DNA Origami Nano-Sheets and Nano-Rods Alter the Orientational Order in a Lyotropic Chromonic Liquid Crystal
Abstract
:1. Introduction
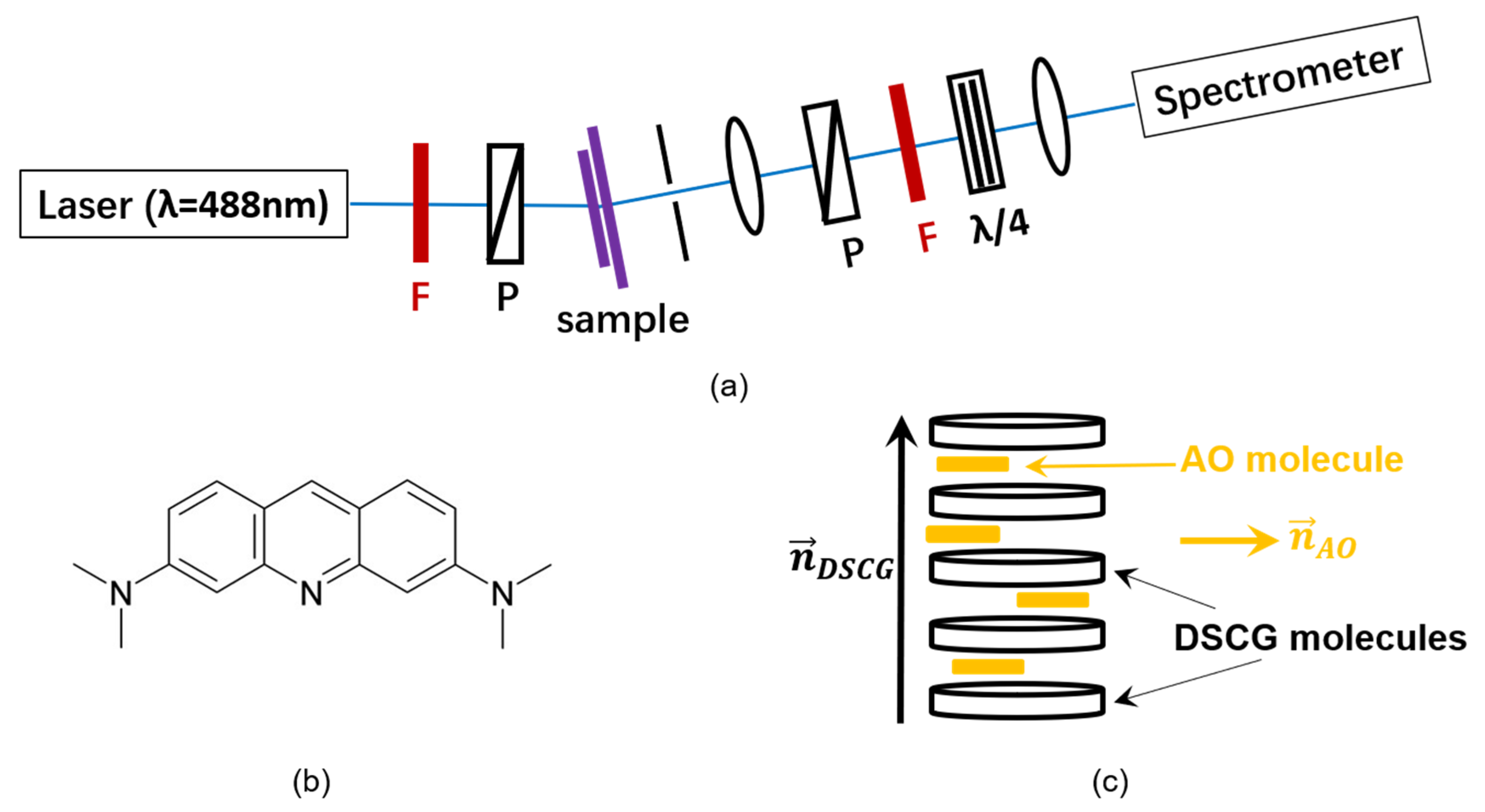
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Experimental Details
Appendix A.1. DNA Origami Synthesis
| Design | No. of Nucleotides of the Scaffold | No. of Staples | Cooling Duration of Folding (h) | Buffer | Concentration of MgCl2 (mM) |
|---|---|---|---|---|---|
| 1LS | 7249 | 184 | 4.8 | TE | 16 |
| 2LS | 8064 | 223 | 19.8 | TE | 16 |
| 24HB | 7560 | 210 | 25.3 | TE | 14 |
| 18HB | 7560 | 196 | 25.3 | TE | 18 |
| 14HB | 8634 | 227 | 24.6 | TE | 18 |
| 6HB | 7249 1 | 170 | 1.5 | TAE | 10 |
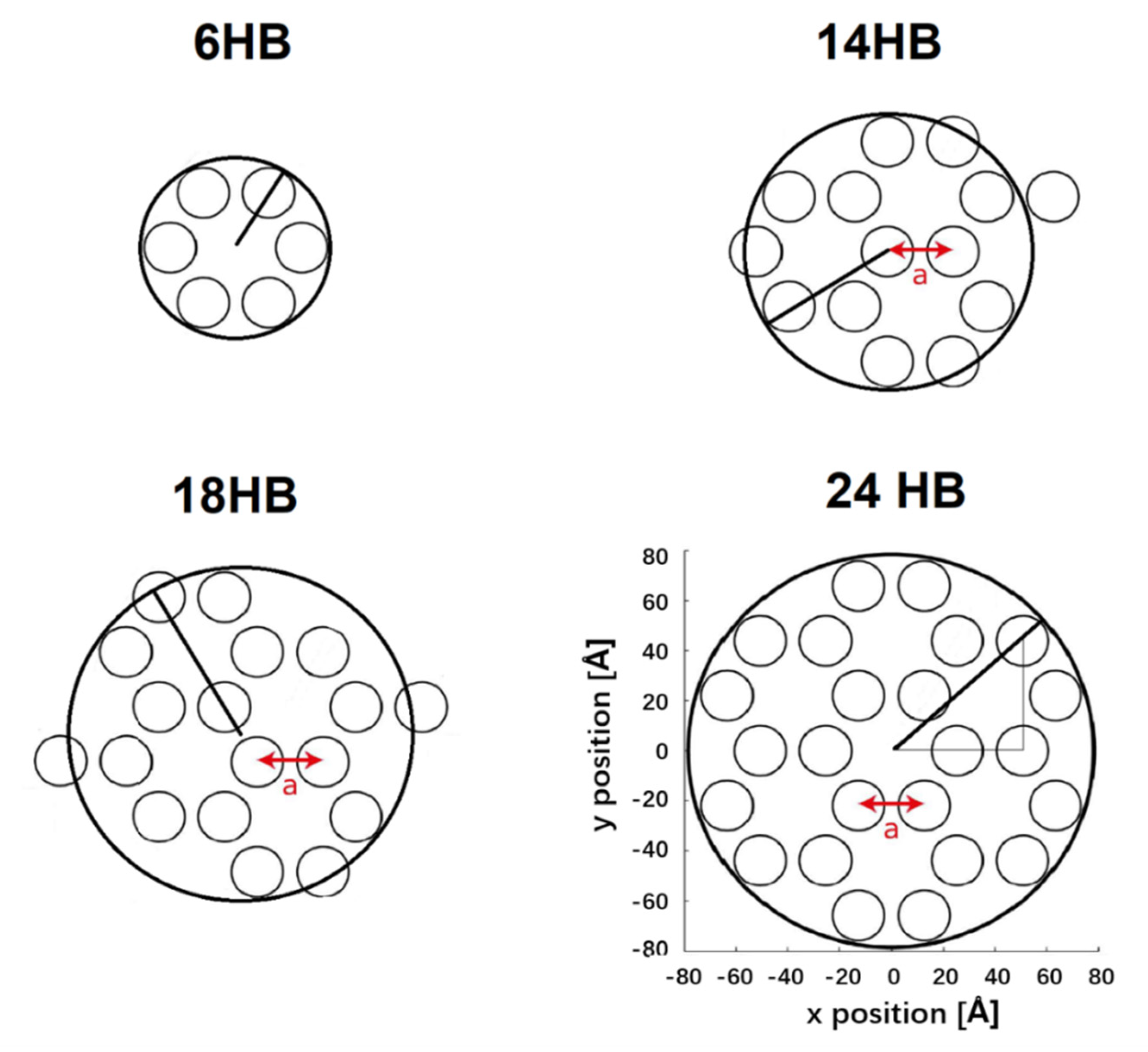
Appendix A.2. Preparation of Liquid Crystal Nanocomposites
| DNA Nanoparticle | Final Concentration of DNA Nanoparticle [nM] | Final Concentration of DSCG [wt.%] | Final Concentration of AO [μM] | Final Concentration of NaCl [M] | Final Concentration of Buffer |
|---|---|---|---|---|---|
| 1LS | 1.81 | 10 | 50 | 0.25 | 1× |
| 2LS | 3.88 | 10 | 50 | 0.25 | 1× |
| 24HB | 8.90 | 10 | 50 | 0.25 | 1× |
| 18HB | 12.00 | 10 | 50 | 0.25 | 1× |
| 14HB | 9.63 | 10 | 50 | 0.25 | 1× |
| 6HB | 12.28 | 10 | 50 | 0.25 | 1× |
Appendix A.3. Preparation of Nanocomposite Test Cells
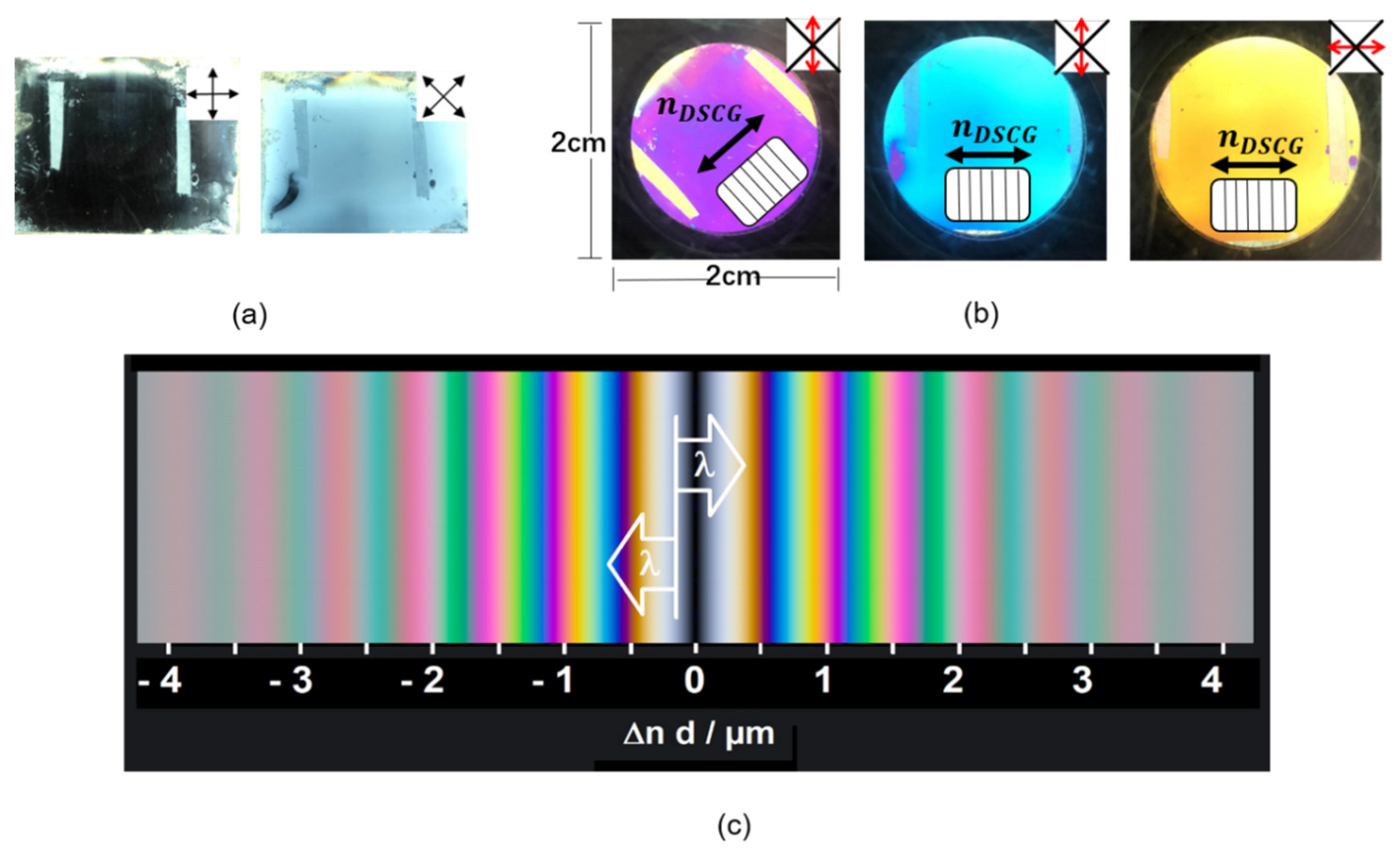
Appendix A.4. Characterization of the Aligned Sample
Appendix A.5. Alignment Study of the Dichroic Intercalating Fluorescent Dye
References
- De Gennes, P.G.; Prost, J. The Physics of Liquid Crystals; Oxford University Press: New York, NY, USA, 1993. [Google Scholar]
- Goodby, J.W.; Collings, P.J.; Kato, T.; Tschierske, C.; Gleeson, H.; Raynes, P. Handbook of Liquid Crystals; Wiley-VCH: Weinheim, Germany, 2014. [Google Scholar]
- Blinov, L.; Chigrinov, V.G. Electro-Optic Effects in Liquid Crystal Materials; Springer: New York, NY, USA, 1993. [Google Scholar]
