Manufacturing of Food Packaging Based on Nanocellulose: Current Advances and Challenges
Abstract
:1. Introduction
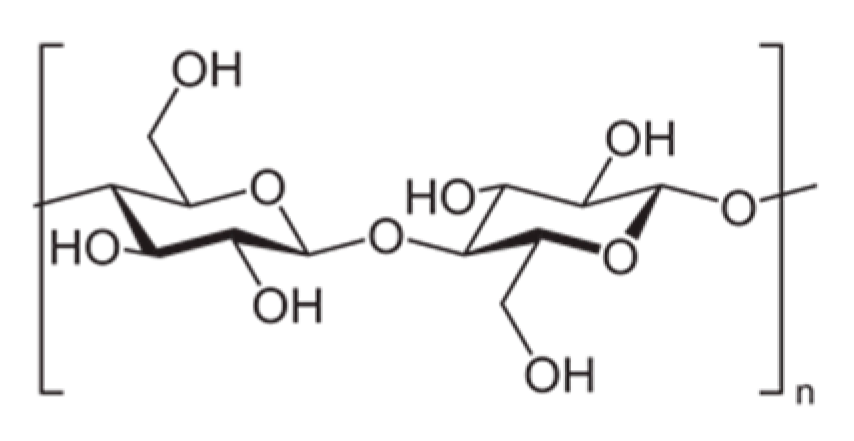
1.1. Cellulose Properties and Capabilities
1.2. Introduction to Nanocellulose
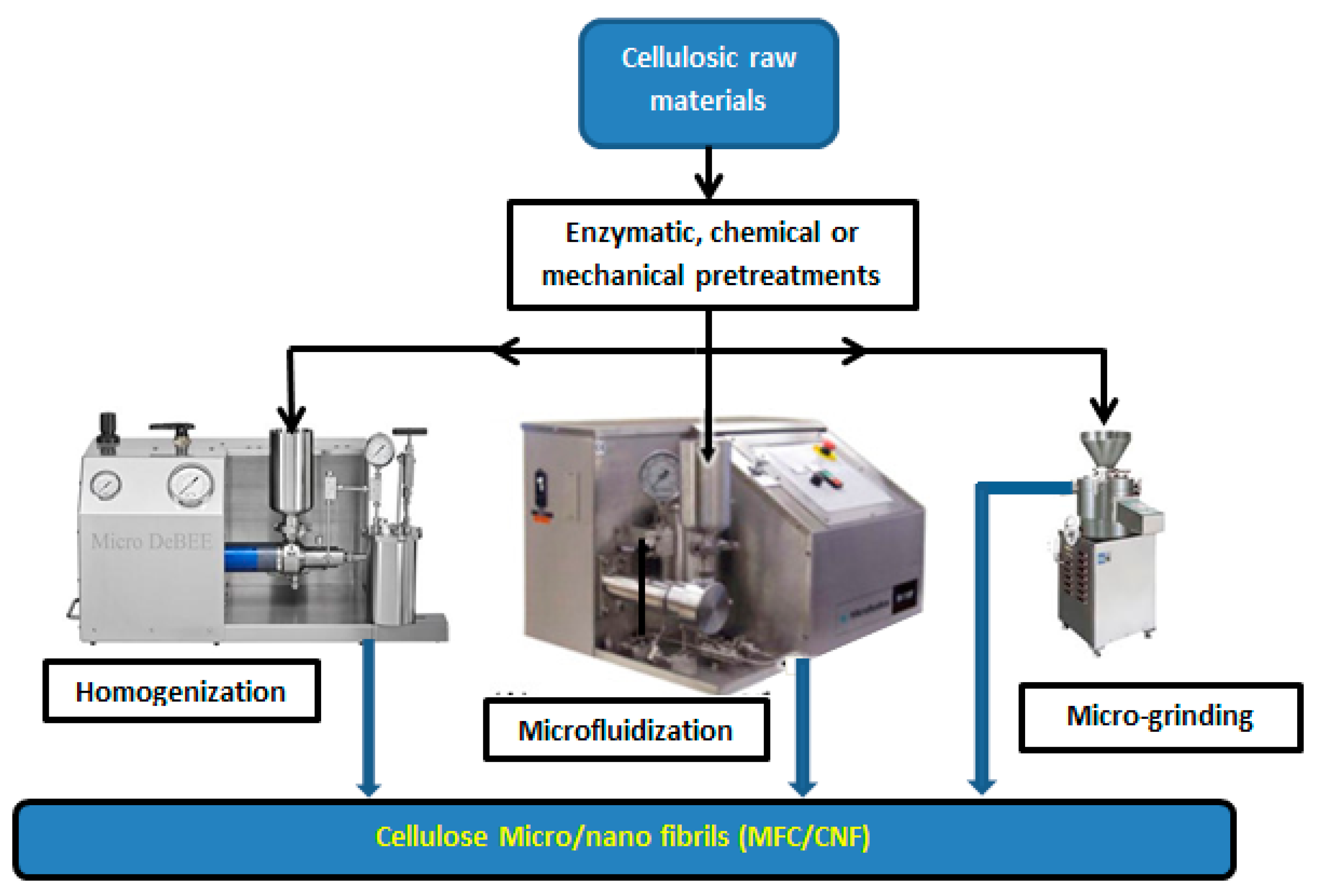
1.3. CNCs and MFC/CNFs Production
2. Chemical Modifications of Nanocellulose
2.1. Nanocellulose Oxidation
2.2. Esterification
2.3. Amidation
2.4. Carbamination
2.5. Grafting “from” and Grafting “onto”
3. Nanocellulose and Food Packaging
3.1. Layer-by-Layer (LbL) Assembly
3.2. Nanocomposite Extrusion
3.3. Electrospinning
3.4. Casting and Evaporation
4. Nanocellulose and Resins Barrier: Properties, Applications, and Market Trends
5. Why Focus on Nanocellulose Coatings?
6. Optimization of NC Coatings for Packaging Applications
6.1. NC Redispersion
6.2. NC Surface Chemistry
6.3. NC Concentration
6.4. Coating Process and Storage of Coated Materials
7. Practical Solutions for the Protection of NC Coatings
8. Lamination of Coated Materials
9. Nanocellulose in Food Packaging Applications: MFC/CNFs or CNCs?
9.1. Yield and Performance
9.2. Regulations about CNCs and MFC/CNFs
9.3. Processability Performance and Production Costs
9.4. Biodegradability and Safety
10. Summary and Conclusions
- (1)
- MFC and CNFs can be regarded as an effective and competitive alternative to packaging including resins barrier like EVOH and PVDC.
