Styrene-Based Elastomer Composites with Functionalized Graphene Oxide and Silica Nanofiber Fillers: Mechanical and Thermal Conductivity Properties
Abstract
:1. Introduction
2. Experimental
3. Results and Discussion
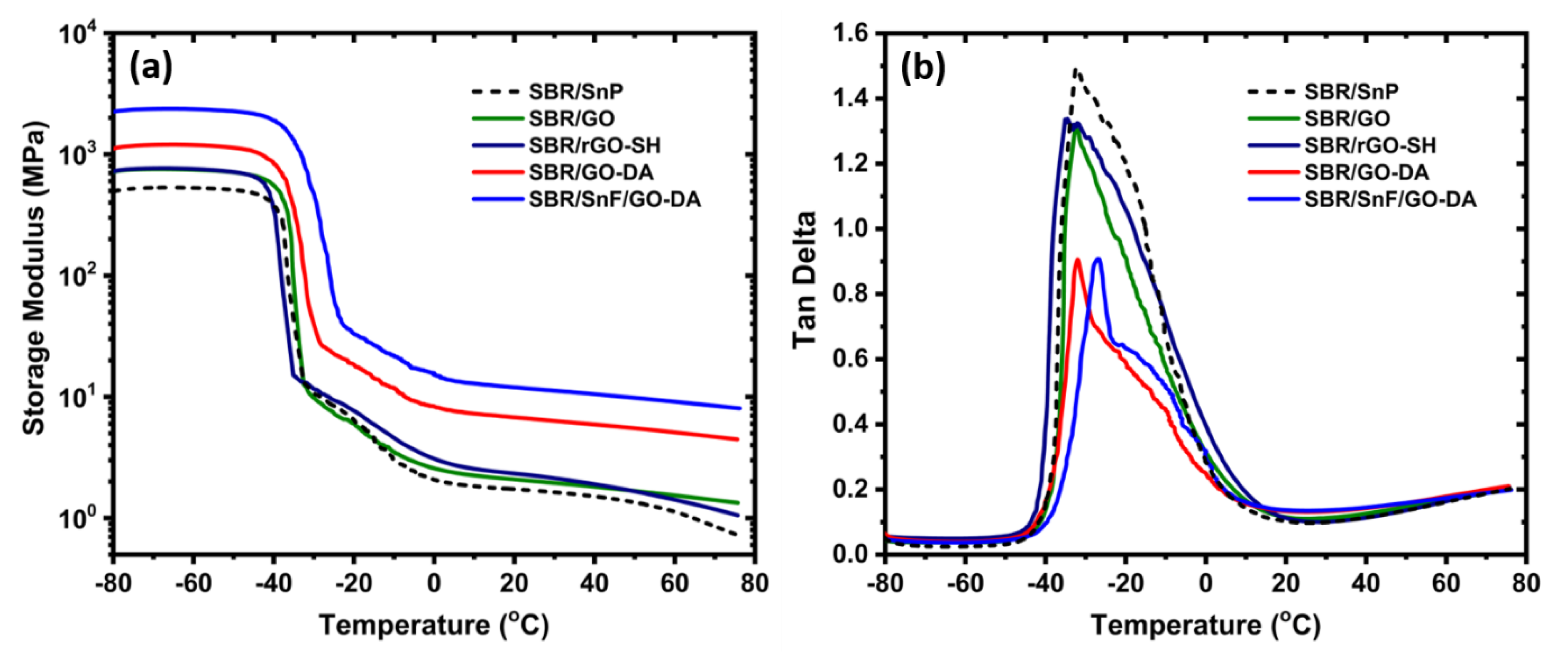
3.1. SBR Composites
3.2. SBS Composites
3.3. Thermal Conductivity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mao, Y.; Li, S.; Fang, R.L.; Ploehn, H.J. Magadiite/styrene-butadiene rubber composites for tire tread applications: Effects of varying layer spacing and alternate inorganic fillers. J. Appl. Polym. Sci. 2017, 134, 44764. [Google Scholar] [CrossRef]
- Mensah, B.; Gupta, K.C.; Kim, H.; Wang, W.; Jeong, K.U.; Nah, C. Graphene-reinforced elastomeric nanocomposites: A review. Polym. Test. 2018, 68, 160–184. [Google Scholar] [CrossRef]
- Zheng, L.; Jerrams, S.; Xu, Z.; Zhang, L.; Liu, L.; Wen, S. Enhanced gas barrier properties of graphene oxide/rubber composites with strong interfaces constructed by graphene oxide and sulfur. Chem. Eng. J. 2020, 383, 123100. [Google Scholar] [CrossRef]
- Milroy, C.; Manthiram, A. An elastic, conductive, electroactive nanocomposite binder for flexible sulfur cathodes in lithium–sulfur batteries. Adv. Mater. 2016, 28, 9744–9751. [Google Scholar] [CrossRef]
- Wu, H.; Thakur, V.K.; Kessler, M.R. Novel low-cost hybrid composites from asphaltene/SBS tri-block copolymer with improved thermal and mechanical properties. J. Mater. Sci. 2016, 51, 2394–2403. [Google Scholar] [CrossRef]
- Pedroni, L.G.; Soto-Oviedo, M.A.; Rosolen, J.M.; Felisberti, M.I.; Nogueira, A.F. Conductivity and mechanical properties of composites based on MWCNTs and styrene-butadiene-styrene blockTM copolymers. J. Appl. Polym. Sci. 2009, 112, 3241–3248. [Google Scholar] [CrossRef]
- Chen, H.; Ling, M.; Hencz, L.; Ling, H.Y.; Li, G.; Lin, Z.; Liu, G.; Zhang, S. Exploring chemical, mechanical, and electrical functionalities of binders for advanced energy-storage devices. Chem. Rev. 2018, 118, 8936–8982. [Google Scholar] [CrossRef]
- Xing, W.; Tang, M.; Wu, J.; Huang, G.; Li, H.; Lei, Z.; Fu, X.; Li, H. Multifunctional properties of graphene/rubber nanocomposites fabricated by a modified latex compounding method. Compos. Sci. Technol. 2014, 99, 67–74. [Google Scholar] [CrossRef]
- Luo, Y.; Zhao, P.; Yang, Q.; He, D.; Kong, L.; Peng, Z. Fabrication of conductive elastic nanocomposites via framing intact interconnected graphene networks. Compos. Sci. Technol. 2014, 100, 143–151. [Google Scholar] [CrossRef]
- Yu, A.; Ramesh, P.; Itkis, M.E.; Bekyarova, E.; Haddon, R.C. Graphite nanoplatelet-epoxy composite thermal interface materials. J. Phys. Chem. Lett. 2007, 111, 7565–7569. [Google Scholar] [CrossRef]
