Abstract
The diversification of pollutants type and concentration in wastewater has underlined the importance of finding new alternatives to traditional treatment methods. Advanced oxidation processes (AOPs), among others, are considered as promising candidate to efficiently remove organic pollutants such as dyes or pharmaceutical active compounds (PhACs). The present minireview resumes several recent achievements on the implementation and optimization of photoactive heterostructures used as photocatalysts for dyes and PhACs removal. The paper is focused on various methods of enhancing the heterostructure photocatalytic properties by optimizing parameters such as synthesis methods, composition, crystallinity, morphology, pollutant concentration and light irradiation.
1. Introduction
The increase of the world population, especially in the last 50 years has stimulated the industrial growth and water consumption. However, with the rapid development of industrialization, the presence of organic pollutants in water and environment is now an important problem, creating hazardous threats to the ecological system [1,2,3].
Dyes and pharmaceutical compounds represent two important classes of organic pollutants affecting the water and human life quality [4,5]. The contamination with dyes substances such as rhodamine B (RhB), malachite green (MG), methyl orange (MO), etc. have raised serious issues related to human health. Most of the dyes are considered harmful due to the high toxicity and carcinogenicity induced by their nonbiodegradable aromatic structure [6,7,8]. Pharmaceutically active compounds (PhACs), such as tetracycline (TC), ciprofloxacin (CIP), triclosan (TCS), carbamazepine (CBZ), salicylic acid (SA), ibuprofen (IBS) and sulfamethazine (SMZ), etc., are helpful to cure human diseases but the pollution from metabolized or partially metabolized pharmaceutical wastes is already recognized as a hazard [9,10,11].
Advanced oxidation processes (AOPs) represent an energy-efficient and green approach suitable for environmental remediation, particularly optimized for wastewater treatment due to the use of solar energy as a driving force to initiate the oxidation reactions [12,13,14]. The photocatalysis is considered as an advanced oxidation process, using stand-alone or coupled semiconductors as well as other composite materials [15,16,17].
Breakthrough heterostructures that benefit from the full range of solar light have attracted the attention of many scientists, the UV, visible or near infrared photoexcitation depending on their band gaps energy and ability to generate (super)oxidative species able to destroy organic pollutants [18,19]. Owing to the controllable band structures and efficient electron-hole separation, the heterostructured photocatalyst exhibits a superior performance to their individual components [20,21,22].
The synergistic effect of coupling photocatalysis with other techniques in order to improve the pollutant degradation efficiency was studied by many research groups. Coupling adsorption with photocatalysis has shown promising results on RhB [23] and MG [24] dyes removal. Encouraging results were obtained on PhACs [25,26] and dyes [27] removal by coupling biodegradation with photocatalysis. Enhanced mineralization conversion efficiencies were obtained by coupling flocculation with photocatalysis [28], ultrasound with photocatalysis [29] and plasma with photocatalysis [30].
The present minireview resumes several recent achievements on the implementation and optimization of photoactive heterostructures used as photocatalysts for dyes and PhACs removal. Many other papers which are not included here have the potential to contain highly innovative work. The paper is focused on the influence of crystallinity, morphology, pollutant concentration, irradiation time and light spectra on the photocatalytic activity of various heterostructures. This minireview make a comparative evaluation of the radiation intensity and spectra required for organic pollutant removal based on the heterostructure charge transport mechanism. The photocatalytic efficiency refers to partial or total pollutant degradation according to the information’s provided in the literature. The lack of standardization regarding the photocatalysis experimental parameters has, as a consequence, the presence of various scientific papers containing data difficult to compare.
2. Heterostructure Mechanisms for Photocatalytic Application
Photocatalysis is a chemical process mediated by one or more semiconductors, which under irradiation increase the reduction and oxidation (redox) reactions rate based on charge carriers’ generation [31,32]. When the chemical potential of the electrons from the conduction-band (CB) is between +0.5 and −2.0 V versus the normal hydrogen electrode (NHE), they act as reductants due to the strong oxidizability [33,34,35]. The photocatalytic process is characterized by three main steps: (i) electron-hole pair’s generation due to the light absorption, (ii) the diffusion of photoexcited charge carriers on the semiconductor surface and (iii) the redox reaction on the semiconductor surface [36,37,38].
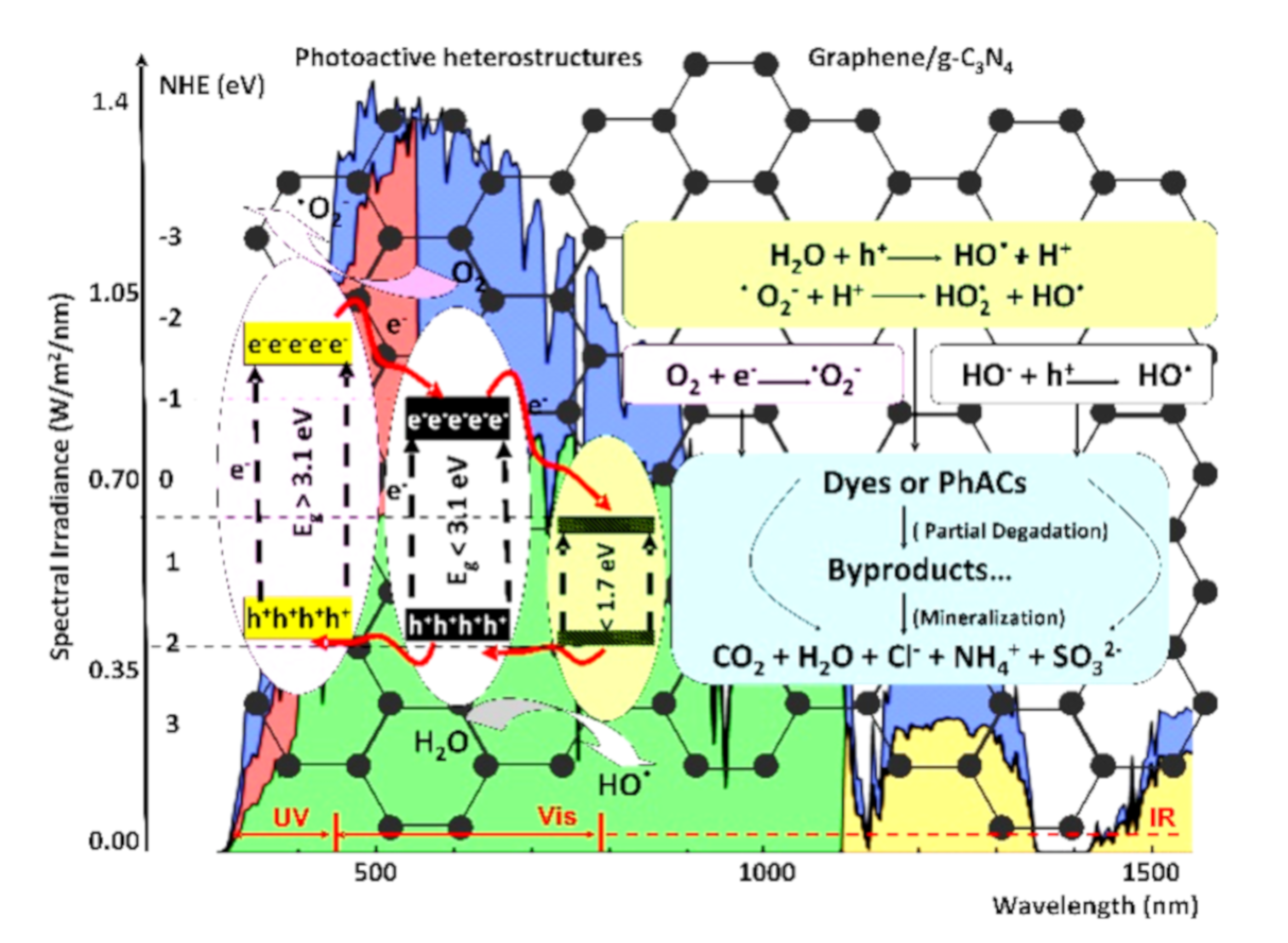
As presented in Figure 1, during the photocatalytic degradation of organic pollutants, the photogenerated electrons and holes are trapped by dissolved O2 and H2O [39,40]. Highly reactive oxygen species, including superoxide (O2–) and hydroxyl radicals (OH), are developed under light irradiation [41,42]. If the organic pollutants contain only carbon, oxygen and hydrogen atoms, it can be degraded to by-products or even completely mineralized (CO2 and H2O). However, the major part of dyes and PhACs contains also other atoms, and the mineralization occurs with the formation of additional products (based on Cl−, NH4+, SO32−, etc.) [43,44].