- (2)
- Nanocellulose coatings of plastic film/papers and castings can be used for barrier to MOSH and MOAHs, gases, and oil; however, the coating technique seems more practical and sustainable for food packaging manufacturing on an industrial level.
- (3)
- The hydrophobic nanocellulose seems not to be effective in blocking the water vapor in standard conditions (90% and 38 °C).
- (4)
- Until now, the nanocellulose has not yet been made heat sealable; therefore, it must be used in combination with thermoplastic films or sealant layers to create packaging materials.
- (5)
- If the packaging is made for the storage of oil or dry products (aw < 0.4) with a high content of fatty acids food in dry environment, the coating of the nanocellulose may not require any protection.
- (6)
- If the one between the product to be conserved and the storage environment is humid (>40% RH), hydrophobic nanocellulose coatings or neat nanocellulose confined in multilayer structures are needed to preserve the integrity of the NC in humid conditions.
- (7)
- It is possible to implement fully compostable and bio-based multilayer’s packaging incorporating the neat nanocellulose; in addition, the bio-based laminates may include hydrorepellent films such as the PP, PE, or OPP to protect the coatings from the humidity.
- (8)
- Castings and coatings of CNCs are clearer and more transparent than those based on MFC.
- (9)
- On the one hand, certain modified nanocelluloses may not be suitable for food packaging applications if the chemical modification makes the NC less biodegradable/nonbiodegradable or if the modifications are implemented with unhealthy, toxic, or dangerous chemical compounds.
- (10)
- Although the CNCs coating appears more practicable than MFC one, only the latter is currently approved by EFSA and therefore can be used in food contact materials for the European Union packaging market.
Author Contributions
Funding
Conflicts of Interest
References
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. Engl. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, Y. Structure and properties of the cellulose microfibril. J. Wood Sci. 2009, 55, 241–249. [Google Scholar] [CrossRef]
- Stenstad, P.; Andresen, M.; Tanem, B.S.; Stenius, P. Chemical surface modifications of microfibrillated cellulose. Cellulose 2007, 15, 35–45. [Google Scholar] [CrossRef]
- Ach, A. Biodegradable Plastics Based on Cellulose Acetate. J. Macromol. Sci. Part A 1993, 30, 733–740. [Google Scholar] [CrossRef]
- Warth, H.; Mülhaupt, R.; Schätzle, J. Thermoplastic cellulose acetate and cellulose acetate compounds prepared by reactive processing. J. Appl. Polym. Sci. 1997, 64, 231–242. [Google Scholar] [CrossRef]
- Barbiroli, A.; Bonomi, F.; Capretti, G.; Iametti, S.; Manzoni, M.; Piergiovanni, L.; Rollini, M. Antimicrobial activity of lysozyme and lactoferrin incorporated in cellulose-based food packaging. Food Control 2012, 26, 387–392. [Google Scholar] [CrossRef] [Green Version]
- Imran, M.; El-Fahmy, S.; Revol-Junelles, A.-M.; Desobry, S. Cellulose derivative based active coatings: Effects of nisin and plasticizer on physico-chemical and antimicrobial properties of hydroxypropyl methylcellulose films. Carbohydr. Polym. 2010, 81, 219–225. [Google Scholar] [CrossRef]
- Sebti, I.; Chollet, E.; Degraeve, P.; Noel, C.; Peyrol, E. Water Sensitivity, Antimicrobial, and Physicochemical Analyses of Edible Films Based on HPMC and/or Chitosan. J. Agric. Food Chem. 2007, 55, 693–699. [Google Scholar] [CrossRef]
- Petersen, K.; Nielsen, P.V.; Bertelsen, G.; Lawther, M.; Olsen, M.B.; Nilsson, N.H.; Mortensen, G. Potential of biobased materials for food packaging. Trends Food Sci. Technol. 1999, 10, 52–68. [Google Scholar] [CrossRef]
- Del Nobile, M.; Fava, P.; Piergiovanni, L. Water transport properties of cellophane flexible films intended for food packaging applications. J. Food Eng. 2002, 53, 295–300. [Google Scholar] [CrossRef]
- Hamedi, M.M.; Karabulut, E.; Marais, A.; Herland, A.; Nyström, G.; Wågberg, L. Nanocellulose Aerogels Functionalized by Rapid Layer-by-Layer Assembly for High Charge Storage and Beyond. Angew. Chem. Int. Ed. 2013, 52, 12038–12042. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, A.; Pal, L.; Hubbe, M.A. Nanocellulose in packaging: Advances in barrier layer technologies. Ind. Crop. Prod. 2017, 95, 574–582. [Google Scholar] [CrossRef]
- Dufresne, A. Nanocellulose: A new ageless bionanomaterial. Mater. Today 2013, 16, 220–227. [Google Scholar] [CrossRef]
- Gardner, D.J.; Oporto-Velasquez, G.; Mills, R.; Samir, M.A.S.A. Adhesion and Surface Issues in Cellulose and Nanocellulose. J. Adhes. Sci. Technol. 2008, 22, 545–567. [Google Scholar] [CrossRef] [Green Version]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J.P. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef]
- Leung, A.C.W.; Hrapovic, S.; Lam, E.; Male, K.B.; Mahmoud, K.A.; Liu, Y.; Luong, J.H.T. Characteristics and Properties of Carboxylated Cellulose Nanocrystals Prepared from a Novel One-Step Procedure. Small 2010, 7, 302–305. [Google Scholar] [CrossRef] [Green Version]
