Molecularly Imprinting Polymers (MIP) Based on Nitrogen Doped Carbon Dots and MIL-101(Fe) for Doxorubicin Hydrochloride Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Apparatus
2.2. Preparation of N-Doped CDs (N-CDs)
2.3. Synthesis of N-CDs@MIL-101(Fe) (N-MIL)
2.4. Synthesis of Template MIP Nanoparticles
2.5. Drug Loading Study
2.6. Release In Vitro
2.7. In Vitro Cytotoxicity Test
3. Results and Discussion
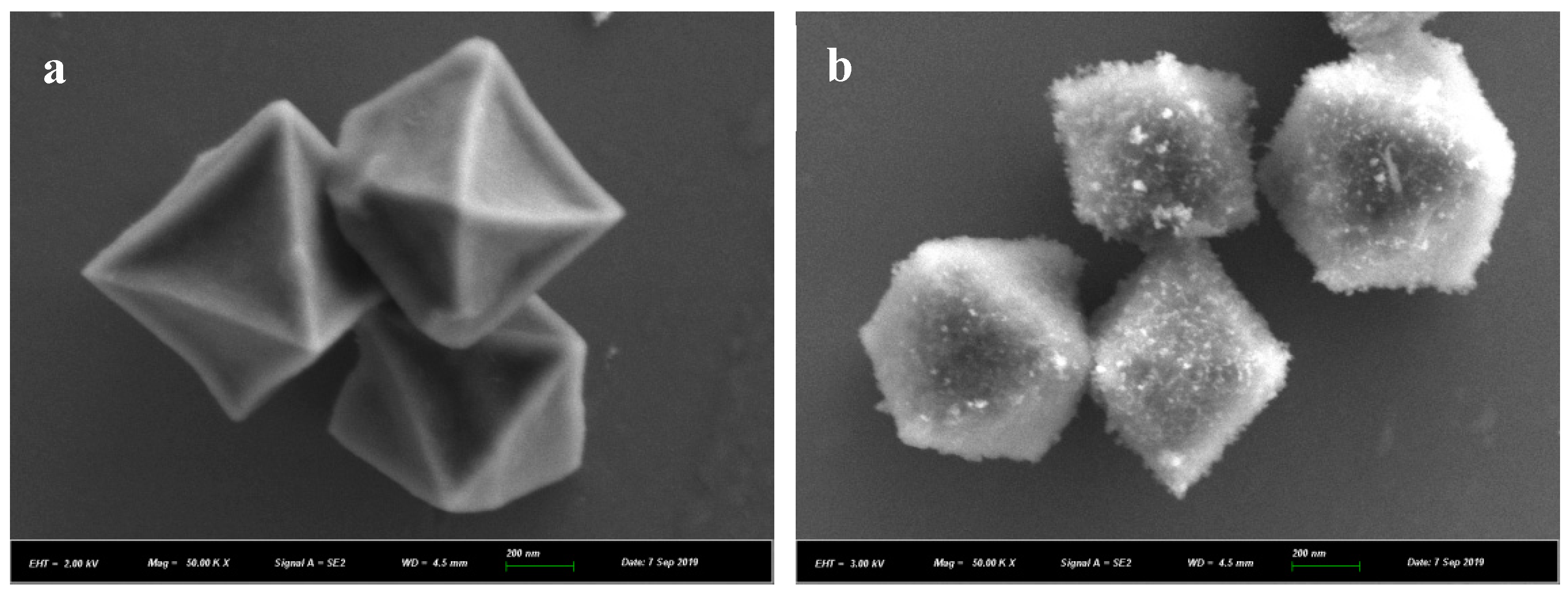
3.1. Structure Characterization
3.2. Specific Adsorption of MIP on Drugs
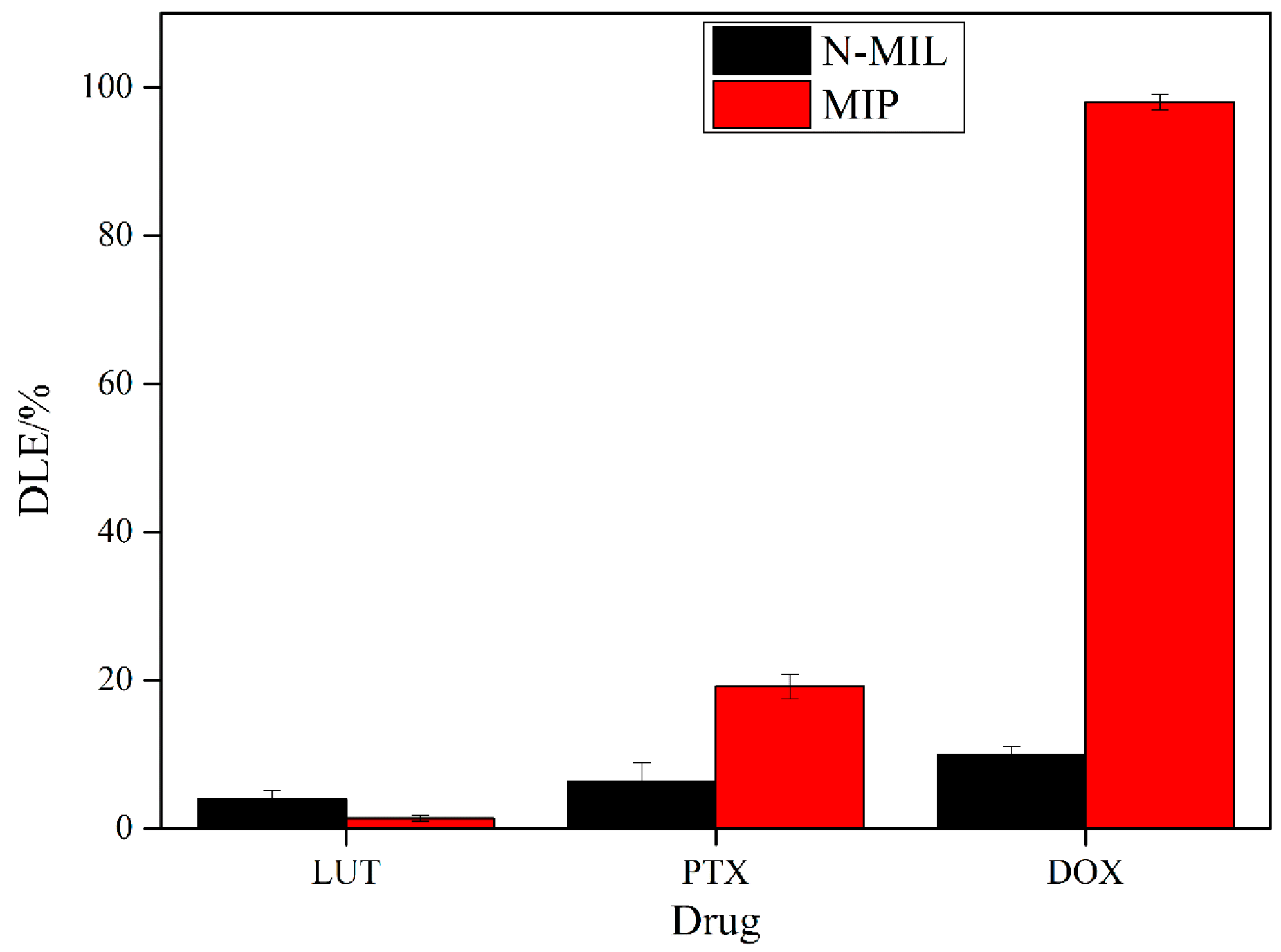
3.3. Drug Loading Studies
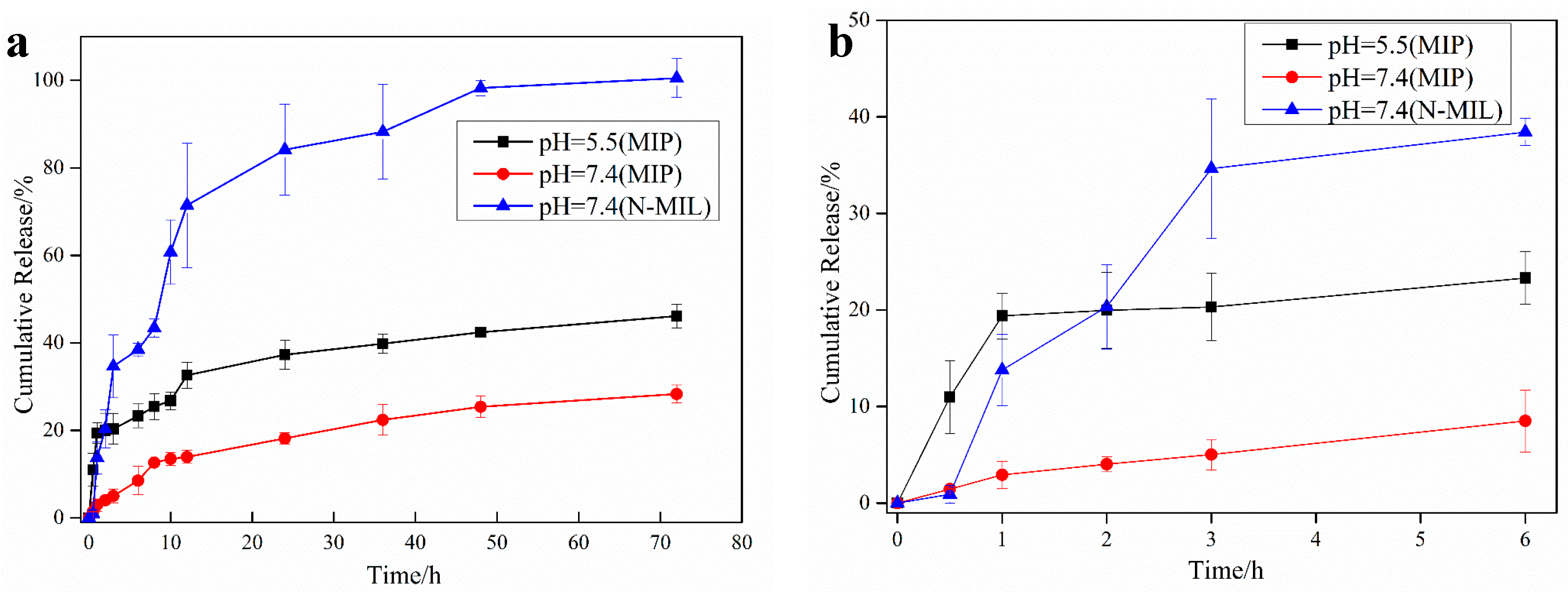
3.4. In Vitro Drug Release
3.5. Cytotoxicity Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tian, J.; Ding, L.; Ju, H.; Yang, Y.; Li, X.; Shen, Z.; Zhu, Z.; Yu, J.S.; Yang, C.J. A multifunctional nanomicelle for real-time targeted imaging and precise near-infrared cancer therapy. Angew. Chem. Int. Ed. 2014, 53, 9544–9549. [Google Scholar] [CrossRef]
- Siegel, R. Cancer statistics. CA Cancer J. Clin 2013, 63, 11–30. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.W.; Zhao, P.; Khan, A.; Raza, F.; Raza, S.M.; Sarfraz, M.; Chen, Y.; Li, M.; Yang, T.; Ma, X.; et al. Synergism of cisplatin-oleanolic acid co-loaded calcium carbonate nanoparticles on hepatocellular carcinoma cells for enhanced apoptosis and reduced hepatotoxicity. Int. J. Nanomed. 2019, 14, 3753. [Google Scholar] [CrossRef] [Green Version]
- Tang, L.-Y.; Wang, Y.-C.; Li, Y.; Du, J.-Z.; Wang, J. Shell-detachable micelles based on disulfide-linked block copolymer as potential carrier for intracellular drug delivery. Bioconjugate Chem. 2009, 20, 1095–1099. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Y.-C.; Dou, S.; Xiong, M.-H.; Sun, T.-M.; Wang, J. Doxorubicin-tethered responsive gold nanoparticles facilitate intracellular drug delivery for overcoming multidrug resistance in cancer cells. ACS Nano 2011, 5, 3679–3692. [Google Scholar] [CrossRef]