- Smalyukh, I.I.; Lavrentovich, O.D.; Kuzmin, A.N.; Kachynski, A.V.; Prasad, P.N. Elasticity-mediated self-organization and colloidal interactions of solid spheres with tangential anchoring in a nematic liquid crystal. Phys. Rev. Lett. 2005, 95, 157801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musevic, I.; Skarabot, M.; Tkalec, U.; Ravnik, M.; Žumer, S. Two-dimensional nematic colloidal crystals self-assembled by topological defects. Science 2006, 313, 954–958. [Google Scholar] [CrossRef] [PubMed]
- Musevic, I. Interactions, topology and photonic properties of liquid crystal colloids and dispersions. Eur. Phys. J. Spec. Top. 2019, 227, 2455–2485. [Google Scholar] [CrossRef]
- Van der Schoot, P.; Popa-Nita, V.; Kralj, S. Alignment of carbon nanotubes in nematic liquid crystals. J. Phys. Chem. B 2008, 112, 4512–4518. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Cui, Y.; Gardner, D.; Li, X.; He, S.; Smalyukh, I.I. Self-alignment of plasmonic gold nanorods in reconfigurable anisotropic fluids for tunable bulk metamaterial applications. Nano Lett. 2010, 10, 1347–1353. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yuan, Y.; Smalyukh, I.I. Electrically and optically tunable plasmonic guest-host liquid crystals with long-range ordered nano-particles. Nano Lett. 2014, 14, 4071–4077. [Google Scholar] [CrossRef]
- Muhammed, R.M.; Ravi, K.P.; Surajit, D. Colloidal analogues of polymer chains, ribbons and 2D crystals employing orientations and interactions of nano-rods dispersed in a nematic liquid crystal. Nat. Sci. Rep. 2019, 9, 4652. [Google Scholar]
- Dierking, I.; Al-Zangana, S. Lyotropic liquid crystal phases from anisotropic nanomaterials. Nanomaterials 2017, 7, 305. [Google Scholar] [CrossRef]
- Nakata, M.; Zanchetta, G.; Chapman, B.D.; Jones, C.D.; Cross, J.O.; Pindak, R.; Bellini, T.; Clark, N.A. End-to-end stacking and liquid crystal condensation of 6-to 20-base pair DNA duplexes. Science 2007, 318, 1276–1279. [Google Scholar] [CrossRef]
