Recent Developments in Plasmonic Nanostructures for Metal Enhanced Fluorescence-Based Biosensing
Abstract
1. Introduction
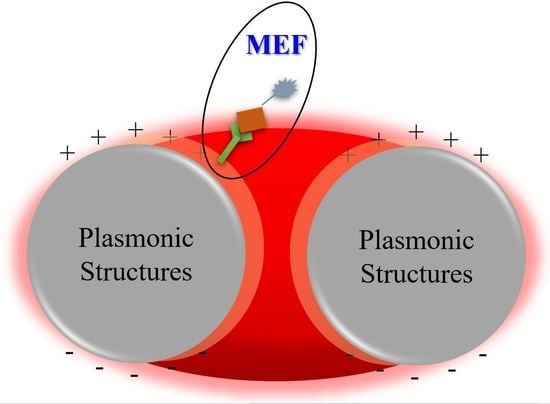
2. Metal Enhanced Fluorescence
2.1. When Metal Enhanced Fluorescence Occur?
2.2. Metal Enhanced Fluorescence Mechanism
2.3. Metal Enhanced Fluorescence from Plasmonic Nanostructures
2.3.1. Conventional Plasmonic Nanostructures for Metal Enhanced Fluorescence
2.3.2. Recent Developments in Plasmonic Nanostructures for Metal Enhanced Fluorescence
Metal Enhanced Fluorescence from Nano-Particles and Nanoclusters Fabricated by Chemical Synthesis Methods
| Material | Configuration of Structures | Structures Feature Size (nm) | Wavelength λ (nm) | Fluorophore | EF | Year | Ref. |
|---|---|---|---|---|---|---|---|
| Au | Nanocomposite | Dia: 20 nm | 375 nm | Amantadine hydrochloride | 1.4 | 2018 | [91] |
| Au | Nanorods | Dia: 17 nm Length: 43 nm | 532/785 nm | Rhodamine B | 2.2 | 2012 | [84] |
| Au | Nanoshells | Dia: 200 nm | 760 nm | Rhodamine 610 | 2.4 | 2011 | [92] |
| Au | Nanoparticle | Dia: 33 nm | 450 nm | PPQ-Zn2+-PPQ | 3 | 2019 | [41] |
| Ag@SiO2@PMOs | Nanocubes | Dia: 50 nm | 465 nm | Cu2+ | 3 | 2016 | [93] |
| Ag | 2D nanoparticle arrays | Dia: 20 nm | 532 nm | Rhodamine 6G | 3 | 2012 | [94] |
| Ag@SiO2-Au | Nanoclusters | Dia: 50 nm | 610 nm | AuNCs | 3.2 | 2019 | [95] |
| Au@SiO2-NH2@Au | Nanoclusters | Dia: 99 nm | 610 nm | AuNCs | 3.7 | 2018 | [60] |
| Au | Nanorods | - | 753 nm | Cy7 | 4.36 | 2020 | [96] |
| Au | Nanobipyramids | - | 751 nm | Cy7 | 5.63 | 2020 | [96] |
| Ag | Colloidal nanoparticles | Dia: 123 nm | 560 nm | Rhodamine 700 | 7 | 2019 | [97] |
| Ag | Nanowires on template | Pores Dia: 200 nm | 550 nm | Rhodamine B | 7.5 | 2018 | [98] |
| Ag | 3D nanoparticle arrays | Dia: 20 nm | 532 nm | Rhodamine 6G | 8.5 | 2012 | [94] |
| Au@SiO2 | Core-shell nanoparticles | Dia: 89.7 | 642 nm | Alexa | 9 | 2020 | [99] |
| Ag/Au@Silca | Nanoclusters | Dia: 37 nm Thickness: 13 nm | 635 nm | Cy5 | 9.4 | 2010 | [100] |
| CuNCs | Nanoclusters | Dia: 40–50 nm | 574 nm | CS-GSH-CuNCs | 10 | 2020 | [101] |
| Ag@SiO2 | Nanoparticle | Dia: 90 nm | 370 nm | Au25 | 12 | 2017 | [102] |
| Ag | Nanoshells | Dia: 5 nm | 420 nm | Rhodamine 123 | 20 | 2010 | [103] |
| Ag/Au | Nanocluster | Dia: 25 nm | 548 nm | Cy5 | 35 | 2010 | [82] |
| Au | Nanorods | Dia: 13 nm | 635 nm | Cy5 | 40 | 2010 | [85] |
| Ag@SiO2 | Core-shell nanoparticles | Dia: 89.7 | 642 nm | Alexa | 70 | 2020 | [99] |
| Cu | Nanospheres | Dia: 462 nm | 650 nm | Porphyrin | 89 | 2013 | [13] |
| Ag | Nanoshells | Dia: 50–80 nm | 514.5 nm | Rhodamine B | 94 | 2012 | [83] |
| Au | Nanorods | Dia: 18.1 | 760 nm | streptavidin-CW800 | 100 | 2018 | [104] |
| Au | Nanocluster | Dia: 20 nm | 365 nm | Eu3+-EUTC | 100 | 2014 | [105] |
| Ag@Au | Naprisms | - | 532 nm | Ir-Zne | 110 | 2017 | [89] |
Metal Enhanced Fluorescence from Non-Periodic Nanostructures Fabricated by Deposition Methods
Metal Enhanced Fluorescence from Periodical Nanostructures Fabricated by Lithography Methods
| Material | Configuration of Structures | Structures Feature Size (nm) | Wavelength λ (nm) | Fluorophore | EF | Year | Ref. |
|---|---|---|---|---|---|---|---|
| Ag | Nano triangles | Dia: 300 nm | 525 nm | Alexa 488 | 7.8 | 2013 | [26] |
| Ag | Concentric gratings | Width: 200 nm Height: 65 nm | 635 nm | Alexa 647 | 10 | 2011 | [123] |
| Ag | Nano gratings | Pitch: 300 nm | 532 nm | Rhodamine 6G | 14 | 2011 | [124] |
| Al2O3@Ag | Nano gratings | Dia: 142 nm Height: 67 nm | 400 nm | Rhodamine 6G | 14 | 2016 | [125] |
| Ag | Nanodots | Dia: 100 nm Height: 30 nm | 560 nm | Cy3 | 15 | 2011 | [126] |
| Au | Nanocylinders | Dia: 100 nm Height: 35 nm | 580 nm | CdSe/ZnS core shells | 26 | 2006 | [18] |
| Ag | Nano gratings | Pitch: 375 nm | 532 nm | Rhodamine 6G | 30 | 2011 | [124] |
| Au | Nanoprisms | Width: 100 nm Height: 35 nm | 580 nm | CdSe/ZnS core shells | 33 | 2006 | [18] |
| Au | Nanogaps | Height: 60 nm Pitch: 400 nm | 670 nm | Cy5 | 47.4 | 2014 | [127] |
| Ag | Nano triangles | Dia: 500 nm | 780 nm | Alexa 790 | 83 | 2013 | [26] |
| Ag | Nano gratings | Height: 44 nm Pitch: 400 nm | 530/550 nm | Rhodamine 6G | 116 | 2015 | [128] |
| Ag | 3D nanodomes | Dia: 250 nm Height: 100 nm Pitch: 500 nm | 635 nm | streptavidin-Cy5 | 128 | 2018 | [30] |
| Ag | 3D nano gratings | Height: 30 nm Pitch: 480 nm | 632.8 nm | Cy5 | 170 | 2017 | [129] |
| ZnO | Nanorods | Dia: 230 nm Height: 1.5 µm Pitch: 390 nm | 532 nm | Rhodamine 6G | 300 | 2019 | [120] |
| Au@SiO2 | Nanopilllar | Dia: 100 nm Pitch: 200 nm | 800 nm | IRDye-800cw-labelled goat antihuman IgG | 910 | 2019 | [130] |
| Au | nanoantenna | Dia: 76 nm Height: 50 nm | 633 nm | Alexa 647 | 1100 | 2013 | [119] |
| Au | bowtie nanoantenna | - | 780/820 nm | TPQDI | 1340 | 2009 | [50] |
| Au | D2PA nanoantenna | Dia: 100 nm Height: 65 nm Pitch: 200 nm | 785 nm | ICG, IgG | 2970, 7400 | 2012, 2012 | [121,122] |
2.4. Metal Enhanced Fluorescence-Based Biosensors Applications
| Detection Analyte | Detection Time | Limit of Detection | Year | Ref. |
|---|---|---|---|---|
| Mouse IgG antigen | - | 0.25 µg/mL | 2015 | [132] |
| Human Semen | 60 min | 0.06 µg/mL | 2018 | [135] |
| Human Vaginal Fluid | 60 min | 0.005 µg/mL | 2018 | [135] |
| Human immunoglobulins | 60 min | 0.0008 µg/mL | 2019 | [137] |