- Bullo, S.; Buskaran, K.; Fakurazi, S. Dual Drugs Anticancer Nanoformulation using Graphene Oxide-PEG as Nanocarrier for Protocatechuic Acid and Chlorogenic Acid. Pharm. Res. 2019, 36, 91. [Google Scholar] [CrossRef]
- Zong, L.; Li, X.; Wang, H.; Cao, Y.; Yin, L.; Li, M.; Wei, Z.; Chen, D.; Pu, X. Formulation and characterization of biocompatible and stable IV itraconazole nanosuspensions stabilized by a new stabilizer polyethylene glycol-poly(β-Benzyl-L-aspartate) (PEG-PBLA). Int. J. Pharm. 2017, 531, 107–118. [Google Scholar] [CrossRef] [Green Version]
- Zheng, H.; Zhang, Y.; Liu, L.; Wan, W.; Guo, P.; Nyström, A.M.; Zou, X. One-pot synthesis of metal–organic frameworks with encapsulated target molecules and their applications for controlled drug delivery. J. Am. Chem. Soc. 2016, 138, 962–968. [Google Scholar] [CrossRef]
- Chowdhuri, A.R.; Singh, T.; Ghosh, S.K.; Sahu, S.K. Carbon dots embedded magnetic nanoparticles@ chitosan@ metal organic framework as a nanoprobe for pH sensitive targeted anticancer drug delivery. ACS Appl. Mater. Interfaces 2016, 8, 16573–16583. [Google Scholar] [CrossRef]
- Qiu, B.; Shi, Y.; Yan, L.; Wu, X.; Zhu, J.; Zhao, D.; Khan, M.Z.H.; Liu, X. Development of an on-line immobilized α-glucosidase microreactor coupled to liquid chromatography for screening of α-glucosidase inhibitors. J. Pharm. Biomed. Anal. 2020, 180, 113047. [Google Scholar] [CrossRef]
- Jiang, L.; Gao, Z.-M.; Ye, L.; Zhang, A.-Y.; Feng, Z.-G. A tumor-targeting nano doxorubicin delivery system built from amphiphilic polyrotaxane-based block copolymers. Polymer 2013, 54, 5188–5198. [Google Scholar] [CrossRef]
- Li, L.; Chen, L.; Zhang, H.; Yang, Y.; Liu, X.; Chen, Y. Temperature and magnetism bi-responsive molecularly imprinted polymers: Preparation, adsorption mechanism and properties as drug delivery system for sustained release of 5-fluorouracil. Mater. Sci. Eng. C 2016, 61, 158–168. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Li, N.; Pan, W.; Yu, Z.; Yang, L.; Tang, B. Hollow mesoporous silica nanoparticles with tunable structures for controlled drug delivery. ACS Appl. Mater. Interfaces 2017, 9, 2123–2129. [Google Scholar] [CrossRef]