- Shahil, K.M.F.; Balandin, A.A. Graphene–multilayer graphene nanocomposites as highly efficient thermal interface materials. Nano Lett. 2012, 12, 861–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shtein, M.; Nadiv, R.; Buzaglo, M.; Regev, O. Graphene-based hybrid composites for efficient thermal management of electronic devices. ACS Appl. Mater. Interfaces 2015, 7, 23725–23730. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.; Goncalves, S.; Mora, H.; Carabineiro, S.A.C.; Viana, J.C.; Lanceros-Mendez, S. Highly sensitive piezoresistive graphene-based stretchable composites for sensing applications. ACS Appl. Mater. Interfaces 2019, 11, 46286–46295. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, J.L. Rubber—Filler interactions and rheological properties in filled compounds. Prog. Polym. Sci. 2002, 27, 627–687. [Google Scholar] [CrossRef]
- Choi, S.S.; Kim, Y.; Kwon, H.M. Microstructural analysis and cis–trans isomerization of BR and SBR vulcanizates reinforced with silica and carbon black using NMR and IR. RSC Adv. 2014, 4, 31113–31119. [Google Scholar] [CrossRef]
- Liu, Y.T.; Xie, X.M.; Ye, X.Y. High-concentration organic solutions of poly(styrene-co-butadiene-co-styrene)-modified graphene sheets exfoliated from graphite. Carbon 2011, 49, 3529–3537. [Google Scholar] [CrossRef]
- Xing, W.; Li, H.; Huang, G.; Cai, L.H.; Wu, J. Graphene oxide induced crosslinking and reinforcement of elastomers. Compos. Sci. Technol. 2017, 144, 223–229. [Google Scholar] [CrossRef]
- Potts, J.R.; Shankar, O.; Du, L.; Ruof, R.S. Processing-morphology-property relationships and composite theory analysis of reduced graphene oxide/natural rubber nanocomposites. Macromolecules 2012, 45, 6045–6055. [Google Scholar] [CrossRef]
- Mao, Y.; Wen, S.; Chen, Y.; Zhang, F.; Panine, P.; Chan, T.W.; Zhang, L.; Liang, Y.; Liu, L. High performance graphene oxide based rubber composites. Sci. Rep. 2013, 3, 2508. [Google Scholar] [CrossRef] [Green Version]
- Morimoto, N.; Kubo, T.; Nishina, Y. Tailoring the oxygen content of graphite and reduced graphene oxide for specific applications. Sci. Rep. 2016, 6, 21715. [Google Scholar] [CrossRef]
- Kang, S.M.; Park, S.; Kim, D.; Park, S.Y.; Ruoff, R.S.; Lee, H. Simultaneous reduction and surface functionalization of graphene oxide by mussel-inspired chemistry. Adv. Funct. Mater. 2011, 21, 108–112. [Google Scholar] [CrossRef]
- Ma, H.L.; Zhang, H.B.; Hu, Q.H.; Li, W.J.; Jiang, Z.G.; Yu, Z.Z.; Dasari, A. Functionalization and reduction of graphene oxide with p-phenylene diamine for electrically conductive and thermally stable polystyrene composites. ACS Appl. Mater. Interfaces 2012, 4, 1948–1953. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fei, G.; Pan, Y.; Zhang, K.; Hao, S.; Zheng, Z.; Xia, H. Simultaneous reduction and surface functionalization of graphene oxide by cystamine dihydrochloride for rubber composites. Compos. Part A Appl. Sci. 2019, 122, 18–26. [Google Scholar] [CrossRef]
- Polizos, G.; Sharma, J.K.; Smith, D.B.; Tuncer, E.; Park, J.; Voylov, D.; Sokolov, A.P.; Meyer III, H.M.; Aman, M. Anti-soiling and highly transparent coatings with multi-scale features. Sol. Energy Mater. Sol. Cells 2018, 188, 255–262. [Google Scholar] [CrossRef]