Figure 1.
Photoactive heterostructures and the corresponding photocatalytic reactions.
Photoactive heterostructures are considered as composite materials developed by using at least two components/compounds with similar or different chemical nature and able to develop charge carriers during the light irradiation [45,46,47]. The literature outlines four typical heterostructures mechanisms (p-n junctions, type II heterostructures, Schottky junctions and Z-scheme heterostructures) mostly used in photocatalytic studies.
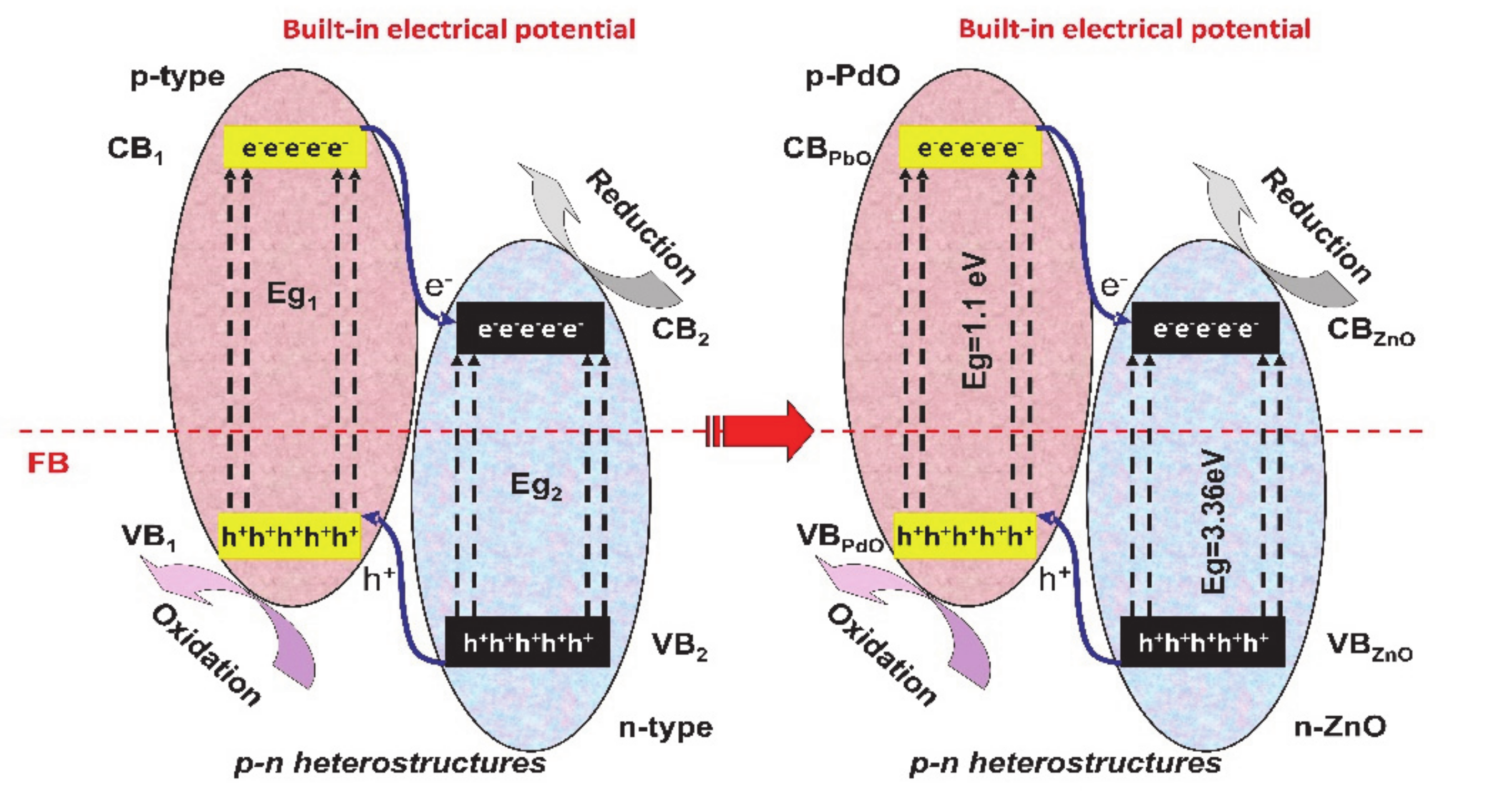
The p-n junctions (see Figure 2) mimics the photovoltaic cell mechanism but with the difference that the charge carriers are used in redox reactions. Between the p-type and n-type semiconductors, an interfacial region is formed and the electrons from the CB of the p-type semiconductor migrate to the n-type semiconductor. The holes follow the reverse direction, from the n-type semiconductor VB to the p-type semiconductor VB forming a space-charge region. Due to the charge carrier’s diffusion, the space-charge region will have an established built-in potential directing the electrons and holes flow in opposite directions [48,49,50].

Figure 2.
The p-n junction’s mechanism.
