Impact of Thermal Treatment of Nb2O5 on Its Performance in Glucose Dehydration to 5-Hydroxymethylfurfural in Water
Abstract
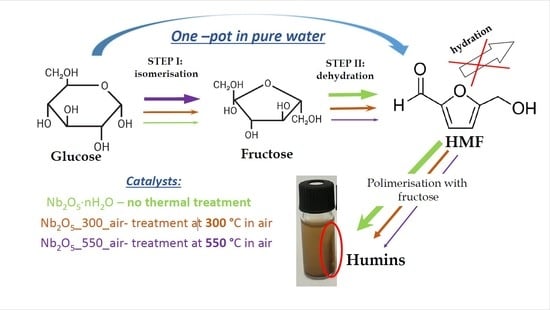
1. Introduction
2. Experimental
2.1. Materials Preparation
2.2. Materials Characterization
2.3. Catalytic Testing
3. Results and Discussion
3.1. Textural Properties
3.2. Crystallinity of the Samples
3.3. Chemical Properties
3.3.1. X-ray Photoelectron Spectroscopy (XPS) Studies
3.3.2. Acidity of the Catalysts
3.4. Thermogravimetric Analysis
3.5. Catalytic Results
Effects of the Reaction Time and Temperature on the Dehydration of Glucose to 5-Hydroxymethylfurfural (HMF)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eblagon, K.M.; Malaika, A.; Pereira, M.F.R.; Figueiredo, J.L. Cutting the Green Waste. Structure-Performance Relationship in Functionalized Carbon Xerogels for Hydrolysis of Cellobiose. ChemCatChem 2018, 10, 4934–4946. [Google Scholar] [CrossRef]
- Kong-Win Chang, J.; Duret, X.; Berberi, V.; Zahedi-Niaki, H.; Lavoie, J.-M. Two-Step Thermochemical Cellulose Hydrolysis with Partial Neutralization for Glucose Production. Front. Chem. 2018, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hegner, J.; Pereira, K.C.; DeBoef, B.; Lucht, B.L. Conversion of cellulose to glucose and levulinic acid via solid-supported acid catalysis. Tetrahedron Lett. 2010, 51, 2356–2358. [Google Scholar] [CrossRef]
- Chang, C.; Xu, G.; Zhu, W.; Bai, J.; Fang, S. One-pot production of a liquid biofuel candidate—Ethyl levulinate from glucose and furfural residues using a combination of extremely low sulfuric acid and zeolite USY. Fuel 2015, 140, 365–370. [Google Scholar] [CrossRef]
- Gallo, J.M.R.; Alonso, D.M.; Mellmer, M.A.; Dumesic, J.A. Production and upgrading of 5-hydroxymethylfurfural using heterogeneous catalysts and biomass-derived solvents. Green Chem. 2013, 15, 85–90. [Google Scholar] [CrossRef]
- Morawa Eblagon, K.; Pereira, M.F.R.; Figueiredo, J.L. One-pot oxidation of cellobiose to gluconic acid. Unprecedented high selectivity on bifunctional gold catalysts over mesoporous carbon by integrated texture and surface chemistry optimization. Appl. Catal. B-Environ. 2016, 184, 381–396. [Google Scholar] [CrossRef]
- Van Putten, R.-J.; van der Waal, J.C.; de Jong, E.; Rasrendra, C.B.; Heeres, H.J.; de Vries, J.G. Hydroxymethylfurfural, A Versatile Platform Chemical Made from Renewable Resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef]
- Rass, H.A.; Essayem, N.; Besson, M. Selective Aerobic Oxidation of 5-HMF into 2,5-Furandicarboxylic Acid with Pt Catalysts Supported on TiO2- and ZrO2-Based Supports. ChemSusChem 2015, 8, 1206–1217. [Google Scholar] [CrossRef]
- Sajid, M.; Zhao, X.; Liu, D. Production of 2,5-furandicarboxylic acid (FDCA) from 5-hydroxymethylfurfural (HMF): Recent progress focusing on the chemical-catalytic routes. Green Chem. 2018, 20, 5427–5453. [Google Scholar] [CrossRef]