- Abitbol, T.; Rivkin, A.; Cao, Y.; Nevo, Y.; Abraham, E.; Ben-Shalom, T.; Lapidot, S.; Shoseyov, O. Nanocellulose, a tiny fiber with huge applications. Curr. Opin. Biotechnol. 2016, 39, 76–88. [Google Scholar] [CrossRef]
- Salas, C.; Nypelö, T.; Rodríguez-Abreu, C.; Carrillo, C.; Rojas, O.J. Nanocellulose properties and applications in colloids and interfaces. Curr. Opin. Colloid Interface Sci. 2014, 19, 383–396. [Google Scholar] [CrossRef]
- Yu, H.-Y.; Qin, Z.; Liang, B.; Liu, N.; Zhou, Z.; Chen, L. Facile extraction of thermally stable cellulose nanocrystals with a high yield of 93% through hydrochloric acid hydrolysis under hydrothermal conditions. J. Mater. Chem. A 2013, 1, 3938. [Google Scholar] [CrossRef]
- Montanari, S.; Roumani, M.; Heux, L.; Vignon, M.R. Topochemistry of Carboxylated Cellulose Nanocrystals Resulting from TEMPO-Mediated Oxidation. Macromolecules 2005, 38, 1665–1671. [Google Scholar] [CrossRef]
- Nechyporchuk, O.; Belgacem, M.N.; Bras, J. Production of cellulose nanofibrils: A review of recent advances. Ind. Crop. Prod. 2016, 93, 2–25. [Google Scholar] [CrossRef]
- Henriksson, G.; Berglund, L.; Lindström, T.; Henriksson, M. An environmentally friendly method for enzyme-assisted preparation of microfibrillated cellulose (MFC) nanofibers. Eur. Polym. J. 2007, 43, 3434–3441. [Google Scholar] [CrossRef]
- Pääkkö, M.; Ankerfors, M.; Kosonen, H.; Nykänen, A.; Ahola, S.; Österberg, M.; Ruokolainen, J.; Laine, J.; Larsson, P.T.; Ikkala, O.; et al. Enzymatic Hydrolysis Combined with Mechanical Shearing and High-Pressure Homogenization for Nanoscale Cellulose Fibrils and Strong Gels. Biomacromolecules 2007, 8, 1934–1941. [Google Scholar] [CrossRef] [PubMed]
- Josset, S.; Orsolini, P.; Siqueira, G.; Tejado, A.; Tingaut, P.; Zimmermann, T. Energy consumption of the nanofibrillation of bleached pulp, wheat straw and recycled newspaper through a grinding process. Nord. Pulp Pap. Res. J. 2014, 29, 167–175. [Google Scholar] [CrossRef] [Green Version]
- Fujisawa, S.; Okita, Y.; Fukuzumi, H.; Saito, T.; Isogai, A. Preparation and characterization of TEMPO-oxidized cellulose nanofibril films with free carboxyl groups. Carbohydr. Polym. 2011, 84, 579–583. [Google Scholar] [CrossRef]
- Filipova, I.; Fridrihsone, V.; Cabulis, U.; Bērziņš, A. Synthesis of Nanofibrillated Cellulose by Combined Ammonium Persulphate Treatment with Ultrasound and Mechanical Processing. Nanomaterials 2018, 8, 640. [Google Scholar] [CrossRef] [Green Version]
- Lavoine, N.; Bergström, L. Nanocellulose-based foams and aerogels: Processing, properties, and applications. J. Mater. Chem. A 2017, 5, 16105–16117. [Google Scholar] [CrossRef] [Green Version]
- Pedrosa, P.; Pomposo, J.A.; Calahorra, E.; Cortazar, M. On the glass transition behavior, interaction energies, and hydrogen-bonding strengths of binary poly (p-vinylphenol)/polyether blends. Macromolecules 1994, 27, 102–109. [Google Scholar] [CrossRef]
- Labuschagne, P.; Germishuizen, W.A.; Verryn, S.M.C.; Moolman, S. Improved oxygen barrier performance of poly(vinyl alcohol) films through hydrogen bond complex with poly(methyl vinyl ether-co-maleic acid). Eur. Polym. J. 2008, 44, 2146–2152. [Google Scholar] [CrossRef]
- Anirudhan, T.; Rejeena, S. Adsorption and hydrolytic activity of trypsin on a carboxylate-functionalized cation exchanger prepared from nanocellulose. J. Colloid Interface Sci. 2012, 381, 125–136. [Google Scholar] [CrossRef]
- Almasi, H.; Ghanbarzadeh, B.; Dehghannya, J.; Entezami, A.A.; Asl, A.K. Novel nanocomposites based on fatty acid modified cellulose nanofibers/poly(lactic acid): Morphological and physical properties. Food Packag. Shelf Life 2015, 5, 21–31. [Google Scholar] [CrossRef]
- Dehnad, D.; Mirzaei, H.; Emam-Djomeh, Z.; Jafari, S.M.; Dadashi, S. Thermal and antimicrobial properties of chitosan–nanocellulose films for extending shelf life of ground meat. Carbohydr. Polym. 2014, 109, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Habibi, Y.; Chanzy, H.; Vignon, M. TEMPO-mediated surface oxidation of cellulose whiskers. Cellulose 2006, 13, 679–687. [Google Scholar] [CrossRef] [Green Version]
- Sun, B.; Hou, Q.; Liu, Z.; Ni, Y. Sodium periodate oxidation of cellulose nanocrystal and its application as a paper wet strength additive. Cellulose 2015, 22, 1135–1146. [Google Scholar] [CrossRef]
- Braun, B.; Dorgan, J.R. Single-Step Method for the Isolation and Surface Functionalization of Cellulosic Nanowhiskers. Biomacromolecules 2009, 10, 334–341. [Google Scholar] [CrossRef]
- De Castro, D.O.; Bras, J.; Gandini, A.; Belgacem, N.; Belgacem, M.N. Surface grafting of cellulose nanocrystals with natural antimicrobial rosin mixture using a green process. Carbohydr. Polym. 2016, 137, 1–8. [Google Scholar] [CrossRef]
- Ramírez, J.A.Á.; Fortunati, E.; Kenny, J.M.; Torre, L.; Foresti, M.L. Simple citric acid-catalyzed surface esterification of cellulose nanocrystals. Carbohydr. Polym. 2017, 157, 1358–1364. [Google Scholar] [CrossRef]
- Fotie, G.; Gazzotti, S.; Ortenzi, M.A.; Piergiovanni, L. Implementation of High Gas Barrier Laminated Films Based on Cellulose Nanocrystals for Food Flexible Packaging. Appl. Sci. 2020, 10, 3201. [Google Scholar] [CrossRef]