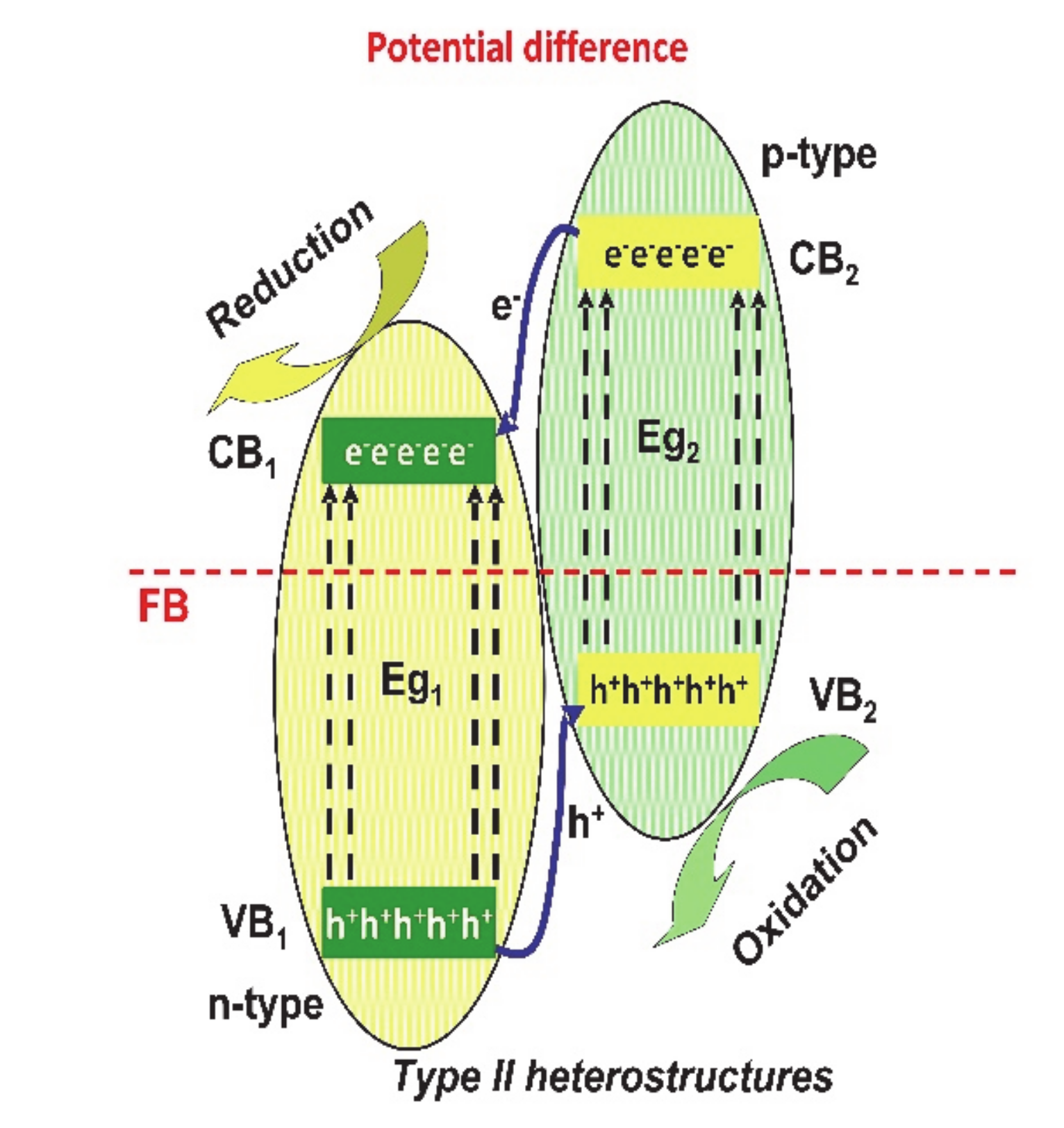
In the type-II (see Figure 3), the potential difference between the semiconductors will easily separate electrons and holes, preventing subsequent carrier recombination. The reason consists on the fact that the CB and VB of one semiconductor are higher than that of the other semiconductor, which assures an effective separation with holes and electrons at different sides of the heterojunction [51,52,53].

Figure 3.
The type-II heterostructures mechanism.
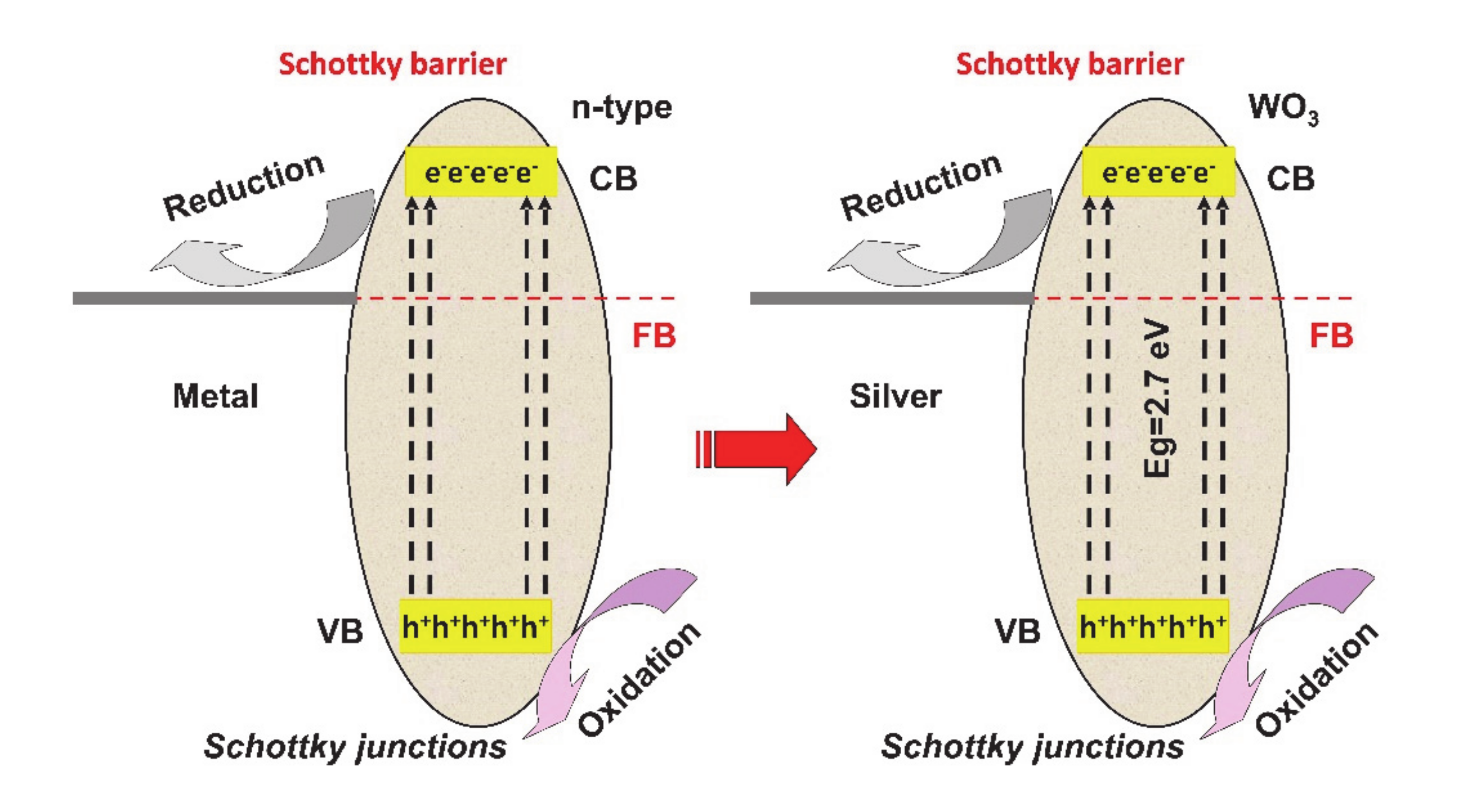
The Schottky junctions (see Figure 4) represented by the semiconductor–metal interface, can induce an effective way to reduce charge carriers’ recombination and extend the spectral light absorption of the semiconductor. One of the most important features is represented by the localized surface plasmonic resonance (LSPR) effect, which assures the visible light absorption and the excitation of active electrons/holes pairs. The LSPR takes place when the work function of the metal surpasses that of the semiconductor, developing a positive space-charge region on the semiconductor surface as well as an upwards of the semiconductor band [54,55,56].

Figure 4.
Schottky junction’s mechanism.