- Zanchetta, G.; Giavazzi, F.; Nakata, M.; Buscaglia, M.; Cerbino, R.; Clark, N.A.; Bellini, T. Right-handed double-helix ultrashort DNA yields chiral nematic phases with both right- and left-handed director twist. Proc. Natl. Acad. Sci. USA 2010, 107, 17497–17502. [Google Scholar] [CrossRef] [Green Version]
- Bellini, T.; Zanchetta, G.; Fraccia, T.P.; Cerbino, R.; Tsai, E.; Smith, G.P.; Moran, M.J.; Walba, D.M.; Clark, N.A. Liquid crystal self-assembly of random-sequence DNA oligomers. Proc. Natl. Acad. Sci. USA 2012, 109, 1110–1115. [Google Scholar] [CrossRef] [Green Version]
- Fraccia, T.P.; Smith, G.P.; Zanchetta, G.; Paraboschi, E.; Yi, Y.; Walba, D.M.; Dieci, G.; Clark, N.A.; Bellini, T. Abiotic ligation of DNA oligomers templated by their liquid crystal ordering. Nat. Commun. 2015, 6, 6424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, K.; Chen, D.; Marcozzi, A.; Zheng, L.; Su, J.; Pesce, D.; Zajaczkowski, W.; Kolbe, A.; Pisula, W.; Müllen, K.; et al. Thermotropic liquid crystals from biomacromolecules. Proc. Natl. Acad. Sci. USA 2014, 111, 18596–18600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douglas, S.M.; Chou, J.J.; Shih, W.M. DNA-nanotube-induced alignment of membrane proteins for NMR structure determination. Proc. Natl. Acad. Sci. USA 2007, 104, 6644–6648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siavashpouri, M.; Wachauf, C.H.; Zakhary, M.J.; Praetorius, F.; Dietz, H.; Dogic, Z. Molecular engineering of chiral colloidal liquid crystals using DNA origami. Nat. Mater. 2017, 16, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Rothemund, P.W.K. Folding DNA to create nanoscale shapes and patterns. Nature 2006, 440, 297–302. [Google Scholar] [CrossRef] [Green Version]
- Douglas, S.M.; Dietz, H.; Liedl, T.; Högberg, B.; Graf, F.; Shih, W.M. Self-assembly of DNA into nanoscale three-dimensional shapes. Nature 2009, 459, 414–418. [Google Scholar] [CrossRef]
- Kuzuya, A.; Komiyama, M. DNA origami: Fold, stick, and beyond. Nanoscale 2010, 2, 310–322. [Google Scholar] [CrossRef]