- Sehaqui, H.; Kulasinski, K.; Pfenninger, N.; Zimmermann, T.; Tingaut, P. Highly Carboxylated Cellulose Nanofibers via Succinic Anhydride Esterification of Wheat Fibers and Facile Mechanical Disintegration. Biomacromolecules 2016, 18, 242–248. [Google Scholar] [CrossRef]
- Eyley, S.; Thielemans, W. Surface modification of cellulose nanocrystals. Nanoscale 2014, 6, 7764–7779. [Google Scholar] [CrossRef] [Green Version]
- Araki, J.; Wada, M.; Kuga, S. Steric Stabilization of a Cellulose Microcrystal Suspension by Poly(ethylene glycol) Grafting. Langmuir 2001, 17, 21–27. [Google Scholar] [CrossRef]
- Navarro, J.; Bergström, L. Labelling of N-hydroxysuccinimide-modified Rhodamine B on cellulose nanofibrils by the amidation reaction. RSC Adv. 2014, 4, 60757–60761. [Google Scholar] [CrossRef]
- Chen, Q.; Zhou, L.-L.; Zou, J.-Q.; Gao, X. The preparation and characterization of nanocomposite film reinforced by modified cellulose nanocrystals. Int. J. Boil. Macromol. 2019, 132, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Dufresne, A.; Belgacem, M.N. Cellulose-reinforced composites: From micro-to nanoscale. Polímeros 2010, 20, 1–10. [Google Scholar] [CrossRef]
- Siqueira, G.; Bras, J.; Dufresne, A. New Process of Chemical Grafting of Cellulose Nanoparticles with a Long Chain Isocyanate. Langmuir 2010, 26, 402–411. [Google Scholar] [CrossRef]
- Ljungberg, N.; Bonini, C.; Bortolussi, F.; Boisson, C.; Heux, L.; Cavaillé, J.-Y. New Nanocomposite Materials Reinforced with Cellulose Whiskers in Atactic Polypropylene: Effect of Surface and Dispersion Characteristics. Biomacromolecules 2005, 6, 2732–2739. [Google Scholar] [CrossRef]
- Habibi, Y.; Goffin, A.-L.; Schiltz, N.; Duquesne, E.; Dubois, P.; Dufresne, A. Bionanocomposites based on poly(ε-caprolactone)-grafted cellulose nanocrystals by ring-opening polymerization. J. Mater. Chem. 2008, 18, 5002–5010. [Google Scholar] [CrossRef]
- Anirudhan, T.; Rejeena, S. Poly(acrylic acid)-modified poly(glycidylmethacrylate)-grafted nanocellulose as matrices for the adsorption of lysozyme from aqueous solutions. Chem. Eng. J. 2012, 187, 150–159. [Google Scholar] [CrossRef]
- Ankerfors, M.; Lindström, T.; Nordmark, G.G. Multilayer assembly onto pulp fibres using oppositely charged microfibrillated celluloses, starches, and wetstrength resins—Effect on mechanical properties of CTMP-sheets. Nord. Pulp Pap. Res. J. 2016, 31, 135–141. [Google Scholar] [CrossRef]
- Aulin, C.; Karabulut, E.; Tran, A.; Wågberg, L.; Lindström, T. Transparent Nanocellulosic Multilayer Thin Films on Polylactic Acid with Tunable Gas Barrier Properties. ACS Appl. Mater. Interfaces 2013, 5, 7352–7359. [Google Scholar] [CrossRef]
- Martin, C.; Jean, B. Nanocellulose/polymer multilayered thin films: Tunable architectures towards tailored physical properties. Nord. Pulp Pap. Res. J. 2014, 29, 19–30. [Google Scholar] [CrossRef]
- Shariki, S.; Liew, S.Y.; Thielemans, W.; Walsh, D.A.; Cummings, C.Y.; Rassaei, L.; Wasbrough, M.J.; Edler, K.J.; Bonné, M.J.; Marken, F. Tuning percolation speed in layer-by-layer assembled polyaniline–nanocellulose composite films. J. Solid State Electrochem. 2010, 15, 2675–2681. [Google Scholar] [CrossRef]
- Li, F.; Mascheroni, E.; Piergiovanni, L. The Potential of NanoCellulose in the Packaging Field: A Review. Packag. Technol. Sci. 2015, 28, 475–508. [Google Scholar] [CrossRef]
- Marais, A.; Utsel, S.; Gustafsson, E.; Wågberg, L. Towards a super-strainable paper using the Layer-by-Layer technique. Carbohydr. Polym. 2014, 100, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Foster, E.J.; Moon, R.J.; Agarwal, U.P.; Bortner, M.J.; Bras, J.; Camarero-Espinosa, S.; Chan, K.J.; Clift, M.J.D.; Cranston, E.D.; Eichhorn, S.J.; et al. Current characterization methods for cellulose nanomaterials. Chem. Soc. Rev. 2018, 47, 2609–2679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, C.; Hamad, W.Y. Cellulose reinforced polymer composites and nanocomposites: A critical review. Cellulose 2013, 20, 2221–2262. [Google Scholar] [CrossRef]
- Kian, L.; Saba, N.; Jawaid, M.; Sultan, M. A review on processing techniques of bast fibers nanocellulose and its polylactic acid (PLA) nanocomposites. Int. J. Boil. Macromol. 2019, 121, 1314–1328. [Google Scholar] [CrossRef]
- Wu, C.-N.; Saito, T.; Fujisawa, S.; Fukuzumi, H.; Isogai, A. Ultrastrong and High Gas-Barrier Nanocellulose/Clay-Layered Composites. Biomacromolecules 2012, 13, 1927–1932. [Google Scholar] [CrossRef]
- De Menezes, A.J.; Siqueira, G.; Curvelo, A.A.; Dufresne, A. Extrusion and characterization of functionalized cellulose whiskers reinforced polyethylene nanocomposites. Polymyer 2009, 50, 4552–4563. [Google Scholar] [CrossRef]
- Frenot, A.; Henriksson, M.W.; Walkenström, P. Electrospinning of cellulose-based nanofibers. J. Appl. Polym. Sci. 2006, 103, 1473–1482. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Park, C.W.; Youe, W.J.; Han, S.Y.; Kim, Y.S.; Lee, S.H. Characteristics of carbon nanofibers produced from lignin/polyacrylonitrile (PAN)/kraft lignin-g-PAN copolymer blends electrospun nanofibers. Holzforschung 2017, 71, 743–750. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Mariano, M.; Huang, J.; Lin, N.; Ahmad, I.; Dufresne, A.; Thomas, S. Recent developments on nanocellulose reinforced polymer nanocomposites: A review. Polymer 2017, 132, 368–393. [Google Scholar] [CrossRef]