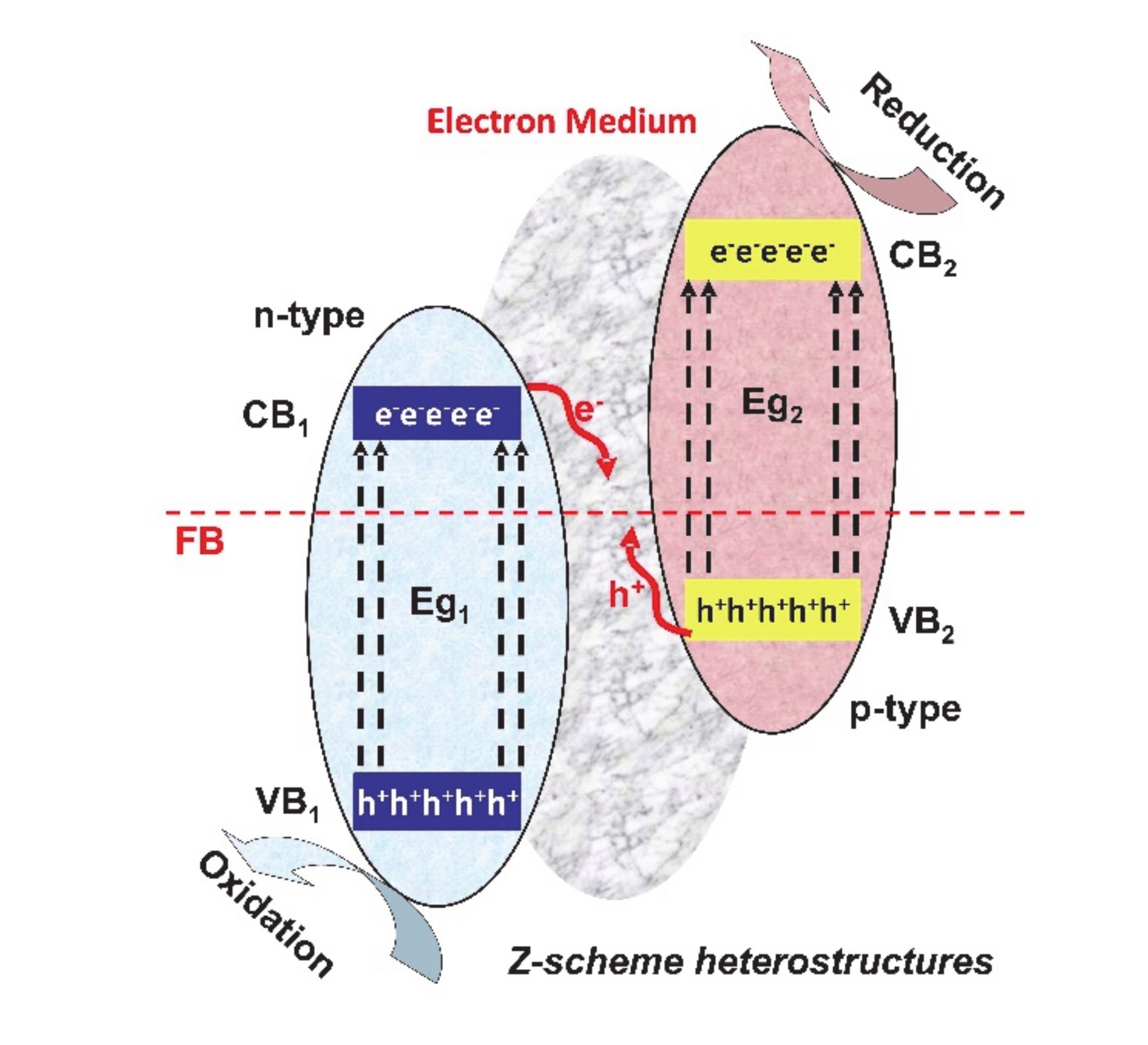
The Z-scheme (see Figure 5) consists of two connected photocatalytic systems, with the advantage of endowing charge carriers with stronger reduction/oxidation properties.

Figure 5.
Z-scheme heterostructures for photocatalytic applications.
These systems can use shuttle redox mediators, solid-state electron mediators or no electron mediators. The photogenerated electrons from the second photocatalytic systems will migrate to the electron medium, in order to recombine with the photogenerated holes from the first photocatalytic system. In this way, the opposite electrons and holes are involved in the reduction and oxidation reactions, which assure a highly efficient charge separation [57,58,59].
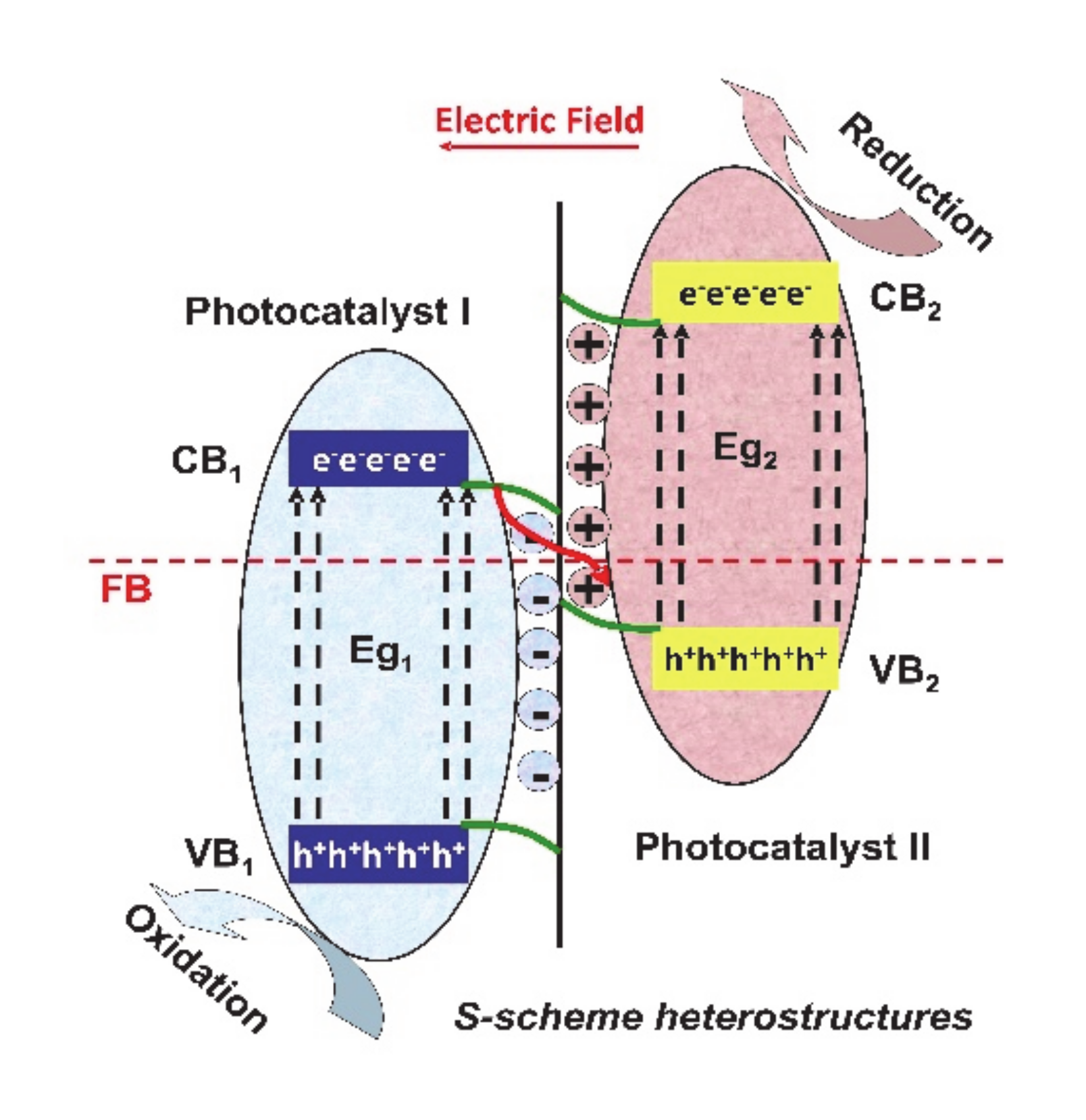
A relatively new type of heterostructure mechanism is represented by S-scheme (see Figure 6), where the photogenerated electrons from photocatalyst I with lower conduction band (CB) potential will combine the photogenerated holes from photocatalyst II with higher valence band (VB) potential based on the driving force of the internal electric field, thereby inducing the spatial separation of charge carriers under irradiation, considering the conditions of ensuring the strong redox ability [60].

Figure 6.
The mechanism of S-scheme heterostructures.