- Demir, B.; Lemberger, M.M.; Panagiotopoulou, M.; Medina Rangel, P.X.; Timur, S.; Hirsch, T.; Tse Sum Bui, B.; Wegener, J.; Haupt, K. Tracking hyaluronan: Molecularly imprinted polymer coated carbon dots for cancer cell targeting and imaging. ACS Appl. Mater. Interfaces 2018, 10, 3305–3313. [Google Scholar] [CrossRef]
- Liu, R.; Cui, Q.; Wang, C.; Wang, X.; Yang, Y.; Li, L. Preparation of sialic acid-imprinted fluorescent conjugated nanoparticles and their application for targeted cancer cell imaging. ACS Appl. Mater. Interfaces 2017, 9, 3006–3015. [Google Scholar] [CrossRef]
- Zhang, W.; Kang, J.; Li, P.; Liu, L.; Wang, H.; Tang, B. Two-photon fluorescence imaging of sialylated glycans in vivo based on a sialic acid imprinted conjugated polymer nanoprobe. Chem. Commun. 2016, 52, 13991–13994. [Google Scholar] [CrossRef]
- Ruela, A.L.M.; Figueiredo, E.C.; Pereira, G.R. Molecularly imprinted polymers as nicotine transdermal delivery systems. Biochem. Eng. J. 2014, 248, 1–8. [Google Scholar] [CrossRef]
- Pawley, C.J.; Perez-Gavilan, A.; Foley, K.S.; Lentink, S.; Welsh, H.N.; Tuijthof, G.; Steen Redeker, E.; Diliën, H.; Eersels, K.; Van Grinsven, B. Studying the Drug Delivery Kinetics of a Nanoporous Matrix Using a MIP-Based Thermal Sensing Platform. Polymers 2017, 9, 560. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Yang, K.; Wei, X.; Ding, S.; Tian, F.; Li, F. One-pot solvothermal synthesis of carbon dots/Ag nanoparticles/TiO2 nanocomposites with enhanced photocatalytic performance. Ceram. Int. 2018, 44, 22481–22488. [Google Scholar] [CrossRef]
- Qin, S.-J.; Yan, B. Dual-emissive ratiometric fluorescent probe based on Eu3+/C-dots@ MOF hybrids for the biomarker diaminotoluene sensing. Sens. Actuators B 2018, 272, 510–517. [Google Scholar] [CrossRef]
