by H2O
2.2.2. Exfoliation of h-BN by L-Lysine Assisted Ball Milling (Lys@BNNP)
2.2.3. Exfoliation of h-BN by L-Glutamic Acid Assisted Ball Milling (Glu@BNNP)
2.2.4. Preparation of Pure PVA Hydrogel
2.2.5. Preparation of h-BN/PVA, BNNP/PVA, Lys@BNNP/PVA and Glu@BNNP/PVA Hydrogels
2.3. Characterization
2.3.1. Structural Characterizations
2.3.2. Performance Characterizations
3. Results and Discussion
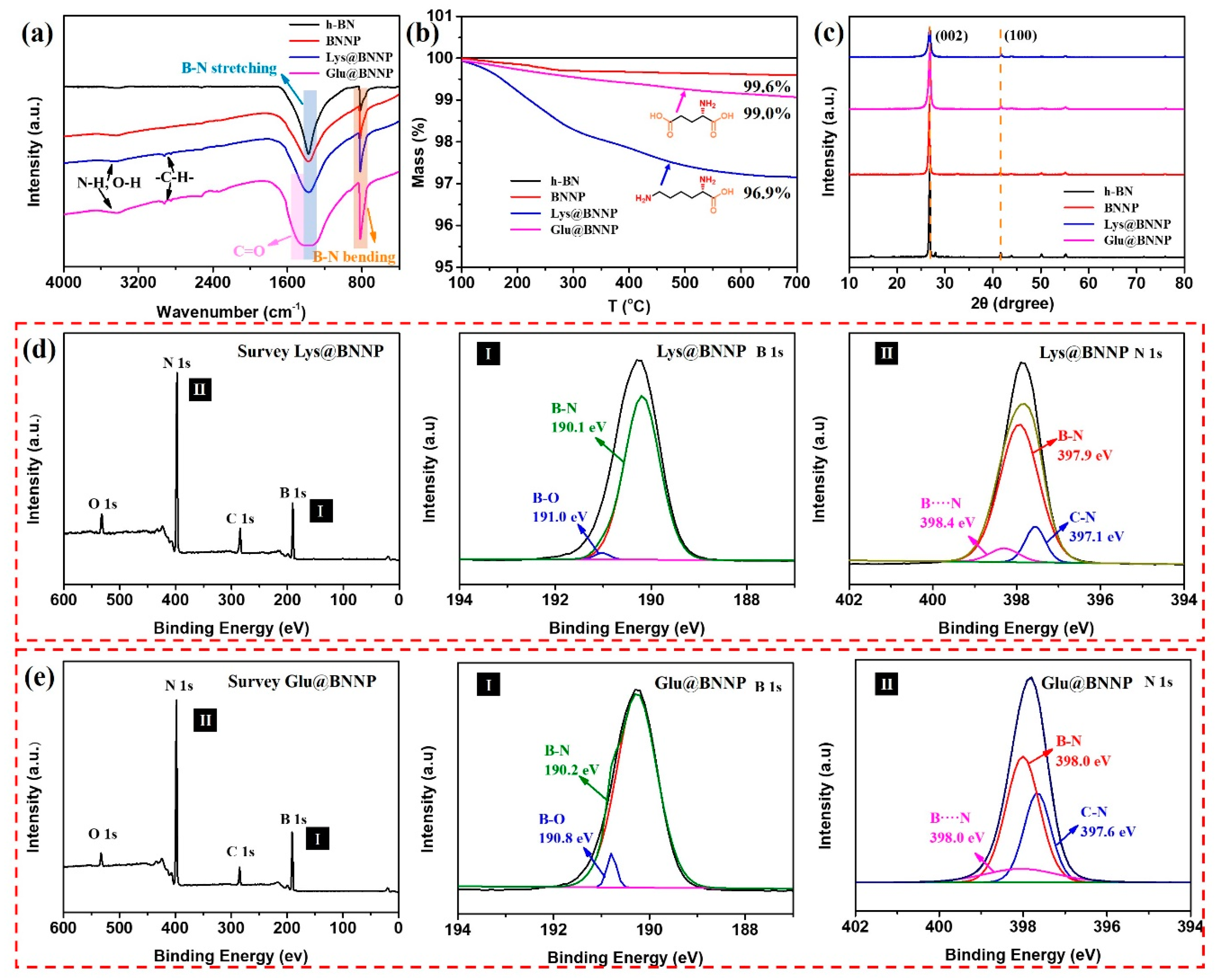
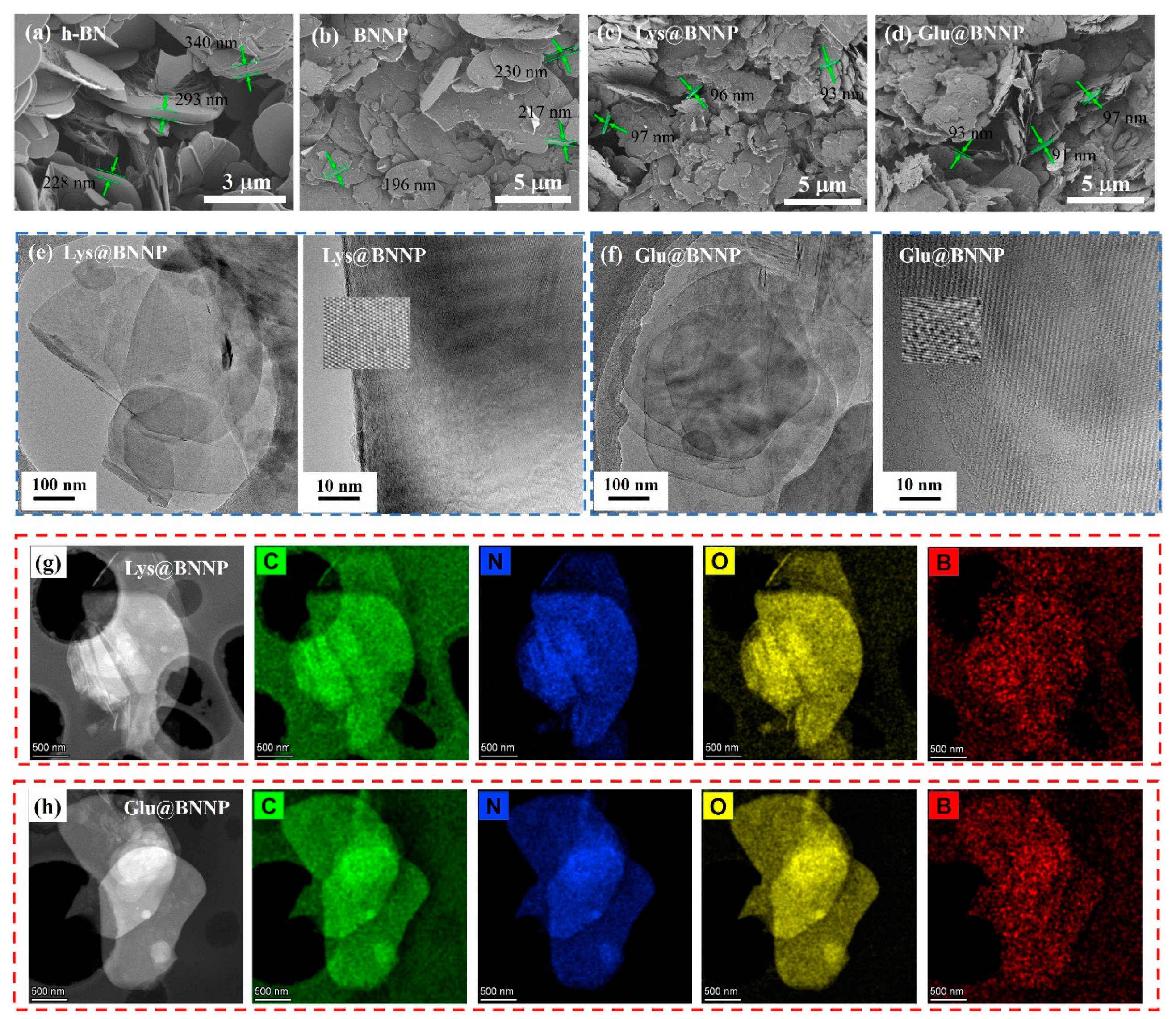
3.1. Characterizations of AA@BNNPs
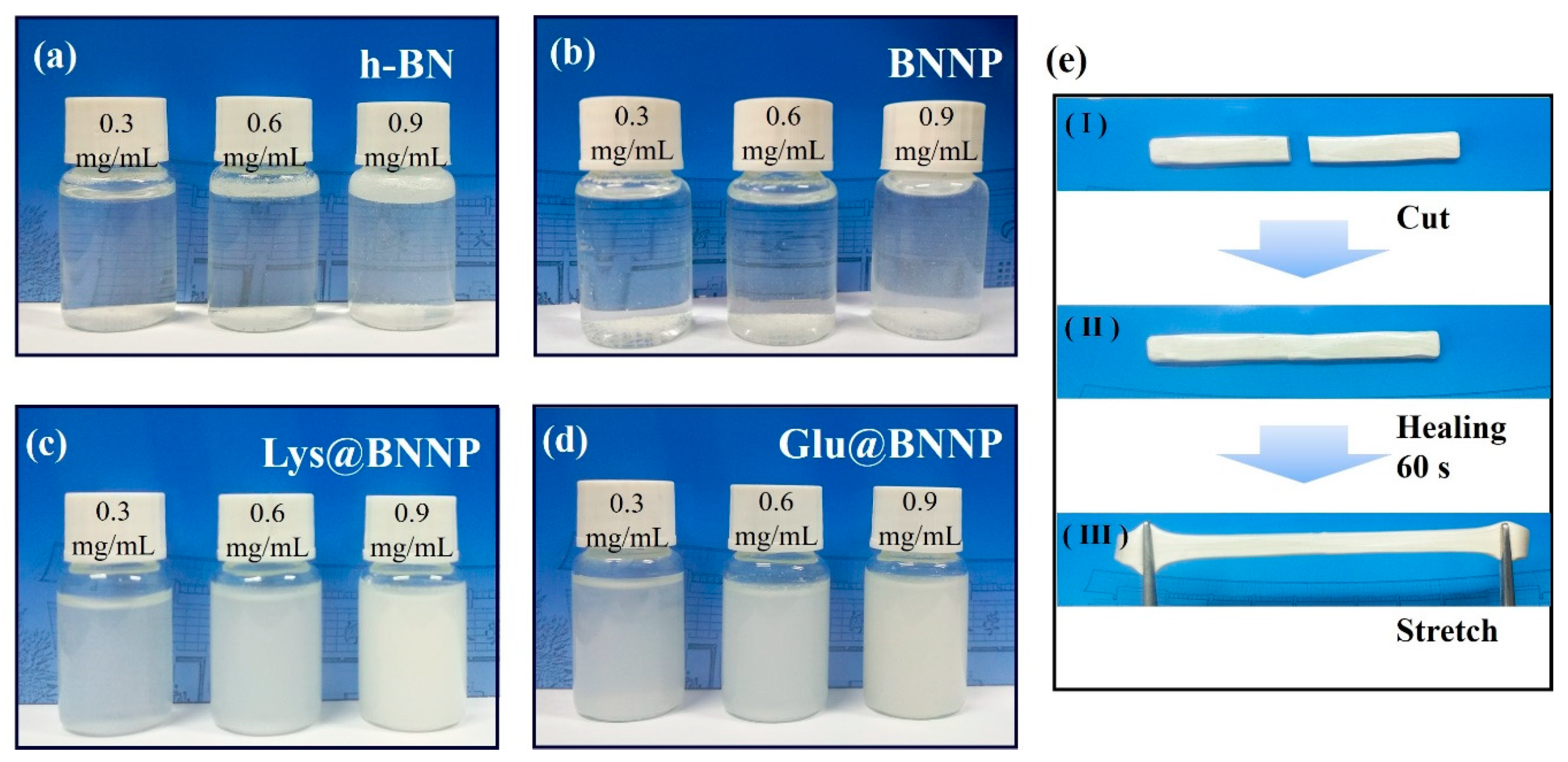
3.2. Measurements of PVA Hydrogel
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Xiao, F.; Naficy, S.; Casillas, G.; Khan, M.H.; Katkus, T.; Jiang, L.; Liu, H.; Li, H.; Huang, Z. Edge-Hydroxylated Boron Nitride Nanosheets as an Effective Additive to Improve the Thermal Response of Hydrogels. Adv. Mater. 2015, 27, 7196–7203. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Jang, S.K.; Jang, W.J.; Kim, M.; Park, S.Y.; Kim, S.W.; Kahng, S.J.; Choi, J.Y.; Ruoff, R.S.; Song, Y.J.; et al. A Platform for Large-Scale Graphene Electronics-CVD Growth of Single-Layer Graphene on CVD-Grown Hexagonal Boron Nitride. Adv. Mater. 2013, 25, 2746–2752. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Chen, G.; Shi, Z.; Liu, C.C.; Zhang, L.; Xie, G.; Cheng, M.; Wang, D.; Yang, R.; Shi, D.; et al. Epitaxial growth of single-domain graphene on hexagonal boron nitride. Nat. Mater. 