- Bui, H.; Onodera, C.; Kidwell, C.; Tan, Y.; Graugnard, E.; Kuang, W.; Lee, J.; Knowlton, W.B.; Yurke, B.; Hughes, W.L. Programmable periodicity of quantum dot arrays with DNA origami nanotubes. Nano Lett. 2010, 10, 3367–3372. [Google Scholar] [CrossRef]
- Tørring, T.; Voigt, N.V.; Nangreave, J.; Yan, H.; Gothelf, K.V. DNA origami: A quantum leap for self-assembly of complex structures. Chem. Soc. Rev. 2011, 40, 5636–5646. [Google Scholar] [CrossRef]
- Douglas, S.M.; Marblestone, A.H.; Teerapittayanon, S.; Vazquez, A.; Church, G.M.; Shih, W.M. Rapid prototyping of 3D DNA-origami shapes with caDNAno. Nucleic Acids Res. 2009, 37, 5001–5006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro, C.E.; Kilchherr, F.; Kim, D.N.; Shiao, E.L.; Wauer, T.; Wortmann, P.; Bathe, M.; Dietz, H. A primer to scaffolded DNA origami. Nat. Methods 2011, 8, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.N.; Kilchherr, F.; Dietz, H.; Bathe, M. Quantitative prediction of 3D solution shape and flexibility of nucleic acid nanostructures. Nucleic Acids Res. 2012, 40, 2862–2868. [Google Scholar] [CrossRef]
- Fischer, S.; Hartl, C.; Frank, K.; Rädler, J.O.; Liedl, T.; Nickel, B. Shape and interhelical spacing of DNA origami nanostructures studied by small-angle X-ray scattering. Nano Lett. 2016, 16, 4282–4287. [Google Scholar] [CrossRef]
- Xia, K.; Shen, J.; Li, Q.; Fan, C.; Gu, H. Near-atomic fabrication with nucleic acids. ACS Nano 2020, 14, 1319–1337. [Google Scholar] [CrossRef]
- Fan, S.; Wang, D.; Kenaan, A.; Cheng, J.; Cui, D.; Song, J. Create nanoscale patterns with DNA origami. Small 2019, 15, 1805554. [Google Scholar] [CrossRef]
- Wang, P.; Meyer, T.A.; Pan, V.; Dutta, P.K.; Ke, Y. The beauty and utility of DNA origami. Chem 2017, 2, 359–382. [Google Scholar] [CrossRef] [Green Version]
- Hong, F.; Zhang, F.; Liu, Y.; Yan, H. DNA origami: Scaffolds for creating higher order structures. Chem. Rev. 2017, 117, 12584–12640. [Google Scholar] [CrossRef]