- Fortunato, G.; Zimmermann, T.; Lübben, J.; Bordeanu, N.; Hufenus, R. Reinforcement of Polymeric Submicrometer-sized Fibers by Microfibrillated Cellulose. Macromol. Mater. Eng. 2012, 297, 576–584. [Google Scholar] [CrossRef]
- Cherpinski, A.; Torres-Giner, S.; Vartiainen, J.; Peresin, M.S.; Lahtinen, P.; Lagaron, J.M. Improving the water resistance of nanocellulose-based films with polyhydroxyalkanoates processed by the electrospinning coating technique. Cellulose 2018, 25, 1291–1307. [Google Scholar] [CrossRef]
- Martínez-Sanz, M.; Lopez-Rubio, A.; Villano, M.; Oliveira, C.; Majone, M.; Reis, M.A.; Lagaron, J.M. Production of bacterial nanobiocomposites of polyhydroxyalkanoates derived from waste and bacterial nanocellulose by the electrospinning enabling melt compounding method. J. Appl. Polym. Sci. 2015, 133. [Google Scholar] [CrossRef]
- Sanders, J.E.; Han, Y.; Rushing, T.S.; Gardner, D.J. Electrospinning of Cellulose Nanocrystal-Filled Poly (Vinyl Alcohol) Solutions: Material Property Assessment. Nanomaterials 2019, 9, 805. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Chen, Y.; Chang, P.R.; Muir, A.D.; Falk, G. Starch-based nanocomposites reinforced with flax cellulose nanocrystals. Express Polym. Lett. 2008, 2, 502–510. [Google Scholar] [CrossRef]
- Wang, L.; Chen, C.; Wang, J.; Gardner, D.J.; Tajvidi, M. Cellulose nanofibrils versus cellulose nanocrystals: Comparison of performance in flexible multilayer films for packaging applications. Food Packag. Shelf Life 2020, 23, 100464. [Google Scholar] [CrossRef]
- Nelson, K.; Retsina, T.; Iakovlev, M.; Van Heiningen, A.; Deng, Y.; Shatkin, J.A.; Mulyadi, A. American Process: Production of Low Cost Nanocellulose for Renewable, Advanced Materials Applications. In Superconductivity; Springer Science and Business Media LLC: Berlin, Germany, 2016; pp. 267–302. [Google Scholar]
- Muramatsu, M.; Okura, M.; Kuboyama, K.; Ougizawa, T.; Yamamoto, T.; Nishihara, Y.; Saito, Y.; Ito, K.; Hirata, K.; Kobayashi, Y. Oxygen permeability and free volume hole size in ethylene–vinyl alcohol copolymer film: Temperature and humidity dependence. Radiat. Phys. Chem. 2003, 68, 561–564. [Google Scholar] [CrossRef]
- Fotie, G.; Amoroso, L.; Muratore, G.; Piergiovanni, L. Carbon dioxide diffusion at different relative humidity through coating of cellulose nanocrystals for food packaging applications. Food Packag. Shelf Life 2018, 18, 62–70. [Google Scholar] [CrossRef]
- Blanchard, A.; Gouanvé, F.; Espuche, E. Effect of humidity on mechanical, thermal and barrier properties of EVOH films. J. Membr. Sci. 2017, 540, 1–9. [Google Scholar] [CrossRef]
- Fotie, G.; Rampazzo, R.; Ortenzi, M.A.; Checchia, S.; Fessas, D.; Piergiovanni, L. The Effect of Moisture on Cellulose Nanocrystals Intended as a High Gas Barrier Coating on Flexible Packaging Materials. Polymers 2017, 9, 415. [Google Scholar] [CrossRef] [PubMed]
- Maes, C.; Junior, G.M.Y.; Teniers, C.; Luyten, W.; Herremans, G.; Peeters, R.; Samyn, P.; Carleer, R.; Buntinx, M. Ethylene Vinyl Alcohol Copolymer (EVOH) as a Functional Barrier against Surrogate Components Migrating from Paperboard. J. Chem. 2019, 2019, 1–7. [Google Scholar] [CrossRef]
- Armstrong, R.B. New EVAL® EVOH Resins for Flexible Packaging. In Proceedings of the EVAL Americas, 2004 PLACE Conference Proceeeding, Atlanta, GA, USA, 3–6 December 2004; pp. 1–12. [Google Scholar]
- Hubbe, M.A.; Ferrer, A.; Tyagi, P.; Yin, Y.; Salas, C.; Pal, L.; Rojas, O.J. Nanocellulose in Thin Films, Coatings, and Plies for Packaging Applications: A Review. BioResources 2017, 12, 2143–2233. [Google Scholar] [CrossRef] [Green Version]
- Mascheroni, E.; Rampazzo, R.; Ortenzi, M.A.; Piva, G.; Bonetti, S.; Piergiovanni, L. Comparison of cellulose nanocrystals obtained by sulfuric acid hydrolysis and ammonium persulfate, to be used as coating on flexible food-packaging materials. Cellulose 2016, 23, 779–793. [Google Scholar] [CrossRef] [Green Version]
- Missio, A.L.; Mattos, B.D.; Ferreira, D.D.F.; Magalhães, W.L.; Bertuol, D.A.; Gatto, D.A.; Petutschnigg, A.; Tondi, G. Nanocellulose-tannin films: From trees to sustainable active packaging. J. Clean. Prod. 2018, 184, 143–151. [Google Scholar] [CrossRef]
- El-Wakil, N.A.; Hassan, M.L.; Abou-Zeid, R.E.; Dufresne, A. Development of wheat gluten/nanocellulose/titanium dioxide nanocomposites for active food packaging. Carbohydr. Polym. 2015, 124, 337–346. [Google Scholar] [CrossRef]
- Lavoine, N.; Desloges, I.; Bras, J. Microfibrillated cellulose coatings as new release systems for active packaging. Carbohydr. Polym. 2014, 103, 528–537. [Google Scholar] [CrossRef]
- Lu, P.; Guo, M.; Xu, Z.; Wu, M. Application of Nanofibrillated Cellulose on BOPP/LDPE Film as Oxygen Barrier and Antimicrobial Coating Based on Cold Plasma Treatment. Coatings 2018, 8, 207. [Google Scholar] [CrossRef] [Green Version]