3. Photocatalytic Organic Pollutants Removal by Heterostructures
3.1. Dyes
Dyes are substances with complex chemical structures involving aromatic cycles and functional groups [61,62,63]. There are several classifications such as acid dyes, alkaline dyes, azo dyes, sulphur dye, etc., most of them considering the composition and the solubility in water. Between others, the literature mentions the following representative dyes: RhB, methylene blue (MB), MO and MG. These dyes molecules are characterized by aromatic structures with high chemical stability toward traditional wastewater treatment processes. Table 1 includes representative studies regarding photocatalysts materials (composition, synthesis method, morphology and crystallinity) and photocatalytic parameters (pollutant concentration, radiation type, intensity and time), influencing the dyes photocatalytic removal process (efficiency and rate constant).

Table 1.
Recent representative studies on heterostructures photocatalytic application for dyes removal.
The RhB photocatalytic removal using g-C3N4-based heterostructures obtained by ultrasonication [64,65] and hydrothermal [66,67] methods were investigated in the presence of Vis irradiation. The Bi12TiO20/g-C3N4 [64] and Bi3.84W0.16O6.24 (BWO)/g-C3N4 [65] heterostructures were evaluated in similar photocatalytic conditions (10 mg/L RhB and 50 min irradiation period), but with different light intensities (500 W for Bi12TiO20/g-C3N4 and 100 W for Bi3.84W0.16O6.24 (BWO)/g-C3N4). Using smaller radiation intensity, the Bi3.84W0.16O6.24 (BWO)/g-C3N4 heterostructure exhibits higher photocatalytic efficiency (99.8%) with lower energy consumption than that of Bi12TiO20/g-C3N4 (97%). Both heterostructures follow the Z-scheme mechanism and have similar crystalline structures, but different morphologies (sheet-like for Bi3.84W0.16O6.24 (BWO)/g-C3N4 and multilayer for Bi12TiO20/g-C3N4). A similar observation is valid for TiO2/g-C3N4 [66] and g-C3N4/ZnO [67] obtained by the hydrothermal method. In this case, the g-C3N4/ZnO heterostructure achieves 98.5% photocatalytic efficiency toward RhB after 70 min of 300 W Vis light irradiation. The TiO2/g-C3N4 reaches only 85% after 100 min of Vis light irradiation (500 W). The g-C3N4/ZnO benefit from the large rods shape interfaces, which improve the energy conversion due to the larger active surface. In TiO2/g-C3N4, there was tensile stress induced by TiO2 and compressive stress from C3N4, with detrimental influence on the heterostructure interfacial area. However, using the same irradiation scenario (Vis, 500 W for 100 min) but employing WS2/BiOBr heterostructure [68], the photocatalytic efficiency of RhB (20 mg/L) has increased up to 95%, due to high Vis absorbance attributed to both partners. Even if the photocatalytic efficiency is lower compared with g-C3N4/ZnO, the RhB concentration was double.
Solvothermal method was used to develop NiO/BiOI S-scheme heterostructure [60], Bi2MoO6/Bi5O7Br/TiO2 Z-scheme heterostructure [69] and Bi2MoO6/Fe3O [70] type II heterostructure. The optimum energy consumption corresponds to NiO/BiOI reaching 90% RhB (5 mg/L) photocatalytic efficiency in 60 min of irradiation with 300 W Vis light source. The NiO/BiOI is characterized by porous morphology and follows the S-scheme mechanism, in which the electrons from CB of BiOI migrate towards NiO in the junction interface due to the formation of an internal electric field. The Bi2MoO6/Fe3O heterostructure has higher photocatalytic efficiency (99.5%) but after a longer irradiation period (120 min) with 350 W Vis light source. However, in this case, the RhB concentration was 20 mg/L, which means that even if the energy consumption was higher, the quantity of pollutant removed from the aqueous solution was also bigger. The Bi2MoO6/Fe3O is a type II heterostructure with flower-like morphology, who benefit from the surface oxygen vacancies, which serve as electron-trapping sites, and have an important role in the charge transmission through the heterostructures by facilitating the migration of the bulk electron-hole. Higher energy consumption corresponds to Bi2MoO6/Bi5O7Br/TiO2, which follows a Z-scheme mechanism and exhibits 73.43% photocatalytic efficiency of RhB (10 mg/L) after 180 min of irradiation with 500 W Vis light source.
Bi2MoO6/Fe3O type II heterostructure [70] was employed to investigate MB removal in the same photocatalytic conditions as RhB removal. However, in the case of MB, the photocatalytic efficiency has increased up to 93.81%, indicating that this heterostructure can be optimized for a particular type of pollutant molecule. Ta3B2@Ta2O5 [71] heterostructure was obtained by in situ growth method, and the photocatalytic properties were tested in the same conditions as Bi2MoO6/Fe3O (180 min irradiation period, 500 W Vis light source). The photocatalytic efficiency was lower (80%) compared with Bi2MoO6/Fe3O, but the quantity of RhB removal was higher, considering that the initial dye concentration was 5 times bigger (50 mg/L). Concluding, for the same amount of energy consumption the quantity of pollutant removal is significantly higher. The Ta3B2@Ta2O5 heterostructure is characterized by irregular shape particles and contains high crystalline Ta3B2 and Ta2O5. The heterostructure mechanism corresponds to Schottky junction and has the advantage of the metallic transition zone, which improves the visible light absorption and the electron collector facilitating the charge transfer from CB of Ta2O5 to surface.
The g-C3N4/ZnO heterostructure [67] previously presented for RhB removal was also tested for MB (10 mg/L) removal in the same photocatalytic conditions (70 min, 300 W Vis light source). In this particular study, there was no significant difference regarding the photocatalytic efficiency values for both dyes (98% MB and 98.5% RhB) meaning that the heterostructure can efficiently remove different organic dye molecules. A similar experiment was done with BiPO4−x/B2S3 [72] using half of the MB concentration (5 mg/L) and 300 W Vis light intensity. The photocatalytic efficiency has reached 98% after 360 min of irradiation, which represents 5 times more energy consumption for a lower pollutant concentration. The BiPO4−x/B2S3 has a sheets morphology containing monoclinic BiPO4 and orthorhombic Bi2S3 and follows a Z-scheme pathway. The lower photocatalytic efficiency can be a consequence of ultraviolet light heterostructure exposure before the photocatalytic experiment, inducing electrons migration during the conversion of oxygen atoms in oxygen vacancy who may act as the recombination centre of photoinduced charge carriers.
UV light was used to evaluate the photocatalytic performance of spherical particles MnFe2O4/rGO [73] and flower-like Ag/hybridized 1T-2H MoS2/TiO2 [74] after 60 min of irradiation. Both heterostructures exhibit similar efficiencies (~97%) but at different MB concentrations. The MnFe2O4/rGO heterostructure obtained by coprecipitation method requires only 40 W light intensity to remove 97% of MB (10 mg/L) due to the reduced graphene oxide insertion (rGO), which induces a decrease of MnFe2O4 crystallite size from 21 to 18 nm and increase the junction interfacial area. The Ag/hybridized 1T-2H MoS2/TiO2 obtained by chemical reduction exhibits a dual Schottky junction and Z-scheme mechanisms, and was able to remove 96.8% of MB (20 mg/L) using 235 W UV light source. Even if the MB concentration was double, the energy consumption has increased almost 6 times which make this system less energy efficient. Another heterostructure obtained by coprecipitation method and tested under UV irradiation using 30 mg/L MB aqueous solution was ZnAl2O4/Bi2MoO6 [75]. The heterostructure exhibits a sheet-like morphology containing koechlinite Bi2MoO6 and gahnite ZnAl2O4, and follow a type II mechanism in which the VB potential of Bi2MoO6 is higher than that of ZnAl2O4 favouring the formation of superoxidative species. After 180 min of UV irradiation (100 W), the photocatalytic efficiency was 86.36%, due to the hinder effect of the ZnAl2O4 on the Bi2MoO6 light absorption through the suspension solution.