| FITC-labeled YebF protein from Escherichia coli | - | 17.2 ng/mL | 2020 | [138] |
| Prostate-Specific Antigen (PSA) | 30 min | 0.20 ng/mL | 2017 | [133] |
| S-OIV | - | 13.9 pg/mL | 2010 | [131] |
| 17-β-estradiol | Real-time | 1 pg/mL | 2017 | [139] |
| SARS-CoV | - | 1 pg/mL | 2009 | [134] |
| Kidney injury molecule-1 | - | 500 fg/mL | 2018 | [104] |
| Ebola virus | 10 s | 220 fg/mL | 2019 | [130] |
| Neutrophil gelatinase-associated lipocalin | - | 0.5 fg/mL | 2018 | [104] |
| DNA-oligonucleotides | - | 2.5 × 104 nM | 2015 | [36] |
| CD4-mRNA expression | 60 mints | 125 nM | 2019 | [140] |
| Glucose | Real-time | 50 nM | 2015 | [141] |
| Intracellular Adenosine triphosphate | - | 35 nM | 2020 | [96] |
| Lysozyme in Human Serum | Real-Time | 1.6 nM | 2020 | [101] |
| Human Immunoglobulin G | - | 10 nM | 2014 | [79] |
| Carbohydrate-lectin | 5 s | 0.87 nM | 2015 | [111] |
| DNA aptamer | 20 min | 0.33 nM | 2015 | [142] |
| Acetylcholinesterase | - | 0.01 nM | 2018 | [143] |
| Streptavidin | 10 min | 0.05 nM | 2011 | [144] |
| Hairpin ssDNA | 30 min | 10 pM | 2017 | [80] |
| miRNA-21-Bladder cancer-related biomarker in Urine | 120 min | 26.3 fM | 2019 | [145] |
| Human NOGGIN | 25 µs | 1.5 × 10−3 nM | 2018 | [146] |
| Alexa 488 labelled oligonucleotide | - | 1 × 10−5 nM | 2016 | [106] |
| Human IgG | 60 min | 1 × 10−7 nM | 2012 | [122] |
2.5. Summary and Future Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ekimov, A.I.; Efros, A.L.; Onushchenko, A.A. Quantum size effect in semiconductor microcrystals. Solid State Commun. 1985, 56, 921–924. [Google Scholar] [CrossRef]
- Gittleman, J.I.; Abeles, B.; Bozowski, S. Superparamsgnetism and relaxation effects in granular Ni-SiO2, and Ni-Al2O3 films. Phys. Rev. B 1974, 9, 3891–3897. [Google Scholar] [CrossRef]
- Maier, S.A. Plasmonics: Fundamentals and Applications; Springer US: New York, NY, USA, 2007; ISBN 0387331506. [Google Scholar]
- Mayer, K.M.; Hafner, J.H. Localized Surface Plasmon Resonance Sensors. Chem. Rev. 2011, 111, 3828–3857. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kim, S.; Nam, J.M. Plasmonically Engineered Nanoprobes for Biomedical Applications. J. Am. Chem. Soc. 2016, 138, 14509–14525. [Google Scholar] [CrossRef]
- Reiner, A.T.; Fossati, S.; Dostalek, J. Biosensor platform for parallel surface plasmon-enhanced epifluorescence and surface plasmon resonance detection. Sensors Actuators B Chem. 2018, 257, 594–601. [Google Scholar] [CrossRef]
- Park, J.-E.; Kim, J.; Nam, J.-M. Emerging plasmonic nanostructures for controlling and enhancing photoluminescence. Chem. Sci. 2017, 8, 4696–4704. [Google Scholar] [CrossRef]
- Li, M.; Cushing, S.K.; Wu, N. Plasmon-enhanced optical sensors: A review. Analyst 2015, 140, 386–406. [Google Scholar] [CrossRef]
- Darvill, D.; Centeno, A.; Xie, F. Plasmonic fluorescence enhancement by metal nanostructures: Shaping the future of bionanotechnology. Phys. Chem. Chem. Phys. 2013, 15, 15709–15726. [Google Scholar] [CrossRef]
- Strobbia, P.; Languirand, E.; Cullum, B.M. Recent advances in plasmonic nanostructures for sensing: A review. Opt. Eng. 2015, 54, 100902. [Google Scholar] [CrossRef]
- Petryayeva, E.; Krull, U.J. Localized surface plasmon resonance: Nanostructures, bioassays and biosensing—A review. Anal. Chim. Acta 2011, 706, 8–24. [Google Scholar] [CrossRef]
- Yu, H.; Peng, Y.; Yang, Y.; Li, Z.Y. Plasmon-enhanced light–matter interactions and applications. NPJ Comput. Mater. 2019, 5, 1–14. [Google Scholar] [CrossRef]
- Sugawa, K.; Tamura, T.; Tahara, H.; Yamaguchi, D.; Akiyama, T.; Otsuki, J.; Kusaka, Y.; Fukuda, N.; Ushijima, H. Metal-enhanced fluorescence platforms based on plasmonic ordered copper arrays: Wavelength dependence of quenching and enhancement effects. ACS Nano 2013, 7, 9997–10010. [Google Scholar] [CrossRef] [PubMed]
- Tittl, A.; Mai, P.; Taubert, R.; Dregely, D.; Liu, N.; Giessen, H. Palladium-based plasmonic perfect absorber in the visible wavelength range and its application to hydrogen sensing. Nano Lett. 2011, 11, 4366–4369. [Google Scholar] [CrossRef] [PubMed]
- Langhammer, C.; Yuan, Z.; Zorić, I.; Kasemo, B. Plasmonic properties of supported Pt and Pd nanostructures. Nano Lett. 2006, 6, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Le Ru, E.C.; Etchegoin, P.G. Introduction to Plasmons and Plasmonics. In Principles of Surface-Enhanced Raman Spectroscopy; Elsevier: Amsterdam, The Netherlands, 2009; pp. 121–183. [Google Scholar]
- Karabchevsky, A. Publisher’s Note: Microspot sensing based on surface-enhanced fluorescence from nanosculptured thin films. J. Nanophotonics 2012, 6, 060105. [Google Scholar] [CrossRef]
- Pompa, P.P.; Martiradonna, L.; Della Torre, A.; Della Sala, F.; Manna, L.; de Vittorio, M.; Calabi, F.; Cinagolani, R.; Rinaldi, R. Metal-enhanced fluorescence of colloidal nanocrystals with nanoscale control. Nat. Nanotechnol. 2006, 1, 126–130. [Google Scholar] [CrossRef]
- Chen, H.; Shao, L.; Li, Q.; Wang, J. Gold nanorods and their plasmonic properties. Chem. Soc. Rev. 2013, 42, 2679–2724. [Google Scholar] [CrossRef]
- Chung, T.; Lee, S.Y.; Song, E.Y.; Chun, H.; Lee, B. Plasmonic nanostructures for nano-scale bio-sensing. Sensors 2011, 11, 10907–10929. [Google Scholar] [CrossRef]