2013, 12, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Weng, Q.; Wang, X.; Wang, X.; Bando, Y.; Golberg, D. Functionalized hexagonal boron nitride nanomaterials: Emerging properties and applications. Chem. Soc. Rev. 2016, 45, 3989–4012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhi, B.C.; Bando, Y.; Terao, T.; Tang, C.; Kuwahara, H.; Golborg, D. Towards Thermoconductive, Electrically Insulating Polymeric Composites with Boron Nitride Nanotubes as Fillers. Adv. Funct. Mater. 2009, 19, 1856–1862. [Google Scholar] [CrossRef]
- Wang, X.B.; Weng, Q.; Wang, X.; Li, X.; Zhang, J.; Liu, F.; Jiang, X.F.; Guo, H.; Xu, N.; Golborg, D.; et al. Biomass-Directed Synthesis of 20 g High-Quality Boron Nitride Nanosheets for Thermoconductive Polymeric Composites. ACS Nano 2014, 8, 9081–9088. [Google Scholar] [CrossRef]
- Sainsbury, T.; Satti, A.; Mar, P.; Wang, Z.; McGovern, I.; Gun’ko, Y.K.; Coleman, J. Oxygen Radical Functionalization of Boron Nitride Nanosheets. J. Am. Chem. Soc. 2012, 134, 18758–18771. [Google Scholar] [CrossRef]
- Wang, T.; Ou, D.; Liu, H.; Jiang, S.; Huang, W.; Fang, X.; Chen, X.; Lu, M. Thermally Conductive Boron Nitride Nanosheet Composite Paper as a Flexible Printed Circuit Board. ACS App. Nano Mater. 2018, 1, 1705–1712. [Google Scholar] [CrossRef]
- Nie, X.; Li, G.; Jiang, Z.; Li, W.; Ouyang, T.; Wang, J. Co-Solvent Exfoliation of Hexagonal Boron Nitride: Effect of Raw Bulk Boron Nitride Size and Co-Solvent Composition. Nanomaterials 2020, 10, 1035. [Google Scholar] [CrossRef]
- Xing, L.; Hu, C.; Zhang, Y.; Wang, X.; Shi, L.; Ran, R. A mechanically robust double-network hydrogel with high thermal responses via doping hydroxylated boron nitride nanosheets. J. Mater. Sci. 2019, 54, 3368–3382. [Google Scholar] [CrossRef]