- Gu, C.; Guo, C.; Li, Z.; Wang, M.; Zhou, N.; He, L.; Zhang, Z.; Du, M. Bimetallic ZrHf-based metal-organic framework embedded with carbon dots: Ultra-sensitive platform for early diagnosis of HER2 and HER2-overexpressed living cancer cells. Biosens. Bioelectron. 2019, 134, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Gao, H.; Yang, M.; Xing, L.; Dong, W.; Li, A.; Zheng, H.; Wang, G. Smart integration of carbon quantum dots in metal-organic frameworks for fluorescence-functionalized phase change materials. Energy Storage Mater. 2019, 18, 349–355. [Google Scholar] [CrossRef]
- Niu, X.; Liu, G.; Li, L.; Fu, Z.; Xu, H.; Cui, F. Green and economical synthesis of nitrogen-doped carbon dots from vegetables for sensing and imaging applications. RSC Adv. 2015, 5, 95223–95229. [Google Scholar] [CrossRef]
- Xie, Q.; Li, Y.; Lv, Z.; Zhou, H.; Yang, X.; Chen, J.; Guo, H. Effective adsorption and removal of phosphate from aqueous solutions and eutrophic water by Fe-based MOFs of MIL-101. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Qian, K.; Deng, Q.; Fang, G.; Wang, J.; Pan, M.; Wang, S.; Pu, Y. Metal-organic frameworks supported surface-imprinted nanoparticles for the sensitive detection of metolcarb. Biosens. Bioelectron. 2016, 79, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Hamon, L.; Serre, C.; Devic, T.; Loiseau, T.; Millange, F.; Férey, G.r.; Weireld, G.D. Comparative study of hydrogen sulfide adsorption in the MIL-53 (Al, Cr, Fe), MIL-47 (V), MIL-100 (Cr), and MIL-101 (Cr) metal− organic frameworks at room temperature. J. Am. Chem. Soc. 2009, 131, 8775–8777. [Google Scholar] [CrossRef]
- Peng, H.-H.; Hong, D.-X.; Guan, Y.-X.; Yao, S.-J. Preparation of pH-responsive DOX-loaded chitosan nanoparticles using supercritical assisted atomization with an enhanced mixer. Int. J. Pharm. 2019, 558, 82–90. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Cao, P.-P.; He, Z.-Y.; He, X.-W.; Li, W.-Y.; Li, Y.-H.; Zhang, Y.-K. Targeted imaging and targeted therapy of breast cancer cells via fluorescent double template-imprinted polymer coated silicon nanoparticles by an epitope approach. Nanoscale 2019, 11, 17018–17030. [Google Scholar] [CrossRef]