- Rycenga, M.; Cobley, C.M.; Zeng, J.; Li, W.; Moran, C.H.; Zhang, Q.; Qin, D.; Xia, Y. Controlling the synthesis and assembly of silver nanostructures for plasmonic applications. Chem. Rev. 2011, 111, 3669–3712. [Google Scholar] [CrossRef]
- Badshah, M.A.; Ju, J.; Lu, X.; Abbas, N.; Kim, S. min Enhancing the sensitivity of DNA microarrays by metal-enhanced fluorescence using vertical nanorod structures. Sensors Actuators B Chem. 2018, 274, 451–457. [Google Scholar] [CrossRef]
- Badshah, M.A.; Lu, X.; Ju, J.; Kim, S. Silver nanorod structures for metal enhanced fluorescence. In Proceedings of the Nanoengineering: Fabrication, Properties, Optics, and Devices XIII, San Diego, CA, USA, 30–31 August 2016; Volume 9927, p. 992715. [Google Scholar]
- Sherry, L.J.; Chang, S.H.; Schatz, G.C.; Van Duyne, R.P.; Wiley, B.J.; Xia, Y. Localized surface plasmon resonance spectroscopy of single silver nanocubes. Nano Lett. 2005, 5, 2034–2038. [Google Scholar] [CrossRef] [PubMed]
- Tavakkoli Yaraki, M.; Daqiqeh Rezaei, S.; Tan, Y.N. Simulation guided design of silver nanostructures for plasmon-enhanced fluorescence, singlet oxygen generation and SERS applications. Phys. Chem. Chem. Phys. 2020, 22, 5673–5687. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Pang, J.S.; Centeno, A.; Ryan, M.P.; Riley, D.J.; Alford, N.M. Nanoscale control of Ag nanostructures for plasmonic fluorescence enhancement of near-infrared dyes. Nano Res. 2013, 6, 496–510. [Google Scholar] [CrossRef]
- Lin, G.; Lewandowska, M. Plasmon-enhanced fluorescence provided by silver nanoprisms for sensitive detection of sulfide. Sensors Actuators B Chem. 2019, 292, 241–246. [Google Scholar] [CrossRef]
- Bukasov, R.; Ali, T.A.; Nordlander, P.; Shumaker-Parry, J.S. Probing the Plasmonic Near-Field of Gold Nanocrescent Antennas. ACS Nano 2010, 4, 6639–6650. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, J.; Badshah, M.A.; Dong, L.; Li, J.; Kim, S.M.; Lu, M. A programmable nanoreplica molding for the fabrication of nanophotonic devices. Sci. Rep. 2016, 6, 22445. [Google Scholar] [CrossRef]
- Badshah, M.A.; Kim, J.; Jang, H.; Kim, S.M. Fabrication of highly packed plasmonic nanolens array using polymer nanoimprinted nanodots for an enhanced fluorescence substrate. Polymers 2018, 10, 649. [Google Scholar] [CrossRef]
- Suzuki, M. Practical applications of thin films nanostructured by shadowing growth. J. Nanophotonics 2013, 7, 073598. [Google Scholar] [CrossRef]
- Drexhage, K.H. Influence of a dielectric interface on fluorescence decay time. J. Lumin. 1970, 1–2, 693–701. [Google Scholar] [CrossRef]
- Bauch, M.; Toma, K.; Toma, M.; Zhang, Q.; Dostalek, J. Plasmon-Enhanced Fluorescence Biosensors: A Review. Plasmonics 2014, 9, 781–799. [Google Scholar] [CrossRef]
- Liaw, J.W.; Tsai, H.Y.; Huang, C.H. Size-Dependent Surface Enhanced Fluorescence of Gold Nanorod: Enhancement or Quenching. Plasmonics 2012, 7, 543–553. [Google Scholar] [CrossRef]
- Ray, K.; Badugu, R.; Lakowicz, J.R. Distance-dependent metal-enhanced fluorescence from Langmuir-Blodgett monolayers of Alkyl-NBD derivatives on silver island films. Langmuir 2006, 22, 8374–8378. [Google Scholar] [CrossRef] [PubMed]
- Puchkova, A.; Vietz, C.; Pibiri, E.; Wünsch, B.; Sanz Paz, M.; Acuna, G.P.; Tinnefeld, P. DNA Origami Nanoantennas with over 5000-fold Fluorescence Enhancement and Single-Molecule Detection at 25 μm. Nano Lett. 2015, 15, 8354–8359. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R.; Ray, K.; Chowdhury, M.; Szmacinski, H.; Fu, Y.; Zhang, J.; Nowaczyk, K. Plasmon-controlled fluorescence: A new paradigm in fluorescence spectroscopy. Analyst 2008, 133, 1308–1346. [Google Scholar] [CrossRef]
- Geddes, C.D. Metal-enhanced fluorescence. Phys. Chem. Chem. Phys. 2013, 15, 19537. [Google Scholar] [CrossRef]
- Karolin, J.O.; Geddes, C.D. Reduced lifetimes are directly correlated with excitation irradiance in metal-enhanced fluorescence (MEF). J. Fluoresc. 2012, 22, 1659–1662. [Google Scholar] [CrossRef]
- Li, J.F.; Li, C.Y.; Aroca, R.F. Plasmon-enhanced fluorescence spectroscopy. Chem. Soc. Rev. 2017, 46, 3962–3979. [Google Scholar] [CrossRef]
- Pawar, S.; Bhattacharya, A.; Nag, A. Metal-Enhanced Fluorescence Study in Aqueous Medium by Coupling Gold Nanoparticles and Fluorophores Using a Bilayer Vesicle Platform. ACS Omega 2019, 4, 5983–5990. [Google Scholar] [CrossRef]
- Jeong, Y.; Kook, Y.M.; Lee, K.; Koh, W.G. Metal enhanced fluorescence (MEF) for biosensors: General approaches and a review of recent developments. Biosens. Bioelectron. 2018, 111, 102–116. [Google Scholar] [CrossRef]
- Hlaing, M.; Gebear-Eigzabher, B.; Roa, A.; Marcano, A.; Radu, D.; Lai, C.Y. Absorption and scattering cross-section extinction values of silver nanoparticles. Opt. Mater. 2016, 58, 439–444. [Google Scholar] [CrossRef]
- Knoblauch, R.; Geddes, C.D. Review of Advances in Metal-Enhanced Fluorescence. In Reviews in Plasmonics 2017; Springer: Cham, Switzerland, 2019; pp. 253–283. [Google Scholar]
- Zhang, Y.; Mali, B.L.; Geddes, C.D. Metal-enhanced fluorescence exciplex emission. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 85, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Zenin, V.A.; Andryieuski, A.; Malureanu, R.; Radko, I.P.; Volkov, V.S.; Gramotnev, D.K.; Lavrinenko, A.V.; Bozhevolnyi, S.I. Boosting Local Field Enhancement by on-Chip Nanofocusing and Impedance-Matched Plasmonic Antennas. Nano Lett. 2015, 15, 8148–8154. [Google Scholar] [CrossRef] [PubMed]