- Lee, D.; Lee, S.; Byun, S.; Paik, K.; Song, S.H. Novel dielectric BN/epoxy nanocomposites with enhanced heat dissipation performance for electronic packaging. Compos. Part A Appl. Sci. Manuf. 2018, 107, 217–223. [Google Scholar] [CrossRef]
- Rousseas, M.; Goldstein, A.P.; Mickelson, W.; Worsley, M.A.; Woo, L.; Zettl, A. Synthesis of Highly Crystalline sp2-Bonded Boron Nitride Aerogels. ACS Nano 2013, 7, 8540–8546. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Li, X.; Zhou, J.; Guo, W. Ultralight Three-Dimensional Boron Nitride Foam with Ultralow Permittivity and Superelasticity. Nano Lett. 2013, 13, 3232–3236. [Google Scholar] [CrossRef]
- Lee, D.; Lee, B.; Park, K.H.; Ryu, H.J.; Jeon, S.; Hong, S.H. Scalable Exfoliation Process for Highly Soluble Boron Nitride Nanoplatelets by Hydroxide-Assisted Ball Milling. Nano Lett. 2015, 15, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Williams, T.V.; Cao, W.; Elsayed-Ali, H.E.; Connell, J.W. Defect Functionalization of Hexagonal Boron Nitride Nanosheets. J. Phys. Chem. C 2010, 114, 17434–17439. [Google Scholar] [CrossRef]
- Lei, W.; Mochalin, V.N.; Liu, D.; Qin, S.; Gogotsi, Y.; Chen, Y. Boron nitride colloidal solutions, ultralight aerogels and freestanding membranes through one-step exfoliation and functionalization. Nat. Commun. 2015, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Williams, T.V.; Connell, J.W. Soluble, Exfoliated Hexagonal Boron Nitride Nanosheets. J. Phys. Chem. Lett. 2009, 1, 277–283. [Google Scholar] [CrossRef]
- Xie, S.Y.; Wang, W.; Fernando, K.A.S.; Wang, X.; Lin, Y.; Sun, Y.P. Solubilization of boron nitride nanotubes. Chem. Commun. 2005, 29, 3670–3672. [Google Scholar] [CrossRef]