- Polizos, G.; Tuncer, E.; Sauers, I.; More, K.L. Physical properties of epoxy resin/titanium dioxide nanocomposites. Polym. Eng. Sci. 2011, 51, 87–93. [Google Scholar] [CrossRef]
- Polizos, G.; Winter, K.; Lance, M.J.; Meyer, H.M.; Armstrong, B.L.; Schaeffer, D.A.; Simpson, J.T.; Hunter, S.R.; Datskos, P.G. Scalable superhydrophobic coatings based on fluorinated diatomaceous earth: Abrasion resistance versus particle geometry. Appl. Surf. Sci. 2014, 292, 563–569. [Google Scholar] [CrossRef]
- Park, J.; Sharma, J.; Goswami, M.; Voylov, D.; Jang, G.G.; Lassiter, M.G.; Rossy, A.M.; Polizos, G. Solution-derived monolithic thin films with low adhesion surface. Sol. Energy Mater. Sol. Cells 2020, 206, 110302. [Google Scholar] [CrossRef]
- Sharma, J.; Cullen, D.A.; Polizos, G.; Nawaz, K.; Wang, H.; Muralidharan, N.; Smith, D.B. Hybrid hollow silica particles: Synthesis and comparison of properties with pristine particles. RSC Adv. 2020, 10, 22331–22334. [Google Scholar] [CrossRef]
- Hung, W.S.; Tsou, C.H.; Guzman, M.D.; An, Q.F.; Liu, Y.L.; Zhang, Y.M.; Hu, C.C.; Lee, K.R.; Lai, J.Y. Cross-linking with diamine monomers to prepare composite graphene oxide-framework membranes with varying d-Spacing. Chem. Mater. 2014, 26, 2983–2990. [Google Scholar] [CrossRef]
- Mungse, H.P.; Singh, R.; Sugimura, H.; Kumar, N.; Khatri, O.P. Molecular pillar supported graphene oxide framework: Conformational heterogeneity and tunable d-spacing. Phys. Chem. Chem. Phys. 2015, 17, 20822–20829. [Google Scholar] [CrossRef]
- Hung, W.S.; Lin, T.J.; Chiao, Y.H.; Sengupta, A.; Hsiao, Y.C.; Wickramasinghe, S.R.; Hu, C.C.; Lee, K.R.; Lai, J.Y. Graphene-induced tuning of the d-spacing of graphene oxide composite nanofiltration membranes for frictionless capillary action-induced enhancement of water permeability. J. Mater. Chem. A 2018, 6, 19445–19454. [Google Scholar] [CrossRef]
- Yap, P.L.; Kabiri, S.; Tran, D.N.H.; Losic, D. Multifunctional binding chemistry on modified graphene composite for selective and highly efficient adsorption of mercury. ACS Appl. Mater. Interfaces 2019, 11, 6350–6362. [Google Scholar] [CrossRef] [PubMed]
- Orth, E.S.; Fonsaca, J.E.S.; Domingues, S.H.; Mehl, H.; Oliveira, M.M.; Zarbin, A.J.G. Targeted thiolation of graphene oxide and its utilization as precursor for graphene/silver nanoparticles composites. Carbon 2013, 61, 543–550. [Google Scholar] [CrossRef]
- Huang, H.H.; De Silva, K.K.H.; Kumara, G.R.A.; Yoshimura, M. Structural evolution of hydrothermally derived reduced graphene oxide. Sci. Rep. 2018, 8, 6849. [Google Scholar] [CrossRef]
- Drewniak, S.; Muzyka, R.; Stolarczyk, A.; Pustelny, T.; Moranska, M.K.; Setkiewicz, M. Studies of reduced graphene oxide and graphite oxide in the aspect of their possible application in gas sensors. Sensors 2016, 16, 103. [Google Scholar] [CrossRef]
- Newsome, T.E.; Olesik, S.V. Electrospinning Silica/Polyvinylpyrrolidone composite nanofibers. J. Appl. Polym. Sci. 2014, 131, 40966. [Google Scholar] [CrossRef]