- Motagamwala, A.H.; Won, W.; Sener, C.; Alonso, D.M.; Maravelias, C.T.; Dumesic, J.A. Toward biomass-derived renewable plastics: Production of 2,5-furandicarboxylic acid from fructose. Sci. Adv. 2018, 4, 1–8. [Google Scholar] [CrossRef]
- Song, B.; Yu, Y.; Wu, H. Tuning glucose decomposition in hot-compressed gamma-valerolactone/water mixtures: From isomerization to dehydration reactions. Fuel 2019, 238, 225–231. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Z.; Huang, K.; Fang, Z. Efficient conversion of carbohydrates into 5-ethoxymethylfurfural in ethanol catalyzed by AlCl3. Fuel 2013, 113, 625–631. [Google Scholar] [CrossRef]
- Nagpure, A.S.; Venugopal, A.K.; Lucas, N.; Manikandan, M.; Thirumalaiswamy, R.; Chilukuri, S. Renewable fuels from biomass-derived compounds: Ru-containing hydrotalcites as catalysts for conversion of HMF to 2,5-dimethylfuran. Catal. Sci. Technol. 2015, 5, 1463–1472. [Google Scholar] [CrossRef]
- Zou, X.; Zhu, C.; Wang, Q.; Yang, G. Catalytic dehydration of hexose sugars to 5-hydroxymethylfural. Biofuels Bioprod. Bior. 2019, 13, 153–173. [Google Scholar] [CrossRef]
- Kreissl, H.T.; Nakagawa, K.; Peng, Y.-K.; Koito, Y.; Zheng, J.; Tsang, S.C.E. Niobium oxides: Correlation of acidity with structure and catalytic performance in sucrose conversion to 5-hydroxymethylfurfural. J. Catal. 2016, 338, 329–339. [Google Scholar] [CrossRef]
- Shahangi, F.; Najafi Chermahini, A.; Saraji, M. Dehydration of fructose and glucose to 5-hydroxymethylfurfural over Al-KCC-1 silica. J. Energy Chem. 2018, 27, 769–780. [Google Scholar] [CrossRef]
- Román-Leshkov, Y.; Chheda, J.N.; Dumesic, J.A. Phase Modifiers Promote Efficient Production of Hydroxymethylfurfural from Fructose. Science 2006, 312, 1933–1937. [Google Scholar] [CrossRef]
- Antal, M.J.; Mok, W.S.L.; Richards, G.N. Mechanism of formation of 5-(hydroxymethyl)-2-furaldehyde from d-fructose and sucrose. Carbohydr. Res. 1990, 199, 91–109. [Google Scholar] [CrossRef]
- Kim, M.; Su, Y.; Fukuoka, A.; Hensen, E.J.M.; Nakajima, K. Aerobic Oxidation of 5-(Hydroxymethyl)furfural Cyclic Acetal Enables Selective Furan-2,5-dicarboxylic Acid Formation with CeO2-Supported Gold Catalyst. Angew. Chem. Int. Ed. 2018, 57, 8235–8239. [Google Scholar] [CrossRef]
- Pagán-Torres, Y.J.; Wang, T.; Gallo, J.M.R.; Shanks, B.H.; Dumesic, J.A. Production of 5-Hydroxymethylfurfural from Glucose Using a Combination of Lewis and Brønsted Acid Catalysts in Water in a Biphasic Reactor with an Alkylphenol Solvent. ACS Catal. 2012, 2, 930–934. [Google Scholar] [CrossRef]
- Dallas Swift, T.; Nguyen, H.; Anderko, A.; Nikolakis, V.; Vlachos, D.G. Tandem Lewis/Brønsted homogeneous acid catalysis: Conversion of glucose to 5-hydoxymethylfurfural in an aqueous chromium(iii) chloride and hydrochloric acid solution. Green Chem. 2015, 17, 4725–4735. [Google Scholar] [CrossRef]