A comparative study regarding the influence of light spectra on the photocatalytic efficiency at low MB concentration (1 mg/L) was done using CuO–TiO2 heterostructure [76], obtained by ultrasonication. The TiO2 fibres sizes were in the range of 150–500 nm with CuO particles on the surface. The study indicates that CuO–TiO2 follow a p-n heterostructure mechanism with superior photocatalytic properties during UVc irradiation when the rate constant was 0.135 min−1, almost ten times higher than that in the presence of Vis irradiation. Consequently, after 45 min of UVc (96 W) irradiation, 99% of MB was removed and the same value was obtained by Vis (240 W) irradiation but after 240 min.
The influence of 300 W Vis light source on the MO photocatalytic removal was tested using LaNiO3/TiO2 S-scheme [77], ZnFe2O4/SnS2 p-n [78] and WO3/g-C3N4 Z-scheme [79] heterostructures. ZnFe2O4/SnS2 heterostructure obtained by the solvothermal method has the lowest energy consumption being able to remove 99% of MO (50 mg/L) in just 20 min. The ZnFe2O4/SnS2 heterostructure exhibits irregular particles shape, and the charges transport mechanism is based on the photogenerated electrons migration into the CB of SnS2 from the CB of ZnFe2O4. The electron-hole recombination is avoided, due to the photogenerated holes transit from VB of SnS2 to VB of ZnFe2O4. LaNiO3/TiO2 and WO3/g-C3N4 show similar photocatalytic activity toward MO (10 mg/L), reaching 100% photocatalytic efficiency in 150 min (LaNiO3/TiO2) and 93% in 120 min (WO3/g-C3N4). The LaNiO3/TiO2 heterostructure with irregular particles morphology was obtained by sol–gel technique and contains anatase/rutile TiO2 as well as crystalline LaNiO3, both being able to develop separate electron-hole pairs under irradiation. The charge carrier’s concentration increases and induces a certain potential difference able to enhance the photocatalytic activity of the S-scheme. The photocatalytic efficiency decreases at 92% when MO concentration is double (20 mg/L), due to the heterostructure limitation to form enough oxidative and superoxidative species in a short period. The porous morphology and small g-C3N4 sheet provide larger surface area and homogenous spread active sites, housing the photochemical reactions and facilitating the mass transport through the WO3/g-C3N4 heterostructure.
The photocatalytic activity decreases when 90 W UVc light was used to irradiate Ag2Mo1−xWxO4 (Ag2WO4/Ag2MoO4) [80] rod-shaped heterostructure, obtained by the microwave-assisted hydrothermal method. Even if the MO concentration was relatively low (5 mg/L), the photocatalytic efficiency after 140 min was 45% due to the available light spectra. The photocatalytic activity can be increased by using an extended light spectrum, considering that both Ag2WO4 and Ag2MoO4 have good Vis absorbance.
Highly concentrated MG (50 mg/L) aqueous solution was used to evaluate the photocatalytic properties of TiO2/WO3 [81] and CdS@ZnS@ZnO [82] heterostructures. Under Vis irradiation, the TiO2/WO3 hollow sphere heterostructure shows a better energy performance reaching 98% photocatalytic efficiency after 60 min using a 300 W light source. The TiO2/WO3 composite can efficiently use the visible light to the electronic structure of composite and the quantum effect arising from small particle size. CdS@ZnS@ZnO spherical shape heterostructure obtained by the hydrothermal method has bigger energy consumption and requires 400 W Vis light source for 180 min to remove 32.5 mg/L (65%) from the 50 mg/L MG initial concentration. However, the situation is drastically changed under UV (125 W) light where the photocatalytic efficiency increases at 95% only after 30 min of irradiation. The CdS@ZnS@ZnO follows a type II mechanism, where the VB and CB potential of ZnO are higher than ZnS allowing the photogenerated holes to be transferred on VB of ZnS. When the CdS was added, the photogenerated electrons could migrate from the CB of ZnS to the CB of CdS and further transferred on the CB of ZnO. It seems reasonable to consider that UV scenario is more energy efficient, but the sustainability issue must be underlined (the available UV sunlight spectra on the Earth surface is rather limited).
La2CuO4-decorated ZnO [83] and MgFe2O4/Bi2MoO6 [84] photocatalytic properties based on Z-scheme mechanism were tested after 120 min of irradiation. The results show that MgFe2O4/Bi2MoO6 with plate morphology reaches 97% photocatalytic efficiency for MG removal (20 mg/L initial concentration) using a 300 W Vis light source. The La2CuO4-decorated ZnO heterostructure obtained by in situ extraction exhibits lower photocatalytic efficiency (91%), but at a higher MG concentration (25 mg/L) and in the presence of 125 W Vis light source. Based on the rate constant provided by the authors, the La2CuO4-decorated ZnO have a higher rate constant corresponding to MG removal due to the La2CuO4 ability to convert visible light and to reduce oxygen molecules to superoxide radicals.
To sum up, the most energy-efficient photocatalytic systems correspond to type II mechanism heterostructures with energy consumption between 62 (47.5 mg/L MG removal by CdS@ZnS@ZnO) and 300 Wh (49 mg/L MG removal by TiO2/WO3). Higher energy consumption is attributed to Z-scheme heterostructure (Bi2MoO6/Bi5O7Br/TiO2), which uses 1500 Wh to remove 7.3 mg/L of RhB and 7.64 mg/L of MO. However, the correlation between the heterostructure mechanism and the energy consumption must consider other factors as well (heterostructure composition, pollutant type and concentration, radiation type, etc.).
3.2. Pharmaceutical Active Compounds
The increase of PhACs concentration raises essential issues to traditional wastewater treatment [85,86,87] and requires the involvement of novel processes such as photocatalysis [88,89,90]. The literature mentions a high number of PhACs investigated in AOP experiments, between the most representative are: TC, CIP, TCS, CBZ, SA, IBS and SMZ. Table 2 includes representative studies regarding photocatalysts materials (composition, synthesis method, morphology and crystallinity) and photocatalytic parameters (pollutant concentration, radiation type, intensity and time) influencing the PhACs photocatalytic removal process (efficiency and rate constant).

Table 2.
Recent representative studies on heterostructures photocatalytic application for pharmaceutical active compounds removal.
The photocatalytic removal of TC under Vis light as irradiation source was evaluated using g-C3N4-based heterostructures. Three heterostructures (BN/B-doped-g-C3N4 [91], WO3/g-C3N4 [79] and g-C3N4-decorated ZrO2−x [92]) following the Z-scheme mechanism were irradiated with a 300 W Vis light source in order to study the photocatalytic activity toward 10 mg/L TC solution. g-C3N4-decorated ZrO2−x with tube morphology obtained by anodic oxidation and physical vapour deposition exhibits lower energy consumption, requiring only 60 min to reach 90.6% photocatalytic efficiency. This result was influenced by the abundant defects states and lattice disorder, allowing ZnO2−x to extend the absorption range to visible spectral region. The interfacial band bending and directed build-in electric field present in the band structure induce an increase of the charge carriers’ mobility and concentration. The sheets-like BN/B-doped-g-C3N4 heterostructure obtained by in situ growth exhibits 88.1% photocatalytic efficiency after 60 min of irradiation. The small difference in the photocatalytic efficiency can be induced by the influence of boron doping concentration on the charge carrier mobility. The BN will canalize the photogenerated charges without recombination, which helps electrons to move to the active sites on photocatalyst surface. The boron nitride has a hexagonal structure and does not significantly affect the crystal structure of B-doped-g-C3N4. The highest photocatalytic efficiency (97%) corresponds to WO3/g-C3N4 heterostructure, who was presented during MO-dedicated subsection. However, the energy consumption is significantly higher, considering that the irradiation time is three times longer (180 min) just to gain an extra 6.4% at the overall photocatalytic efficiency.