- Scotti, R.; Conzatti, L.; D’Arienzo, M.; Credico, B.D.; Giannini, L.; Hanel, T.; Stagnaro, P.; Susanna, A.; Tadiello, L.; Morazzoni, F. Shape controlled spherical (0D) and rod-like (1D) silica nanoparticles in silica/styrene butadiene rubber nanocomposites: Role of the particle morphology on the filler reinforcing effect. Polymer 2014, 55, 1497–1506. [Google Scholar] [CrossRef]
- Spence, D.; Park, J.; Cullen, D.A.; Ho, H.C.; Polizos, G.; Sharma, J. Solution-phase synthesis of silica fibers and their use in making transparent high-strength silica-polymer composites. ChemistrySelect 2018, 3, 13427–13431. [Google Scholar] [CrossRef]
- Cox, H.L. The elasticity and strength of paper and other fibrous materials. Br. J. Appl. Phys. 1952, 3, 72–79. [Google Scholar] [CrossRef]
- Carman, G.P.; Reifsnider, K.L. Micromechanics of short-fiber composites. Compos. Sci. Technol. 1992, 43, 137–146. [Google Scholar] [CrossRef]
- Tucker, C.L.; Liang, E. Stiffness predictions for unidirectional short-fiber composites: Review and evaluation. Compos. Sci. Technol. 1999, 59, 655–671. [Google Scholar] [CrossRef]
- Coleman, J.N.; Khan, U.; Blau, W.J.; Gun’ko, Y.K. Small but strong: A review of the mechanical properties of carbon nanotube–polymer composites. Carbon 2006, 44, 1624–1652. [Google Scholar] [CrossRef]
- Krenchel, H. Fibre Reinforcement: Theoretical and Practical Investigations of the Elasticity and Strength of Fibre-Reinforced Materials; Akademisk Forlag: Copenhagen, Denmark, 1964. [Google Scholar]
- Wellenberger, F.T.; Watson, J.C.; Li, H. Glass Fibers. ASM Handb. 2001, 21, 06781G. [Google Scholar]
- Meyers, M.A.; Chawla, K.K. Mechanical Behavior of Materials; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Dikin, D.A.; Chen, X.; Ding, W.; Wagner, G.; Ruoff, R.S. Resonance vibration of amorphous SiO2 nanowires driven by mechanical or electrical field excitation. J. Appl. Phys. 2003, 93, 226–230. [Google Scholar] [CrossRef]
- Polizos, G.; Tuncer, E.; Sauers, I.; More, K.L. Properties of a nanodielectric cryogenic resin. Appl. Phys. Lett. 2010, 96, 152903. [Google Scholar] [CrossRef]
- Polizos, G.; Tuncer, E.; Agapov, A.L.; Stevens, D.; Sokolov, A.P.; Kidder, M.K.; Jacobs, J.D.; Koerner, H.; Vaia, R.A.; More, K.L.; et al. Effect of polymer-nanoparticle interactions on the glass transition dynamics and the conductivity mechanism in polyurethane titanium dioxide nanocomposites. Polymer 2012, 53, 595–603. [Google Scholar] [CrossRef]
- Manias, E.; Polizos, G.; Nakajima, H.; Heidecker, M.J. Fundamentals of polymer nanocomposite technology. In Flame Retardant Polymer Nanocomposites; Alexander, B.M., Charles, A.W., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; pp. 31–66. [Google Scholar]
- Ferrer, G.G.; Melia, J.M.S.; Canales, J.H.; Duenas, J.M.M.; Colomer, F.R.; Pradas, M.M.; Ribelles, J.L.G.; Pissis, P.; Polizos, G. Poly(2-hydroxyethyl acrylate) hydrogel confined in a hydrophobous porous matrix. Colloid Polym. Sci. 2005, 283, 681–690. [Google Scholar] [CrossRef]