- Swift, T.D.; Bagia, C.; Choudhary, V.; Peklaris, G.; Nikolakis, V.; Vlachos, D.G. Kinetics of Homogeneous Brønsted Acid Catalyzed Fructose Dehydration and 5-Hydroxymethyl Furfural Rehydration: A Combined Experimental and Computational Study. ACS Catal. 2014, 4, 259–267. [Google Scholar] [CrossRef]
- Vieira, J.L.; Almeida-Trapp, M.; Mithöfer, A.; Plass, W.; Gallo, J.M.R. Rationalizing the conversion of glucose and xylose catalyzed by a combination of Lewis and Brønsted acids. Catal. Today 2020, 344, 92–101. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, Z.; Zhou, Y.; Han, B.; Fan, H.; Li, W.; Song, J.; Xie, Y. Conversion of fructose to 5-hydroxymethylfurfural using ionic liquids prepared from renewable materials. Green Chem. 2008, 10, 1280–1283. [Google Scholar] [CrossRef]
- Zhao, H.; Holladay, J.E.; Brown, H.; Zhang, Z.C. Metal Chlorides in Ionic Liquid Solvents Convert Sugars to 5-Hydroxymethylfurfural. Science 2007, 316, 1597–1600. [Google Scholar] [CrossRef]
- Esteban, J.; Vorholt, A.J.; Leitner, W. An overview of the biphasic dehydration of sugars to 5-hydroxymethylfurfural and furfural: A rational selection of solvents using COSMO-RS and selection guides. Green Chem. 2020. [Google Scholar] [CrossRef]
- Xiong, H.; Wang, T.; Shanks, B.H.; Datye, A.K. Tuning the Location of Niobia/Carbon Composites in a Biphasic Reaction: Dehydration of d-Glucose to 5-Hydroxymethylfurfural. Catal. Lett. 2013, 143, 509–516. [Google Scholar] [CrossRef]
- Román-Leshkov, Y.; Dumesic, J.A. Solvent Effects on Fructose Dehydration to 5-Hydroxymethylfurfural in Biphasic Systems Saturated with Inorganic Salts. Top. Catal. 2009, 52, 297–303. [Google Scholar] [CrossRef]
- Murayama, T.; Chen, J.; Hirata, J.; Matsumoto, K.; Ueda, W. Hydrothermal synthesis of octahedra-based layered niobium oxide and its catalytic activity as a solid acid. Catal. Sci. Technol. 2014, 4, 4250–4257. [Google Scholar] [CrossRef]
- Nowak, I.; Ziolek, M. Niobium Compounds: Preparation, Characterization, and Application in Heterogeneous Catalysis. Chem. Rev. 1999, 99, 3603–3624. [Google Scholar] [CrossRef]
- Luisa Marin, M.; Hallett-Tapley, G.L.; Impellizzeri, S.; Fasciani, C.; Simoncelli, S.; Netto-Ferreira, J.C.; Scaiano, J.C. Synthesis, acid properties and catalysis by niobium oxide nanostructured materials. Catal. Sci. Technol. 2014, 4, 3044–3052. [Google Scholar] [CrossRef]
- Santos, K.M.A.; Albuquerque, E.M.; Innocenti, G.; Borges, L.E.P.; Sievers, C.; Fraga, M.A. The Role of Brønsted and Water-Tolerant Lewis Acid Sites in the Cascade Aqueous-Phase Reaction of Triose to Lactic Acid. ChemCatChem 2019, 11, 3054–3063. [Google Scholar] [CrossRef]
- Nakajima, K.; Baba, Y.; Noma, R.; Kitano, M.N.; Kondo, J.; Hayashi, S.; Hara, M. Nb2O5·nH2O as a Heterogeneous Catalyst with Water-Tolerant Lewis Acid Sites. J. Am. Chem. Soc. 2011, 133, 4224–4227. [Google Scholar] [CrossRef] [PubMed]