- Aslan, K.; Leonenko, Z.; Lakowicz, J.R.; Geddes, C.D. Annealed silver-island films for applications in metal-enhanced fluorescence: Interpretation in terms of radiating plasmons. J. Fluoresc. 2005, 15, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Abadeer, N.S.; Brennan, M.R.; Wilson, W.L.; Murphy, C.J. Distance and plasmon wavelength dependent fluorescence of molecules bound to silica-coated gold nanorods. ACS Nano 2014, 8, 8392–8406. [Google Scholar] [CrossRef]
- Mishra, H.; Zhang, Y.; Geddes, C.D. Metal enhanced fluorescence of the fluorescent brightening agent Tinopal-CBX near silver island film. Dye. Pigment. 2011, 91, 225–230. [Google Scholar] [CrossRef]
- Kinkhabwala, A.; Yu, Z.; Fan, S.; Avlasevich, Y.; Müllen, K.; Moerner, W.E. Large single-molecule fluorescence enhancements produced by a bowtie nanoantenna. Nat. Photonics 2009, 3, 654–657. [Google Scholar] [CrossRef]
- Govorov, A.; Hernández Martínez, P.L.; Demir, H.V. Förster-Type Nonradiative Energy Transfer Models. In SpringerBriefs in Applied Sciences and Technology; Springer: Berlin/Heidelberg, Germany, 2016; pp. 19–27. [Google Scholar]
- Jones, G.A.; Bradshaw, D.S. Resonance energy transfer: From fundamental theory to recent applications. Front. Phys. 2019, 7. [Google Scholar] [CrossRef]
- Khurgin, J.B.; Sun, G. Enhancement of optical properties of nanoscaled objects by metal nanoparticles. J. Opt. Soc. Am. B 2009, 26, B83–B95. [Google Scholar] [CrossRef]
- Lakowicz, J.R.; Shen, Y.; D’Auria, S.; Malicka, J.; Fang, J.; Gryczynski, Z.; Gryczynski, I. Radiative decay engineering: 2. Effects of silver island films on fluorescence intensity, lifetimes, and resonance energy transfer. Anal. Biochem. 2002, 301, 261–277. [Google Scholar] [CrossRef]
- Lakowicz, J.R.; Malicka, J.; Gryczynski, I.; Gryczynski, Z.; Geddes, C.D. Radiative decay engineering: The role of photonic mode density in biotechnology. J. Phys. D. Appl. Phys. 2003, 36, R240. [Google Scholar] [CrossRef]
- Anger, P.; Bharadwaj, P.; Novotny, L. Enhancement and quenching of single-molecule fluorescence. Phys. Rev. Lett. 2006, 96, 113002. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, P.; Novotny, L. Spectral dependence of single molecule fluorescence enhancement. Opt. Express 2007, 15, 14266–14274. [Google Scholar] [CrossRef] [PubMed]
- Vietz, C.; Lalkens, B.; Acuna, G.P.; Tinnefeld, P. Synergistic Combination of Unquenching and Plasmonic Fluorescence Enhancement in Fluorogenic Nucleic Acid Hybridization Probes. Nano Lett. 2017, 17, 6496–6500. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.A.; Wang, J.; Jasinski, J.B.; Achilefu, S. Fluorescence Manipulation by Gold Nanoparticles: From Complete Quenching to Extensive Enhancement. J. Nanobiotechnol. 2011, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Ma, D.; Du, J. Distance dependent fluorescence quenching and enhancement of gold nanoclusters by gold nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 189, 161–166. [Google Scholar] [CrossRef]
- Chen, Y.; Munechika, K.; Ginger, D.S. Dependence of fluorescence intensity on the spectral overlap between fluorophores and plasmon resonant single silver nanoparticles. Nano Lett. 2007, 7, 690–696. [Google Scholar] [CrossRef]
- Jain, P.K.; Lee, K.S.; El-Sayed, I.H.; El-Sayed, M.A. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: Applications in biological imaging and biomedicine. J. Phys. Chem. B 2006, 110, 7238–7248. [Google Scholar] [CrossRef]
- Liz-Marzán, L.M. Tailoring surface plasmons through the morphology and assembly of metal nanoparticles. Langmuir 2006, 22, 32–41. [Google Scholar] [CrossRef]
- Shabaninezhad, M.; Ramakrishna, G. Theoretical investigation of size, shape, and aspect ratio effect on the LSPR sensitivity of hollow-gold nanoshells. J. Chem. Phys. 2019, 150, 144116. [Google Scholar] [CrossRef]
- Jin, R.; Cao, Y.C.; Hao, E.; Métraux, G.S.; Schatz, G.C.; Mirkin, C.A. Controlling anisotropic nanoparticle growth through plasmon excitation. Nature 2003, 425, 487–490. [Google Scholar] [CrossRef]
- Glass, A.M.; Liao, P.F.; Bergman, J.G.; Olson, D.H. Interaction of metal particles with adsorbed dye molecules: Absorption and luminescence. Opt. Lett. 1980, 5, 368–370. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Goldys, E.M. Plasmonic approach to enhanced fluorescence for applications in biotechnology and the life sciences. Langmuir 2012, 28, 10152–10163. [Google Scholar] [CrossRef] [PubMed]
- Szmacinski, H.; Toshchakov, V.; Piao, W.; Lakowicz, J.R. Imaging of Protein Secretion from a Single Cell Using Plasmonic Substrates. Bionanoscience 2013, 3, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Aslan, K.; Previte, M.J.R.; Geddes, C.D. Metal-enhanced fluorescence from paper substrates: Modified spectral properties of dyes for potential high-throughput surface analysis and assays and as an anti-counterfeiting technology. Dye. Pigment. 2008, 77, 545–549. [Google Scholar] [CrossRef]
- Cui, Q.; He, F.; Li, L.; Möhwald, H. Controllable metal-enhanced fluorescence in organized films and colloidal system. Adv. Colloid Interface Sci. 2014, 207, 164–177. [Google Scholar] [CrossRef]
- Jung, D.W.; Kim, J.M.; Yun, H.J.; Yi, G.R.; Cho, J.Y.; Jung, H.; Lee, G.; Chae, W.S.; Nam, K.M. Understanding metal-enhanced fluorescence and structural properties in Au@Ag core-shell nanocubes. RSC Adv. 2019, 9, 29232–29237. [Google Scholar] [CrossRef]