- Liu, J.; Gu, J.; Luo, J.; Wang, S.; Zhang, H.; Tao, Y. Controlled Synthesis of Thermoresponsive Polymers Derived from L-Lysine, a Biorenewable Resource. J. Polym. Sci. Pol. Chem. 2019, 57, 862–868. [Google Scholar] [CrossRef]
- Pati, D.; Shaikh, A.Y.; Hotha, S.; Gupta, S.S. Synthesis of glycopolypeptides by the ring opening polymerization of O-glycosylated-a-amino acid N-carboxyanhydride (NCA). Polym. Chem. 2011, 2, 805–811. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Z.; Li, Z. Thermoresponsive Polypeptides from Pegylated Poly-L-glutamates. Biomacromolecules 2011, 18, 2859–2863. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, X.; Li, M.; Ren, X.; Tao, Y. Precision Synthesis of Sustainable Thermoplastic Elastomers from Lysine-Derived Monomers. J. Polym. Sci. Pol. Chem. 2017, 52, 349–355. [Google Scholar] [CrossRef]
- Chen, W.P.; Hao, D.Z.; Hao, W.J.; Guo, X.L.; Jiang, L. Hydrogel with Ultrafast Self-Healing Property Both in Air and Underwater. ACS Appl. Mater. Interfaces 2018, 10, 1258–1265. [Google Scholar] [CrossRef] [PubMed]
- Jun, H.; Li, G.H.; Yang, N.; Qin, L.L.; Grami, M.E.; Zhang, Q.X.; Wang, N.Y.; Qu, X.W. Preparation and characterization of surface modified boron nitride epoxy composites with enhanced thermal conductivity. RSC Adv. 2014, 4, 44282–44290. [Google Scholar]
- Shao, C.; Chang, H.; Wang, M. High-Strength, Tough, and Self-Healing Nanocomposite Physical Hydrogels Based on the Synergistic Effects of Dynamic Hydrogen Bond and Dual Coordination Bonds. ACS Appl. Mater. Interfaces 2017, 9, 28305–28318. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhan, C.; You, Y.; Tong, L.; Wei, R.; Liu, X. Preparation and thermal conductivity of copper phthalocyanine grafted boron nitride nanosheets. Mater. Lett. 2018, 227, 33–36. [Google Scholar] [CrossRef]