- Lebarbier, V.; Houalla, M.; Onfroy, T. New insights into the development of Brønsted acidity of niobic acid. Catal. Today 2012, 192, 123–129. [Google Scholar] [CrossRef]
- Siddiki, S.M.A.H.; Rashed, M.N.; Ali, M.A.; Toyao, T.; Hirunsit, P.; Ehara, M.; Shimizu, K.-I. Lewis Acid Catalysis of Nb2O5 for Reactions of Carboxylic Acid Derivatives in the Presence of Basic Inhibitors. ChemCatChem 2019, 11, 383–396. [Google Scholar] [CrossRef]
- De Carvalho, G.S.G.; Chagas, L.H.; Fonseca, C.G.; de Castro, P.P.; Sant’Ana, A.C.; Leitão, A.A.; Amarante, G.W. Nb2O5 supported on mixed oxides catalyzed oxidative and photochemical conversion of anilines to azoxybenzenes. New J. Chem. 2019, 43, 5863–5871. [Google Scholar] [CrossRef]
- Li, X.; Peng, K.; Xia, Q.; Liu, X.; Wang, Y. Efficient conversion of cellulose into 5-hydroxymethylfurfural over niobia/carbon composites. Chem. Eng. J. 2018, 332, 528–536. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Li, X.; Liu, X.; Xia, Y.; Hu, B.; Lu, G.; Wang, Y. Direct conversion of biomass-derived carbohydrates to 5-hydroxymethylfurural over water-tolerant niobium-based catalysts. Fuel 2015, 139, 301–307. [Google Scholar] [CrossRef]
- Catrinck, M.N.; Ribeiro, E.S.; Monteiro, R.S.; Ribas, R.M.; Barbosa, M.H.P.; Teófilo, R.F. Direct conversion of glucose to 5-hydroxymethylfurfural using a mixture of niobic acid and niobium phosphate as a solid acid catalyst. Fuel 2017, 210, 67–74. [Google Scholar] [CrossRef]
- Wang, F.; Wu, H.-Z.; Liu, C.-L.; Yang, R.-Z.; Dong, W.-S. Catalytic dehydration of fructose to 5-hydroxymethylfurfural over Nb2O5 catalyst in organic solvent. Carbohydr. Res. 2013, 368, 78–83. [Google Scholar] [CrossRef]
- Raba, A.M.; Bautista-Ruíz, J.; Joya, M.R. Synthesis and Structural Properties of Niobium Pentoxide Powders: A Comparative Study of the Growth Process. Mater. Res. 2016, 19, 1381–1387. [Google Scholar] [CrossRef]
- Pan, L.; Wang, Y.; Wang, X.; Qu, H.; Zhao, J.; Li, Y.; Gavrilyuk, A. Hydrogen photochromism in Nb2O5 powders. Phys. Chem. Chem. Phys. 2014, 16, 20828–20833. [Google Scholar] [CrossRef] [PubMed]
- Florentino, A.; Cartraud, P.; Magnoux, P.; Guisnet, M. Textural, acidic and catalytic properties of niobium phosphate and of niobium oxide: Influence of the pretreatment temperature. Appl. Catal. A-Gen. 1992, 89, 143–153. [Google Scholar] [CrossRef]
- Ni, J.; Wang, W.; Wu, C.; Liang, H.; Maier, J.; Yu, Y.; Li, L. Highly Reversible and Durable Na Storage in Niobium Pentoxide through Optimizing Structure, Composition, and Nanoarchitecture. Adv. Mater. 2017, 29, 1605607–1605613. [Google Scholar] [CrossRef] [PubMed]
- Ristić, M.; Popović, S.; Musić, S. Sol–gel synthesis and characterization of Nb2O5 powders. Mater. Lett. 2004, 58, 2658–2663. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, X.; Ye, L.; Chi Edman Tsang, S. Nanostructured Nb2O5 catalysts. Nano Rev. 2012, 3, 17631–17642. [Google Scholar] [CrossRef]