- Fayyaz, S.; Tabatabaei, M.; Hou, R.; Lagugné-Labarthet, F. Surface-enhanced fluorescence: Mapping individual hot spots in silica-protected 2D gold nanotriangle arrays. J. Phys. Chem. C 2012, 116, 11665–11670. [Google Scholar] [CrossRef]
- Geddes, C.D.; Parfenov, A.; Roll, D.; Gryczynski, I.; Malicka, J.; Lakowicz, J.R. Silver Fractal-like Structures for Metal-Enhanced Fluorescence: Enhanced Fluorescence Intensities and Increased Probe Photostabilities. J. Fluoresc. 2003, 13, 267–276. [Google Scholar] [CrossRef]
- Waxenegger, J.; Trügler, A.; Hohenester, U. Plasmonics simulations with the MNPBEM toolbox: Consideration of substrates and layer structures. Comput. Phys. Commun. 2015, 193, 138–150. [Google Scholar] [CrossRef]
- Knoblauch, R.; Ben Hamo, H.; Marks, R.; Geddes, C.D. Spectral Distortions in Metal-Enhanced Fluorescence: Experimental Evidence for Ultra-Fast and Slow Transitions. J. Phys. Chem. C 2020, 124, 4723–4737. [Google Scholar] [CrossRef]
- Asian, K.; Lakowicz, J.R.; Szmacinski, H.; Geddes, C.D. Metal-enhanced fluorescence solution-based sensing platform. J. Fluoresc. 2004, 14, 677–679. [Google Scholar] [PubMed]
- Feng, A.L.; You, M.L.; Tian, L.; Singamaneni, S.; Liu, M.; Duan, Z.; Lu, T.J.; Xu, F.; Lin, M. Distance-dependent plasmon-enhanced fluorescence of upconversion nanoparticles using polyelectrolyte multilayers as tunable spacers. Sci. Rep. 2015, 5, 7779. [Google Scholar] [CrossRef] [PubMed]
- Khatua, S.; Paulo, P.M.R.; Yuan, H.; Gupta, A.; Zijlstra, P.; Orrit, M. Resonant plasmonic enhancement of single-molecule fluorescence by individual gold nanorods. ACS Nano 2014, 8, 4440–4449. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.; Son, K.J.; Koh, W.G. Metal-enhanced fluorescence using silver nanoparticles-embedded polyelectrolyte multilayer films for microarray-based immunoassays. Colloid Polym. Sci. 2014, 292, 1355–1364. [Google Scholar] [CrossRef]
- Mei, Z.; Tang, L. Surface-Plasmon-Coupled Fluorescence Enhancement Based on Ordered Gold Nanorod Array Biochip for Ultrasensitive DNA Analysis. Anal. Chem. 2017, 89, 633–639. [Google Scholar] [CrossRef]
- Yang, B.; Lu, N.; Qi, D.; Ma, R.; Wu, Q.; Hao, J.; Liu, X.; Mu, Y.; Reboud, V.; Kehagias, N.; et al. Tuning the Intensity of Metal-Enhanced Fluorescence by Engineering Silver Nanoparticle Arrays. Small 2010, 6, 1038–1043. [Google Scholar] [CrossRef]
- Touahir, L.; Galopin, E.; Boukherroub, R.; Gouget-Laemmel, A.C.; Chazalviel, J.N.; Ozanam, F.; Szunerits, S. Localized surface plasmon-enhanced fluorescence spectroscopy for highly-sensitive real-time detection of DNA hybridization. Biosens. Bioelectron. 2010, 25, 2579–2585. [Google Scholar] [CrossRef]
- Guerrero, A.R.; Zhang, Y.; Aroca, R.F. Experimental confirmation of local field enhancement determining far-field measurements with shell-isolated silver nanoparticles. Small 2012, 8, 2964–2967. [Google Scholar] [CrossRef]
- Gabudean, A.M.; Focsan, M.; Astilean, S. Gold nanorods performing as dual-modal nanoprobes via metal-enhanced fluorescence (MEF) and surface-enhanced Raman scattering (SERS). J. Phys. Chem. C 2012, 116, 12240–12249. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, J.; Lakowicz, J.R. Plasmon-enhanced fluorescence from single fluorophores end-linked to gold nanorods. J. Am. Chem. Soc. 2010, 132, 5540–5541. [Google Scholar] [CrossRef]
- Tserkezis, C.; Mortensen, N.A.; Wubs, M. How nonlocal damping reduces plasmon-enhanced fluorescence in ultranarrow gaps. Phys. Rev. B 2017, 96, 85413. [Google Scholar] [CrossRef]
- Sugawa, K.; Takeshima, N.; Uchida, K.; Tahara, H.; Jin, S.; Tsunenari, N.; Akiyama, T.; Kusaka, Y.; Fukuda, N.; Ushijima, H.; et al. Photocurrent enhancement of porphyrin molecules over a wide-wavelength region based on combined use of silver nanoprisms with different aspect ratios. J. Mater. Chem. C 2015, 3, 11439–11448. [Google Scholar] [CrossRef]
- Peng, M.; Sun, F.; Na, N.; Ouyang, J. Target-Triggered Assembly of Nanogap Antennas to Enhance the Fluorescence of Single Molecules and Their Application in MicroRNA Detection. Small 2020, 16, 2000460. [Google Scholar] [CrossRef]
- Huang, P.H.; Hong, C.P.; Zhu, J.F.; Chen, T.T.; Chan, C.T.; Ko, Y.C.; Lin, T.L.; Pan, Z.B.; Sun, N.K.; Wang, Y.C.; et al. Ag@Au nanoprism-metal organic framework-based paper for extending the glucose sensing range in human serum and urine. Dalt. Trans. 2017, 46, 6985–6993. [Google Scholar] [CrossRef] [PubMed]
- Luan, J.; Seth, A.; Gupta, R.; Wang, Z.; Rathi, P.; Cao, S.; Gholami Derami, H.; Tang, R.; Xu, B.; Achilefu, S.; et al. Ultrabright fluorescent nanoscale labels for the femtomolar detection of analytes with standard bioassays. Nat. Biomed. Eng. 2020, 4, 518–530. [Google Scholar] [CrossRef]
- Wang, X.; Wang, C.; Yang, N.; Xia, J.; Li, L. Preparation of fluorescent nanocomposites based on gold nanoclusters self-assembly. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 548, 27–31. [Google Scholar] [CrossRef]
- Zaiba, S.; Lerouge, F.; Gabudean, A.M.; Focsan, M.; Lermé, J.; Gallavardin, T.; Maury, O.; Andraud, C.; Parola, S.; Baldeck, P.L. Transparent plasmonic nanocontainers protect organic fluorophores against photobleaching. Nano Lett. 2011, 11, 2043–2047. [Google Scholar] [CrossRef]