- Kuzyk, A.; Jungmann, R.; Acuna, G.P.; Liu, N. DNA origami route for nanophotonics. ACS Photonics 2018, 5, 1151–1163. [Google Scholar] [CrossRef] [Green Version]
- Liu, N.; Liedl, T. DNA-assembled advanced plasmonic architectures. Chem. Rev. 2018, 118, 3032–3053. [Google Scholar] [CrossRef]
- Balakrishnan, D.; Wilkens, G.D.; Heddle, J.G. Delivering DNA origami to cells. Nanomedicine 2019, 14, 911–925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, X.; Liu, J.; Wu, X.; Ding, B. Multifunctional DNA origami nanoplatforms for drug delivery. Chem. Asian J. 2019, 14, 2193–2202. [Google Scholar] [CrossRef] [PubMed]
- Heuer-Jungemann, A.; Liedl, T. From DNA tiles to functional DNA materials. Trends Chem. 2019, 1, 799–814. [Google Scholar] [CrossRef]
- Bila, H.; Kurisinkal, E.E.; Bastings, M.M.C. Engineering a stable future for DNA-origami as a biomaterial. Biomater. Sci. 2019, 7, 532–541. [Google Scholar] [CrossRef]
- Ijäs, H.; Nummelin, S.; Shen, B.; Kostiainen, M.A.; Linko, V.L. Dynamic DNA origami devices: From strand-displacement reactions to external-stimuli responsive systems. Int. J. Mol. Sci. 2018, 19, 2114. [Google Scholar] [CrossRef] [Green Version]
- Endo, M.; Sugiyama, H. DNA origami nanomachines. Molecules 2018, 23, 1766. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.; Zhou, C.; Wang, Q. Towards active self-assembly through DNA nanotechnology. Top. Curr. Chem. 2020, 378, 33. [Google Scholar] [CrossRef]
- Lydon, L. Chromonic review. J. Mater. Chem. 2010, 20, 10071–10099. [Google Scholar] [CrossRef]
- Lydon, L. Chromonic liquid crystalline phase. Liq. Cryst. 2011, 38, 11–12. [Google Scholar] [CrossRef]
- Horowitz, V.R.; Janowitz, L.A.; Modic, L.A.; Heiney, P.A.; Collings, P.J. Aggregation behavior and chromonic liquid crystal properties of an anionic monoazo dye. Phys. Rev. E 2005, 72, 041710. [Google Scholar] [CrossRef] [Green Version]