- Tokio, I.; Kazuharu, O.; Kozo, T. Acidic and Catalytic Properties of Niobium Pentaoxide. Bull. Chem. Soc. Jpn. 1983, 56, 2927–2931. [Google Scholar]
- Zhou, Y.; Qiu, Z.; Lü, M.; Zhang, A.; Ma, Q. Preparation and characterization of porous Nb2O5 nanoparticles. Mater. Res. Bull. 2008, 43, 1363–1368. [Google Scholar] [CrossRef]
- Chan, X.; Pu, T.; Chen, X.; James, A.; Lee, J.; Parise, J.B.; Kim, D.H.; Kim, T. Effect of niobium oxide phase on the furfuryl alcohol dehydration. Catal. Commun. 2017, 97, 65–69. [Google Scholar] [CrossRef]
- Pilarek, B.; Pelczarska, A.J.; Szczygieł, I. Characterization of niobium(v) oxide received from different sources. J. Therm. Anal. Calorim. 2017, 130, 77–83. [Google Scholar] [CrossRef]
- Kodama, R.; Terada, Y.; Nakai, I.; Komaba, S.; Kumagai, N. Electrochemical and In Situ XAFS-XRD Investigation of Nb2O5 for Rechargeable Lithium Batteries. J. Electrochem. Soc. 2006, 153, A583–A588. [Google Scholar] [CrossRef]
- Li, S.; Qian, X.; Uchaker, E.; Cao, X.; Cao, G. Comparison of amorphous, pseudohexagonal and orthorhombic Nb2O5 for high-rate lithium ion insertion. CrystEngComm 2016, 18, 2532–2540. [Google Scholar] [CrossRef]
- Silva, Â.; Wilson, K.; Lee, A.F.; dos Santos, V.C.; Cons Bacilla, A.C.; Mantovani, K.M.; Nakagaki, S. Nb2O5/SBA-15 catalyzed propanoic acid esterification. Appl. Catal. B-Environ. 2017, 205, 498–504. [Google Scholar] [CrossRef]
- Skrodczky, K.; Antunes, M.M.; Han, X.; Santangelo, S.; Scholz, G.; Valente, A.A.; Pinna, N.; Russo, P.A. Niobium pentoxide nanomaterials with distorted structures as efficient acid catalysts. Commun. Chem. 2019, 2, 129–140. [Google Scholar] [CrossRef]
- Kreissl, H.T.; Li, M.M.J.; Peng, Y.-K.; Nakagawa, K.; Hooper, T.J.N.; Hanna, J.V.; Shepherd, A.; Wu, T.-S.; Soo, Y.-L.; Tsang, S.C.E. Structural Studies of Bulk to Nanosize Niobium Oxides with Correlation to Their Acidity. J. Am. Chem. Soc. 2017, 139, 12670–12680. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Aghamohammadi, S.; Li, D.; Li, K.; Farrauto, R. Structure dependence of Nb2O5-X supported manganese oxide for catalytic oxidation of propane: Enhanced oxidation activity for MnOx on a low surface area Nb2O5-X. Appl. Catal. B-Environ. 2019, 244, 438–447. [Google Scholar] [CrossRef]
- Teles, C.A.; de Souza, P.M.; Rabelo-Neto, R.C.; Griffin, M.B.; Mukarakate, C.; Orton, K.A.; Resasco, D.E.; Noronha, F.B. Catalytic upgrading of biomass pyrolysis vapors and model compounds using niobia supported Pd catalyst. Appl. Catal. B-Environ. 2018, 238, 38–50. [Google Scholar] [CrossRef]
- Chary, K.V.R.; Kumar, C.P.; Murali, A.; Tripathi, A.; Clearfield, A. Studies on catalytic functionality of V2O5/Nb2O5 catalysts. J. Mol. Catal. A Chem. 2004, 216, 139–146. [Google Scholar] [CrossRef]