- Jiang, F.; Hsieh, Y.-L. Chemically and mechanically isolated nanocellulose and their self-assembled structures. Carbohydr. Polym. 2013, 95, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Beck, S.; Bouchard, J.; Berry, R. Dispersibility in Water of Dried Nanocrystalline Cellulose. Biomacromolecules 2012, 13, 1486–1494. [Google Scholar] [CrossRef] [PubMed]
- Cherhal, F.; Cousin, F.; Capron, I. Influence of Charge Density and Ionic Strength on the Aggregation Process of Cellulose Nanocrystals in Aqueous Suspension, as Revealed by Small-Angle Neutron Scattering. Langmuir 2015, 31, 5596–5602. [Google Scholar] [CrossRef] [PubMed]
- Eyholzer, C.; Bordeanu, N.; López-Suevos, F.; Rentsch, D.; Zimmermann, T.; Oksman, K. Preparation and characterization of water-redispersible nanofibrillated cellulose in powder form. Cellulose 2009, 17, 19–30. [Google Scholar] [CrossRef] [Green Version]
- Missoum, K.; Bras, J.; Belgacem, M.N. Water Redispersible Dried Nanofibrillated Cellulose by Adding Sodium Chloride. Biomacromolecules 2012, 13, 4118–4125. [Google Scholar] [CrossRef]
- Cranston, E.D.; Gray, D.G. Morphological and Optical Characterization of Polyelectrolyte Multilayers Incorporating Nanocrystalline Cellulose. Biomacromolecules 2006, 7, 2522–2530. [Google Scholar] [CrossRef]
- Kan, K.H.M.; Li, J.; Wijesekera, K.; Cranston, E.D. Polymer-Grafted Cellulose Nanocrystals as pH-Responsive Reversible Flocculants. Biomacromolecules 2013, 14, 3130–3139. [Google Scholar] [CrossRef]
- Karabulut, E.; Wågberg, L. Design and characterization of cellulose nanofibril-based freestanding films prepared by layer-by-layer deposition technique. Soft Matter 2011, 7, 3467. [Google Scholar] [CrossRef]
- Lavoine, N.; Desloges, I.; Sillard, C.; Bras, J. Controlled release and long-term antibacterial activity of chlorhexidine digluconate through the nanoporous network of microfibrillated cellulose. Cellulose 2014, 21, 4429–4442. [Google Scholar] [CrossRef]
- Richmond, F. Cellulose Nanofibers Use in Coated Paper. Ph.D. Thesis, The University of Maine, Orono, ME, USA, 2014. [Google Scholar]
- Koppolu, R.; Abitbol, T.; Kumar, V.; Jaiswal, A.K.; Swerin, A.; Toivakka, M. Continuous roll-to-roll coating of cellulose nanocrystals onto paperboard. Cellulose 2018, 25, 6055–6069. [Google Scholar] [CrossRef]
- Kimpimäki, T.; Lahtinen, K.; Avellan, J. Dispersion coating. In Paper and Paperboard Converting; Kuusipalo, J., Ed.; Finnish Paper Engineers’ Association: Helsinki, Finland, 2008; Volume 58, p. 105. [Google Scholar]
- Yan, Y.; Amer, H.; Rosenau, T.; Zollfrank, C.; Dörrstein, J.; Jobst, C.; Zimmermann, T.; Keckes, J.; Veigel, S.; Gindl-Altmutter, W.; et al. Dry, hydrophobic microfibrillated cellulose powder obtained in a simple procedure using alkyl ketene dimer. Cellulose 2016, 23, 1189–1197. [Google Scholar] [CrossRef] [Green Version]
- Österberg, M.; Vartiainen, J.; Lucenius, J.; Hippi, U.; Seppälä, J.; Serimaa, R.; Laine, J. A Fast Method to Produce Strong NFC Films as a Platform for Barrier and Functional Materials. ACS Appl. Mater. Interfaces 2013, 5, 4640–4647. [Google Scholar] [CrossRef] [PubMed]
- Rodionova, G.; Lenes, M.; Eriksen, Ø.; Gregersen, Ø. Surface chemical modification of microfibrillated cellulose: Improvement of barrier properties for packaging applications. Cellulose 2010, 18, 127–134. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, J.-J.; Han, C.-R.; Duan, J.-F.; Xu, F.; Sun, R.-C. Tough nanocomposite hydrogels from cellulose nanocrystals/poly(acrylamide) clusters: Influence of the charge density, aspect ratio and surface coating with PEG. Cellulose 2013, 21, 541–551. [Google Scholar] [CrossRef]
- Song, Z.; Xiao, H.; Zhao, Y. Hydrophobic-modified nano-cellulose fiber/PLA biodegradable composites for lowering water vapor transmission rate (WVTR) of paper. Carbohydr. Polym. 2014, 111, 442–448. [Google Scholar] [CrossRef]
- Korhonen, J.T.; Kettunen, M.; Ras, R.H.A.; Ikkala, O. Hydrophobic Nanocellulose Aerogels as Floating, Sustainable, Reusable, and Recyclable Oil Absorbents. ACS Appl. Mater. Interfaces 2011, 3, 1813–1816. [Google Scholar] [CrossRef]
- Kisonen, V.; Prakobna, K.; Xu, C.; Salminen, A.; Mikkonen, K.S.; Valtakari, D.; Eklund, P.; Seppälä, J.; Tenkanen, M.; Willför, S.M. Composite films of nanofibrillated cellulose and O-acetyl galactoglucomannan (GGM) coated with succinic esters of GGM showing potential as barrier material in food packaging. J. Mater. Sci. 2015, 50, 3189–3199. [Google Scholar] [CrossRef]
- Peng, Y.; Gardner, D.J.; Han, Y.; Kiziltas, A.; Cai, Z.; Tshabalala, M.A. Influence of drying method on the material properties of nanocellulose I: Thermostability and crystallinity. Cellulose 2013, 20, 2379–2392. [Google Scholar] [CrossRef]
- Oever, M.V.D.; Molenveld, K.; Van Der Zee, M.; Bos, H.; Products, B.; FBR Sustainable Chemistry & Technology. Bio-Based and Biodegradable Plastics: Facts and Figures: Focus on Food Packaging in the Netherlands; Wageningen Food & Biobased Research: Wageningen, The Netherlands, 2017. [Google Scholar]