Using a combined type II and Z-scheme mechanisms, the photocatalytic activity of Ag3PO4/Co3 (PO4)2/g-C3N4 [93] heterostructure was tested using a 10 mg/L TC solution. The Ag3PO4/Co3 (PO4)2/g-C3N4 heterostructure was obtained by precipitation method and exhibited flower-like morphology. The photocatalytic efficiency (88%) is similar to BN/B-doped-g-C3N4 (88.1%) but at longer irradiation period (120 min, 300 W Vis light source), which requires higher energy consumption. The dual electron transfer induced by the combined mechanisms is still a subject of optimization, in order to have a rational design of ternary heterostructure that facilitates multilevel electron transfer.
The TC solution with 20 mg/L concentration was used to study the photocatalytic activity of two Z-mechanism heterostructures (MoS2/g-C3N4/Bi24O31Cl10 [94] and CuBi2O4/Bi2WO6 [95]), one type II heterostructure (La(OH)3/BiOCl [96]) and one p-n heterostructure (WS2/BiOBr [68]). La (OH)3/BiOCl type II heterostructure with sheets morphology obtained by microwave method shows very low energy consumption and reach 85% photocatalytic efficiency in 60 min of irradiation with a 5 W Vis source. The photocatalytic activity is attributed to the shorted mitigation distance (due to the small BiOCl thickness ~18 nm) followed by the photogenerated electrons during the migration process. The two heterostructures based on Z-mechanism show photocatalytic efficiencies above 90% in the presence of 300 W Vis light source. MoS2/g-C3N4/Bi24O31Cl10 has lower energy consumption and higher photocatalytic efficiency, reaching 97.5% in 60 min while CuBi2O4/Bi2WO6 requires double irradiation period (120 min) to remove 93% of TC. The higher MoS2/g-C3N4/Bi24O31Cl10 photocatalytic efficiency is explained by the ability to work as a dual Z-scheme ternary heterostructure, in which each component will generate charge carriers under irradiation. Consequently, the charge carriers’ concentration will be higher in the ternary heterostructure compared with the binary heterostructure. The p-n heterostructure (WS2/BiOBr) previously described in correlation with RhB dye was tested for two pharmaceutical molecules, and the photocatalytic efficiency was 96% for TC and 92% for CIP (20 mg/L). In order to reach these values, the WS2/BiOBr requires 100 min of irradiation using high-intensity (500 W) Vis light source. At the same CIP concentration and irradiation period, the BiOCl/CQDs/rGO [97] sheets-like heterostructure exhibits 87% photocatalytic efficiency using a lower intensity (300 W) Vis light source. Light-harvesting enhancement by CQDs (carbon quantum dots) and rGO (reduced graphene oxide) was not a crucial factor for increasing the photocatalytic activity, but they have a significant influence on accelerating the charge transfer and suppressing the recombination of photogenerated charge carriers.
An analogue evaluation was done using two heterostructures (ZnIn2S4/BiPO4 [98] and AgI/Bi2MoO6/AgBi (MoO4)2 [99]) obtained by hydrothermal method and tested using low TC (5 mg/L) and high TC (40 mg/L) concentrations. Both heterostructures were irradiated with Vis light for 90 min. AgI/Bi2MoO6/AgBi (MoO4)2 with sheets morphology was tested in 5 mg/L TC solution using a 400 W Vis source, and the photocatalytic efficiency was 91.9%. Contrary, the ZnIn2S4/BiPO4 with flower-like morphology was tested at a higher TC concentration (40 mg/L) but using lower Vis light source intensity (300 W), and the photocatalytic efficiency was 84%. These results show that by optimizing the heterostructure composition, it is possible to remove higher (eight times) pollutant concentrations with lower energy consumption. AgI/Bi2MoO6/AgBi (MoO4)2 follows a Z-scheme mechanism and has the advantage of ternary structure with multiple charge carriers’ injection. ZnIn2S4/BiPO4 is a type II heterostructure who benefit from the high specific surface (~100 m2/g) due to the dandelion-like microflower structure.
Z-scheme (PVPbiochar@ZnF2O4/BiOBr [100]) and S-scheme (LaNiO3/TiO2 [77]) mechanisms were used to evaluate the CIP photocatalytic removal under Vis light (300 W). The PVPbiochar@ZnF2O4/BiOBr heterostructure with sheets-like morphology was obtained by the solvothermal method and contains tetragonal BiOBr and spinel ZnFe2O4. The Z-scheme heterostructure exhibits 84% photocatalytic efficiency (15 mg/L CIP concentration and 60 min irradiation period). Biochar and graphene have similar electrical properties, and the photogenerated holes are trapped by BiOBr reacting directly with CIP or H2O to form HO− species. LaNiO3/TiO2 heterostructure has a granular morphology and was obtained by in situ sol–gel process. The S-scheme requires 180 min of irradiation in order to achieve 55% photocatalytic efficiency in a less concentrated CIP solution (10 mg/L). The higher energy consumption and lower photocatalytic efficiency reside on the low TiO2 activation under in visible spectra. However, under UV light (300 W), there is a significant increase of the photocatalytic efficiency, up to 90%, underlining the significance of matching the electronic structure of each component with the radiation source. Based on the potential difference of CB and VB in TiO2 and LaNiO3, they can form excellent S-scheme heterojunction.
The photocatalytic activity of type II heterostructure (UiO-66/CdIn2S4 [101]), p-n heterostructures (SnO2@ZnS [102]) and Schottky heterojunction (Ag/BiVO4/rGO [103]) was tested in 10 mg/L TCS solution. The optimum energy consumption corresponds to UiO-66/CdIn2S4, able to reach 92% photocatalytic efficiency after 180 min of irradiation with 150 W Vis light source. The UiO-66/CdIn2S4 type II heterostructure uses the combined advantages of better charge carrier’s channelization, high resistance to charge recombination (due to the favourable band alignment) and high specific 3D microflower-like morphology. Using Ag/BiVO4/rGO heterostructure obtained by a hydrothermal process, the TCS was completely removed after 120 min of irradiation with 300 W Vis light source. The high photocatalytic activity of Ag/BiVO4/rGO is the result of the three-way synergy mechanism, in which the photogenerated electrons from the CB of BiVO4 will benefit from the graphene transfer channels and the enhanced migration through Schottky Ag/BiVO4 barrier. SnO2@ZnS heterostructure with sheets morphology containing cubic ZnS and tetragonal SnO2 crystalline structures is able to attempt 40% photocatalytic efficiency after 120 min of irradiation with 500 W Vis light source. In this case, the energy consumption is higher due to interactions between TCS and photocatalyst surface. It was found that hydrated PhACs molecules are preferably adsorbed on the SnO2 surface rather than on the ZnS surface, which decreases the number of active sites.
The photocatalytic properties of Bi7O9I3/Bi5O7I [104] heterostructure were investigated using a 500 W Vis light source and 20 mg/L TCS aqueous solution. Bi7O9I3/Bi5O7I heterostructure with bone-stick-like morphology was obtained by in situ thermal treatment and exhibited 89.28% photocatalytic efficiency after 180 min of irradiation. The heterostructure contains two n-type semiconductors, where the photogenerated electrons are transferred from the CB of Bi7O9I3 to the CB of Bi5O7I and can be trapped by the molecular oxygen to form superoxidative radicals. The influence of the light spectra on the photocatalytic removal of highly concentrated TCS (50 mg/L) solution was studied on p-ZnIn2S4/rGO/n-g-C3N4 [105] heterostructure. The irradiation was done with a 20 W UV source and 2 W Vis source for 120 min. ZnIn2S4/rGO/g-C3N4 heterostructure with sheet-like morphology was obtained by hydrothermal process and exhibited similar photocatalytic efficiencies in UV (100%) and Vis (97%) radiation. It was concluded that it is possible to have ten times lower energy consumption using the same heterostructure, by optimizing the light spectra and intensity accordingly with the semiconductor components. The ternary system follows a Z-scheme charges transport, where the rGO accelerate the electron transfer and the band energy difference between g-C3N4 and ZnIn2S4 prolongs the charge lifetime and promotes electron-hole separation.