- Sun, B.; Wang, C.; Han, S.; Hu, Y.; Zhang, L. Metal-enhanced fluorescence-based multilayer core-shell Ag-nanocube@SiO2@PMOs nanocomposite sensor for Cu2+ detection. RSC Adv. 2016, 6, 61109–61118. [Google Scholar] [CrossRef]
- Dong, J.; Qu, S.; Zhang, Z.; Liu, M.; Liu, G.; Yan, X.; Zheng, H. Surface enhanced fluorescence on three dimensional silver nanostructure substrate. J. Appl. Phys. 2012, 111, 093101. [Google Scholar] [CrossRef]
- Xu, D.D.; Zheng, B.; Song, C.Y.; Lin, Y.; Pang, D.W.; Tang, H.W. Metal-enhanced fluorescence of gold nanoclusters as a sensing platform for multi-component detection. Sensors Actuators B Chem. 2019, 282, 650–658. [Google Scholar] [CrossRef]
- Zheng, M.; Kang, Y.; Liu, D.; Li, C.; Zheng, B.; Tang, H. Detection of ATP from “fluorescence” to “enhanced fluorescence” based on metal-enhanced fluorescence triggered by aptamer nanoswitch. Sensors Actuators B Chem. 2020, 319, 128263. [Google Scholar] [CrossRef]
- Lee, D.; Lee, J.; Song, J.; Jen, M.; Pang, Y. Homogeneous silver colloidal substrates optimal for metal-enhanced fluorescence. Phys. Chem. Chem. Phys. 2019, 21, 11599–11607. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Zhou, X.; He, X.; Cao, H.; Liu, J.; Qiu, H.; Jin, Z. Distance dependent fluorescence enhancement of silver nanowires deposited on AAO. Opt. Mater. 2018, 83, 241–244. [Google Scholar] [CrossRef]
- Sun, S.; Rasskazov, I.L.; Carney, P.S.; Zhang, T.; Moroz, A. Critical Role of Shell in Enhanced Fluorescence of Metal–Dielectric Core–Shell Nanoparticles. J. Phys. Chem. C 2020, 124, 13365–13373. [Google Scholar] [CrossRef]
- Renier, A.; Mangeat, T.; Benalia, H.; Elie-Caille, C.; Pieralli, C.; Wacogne, B. Gold/silica thin film for biosensors applications: Metal enhanced fluorescence. Laser Phys. 2010, 20, 591–595. [Google Scholar] [CrossRef]
- Chen, S.; Huang, Z.; Jia, Q. Electrostatically confined in-situ preparation of stable glutathione-capped copper nanoclusters for fluorescence detection of lysozyme. Sensors Actuators, B Chem. 2020, 319, 128305. [Google Scholar] [CrossRef]
- Kim, J.K.; Jang, D.J. Metal-enhanced fluorescence of gold nanoclusters adsorbed onto Ag@SiO2 core-shell nanoparticles. J. Mater. Chem. C 2017, 5, 6037–6046. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, Y.; Jiang, F.; Lakowicz, J.R. Metal nanoshell-capsule for light-driven release of a small molecule. J. Phys. Chem. C 2010, 114, 7653–7659. [Google Scholar] [CrossRef]
- Luan, J.; Morrissey, J.J.; Wang, Z.; Derami, H.G.; Liu, K.K.; Cao, S.; Jiang, Q.; Wang, C.; Kharasch, E.D.; Naik, R.R.; et al. Add-on plasmonic patch as a universal fluorescence enhancer. Light Sci. Appl. 2018, 7, 29. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, S.; Dou, Y.; Zhuo, Y.; Luo, Y.; Feng, Y. Novel and remarkable enhanced-fluorescence system based on gold nanoclusters for detection of tetracycline. Talanta 2014, 122, 36–42. [Google Scholar] [CrossRef]
- Ji, X.; Xiao, C.; Lau, W.F.; Li, J.; Fu, J. Metal enhanced fluorescence improved protein and DNA detection by zigzag Ag nanorod arrays. Biosens. Bioelectron. 2016, 82, 240–247. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Fu, J.; Zhao, Y. Oblique angle deposition and its applications in plasmonics. Front. Phys. 2014, 9, 47–59. [Google Scholar] [CrossRef]
- Ju, J.; Byeon, E.; Han, Y.A.; Kim, S.M. Fabrication of a substrate for Ag-nanorod metal-enhanced fluorescence using the oblique angle deposition process. Micro Nano Lett. 2013, 8, 370–373. [Google Scholar] [CrossRef]
- Singh, D.P.; Kumar, S.; Singh, J.P. Morphology dependent surface enhanced fluorescence study on silver nanorod arrays fabricated by glancing angle deposition. RSC Adv. 2015, 5, 31341–31346. [Google Scholar] [CrossRef]
- Wang, T.; Costan, J.; Centeno, A.; Pang, J.S.; Darvill, D.; Ryan, M.P.; Xie, F. Broadband enhanced fluorescence using zinc-oxide nanoflower arrays. J. Mater. Chem. C 2015, 3, 2656–2663. [Google Scholar] [CrossRef]
- Yang, J.; Moraillon, A.; Siriwardena, A.; Boukherroub, R.; Ozanam, F.; Gouget-Laemmel, A.C.; Szunerits, S. Carbohydrate Microarray for the Detection of Glycan-Protein Interactions Using Metal-Enhanced Fluorescence. Anal. Chem. 2015, 87, 3721–3728. [Google Scholar] [CrossRef]
- Karabchevsky, A.; Patzig, C.; Rauschenbach, B.; Abdulhalim, I. Microspot Surface Enhanced Fluorescence from Sculptured Thin Films for Control of Antibody Immobilization. In Proceedings of the Nanostructured Thin Films IV, San Diego, CA, USA, 23–25 August 2011; Martín-Palma, R.J., Jen, Y.-J., Mackay, T.G., Eds.; SPIE: Washinton, DC, USA, 2011; Volume 8104, p. 81040L. [Google Scholar]
- Xu, Z.; Chen, Y.; Gartia, M.R.; Jiang, J.; Liu, G.L. Surface plasmon enhanced broadband spectrophotometry on black silver substrates. Appl. Phys. Lett. 2011, 98, 241904. [Google Scholar] [CrossRef]
- Loya, M.C.; Brammer, K.S.; Choi, C.; Chen, L.H.; Jin, S. Plasma-induced nanopillars on bare metal coronary stent surface for enhanced endothelialization. Acta Biomater. 2010, 6, 4589–4595. [Google Scholar] [CrossRef]
- Xiao, C.; Cao, Z.; Deng, J.; Huang, Z.; Xu, Z.; Fu, J.; Yobas, L. Microfluidic-Based Metal Enhanced Fluorescence for Capillary Electrophoresis by Ag Nanorod Arrays. Available online: https://iopscience.iop.org/article/10.1088/0957-4484/25/22/225502 (accessed on 28 July 2020).