- Nastishin, Y.A.; Liu, H.; Schneider, T.; Nazarenko, V.; Vasyuta, R.; Shiyanovskii, S.V.; Lavrentovich, O.D. Optical characterization of the nematic lyotropic chromonic liquid crystals: Light absorption, birefringence, and scalar order parameter. Phys. Rev. E 2005, 72, 041711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiyanovskii, S.V.; Schneider, T.; Smalyukh, I.I.; Ishikawa, T.; Niehaus, G.D.; Doane, K.J.; Woolverton, C.J.; Lavrentovich, O.D. Real-time microbe detection based on director distortions around growing immune complexes in lyotropic chromonic liquid crystals. Phys. Rev. E 2005, 71, 020702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tam-Chang, S.-W.; Huang, L. Chromonic liquid crystals: Properties and applications as functional materials. Chem. Commun. 2008, 17, 1957–1967. [Google Scholar] [CrossRef] [PubMed]
- Collings, P.J.; Dickinson, A.J.; Smith, E.C. Molecular aggregation and chromonic liquid crystals. Liq. Cryst. 2010, 37, 701–710. [Google Scholar] [CrossRef]
- Agra-Kooijman, D.M.; Singh, G.; Lorenz, A.; Collings, P.J.; Kitzerow, H.-S.; Kumar, S. Columnar molecular aggregation in the aqueous solutions of disodium cromoglycate. Phys. Rev. E 2014, 89, 062504. [Google Scholar] [CrossRef] [Green Version]
- Gao, M.; Kim, Y.-K.; Zhang, C.; Borshch, V.; Zhou, S.; Park, H.-S.; Jákli, A.; Lavrentovich, O.D.; Tamba, M.-G.; Kohlmeier, A.; et al. Direct observation of liquid crystals using cryo-TEM: Specimen preparation and low-dose imaging. Microsc. Res. Tech. 2014, 77, 754–772. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.; Nastishin, Y.A.; Omelchenko, M.M.; Tortora, L.; Nazarenko, V.G.; Boiko, O.P.; Ostapenko, T.; Hu, T.; Almasan, C.C.; Sprunt, S.N.; et al. Elasticity of lyotropic chromonic liquid crystals probed by director reorientation in a magnetic field. Phys. Rev. Lett. 2012, 109, 037801. [Google Scholar] [CrossRef] [Green Version]