- Wu, X.; Liu, H.; Tang, Z.; Guo, B. Scalable fabrication of thermally conductive elastomer/boron nitride nanosheets composites by slurry compounding. Compos. Sci. Technol. 2016, 123, 179–186. [Google Scholar] [CrossRef]
- Lei, W.; Liu, D.; Chen, Y. ighly Crumpled Boron Nitride Nanosheets as Adsorbents: Scalable Solvent-Less Production. Adv. Mater. Interfaces 2015, 2, 1400529. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, Z.; Wu, J.; Ma, C.; Qiu, C.; Zhao, Y.; Shao, F.; Wang, H.; Zheng, J.; Huang, G. Mechanically robust, ultrastretchable and thermal conducting composite hydrogel and its biomedical applications. Chem. Eng. J. 2019, 360, 231–242. [Google Scholar] [CrossRef]
- Lin, H.; Mehra, N.; Li, Y.; Zhu, J. Graphite oxide/boron nitride hybrid membranes: The role of cross-plane laminar bonding for a durable membrane with large water flux and high rejection rate. J. Membr. Sci. 2020, 593, 117401. [Google Scholar] [CrossRef]
- Lin, Z.; Mcnamara, A.; Liu, Y.; Moon, K.; Wong, C. Exfoliated hexagonal boron nitride-based polymer nanocomposite with enhanced thermal conductivity for electronic encapsulation. Compos. Sci. Technol. 2014, 90, 123–128. [Google Scholar] [CrossRef]
- Shahil, K.M.F.; Balandin, A.A. Graphene–Multilayer Graphene Nanocomposites as Highly Efficient Thermal Interface Materials. Nano Lett. 2012, 12, 861–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, K.; Fang, J.; Ma, J.; Huang, R.; Chai, S.; Chen, F.; Fu, Q. Achieving a Collapsible, Strong, and Highly Thermally Conductive Film Based on Oriented Functionalized Boron Nitride Nanosheets and Cellulose Nanofiber. ACS Appl. Mater. Interfaces 2017, 9, 30035–30045. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Li, H.; Tay, R.Y.; Sun, B.; Tsang, S.H.; Cometto, O.; Lin, J.; Teo, E.H.T.; Tok, A.I.Y. Biocompatible Hydroxylated Boron Nitride Nanosheets/Poly(vinyl alcohol) Interpenetrating Hydrogels with Enhanced Mechanical and Thermal Responses. ACS Nano 2017, 11, 3742–3751. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, N.; Ji, H.; Jiang, X.; Qu, X.; Zhang, X.; Zhang, Y.; Liu, B. Preparation of Boron Nitride Nanoplatelets via Amino Acid Assisted Ball Milling: Towards Thermal Conductivity Application. Nanomaterials 2020, 10, 1652. https://doi.org/10.3390/nano10091652
Yang N, Ji H, Jiang X, Qu X, Zhang X, Zhang Y, Liu B. Preparation of Boron Nitride Nanoplatelets via Amino Acid Assisted Ball Milling: Towards Thermal Conductivity Application. Nanomaterials. 2020; 10(9):1652. https://doi.org/10.3390/nano10091652
Chicago/Turabian StyleYang, Nan, Haifeng Ji, Xiaoxia Jiang, Xiongwei Qu, Xiaojie Zhang, Yue Zhang, and Binyuan Liu. 2020. "Preparation of Boron Nitride Nanoplatelets via Amino Acid Assisted Ball Milling: Towards Thermal Conductivity Application" Nanomaterials 10, no. 9: 1652. https://doi.org/10.3390/nano10091652
APA StyleYang, N., Ji, H., Jiang, X., Qu, X., Zhang, X., Zhang, Y., & Liu, B. (2020). Preparation of Boron Nitride Nanoplatelets via Amino Acid Assisted Ball Milling: Towards Thermal Conductivity Application. Nanomaterials, 10(9), 1652. https://doi.org/10.3390/nano10091652