- Qian, K.; Fang, G.; Wang, S. A novel core-shell molecularly imprinted polymer based on metal-organic frameworks as a matrix. Chem. Commun. 2011, 47, 10118–10120. [Google Scholar] [CrossRef]
- Bene, L.; Bagdány, M.; Ungvári, T.; Damjanovich, L. Dual-Laser Tetra-Polarization FRET (4polFRET) for Site-Selective Control of Homo-FRET in Hetero-FRET Systems on the Cell Surface: The Homo-FRET Gate. Anal. Chem. 2018, 90, 10159–10170. [Google Scholar] [CrossRef]
- Lovell, J.F.; Chan, M.W.; Qi, Q.; Chen, J.; Zheng, G. Porphyrin FRET acceptors for apoptosis induction and monitoring. J. Am. Chem. Soc. 2011, 133, 18580–18582. [Google Scholar] [CrossRef] [PubMed]
- Santamaría, E.; Maestro, A.; Porras, M.; Gutiérrez, J.; González, C. Controlled release of ibuprofen by meso-macroporous silica. J. Solid State Chem. 2014, 210, 242–250. [Google Scholar] [CrossRef]
- Freundlich, H. Of the adsorption of gases. Section II. Kinetics and energetics of gas adsorption. Introductory paper to section II. Trans. Faraday Soc. 1932, 28, 195–201. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Narisawa, S.; Nagata, M.; Hirakawa, Y.; Kobayashi, M.; Yoshino, H. An organic acid-induced sigmoidal release system for oral controlled-release preparations. 2. Permeability enhancement of eudragit RS coating led by the physicochemical interactions with organic acid. J. Pharm. Sci. 1996, 85, 184–188. [Google Scholar] [CrossRef]
- Ávila, M.I.; Alonso-Morales, N.; Baeza, J.A.; Rodriguez, J.J.; Gilarranz, M.A. High load drug release systems based on carbon porous nanocapsule carriers. Ibuprofen case study. J. Mater. Chem. B 2020, 8, 5293–5304. [Google Scholar] [CrossRef]
- Kim, A.R.; Lee, S.L.; Park, S.N. Properties and in vitro drug release of pH-and temperature-sensitive double cross-linked interpenetrating polymer network hydrogels based on hyaluronic acid/poly (N-isopropylacrylamide) for transdermal delivery of luteolin. Int. J. Biol. Macromol. 2018, 118, 731–740. [Google Scholar] [CrossRef]