- McGinn, C.K.; Laderman, L.I.; Zimmermann, N.; Kitzerow, H.-S.; Collings, P.J. Planar anchoring strength and pitch measurements in achiral and chiral chromonic liquid crystals using 90-degree twist cells. Phys. Rev. E 2013, 88, 062513. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.; Neupane, K.; Nastishin, Y.A.; Baldwin, A.R.; Shiyanovskii, S.V.; Lavrentovich, O.D.; Sprunt, S. Elasticity, viscosity, and orientational fluctuations of a lyotropic chromonic nematic liquid crystal disodium cromoglycate. Soft Matter 2014, 10, 6571–6581. [Google Scholar] [CrossRef]
- Zimmermann, N.; Jünnemann-Held, G.; Collings, P.J.; Kitzerow, H.-S. Self-organized assemblies of colloidal particles obtained from an aligned chromonic liquid crystal dispersion. Soft Matter 2015, 11, 1547–1553. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Kitzerow, H.-S. Influence of proton and salt concentration on the chromonic liquid crystal phase diagram of disodium cromoglycate solutions: Prospects and limitations of a host for DNA nanostructures. J. Phys. Chem. B 2016, 120, 3250–3256. [Google Scholar] [CrossRef]
- Martens, K.; Funck, T.; Kempter, S.; Roller, E.-M.; Liedl, T.; Blaschke, B.M.; Knecht, P.; Garrido, J.A.; Zhang, B.; Kitzerow, H.-S. Alignment and graphene-assisted decoration of lyotropic chromonic liquid crystals containing DNA origami nanostructures. Small 2016, 12, 1658–1666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atorf, A.; Funck, T.; Hegmann, T.; Kempter, S.; Liedl, T.; Martens, H.; Mühlenbernd, H.; Zentgraf, T.; Zhang, B.; Kitzerow, H.; et al. Liquid crystals and precious metal: From nano-particle dispersions to functional plasmonic nanostructures. Liquid Cryst. 2017, 44, 1929–1947. [Google Scholar] [CrossRef]
- Martin, T.G.; Dietz, H. Magnesium-free self-assembly of multi-layer DNA objects. Nat. Commun. 