- Levene, H.J.; Korlach, J.; Turner, S.W.; Foquet, M.; Craighead, H.G.; Webb, W.W. Zero-mode waveguides for single-molecule analysis at high concentrations. Science 2003, 299, 682–686. [Google Scholar] [CrossRef]
- Eid, J.; Fehr, A.; Gray, J.; Luong, K.; Lyle, J.; Otto, G.; Peluso, P.; Rank, D.; Baybayan, P.; Bettman, B.; et al. Real-time DNA sequencing from single polymerase molecules. Science 2009, 323, 133–138. [Google Scholar] [CrossRef]
- Ponzellini, P.; Zambrana-Puyalto, X.; Maccaferri, N.; Lanzanò, L.; De Angelis, F.; Garoli, D. Plasmonic zero mode waveguide for highly confined and enhanced fluorescence emission. Nanoscale 2018, 10, 17362–17369. [Google Scholar] [CrossRef] [PubMed]
- Punj, D.; Mivelle, M.; Moparthi, S.B.; Van Zanten, T.S.; Rigneault, H.; Van Hulst, N.F.; García-Parajó, M.F.; Wenger, J. A plasmonic “antenna-in-box” platform for enhanced single-molecule analysis at micromolar concentrations. Nat. Nanotechnol. 2013, 8, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Tang, C.; Zhao, D.; Zhang, H.; Yu, D.; Yu, M.; Balram, K.C.; Gersen, H.; Yang, B.; Cao, W.; et al. Diameter-optimized high-order waveguide nanorods for fluorescence enhancement applied in ultrasensitive bioassays. Nanoscale 2019, 11, 14322–14329. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ding, F.; Li, W.D.; Wang, Y.; Hu, J.; Chou, S.Y. Giant and uniform fluorescence enhancement over large areas using plasmonic nanodots in 3D resonant cavity nanoantenna by nanoimprinting. Nanotechnology 2012, 23, 225301. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Ding, F.; Chen, H.; Ding, W.; Zhang, W.; Chou, S.Y. Enhancement of immunoassay’s fluorescence and detection sensitivity using three-dimensional plasmonic nano-antenna-dots array. Anal. Chem. 2012, 84, 4489–4495. [Google Scholar] [CrossRef]
- Aouani, H.; Mahboub, O.; Bonod, N.; Devaux, E.; Popov, E.; Rigneault, H.; Ebbesen, T.W.; Wenger, J. Bright unidirectional fluorescence emission of molecules in a nanoaperture with plasmonic corrugations. Nano Lett. 2011, 11, 637–644. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, H.Y.; Weng, H.; Gao, B.R.; Hao, Y.W.; Jin, Y.; Chen, Q.D.; Sun, H.B. Surface plasmon enhanced fluorescence of dye molecules on metal grating films. J. Phys. Chem. C 2011, 115, 12636–12642. [Google Scholar] [CrossRef]
- Pale, V.; Kauppinen, C.; Selin, J.; Sopanen, M.; Tittonen, I. Fluorescence-enhancing plasmonic silver nanostructures using azopolymer lithography. RSC Adv. 2016, 6, 48129–48136. [Google Scholar] [CrossRef]
- Yoo, H.W.; Jung, J.M.; Lee, S.K.; Jung, H.T. The fabrication of highly ordered silver nanodot patterns by platinum assisted nanoimprint lithography. Nanotechnology 2011, 22, 095304. [Google Scholar] [CrossRef]
- Wood, A.; Grant, S.; Basuray, S.; Pathak, A.; Bok, S.; Mathai, C.; Gangopadhyay, K.; Gangopadhyay, S. Enhanced Fluorescence Through the Incorporation of Nanocones/Gaps into a Plasmonic Gratings Sensor Platform. In Proceedings of the IEEE Sensors, Valencia, Spain, 2–5 November 2014; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2014; pp. 1479–1482. [Google Scholar]
- Wood, A.J.; Chen, B.; Pathan, S.; Bok, S.; Mathai, C.J.; Gangopadhyay, K.; Grant, S.A.; Gangopadhyay, S. Influence of silver grain size, roughness, and profile on the extraordinary fluorescence enhancement capabilities of grating coupled surface plasmon resonance. RSC Adv. 2015, 5, 78534–78544. [Google Scholar] [CrossRef]
- Tawa, K.; Nakayama, T.; Kintaka, K. Optimal structure of a plasmonic chip for sensitive bio-detection with the grating-coupled surface plasmon-field enhanced fluorescence (GC-SPF). Materials 2017, 10, 1063. [Google Scholar] [CrossRef] [PubMed]
- Zang, F.; Su, Z.; Zhou, L.; Konduru, K.; Kaplan, G.; Chou, S.Y. Ultrasensitive Ebola Virus Antigen Sensing via 3D Nanoantenna Arrays. Adv. Mater. 2019, 31, 1902331. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.F.; Wang, S.F.; Huang, J.C.; Su, L.C.; Yao, L.; Li, Y.C.; Wu, S.C.; Chen, Y.M.A.; Hsieh, J.P.; Chou, C. Detection of swine-origin influenza A (H1N1) viruses using a localized surface plasmon coupled fluorescence fiber-optic biosensor. Biosens. Bioelectron. 2010, 26, 1068–1073. [Google Scholar] [CrossRef] [PubMed]
- Oubaha, M.; Gorin, A.; McDonagh, C.; Duffy, B.; Copperwhite, R. Development of a multianalyte optical sol-gel biosensor for medical diagnostic. Sensors Actuators B Chem. 2015, 221, 96–103. [Google Scholar] [CrossRef]
- Deng, Y.-L.; Xu, D.-D.; Pang, D.-W.; Tang, H.-W. Target-triggered signal turn-on detection of prostate specific antigen based on metal-enhanced fluorescence of Ag@SiO2@SiO2-RuBpy composite nanoparticles. Nanotechnology 2017, 28, 065501. [Google Scholar] [CrossRef]
- Huang, J.C.; Chang, Y.F.; Chen, K.H.; Su, L.C.; Lee, C.W.; Chen, C.C.; Chen, Y.M.A.; Chou, C. Detection of severe acute respiratory syndrome (SARS) coronavirus nucleocapsid protein in human serum using a localized surface plasmon coupled fluorescence fiber-optic biosensor. Biosens. Bioelectron. 2009, 25, 320–325. [Google Scholar] [CrossRef]
- Abbas, N.; Lu, X.; Badshah, M.A.; In, J.B.; Heo, W.I.; Park, K.Y.; Lee, M.-K.; Kim, C.H.; Kang, P.; Chang, W.-J.; et al. Development of a Protein Microarray Chip with Enhanced Fluorescence for Identification of Semen and Vaginal Fluid. Sensors 2018, 18, 3874. [Google Scholar] [CrossRef]
- Ganguli, A.; Mostafa, A.; Berger, J.; Aydin, M.; Sun, F.; Valera, E.; Cunningham, B.T.; King, W.P.; Bashir, R. Rapid Isothermal Amplification and Portable Detection System for SARS-CoV-2. bioRxiv Prepr. Serv. Biol. 2020. [Google Scholar] [CrossRef]
- Della Ventura, B.; Gelzo, M.; Battista, E.; Alabastri, A.; Schirato, A.; Castaldo, G.; Corso, G.; Gentile, F.; Velotta, R. Biosensor for Point-of-Care Analysis of Immunoglobulins in Urine by Metal Enhanced Fluorescence from Gold Nanoparticles. ACS Appl. Mater. Interfaces 2019, 11, 3753–3762. [Google Scholar] [CrossRef]