- Fotie, G.; Amoroso, L.; Limbo, S.; Muratore, G.; Piergiovanni, L. Food life extension by cellulose nanocrystals coatings. Ital. J. Food Sci. 2019, 27, 8–14. [Google Scholar]
- Matikainen, L. Nanocellulose as barrier coating deposited using a laboratory rod coater. Master’s Thesis, Aalto University, Espoo, Finland, 2017; pp. 26–34. [Google Scholar]
- Tang, Y.; Shen, X.; Zhang, J.; Guo, D.; Kong, F.; Zhang, N. Extraction of cellulose nano-crystals from old corrugated container fiber using phosphoric acid and enzymatic hydrolysis followed by sonication. Carbohydr. Polym. 2015, 125, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Liu, C.; Mu, X.; Gong, W.; Lv, D.; Hong, Y.; Si, C.; Li, B. Preparation and characterization of thermally stable cellulose nanocrystals via a sustainable approach of FeCl3-catalyzed formic acid hydrolysis. Cellulose 2016, 23, 2389–2407. [Google Scholar] [CrossRef]
- Boujemaoui, A.; Mongkhontreerat, S.; Malmström, E.E.; Carlmark, A. Preparation and characterization of functionalized cellulose nanocrystals. Carbohydr. Polym. 2015, 115, 457–464. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, J.; Baez, C.; Kitin, P.; Elder, T. Highly thermal-stable and functional cellulose nanocrystals and nanofibrils produced using fully recyclable organic acids. Green Chem. 2016, 18, 3835–3843. [Google Scholar] [CrossRef]
- Qing, Y.; Sabo, R.; Zhu, J.; Agarwal, U.; Cai, Z.; Wua, Y. A comparative study of cellulose nanofibrils disintegrated via multiple processing approaches. Carbohydr. Polym. 2013, 97, 226–234. [Google Scholar] [CrossRef]
- Brodin, F.W.; Gregersen, Ø.K.; Syverud, K. Cellulose nanofibrils: Challenges and possibilities as a paper additive or coating material—A review. Nord. Pulp Pap. Res. J. 2014, 29, 156–166. [Google Scholar] [CrossRef]
- Aulin, C.; Gällstedt, M.; Lindström, T. Oxygen and oil barrier properties of microfibrillated cellulose films and coatings. Cellulose 2010, 17, 559–574. [Google Scholar] [CrossRef]
- European Commission. Commission recommendation of 18 October 2011 on the definition of nanomaterial. Off. J. Eur. Union 2011, 275, 38. [Google Scholar]
- EU. Commission Regulation (EU) No 1183/2012 of 30 November 2012 amending and correcting Regulation (EU) No 10/2011 on plastic materials and articles intended to come into contact with food. Off. J. Eur. Union L 2012, 338, 11. [Google Scholar]
- EU. Commission Regulation (EU) No 2016/1416 of 24 August 2016 amending and correcting Regulation (EU) No 10/2011 on plastic materials and articles intended to come into contact with food. Off. J. Eur. Union L 2016, 230, 22–42. [Google Scholar]
- Piergiovanni, L.; Fotie, G.; Amoroso, L.; Akgun, B.; Limbo, S. Are cellulose nanocrystals “alien particles” to human experience? Packag. Technol. Sci. 2019, 32, 637–640. [Google Scholar] [CrossRef]
- Bott, J.; Störmer, A.; Franz, R. Migration of nanoparticles from plastic packaging materials containing carbon black into foodstuffs. Food Addit. Contam. Part A 2014, 31, 1769–1782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delgado-Aguilar, M.; Tovar, I.G.; Tarrés, Q.; Alcalà, M.; Pèlach, M.À.; Mutjé, P. Approaching a Low-Cost Production of Cellulose Nanofibers for Papermaking Applications. Bioresources 2015, 10, 5345–5355. [Google Scholar] [CrossRef]
- Novo, L.P.; Bras, J.; García, A.; Belgacem, N.; Curvelo, A.A.S.; Belgacem, M.N. Subcritical Water: A Method for Green Production of Cellulose Nanocrystals. ACS Sustain. Chem. Eng. 2015, 3, 2839–2846. [Google Scholar] [CrossRef]
- De Assis, C.A.; Houtman, C.; Phillips, R.; Rojas, O.J.; Pal, L.; Peresin, M.S.; Jameel, H.; Gonzalez, R.; Bilek, E.T. Conversion Economics of Forest Biomaterials: Risk and Financial Analysis of CNC Manufacturing. Biofuels Bioprod. Biorefining 2017, 11, 682–700. [Google Scholar] [CrossRef]
- Miller, J. Cellulose Nanomaterials: State of the Industry the Road to Commercialization. PAPER DAYS 2017, 2, 15. [Google Scholar]
- Coma, R.; Gili, J.M.; Zabala, M.; Riera, T. Feeding and Prey Capture Cycles in the Aposymbiontic Gorgonian Paramuricea Clavata. Mar. Ecol. Prog. Ser. 1994, 115, 257–270. [Google Scholar] [CrossRef]
- Kümmerer, K.; Menz, J.; Schubert, T.; Thielemans, W. Biodegradability of organic nanoparticles in the aqueous environment. Chemosphere 2011, 82, 1387–1392. [Google Scholar] [CrossRef]
- Kovacs, T.G.; Naish, V.; O’Connor, B.; Blaise, C.; Gagné, F.; Hall, L.; Trudeau, V.L.; Martel, P. An ecotoxicological characterization of nanocrystalline cellulose (NCC). Nanotoxicology 2010, 4, 255–270. [Google Scholar] [CrossRef]
- Moreira, S.; Silva, N.B.; Almeida-Lima, J.; Rocha, H.A.O.; Medeiros, S.R.B.; Alves, C.; Gama, F.M.; Gama, M. BC nanofibres: In vitro study of genotoxicity and cell proliferation. Toxicol. Lett. 2009, 189, 235–241. [Google Scholar] [CrossRef] [Green Version]
- Vartiainen, J.; Pöhler, T.; Sirola, K.; Pylkkänen, L.; Alenius, H.; Hokkinen, J.; Tapper, U.; Lahtinen, P.; Kapanen, A.; Putkisto, K.; et al. Health and environmental safety aspects of friction grinding and spray drying of microfibrillated cellulose. Cellulose 2011, 18, 775–786. [Google Scholar] [CrossRef]