2012, 3, 1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, M.A.B.; Tuckwell, A.J.; Berengut, J.F.; Bath, J.; Benn, F.; Duff, A.P.; Whitten, A.E.; Dunn, K.E.; Hynson, R.M.; Turberfield, A.J.; et al. Dimensions and global twist of single-layer DNA origami measured by small-angle X-ray scattering. ACS Nano 2018, 12, 5791–5799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snodin, B.E.K.; Schreck, J.S.; Romano, F.; Louis, A.A.; Doye, J.P.K. Coarse-grained modelling of the structural properties of DNA origami. Nucleic Acids Res. 2019, 47, 1585–1597. [Google Scholar] [CrossRef] [Green Version]
- Cadnano. Available online: https://cadnano.org (accessed on 10 March 2020).
- CanDo. Available online: https://cando-dna-origami.org (accessed on 10 March 2020).

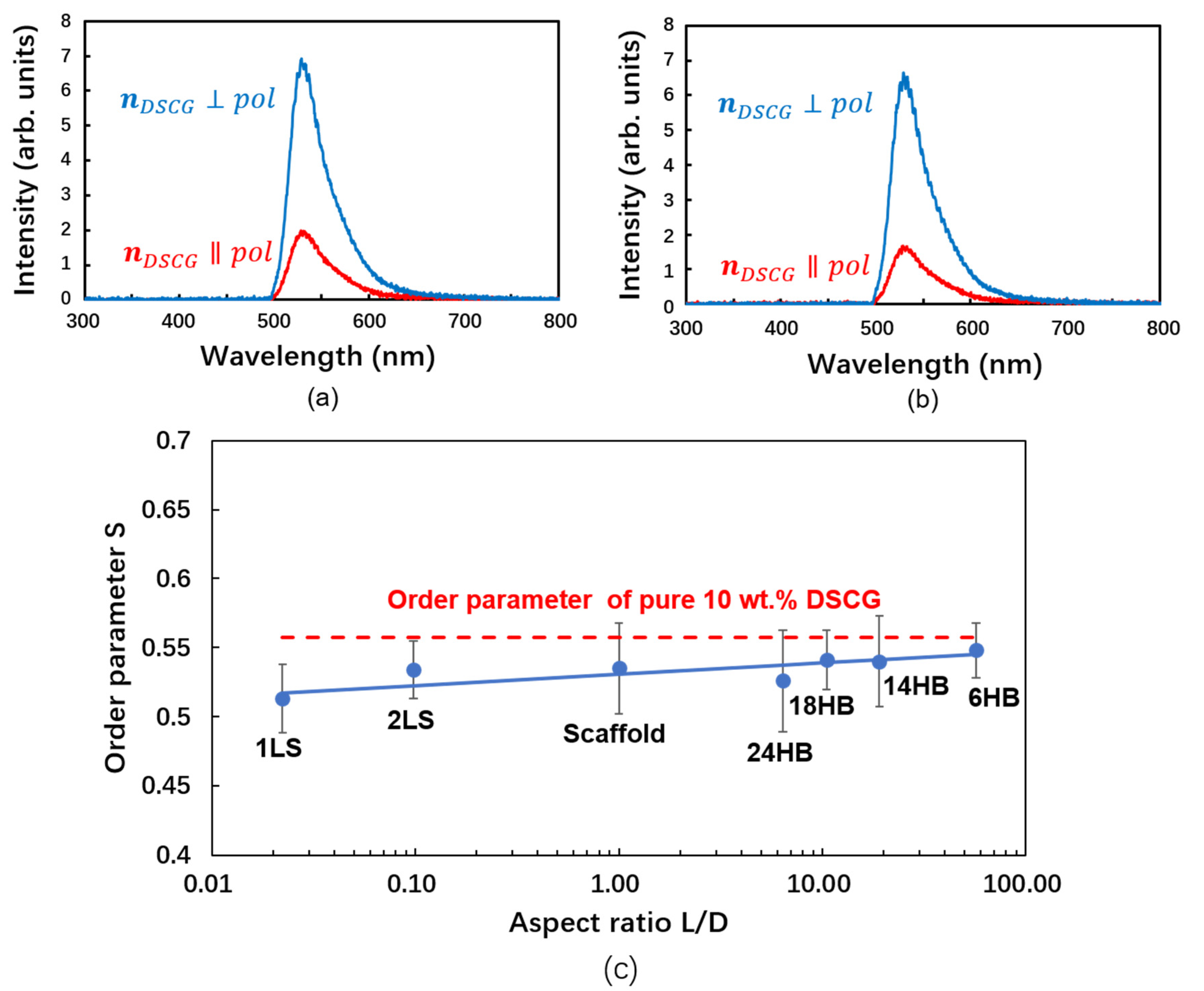
| DNA Origami Design | Lth (nm) | Lexp (nm) | Dth (nm) | Dexp (nm) | Lth/Dth | S |
|---|---|---|---|---|---|---|
| 1LS | 2.00 | 1.99 | 89.92 | 43.88 | 0.02 | 0.51 |
| 2LS | 4.60 | 3.23 | 47.14 | 34.70 | 0.10 | 0.53 |
| 24HB | 107.10 | 105.00 | 16.76 | 4.66 | 6.39 | 0.53 |
| 18HB | 142.80 | 139.78 | 13.60 | 4.46 | 10.50 | 0.54 |
| 14HB | 209.68 | 237.00 | 11.00 | 4.09 | 19.06 | 0.54 |
| 6HB | 410.78 | 405.00 | 7.20 | 1.58 | 57.05 | 0.55 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Martens, K.; Kneer, L.; Funck, T.; Nguyen, L.; Berger, R.; Dass, M.; Kempter, S.; Schmidtke, J.; Liedl, T.; et al. DNA Origami Nano-Sheets and Nano-Rods Alter the Orientational Order in a Lyotropic Chromonic Liquid Crystal. Nanomaterials 2020, 10, 1695. https://doi.org/10.3390/nano10091695
Zhang B, Martens K, Kneer L, Funck T, Nguyen L, Berger R, Dass M, Kempter S, Schmidtke J, Liedl T, et al. DNA Origami Nano-Sheets and Nano-Rods Alter the Orientational Order in a Lyotropic Chromonic Liquid Crystal. Nanomaterials. 2020; 10(9):1695. https://doi.org/10.3390/nano10091695
Chicago/Turabian StyleZhang, Bingru, Kevin Martens, Luisa Kneer, Timon Funck, Linh Nguyen, Ricarda Berger, Mihir Dass, Susanne Kempter, Jürgen Schmidtke, Tim Liedl, and et al. 2020. "DNA Origami Nano-Sheets and Nano-Rods Alter the Orientational Order in a Lyotropic Chromonic Liquid Crystal" Nanomaterials 10, no. 9: 1695. https://doi.org/10.3390/nano10091695