- Lucas, E.; Knoblauch, R.; Combs-Bosse, M.; Broedel, S.E.; Geddes, C.D. Low-concentration trypsin detection from a metal-enhanced fluorescence (MEF) platform: Towards the development of ultra-sensitive and rapid detection of proteolytic enzymes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 228, 117739. [Google Scholar] [CrossRef]
- Lee, W.I.; Shrivastava, S.; Duy, L.T.; Yeong Kim, B.; Son, Y.M.; Lee, N.E. A smartphone imaging-based label-free and dual-wavelength fluorescent biosensor with high sensitivity and accuracy. Biosens. Bioelectron. 2017, 94, 643–650. [Google Scholar] [CrossRef]
- Narasimhan, V.; Siddique, R.H.; Hoffmann, M.; Kumar, S.; Choo, H. Enhanced broadband fluorescence detection of nucleic acids using multipolar gap-plasmons on biomimetic Au metasurfaces. Nanoscale 2019, 11, 13750–13757. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.; Kim, M.; Koh, W.-G. Ag@SiO2-entrapped hydrogel microarray: A new platform for a metal-enhanced fluorescence-based protein assay. Analyst 2015, 140, 3375–3383. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Rong, Z.; Xiao, R.; Wang, S. “Turn on” and label-free core−shell Ag@SiO2 nanoparticles-based metal-enhanced fluorescent (MEF) aptasensor for Hg2+. Sci. Rep. 2015, 5, 9451. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kwon, J.E.; Lee, K.; Koh, W.-G. Signal-amplifying nanoparticle/hydrogel hybrid microarray biosensor for metal-enhanced fluorescence detection of organophosphorus compounds. Biofabrication 2018, 10, 35002. [Google Scholar] [CrossRef]
- Tawa, K.; Yokota, Y.; Kintaka, K.; Nishii, J.; Nakaoki, T. An application of a plasmonic chip with enhanced fluorescence to a simple biosensor with extended dynamic range. Sens. Actuators B Chem. 2011, 157, 703–709. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Lin, Q.; Wei, Y.; Wang, J.; Li, Y.; Yang, R.; Yuan, Q. Highly Sensitive Detection of Bladder Cancer-Related miRNA in Urine Using Time-Gated Luminescent Biochip. ACS Sens. 2019, 4, 2124–2130. [Google Scholar] [CrossRef] [PubMed]
- Hawa, G.; Sonnleitner, L.; Missbichler, A.; Prinz, A.; Bauer, G.; Mauracher, C. Single step, direct fluorescence immunoassays based on metal enhanced fluorescence (MEF-FIA) applicable as micro plate-, array-, multiplexing- or point of care-format. Anal. Biochem. 2018, 549, 39–44. [Google Scholar] [CrossRef]






| Metals | Plasmonic Characteristics | Chemical Reactivity | Reference | ||||
|---|---|---|---|---|---|---|---|
| UV | VIS | NIR | IB | Q Factor | |||
| Silver (Ag) | - | - | High | Biocompatible; easily oxidized | [12] | ||
| Copper (Cu) | - <600 nm | Low | Easily oxidized | [13,16] | |||
| Gold (Au) | - | - | - <500 nm | High | Biocompatible; Stable | [18,19] | |
| Aluminium (Al) | - | - | Low | Stable after surface passivation | [17] | ||
| Palladium (Pd) | - | Low | Stable | [14,15] | |||
| Platinum (Pt) | - | Low | Stable | [15] | |||
| Material | Configuration of Structures | Structures Feature Size (nm) | Wavelength λ (nm) | Fluorophore | EF | Year | Ref. |
|---|---|---|---|---|---|---|---|
| Cu | Structured thin-film nanorods | Height: 550 nm | 590 nm | Rhodamine 123 | 02 | 2012 | [17] |
| Au | Structured thin-film nanorods | Dia: 40 nm Height: 285 nm | 590 nm | Rhodamine 123 | 3.9 | 2012 | [17] |
| Zno | Vertical nanorods | Dia: 83.2 nm Height: 170 nm | 645 nm | Alexa Fluor 647 | 5.7 | 2015 | [110] |
| Au | Nanorods | Dia: 30 nm Height: 13 nm | 650 nm | Alexa 647 | 10 | 2015 | [111] |
| Ag | Structured thin-film nanorods | Dia: 75 nm Height: 400 nm | 590 nm | Rhodamine 123 | 20 | 2011 | [112] |
| Ag | Slanted nanorods | Height: 1000 nm | 635 nm | Cy5 | 23 | 2013 | [108] |
| Ag | Structured thin-film nanorods | Dia: 75 nm Height: 400 nm | 590 nm | Rhodamine 123 | 23 | 2012 | [17] |
| ZnO | Flower shape nanorods | Dia: 718.5 nm Height: 200 nm | 515 nm | Alexa Fluor 532 | 25 | 2015 | [110] |
| Ag | Zigzag nanorods | Height: 2000 nm | 525 nm | Alexa 488 | 28 | 2016 | [106] |
| Ag | Nanocone | Diabase: 180 nm Height: 500 nm | 528 nm | Rhodamine 6G | 30 | 2011 | [113] |
| Ag | Slanted nanorods | Length: 635 nm | 555 nm | Rhodamine 6G | 32 | 2015 | [109] |
| Al | Structured thin-film nanorods | Dia: 30 nm Height: 1000 nm | 590 nm | Rhodamine 123 | 37 | 2012 | [17] |
| Zno | Flower shape nanorods | Dia: 718.5 nm Height: 200 nm | 645 nm | Alexa Fluor 647 | 45 | 2015 | [110] |
| Ag | Structured thin-film nanorods | Dia: 75 nm Height: 400 nm | 590 nm | Rhodamine 123 | 71 | 2012 | [17] |
| Ag | Slanted nanorods | Dia: 220 nm Height: 3000 nm | - | Bovine aortic endothelial cell | - | 2010 | [114] |
| Ag | Vertical nanorods structures | Dia: 120 nm Height: 500 nm | 635 nm | Cy5 | 200 | 2018 | [22] |
| Ag | nanorods | Dia: 89 nm Height: 3000 nm | 520 nm | fluorescein-5-isothiocyanate | 494 | 2014 | [115] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badshah, M.A.; Koh, N.Y.; Zia, A.W.; Abbas, N.; Zahra, Z.; Saleem, M.W. Recent Developments in Plasmonic Nanostructures for Metal Enhanced Fluorescence-Based Biosensing. Nanomaterials 2020, 10, 1749. https://doi.org/10.3390/nano10091749
Badshah MA, Koh NY, Zia AW, Abbas N, Zahra Z, Saleem MW. Recent Developments in Plasmonic Nanostructures for Metal Enhanced Fluorescence-Based Biosensing. Nanomaterials. 2020; 10(9):1749. https://doi.org/10.3390/nano10091749
Chicago/Turabian StyleBadshah, Mohsin Ali, Na Yoon Koh, Abdul Wasy Zia, Naseem Abbas, Zahra Zahra, and Muhammad Wajid Saleem. 2020. "Recent Developments in Plasmonic Nanostructures for Metal Enhanced Fluorescence-Based Biosensing" Nanomaterials 10, no. 9: 1749. https://doi.org/10.3390/nano10091749
APA StyleBadshah, M. A., Koh, N. Y., Zia, A. W., Abbas, N., Zahra, Z., & Saleem, M. W. (2020). Recent Developments in Plasmonic Nanostructures for Metal Enhanced Fluorescence-Based Biosensing. Nanomaterials, 10(9), 1749. https://doi.org/10.3390/nano10091749