- Saxena, A.; Kaloti, M.; Bohidar, H. Rheological properties of binary and ternary protein–polysaccharide co-hydrogels and comparative release kinetics of salbutamol sulphate from their matrices. Int. J. Biol. Macromol. 2011, 48, 263–270. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, M.; Shen, R.; Shao, S.; Chen, L.; Gong, W.; Shan, L.; Gao, C. Development of metoprolol tartrate-loaded sustained-release pellets: Effect of talc on the mechanism of drug release. Pharm. Dev. Technol. 2018, 23, 664–673. [Google Scholar] [CrossRef]
- Shi, Y.; Qiu, B.; Wu, X.; Wang, Y.; Zhu, J.; Liu, X.; Zhao, D. Drug delivery system and in vitro release of luteolin based on magnetic nanocomposite (Fe3O4@ ZIF-67). Micro Nano Lett. 2020, 15, 425–429. [Google Scholar]
- Yang, Y.; Yu, B.; Zhao, T.; Xu, S.; Zhang, H. Cytotoxicity of gold nanoclusters in human liver cancer cells. Int. J. Nanomed. 2014, 9, 5441–5448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.; Wang, W.; Li, H.; Hu, Y. A 54 peptide-mediated functionalized gold nanocages for targeted delivery of DOX as a combinational photothermal-chemotherapy for liver cancer. Int. J. Nanomed. 2017, 12, 5163–5176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, M.; Hou, L.; Huang, S.; Li, Y.; Liu, Y.; Hu, Y. Construction and application of a liver cancer-targeting drug delivery system based on core-shell gold nanocages. Int. J. Nanomed. 2018, 13, 1773–1789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Z.; Huang, G. Synthesis of theranostic Anti-EGFR ligand conjugate iron oxide nanoparticles for magnetic resonance imaging for treatment of liver cancer. J. Drug Delivery Sci. Technol. 2020, 55, 101367. [Google Scholar] [CrossRef]
- Du, L.; Wu, L.; Jin, Y.; Jia, J.; Li, M.; Wang, Y. Self-assembled drug delivery systems. Part 7: Hepatocyte-targeted nanoassemblies of an adefovir lipid derivative with cytochrome P-450-triggered drug release. Int. J. Pharm. 2014, 472, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Huang, S.; Yuan, J.; Xu, X.; Li, H.; Lv, Z.; Yu, W.; Hu, Y. Reverse Multidrug Resistance in Human HepG2/ADR by Anti-miR-21 Combined with Hyperthermia Mediated by Functionalized Gold Nanocages. Mol. Pharm. 2018, 15, 3767–3776. [Google Scholar] [CrossRef]