- Nicolosi, V.; Chhowalla, M.; Kanatzidis, M.G.; Strano, M.S.; Coleman, J.N. Liquid exfoliation of layered materials. Science 2013, 340, 1226419. [Google Scholar] [CrossRef] [Green Version]
- Paton, K.R.; Varrla, E.; Backes, C.; Smith, R.J.; Khan, U.; O’Neill, A.; Boland, C.; Lotya, M.; Istrate, O.M.; King, P.; et al. Scalable production of large quantities of defect-free few-layer graphene by shear exfoliation in liquids. Nat. Mater. 2014, 13, 624–630. [Google Scholar] [CrossRef]
- Vo, L.T.; Anastasiadis, S.H.; Giannelis, E.P. Dielectric study of poly (styrene-co-butadiene) composites with carbon black, silica, and nanoclay. Macromolecules 2011, 44, 6162–6171. [Google Scholar] [CrossRef]
- Tang, Z.; Zhang, L.; Feng, W.; Guo, B.; Liu, F.; Jia, D. Rational design of graphene surface chemistry for high-performance rubber/graphene composites. Macromolecules 2014, 47, 8663–8673. [Google Scholar] [CrossRef] [Green Version]
- Cai, F.; You, G.; Luo, K.; Zhang, H.; Zhao, X.; Wu, S. Click chemistry modified graphene oxide/styrene-butadiene rubber composites and molecular simulation study. Compos. Sci. Technol. 2020, 190, 108061. [Google Scholar] [CrossRef]
- Wang, C. Tear strength of styrene-butadiene-styrene block copolymers. Macromolecules 2001, 34, 9006–9014. [Google Scholar] [CrossRef]











| Name | GO at.% | SH-1 at.% | SH-2 at.% |
|---|---|---|---|
| C (sp2) | 7.9 | 43.3 | 39.6 |
| C (sp3) | 34.7 | 4.5 | 8.7 |
| C-O/C-S/C-N | 24.2 | 18.0 | 19.0 |
| O=C-OH | 6.0 | 1.3 | 1.8 |
| C=O | 0.0 | 57.7 | 6.6 |
| O-C | 22.3 | 8.6 | 11.4 |
| O=C | 3.5 | 9.5 | 5.0 |
| S-O | 0.2 | 0.5 | 0.5 |
| H-S-C | 0.0 | 3.4 | 3.7 |
| N-Aniline | 0.0 | 2.5 | 2.5 |
| N-Aniline+ | 0.0 | 0.7 | 0.6 |
| Si-O | 1.2 | 2.1 | 0.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Sharma, J.; Monaghan, K.W.; Meyer, H.M., III; Cullen, D.A.; Rossy, A.M.; Keum, J.K.; Wood, D.L., III; Polizos, G. Styrene-Based Elastomer Composites with Functionalized Graphene Oxide and Silica Nanofiber Fillers: Mechanical and Thermal Conductivity Properties. Nanomaterials 2020, 10, 1682. https://doi.org/10.3390/nano10091682
Park J, Sharma J, Monaghan KW, Meyer HM III, Cullen DA, Rossy AM, Keum JK, Wood DL III, Polizos G. Styrene-Based Elastomer Composites with Functionalized Graphene Oxide and Silica Nanofiber Fillers: Mechanical and Thermal Conductivity Properties. Nanomaterials. 2020; 10(9):1682. https://doi.org/10.3390/nano10091682
Chicago/Turabian StylePark, Jaehyeung, Jaswinder Sharma, Kyle W. Monaghan, Harry M. Meyer, III, David A. Cullen, Andres M. Rossy, Jong K. Keum, David L. Wood, III, and Georgios Polizos. 2020. "Styrene-Based Elastomer Composites with Functionalized Graphene Oxide and Silica Nanofiber Fillers: Mechanical and Thermal Conductivity Properties" Nanomaterials 10, no. 9: 1682. https://doi.org/10.3390/nano10091682