- DeLoid, G.M.; Cao, X.; Molina, R.M.; Silva, D.I.; Bhattacharya, K.; Ng, K.W.; Loo, S.C.J.; Brain, J.D.; Demokritou, P.; Loo, S.C.J. Toxicological effects of ingested nanocellulose in in vitro intestinal epithelium and in vivo rat models. Environ. Sci. Nano 2019, 6, 2105–2115. [Google Scholar] [CrossRef] [PubMed]
- Lam, E.; Male, K.B.; Chong, J.H.; Leung, A.C.; Luong, J.H.T. Applications of functionalized and nanoparticle-modified nanocrystalline cellulose. Trends Biotechnol. 2012, 30, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Fukuzumi, H.; Saito, T.; Iwata, T.; Kumamoto, Y.; Isogai, A. Transparent and High Gas Barrier Films of Cellulose Nanofibers Prepared by TEMPO-Mediated Oxidation. Biomacromolecules 2009, 10, 162–165. [Google Scholar] [CrossRef]
- Padberg, J.; Gliese, T.; Bauer, W. The influence of fibrillation on the oxygen barrier properties of films from microfibrillated cellulose. Nord. Pulp Pap. Res. J. 2016, 31, 548–560. [Google Scholar] [CrossRef]




| Structures | Requirements | Applications |
|---|---|---|
| PE/paper/tie/EVOH/sealant | Gas and flavor barrier, pinhole resistance | Juices, jam, snacks |
| PE/paper/tie/EVOH/tie/EVOH | Gas and flavor barrier, pinhole resistance | Juices, soups |
| PA/EVOH/PE | Gas and flavor barrier | Jam, raw meat |
| OPET/tie/EVOH/tie/sealant | Gas barrier, transparency | Snacks, lid stock |
| OPP/tie/EVOH/sealant | Gas and flavor barrier, water barrier | Snacks, spices, jam, rice paste |
| Producers | Process | Capacity (tons/year) |
|---|---|---|
| Asia | Modified hydrophobic, oblique collision, TEMPO, phosphate esterification, aqueous counter collision | 754 |
| Europe | Chemical, enzymatic | 10 |
| UK | Chemical pretreatment | 100 |
| USA | TEMPO, SO2 fractionation, chemical | 391 |
| Producers | Process | Capacity (tons/year) |
|---|---|---|
| Asia | High-pressure homogenizer, TEMPO | 200 |
| Europe | Enzymatic, chemical, and mechanical | 1635 |
| UK | Mechanical and minerals | 8800 |
| America | Mechanical | 25 |
| Producers | Process | Capacity (tons/year) |
|---|---|---|
| Asia | Unmodified and modified, proprietary | Pilot |
| Europe | Enzymatic, chemical hydrolysis | 35 |
| America | SO2 fractionation, reactive extrusion | 130 |
| Canada | Sulfuric acid hydrolysis, catalytic conversion | 267 |
| Packaging Requirements | ONLY Coating (i.e., LbL) | ONLY Casting | Coating and Laminating | Casting and Laminating | Extrusion-ES | |
|---|---|---|---|---|---|---|
| O2 and CO2 barrier | 0% RH | +++ | +++ | +++ | +++ | + |
| >40% RH | + | + | +++ | +++ | - | |
| Water vapor barrier | − | + | +++ | +++ | + | |
| Grease barrier | +++ | +++ | +++ | +++ | ++ | |
| MOSH and MOAHs barrier | +++ | +++ | +++ | +++ | + | |
| Antimicrobial activity | +++ | +/- | +/- | +/- | +/- | |
| Mechanical and thermal properties | +/- | +++ | +/- | +++ | +++ | |
| Transparency | CNCs | +++ | + | +++ | +/- | - |
| MFC/CNFs | + | +/- | +/- | - | - | |
| Regulation requirements | CNCs | - | - | - | - | - |
| MFC/CNFs | ++ | ++ | ++ | ++ | ++ | |
| Low production cost and less time consuming | CNCs | +++ | + | +++ | +/- | - |
| MFC/CNFs | ++ | + | ++ | +/- | - | |
| NC Type | NC Thickness | Conditions | KPO2 [cm3 µm/(kPam2day)] | WVTR (g/(daym2) | References | ||
|---|---|---|---|---|---|---|---|
| CNCs | PET-coating | PET-CNCs A | 1 | 50% RH, 23 °C | 0.36 | [38] | |
| PET-CNCs B PLA-CNCsB | 1 1 | 50% RH, 23 °C 50% RH, 23 °C | 0.55 1.13 | [38] | |||
| Laminating (*) | PET/CNCs A/Tie/PE PET/CNCs B/Tie/PE | 1 1 | 80% RH, 23 °C 80% RH, 23 °C | 0.0006 0.0025 | [38] [38] | ||
| Cellophane/metalized aluminum (<1 μm)/tie/CNCs B/Tie//PLA | 1 | 35% RH, 23 °C | 0.0047 | 6.31 S | [104] | ||
| Casting | CNCs B | 36 | 20 | 452 S | [69] | ||
| Laminating (**) | BOPP/tie/CNCs/tie/BOPP | 36 | 80% RH, 23 °C | 10.4 | 0.9 S | [69] | |
| MFC/MFC | PLA-coating | MFC | |||||
| MFC-TEMPO | 0.1 0.4 | 0% RH, 23 °C 50% RH, 23 °C | 1.33 18 | [129] | |||
| Casting | MFC | 37.7 39 | 50% RH, 23 °C 80% RH, 23 °C | <0.011 11.4 | 407.6 S | [130] [71] | |
| MFC-TEMPO | 23.3 3.19 3.19 | 0% RH, 23 °C 0% RH, 23 °C 50% RH, 23 °C | <0.007 0.0006 0.85 | [130] [112] [112] | |||
| Laminating (**) | BOPP/tie/MFC/tie/BOPP | 80% RH, 23 °C | 42.2 | 0.8 S | [69] | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fotie, G.; Limbo, S.; Piergiovanni, L. Manufacturing of Food Packaging Based on Nanocellulose: Current Advances and Challenges. Nanomaterials 2020, 10, 1726. https://doi.org/10.3390/nano10091726
Fotie G, Limbo S, Piergiovanni L. Manufacturing of Food Packaging Based on Nanocellulose: Current Advances and Challenges. Nanomaterials. 2020; 10(9):1726. https://doi.org/10.3390/nano10091726
Chicago/Turabian StyleFotie, Ghislain, Sara Limbo, and Luciano Piergiovanni. 2020. "Manufacturing of Food Packaging Based on Nanocellulose: Current Advances and Challenges" Nanomaterials 10, no. 9: 1726. https://doi.org/10.3390/nano10091726
APA StyleFotie, G., Limbo, S., & Piergiovanni, L. (2020). Manufacturing of Food Packaging Based on Nanocellulose: Current Advances and Challenges. Nanomaterials, 10(9), 1726. https://doi.org/10.3390/nano10091726