| Author | Drug Delivery System | Drug Loading Rate | Reference |
|---|---|---|---|
| Jiang Lan | Amphiphilic polyrotaxane-based block copolymer | 25.5% | [11] |
| Peng Huhong | Chitosan nanoparticle | 90% | [27] |
| Wang Haiyan | Fluorescent double template imprinted polymer | 11.05% | [28] |
| Shi Yuqiong | N-MIL-MIP | 97.9% | This work |
| Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|
| qm (mg/g) | KL (L/mg) | R2 | KF (L/mg) | 1/n | R2 | |
| N-MIL | 140.85 | 0.00332 | 0.7316 | 2.9060 | 0.5571 | 0.8415 |
| MIP | 5000 | 0.000669 | 0.7523 | 5.7227 | 0.8688 | 0.9877 |
| Condition | Korsmeyer–Peppas | Sigmoidal Model | ||||||
|---|---|---|---|---|---|---|---|---|
| K | n | R2 | ks | Rs | t50 | R2 | ||
| MIP | pH = 5.5 | 15.7 | 0.26 | 0.95 | 0.18 | 41.7 | 4.89 | 0.85 |
| pH = 7.4 | 2.63 | 0.59 | 0.97 | 0.20 | 24.1 | 9.55 | 0.91 | |
| N-MIL | pH = 7.4 | 7.89 | 0.26 | 0.76 | 0.27 | 93.1 | 7.68 | 0.95 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Wang, Y.; Zhu, J.; Liu, W.; Khan, M.Z.H.; Liu, X. Molecularly Imprinting Polymers (MIP) Based on Nitrogen Doped Carbon Dots and MIL-101(Fe) for Doxorubicin Hydrochloride Delivery. Nanomaterials 2020, 10, 1655. https://doi.org/10.3390/nano10091655
Shi Y, Wang Y, Zhu J, Liu W, Khan MZH, Liu X. Molecularly Imprinting Polymers (MIP) Based on Nitrogen Doped Carbon Dots and MIL-101(Fe) for Doxorubicin Hydrochloride Delivery. Nanomaterials. 2020; 10(9):1655. https://doi.org/10.3390/nano10091655
Chicago/Turabian StyleShi, Yuqiong, Yuxuan Wang, Jinhua Zhu, Wei Liu, Md. Zaved H. Khan, and Xiuhua Liu. 2020. "Molecularly Imprinting Polymers (MIP) Based on Nitrogen Doped Carbon Dots and MIL-101(Fe) for Doxorubicin Hydrochloride Delivery" Nanomaterials 10, no. 9: 1655. https://doi.org/10.3390/nano10091655