- Candu, N.; El Fergani, M.; Verziu, M.; Cojocaru, B.; Jurca, B.; Apostol, N.; Teodorescu, C.; Parvulescu, V.I.; Coman, S.M. Efficient glucose dehydration to HMF onto Nb-BEA catalysts. Catal. Today 2019, 325, 109–116. [Google Scholar] [CrossRef]
- Amaniampong, P.N.; Li, K.; Jia, X.; Wang, B.; Borgna, A.; Yang, Y. Titania-Supported Gold Nanoparticles as Efficient Catalysts for the Oxidation of Cellobiose to Organic Acids in Aqueous Medium. ChemCatChem 2014, 6, 2105–2114. [Google Scholar] [CrossRef]
- Wang, S.-X.; Guo, R.-T.; Pan, W.-G.; Chen, Q.-L.; Sun, P.; Li, M.-Y.; Liu, S.-M. The deactivation of Ce/TiO2 catalyst for NH3-SCR reaction by alkali metals: TPD and DRIFT studies. Catal. Commun. 2017, 89, 143–147. [Google Scholar] [CrossRef]
- Aegerter, M.A. Sol–gel niobium pentoxide: A promising material for electrochromic coatings, batteries, nanocrystalline solar cells and catalysis. Sol. Energy Mater. Sol. Cells 2001, 68, 401–422. [Google Scholar] [CrossRef]
- Okazaki, S.; Kurosaki, A. Acidic properties and catalytic activities of niobic acid treated with phosphoric acid. Catal. Today 1990, 8, 113–122. [Google Scholar] [CrossRef]
- Ushikubo, T. Recent topics of research and development of catalysis by niobium and tantalum oxides. Catal. Today 2000, 57, 331–338. [Google Scholar] [CrossRef]
- Portillo Perez, G.; Mukherjee, A.; Dumont, M.-J. Insights into HMF catalysis. J. Ind. Eng.Chem. 2019, 70, 1–34. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Ren, J.; Liu, X.; Li, X.; Xia, Y.; Lu, G.; Wang, Y. Mesoporous niobium phosphate: An excellent solid acid for the dehydration of fructose to 5-hydroxymethylfurfural in water. Catal. Sci. Technol. 2012, 2, 2485–2491. [Google Scholar] [CrossRef]
- Jiang, C.; Zhu, J.; Wang, B.; Li, L.; Zhong, H. One-pot synthesis of 5-hydroxymethylfurfural from glucose over zirconium doped mesoporous KIT-6. Chin. J. Chem. Eng. 2018, 26, 1270–1277. [Google Scholar] [CrossRef]
- Guo, B.; Ye, L.; Tang, G.; Zhang, L.; Yue, B.; Tsang, S.C.E.; He, H. Effect of Brønsted/Lewis Acid Ratio on Conversion of Sugars to 5-Hydroxymethylfurfural over Mesoporous Nb and Nb-W Oxides. Chin. J. Chem. 2017, 35, 1529–1539. [Google Scholar] [CrossRef]
- Noma, R.; Nakajima, K.; Kamata, K.; Kitano, M.; Hayashi, S.; Hara, M. Formation of 5-(Hydroxymethyl)furfural by Stepwise Dehydration over TiO2 with Water-Tolerant Lewis Acid Sites. J. Phys. Chem. C 2015, 119, 17117–17125. [Google Scholar] [CrossRef]
- Ordomsky, V.V.; van der Schaaf, J.; Schouten, J.C.; Nijhuis, T.A. Fructose Dehydration to 5-Hydroxymethylfurfural over Solid Acid Catalysts in a Biphasic System. ChemSusChem 2012, 5, 1812–1819. [Google Scholar] [CrossRef]
- Choudhary, V.; Mushrif, S.H.; Ho, C.; Anderko, A.; Nikolakis, V.; Marinkovic, N.S.; Frenkel, A.I.; Sandler, S.I.; Vlachos, D.G. Insights into the Interplay of Lewis and Brønsted Acid Catalysts in Glucose and Fructose Conversion to 5-(Hydroxymethyl)furfural and Levulinic Acid in Aqueous Media. J. Am. Chem. Soc. 2013, 135, 3997–4006. [Google Scholar] [CrossRef] [PubMed]
- Ordomsky, V.V.; Sushkevich, V.L.; Schouten, J.C.; van der Schaaf, J.; Nijhuis, T.A. Glucose dehydration to 5-hydroxymethylfurfural over phosphate catalysts. J. Catal. 2013, 300, 37–46. [Google Scholar] [CrossRef]
- Hansen, T.S.; Mielby, J.; Riisager, A. Synergy of boric acid and added salts in the catalytic dehydration of hexoses to 5-hydroxymethylfurfural in water. Green Chem. 2011, 13, 109–114. [Google Scholar] [CrossRef]












| Sample | SBET (m2/g) | Smeso (m2/g) | Vtot (cm3/g) |
|---|---|---|---|
| Nb2O5·nH2O | 366 | 366 | 0.27 |
| Nb2O5_300_air | 209 | 209 | 0.23 |
| Nb2O5_550_air | 27 | 27 | 0.20 |
| Nb2O5_550_N2 | 39 | 39 | 0.13 |
| Nb2O5_SA | 4 | 4 | 0.12 |
| Sample | Nb3d (At%) | O1s (At%) | O/Nb | Atot (mmol/g) |
|---|---|---|---|---|
| Nb2O5·nH2O | 25.76 | 74.24 | 2.88 | 2.35 |
| Nb2O5_300_air | 25.97 | 74.03 | 2.85 | 1.69 |
| Nb2O5_550_air | 26.61 | 73.39 | 2.76 | 0.19 |
| Nb2O5_550_N2 | 26.69 | 73.31 | 2.75 | 0.05 |
| Nb2O5_SA | 26.81 | 73.19 | 2.73 | 0.02 |
| Sample | O(I) (%) | O(II) (%) | O(III) (%) | O(II)/O(I) |
|---|---|---|---|---|
| Nb2O5·nH2O | 83.96 | 10.08 | 5.96 | 0.12 |
| Nb2O5_300_air | 83.26 | 11.33 | 5.41 | 0.14 |
| Nb2O5_550_air | 86.84 | 8.18 | 4.98 | 0.09 |
| Nb2O5_550_N2 | 85.81 | 8.96 | 5.23 | 0.10 |
| Nb2O5_SA | 85.26 | 9.80 | 4.94 | 0.11 |
| Sample | Tmax (°C) | Weak (%) | Tmax (°C) | Medium (%) | Tmax (°C) | Strong (%) |
|---|---|---|---|---|---|---|
| Nb2O5_SA | 0 | 325 | 53 | 410 | 47 | |
| Nb2O5_300_air | 270 | 22 | 315 | 33 | 394 | 45 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morawa Eblagon, K.; Malaika, A.; Ptaszynska, K.; Pereira, M.F.R.; Figueiredo, J.L. Impact of Thermal Treatment of Nb2O5 on Its Performance in Glucose Dehydration to 5-Hydroxymethylfurfural in Water. Nanomaterials 2020, 10, 1685. https://doi.org/10.3390/nano10091685
Morawa Eblagon K, Malaika A, Ptaszynska K, Pereira MFR, Figueiredo JL. Impact of Thermal Treatment of Nb2O5 on Its Performance in Glucose Dehydration to 5-Hydroxymethylfurfural in Water. Nanomaterials. 2020; 10(9):1685. https://doi.org/10.3390/nano10091685
Chicago/Turabian StyleMorawa Eblagon, Katarzyna, Anna Malaika, Karolina Ptaszynska, Manuel Fernando R. Pereira, and José Luís Figueiredo. 2020. "Impact of Thermal Treatment of Nb2O5 on Its Performance in Glucose Dehydration to 5-Hydroxymethylfurfural in Water" Nanomaterials 10, no. 9: 1685. https://doi.org/10.3390/nano10091685
APA StyleMorawa Eblagon, K., Malaika, A., Ptaszynska, K., Pereira, M. F. R., & Figueiredo, J. L. (2020). Impact of Thermal Treatment of Nb2O5 on Its Performance in Glucose Dehydration to 5-Hydroxymethylfurfural in Water. Nanomaterials, 10(9), 1685. https://doi.org/10.3390/nano10091685