Three Z-scheme heterostructures (Ag/AgCl/BiVO4 [106], Ag/AgBr/ZnFe2O4 [107] and g-C3N4/TiO2 [108]) were employed to investigate CBZ (10 mg/L) photocatalytic removal under Vis light. Ag/AgCl/BiVO4 and Ag/AgBr/ZnFe2O4 were obtained by ultrasonication, and the photocatalytic experiments were done using similar parameters (93.38 W Vis light source and 240 min irradiation period). Three times more CBZ was removed using the same energy consumption by Ag/AgCl/BiVO4 heterostructure (70.6%), compared with Ag/AgBr/ZnFe2O4. The photocatalytic activity difference was attributed to the band energy positions, which are more close in Ag/AgCl/BiVO4 (−0.16 eV CB AgCl/0.33 eV CB BiVO4) heterostructure than that of Ag/AgBr/ZnFe2O4 (0.1 eV AgBr/−1.5 eV ZnFe2O4) heterostructure resulting in shorter transition time and lower recombination rate. g-C3N4/TiO2 heterostructure with sheets-like morphology obtained by calcination method shows better photocatalytic efficiency (99.77%), using lower radiation intensity (50 W) for a longer period (360 min). The high photocatalytic activity is attributed to g-C3N4, which has a high specific area and can enlarge the light absorbance spectra.
The SA photocatalytic removal in the presence of 300 W light source was studied using two WO3-based heterostructures obtained by one-pot (TiO2/WO3 [81]) and hydrothermal (WO3/Bi2WO6 [109]) processes. WO3/Bi2WO6 heterostructure with flower-like morphology was tested using 5 mg/L SA solution and Vis light source. After 360 min of irradiation, the WO3/Bi2WO6 heterostructure following a Z-scheme mechanism has reached 74.5% photocatalytic efficiency, due to the dramatic increases of visible light absorption induced by (1 1 0) facet of WO3. In this case, the active sites of the photocatalyst have been transferred from (0 1 0) facet of Bi2WO6 to (1 1 0) facet of WO3. The TiO2/WO3 heterostructure mechanism was previously presented in relation to MG dye. The photocatalytic evaluation was done under UV irradiation for 60 min, and the photocatalytic efficiency was 42%. The photocatalytic efficiency was correlated with the ten times higher SA concentration (50 mg/L) and five times shorter irradiation period. Compared with WO3/Bi2WO6, the TiO2/WO3 heterostructure has optimized energy consumption in correlation with the photocatalytic experimental conditions.
TiO2-NT’s@Ag-HA heterostructure [110] obtained by photoreduction was used to evaluate the influence of visible (100 W) and full spectrum (120 W) light on the photocatalytic activity toward 28 mg/L SA solution. As expected, due to the TiO2 content, after 240 min of irradiation, the photocatalytic efficiency under full spectrum (75%) was significantly higher than that under Vis spectrum (30%). The superior photocatalytic efficiency was attributed to the combined effect of a local surface plasmon resonance, induced by silver nanoparticles and the formation of additional levels in TiO2 band gap due to Ti3+ oxidation state at nanotubes surface. Bi12TiO20/g-C3N4 heterostructure [64] previously presented in relation to RhB dye was also tested for SA (10 mg/L) removal, in similar photocatalytic conditions (500 W Vis light source, 50 min irradiation period). However, the photocatalytic efficiency is considerably lower (50%) for SA compared with RhB (97%) due to the smaller SA adsorption on the heterostructure surface.
The removal of IBF (10 mg/L) was investigated using 60 W (Co3O4/BiOI [111]), 300 W (W18O49/g-C3N4 [112]) and 500 W (Fe3O4@MIL-53(Fe) [113]) Vis light sources intensities. Plates-like Co3O4/BiOI heterostructure obtained by solvothermal process exhibits 93.87% photocatalytic efficiency after 60 min of irradiation. The Co3O4/BiOI Z-scheme mechanism benefits from the improved separation of the photoexcited charge carriers, induced by the built-in electric field formed between the BiOI microspheres and the Co3O4 wormy epitaxy. In the presence of 300 W radiation source and 60 min irradiation period, the W18O49/g-C3N4 with sheets-like morphology exhibits 96.3% photocatalytic efficiency. Compared with Co3O4/BiOI, the photocatalytic efficiency improvement was moderate, considering that the radiation intensity was significantly higher. The same heterostructure was tested under NIR irradiation for 120 min, and the results indicate low photocatalytic efficiency (39.2%) due to the limited absorbance range. However, this value is promising, considering that most of the photocatalytic materials exhibit neither or very low photocatalytic activity under NIR irradiation. W18O49/g-C3N4 heterostructure follows a Z-scheme mechanism where the oxygen vacancies in W18O49 can lead to LSPR effect and induce the formation of hot electrons, which can significantly improve the amount of effective photogenerated electrons and provide unique hot electrons injection. Fe3O4@MIL-53(Fe) heterostructure with polyhedron particles morphology was obtained by calcinations, and the photocatalytic investigations were done using a 500 W Vis light source. The IBF was completely removed after 60 min irradiation period, due to high MIL-53(Fe) Vis absorbance induced by the spin-allowed d transition Fe3+ on Fe–O cluster. The energy consumption is higher compared with the previously presented studies. The calcination process was also used to produce Bi2O3-TiO2/carbon [114] heterostructure with S-scheme charges transport. The photocatalytic activity was evaluated using 20 mg/L IBF solution and 120 min irradiation with 300 W Vis light source. Bi2O3-TiO2/carbon heterostructure was able to completely remove the IBF due to the synergic role of Bi2O3 and TiO2 on photogenerated electrons and the tuneable charge carrier’s mobility through carbon channels.
High (100 mg/L) and low (2 mg/L) IBF concentrations were used to evaluate the photocatalytic activity of CdS–SnS–SnS2/rGO [115] and g-C3N4/TiO2/Fe3O4@SiO2 [116] heterostructures. CdS–SnS–SnS2/rGO containing hexagonal CdS, SnS2, and orthorhombic SnS was obtained by solvothermal process and exhibits sheets-like morphology. After 60 min of irradiation with 300 W visible light source, the photocatalytic efficiency achieved was 84.4%. The energy consumption is reasonable considering that the IBF concentration was 100 mg/L. CdS–SnS–SnS2/rGO benefits from a double heterojunction where rGO works as a mediator transfer for photogenerated electrons from CB of SnS to CB of SnS2 and finally to the CB of CdS. The lower IBF concentration (2 mg/L) was used to evaluate g-C3N4/TiO2/Fe3O4@SiO2 photocatalytic performance, during 15 min of irradiation with 64 W Vis source. g-C3N4/TiO2/Fe3O4@SiO2 heterostructure with sheets-like morphology was obtained by sol–gel method. The photocatalytic efficiency was 97%, and the energy consumption fits the photocatalytic parameters. Compared with CdS–SnS–SnS2/rGO photocatalytic parameters, the g-C3N4/TiO2/Fe3O4@SiO2 heterostructure uses not only 50 times lower IBF concentration but also 4.7 lower light intensity and 4 times shorter irradiation to exhibit good photocatalytic efficiency.
A 5 mg/L SMZ solution was employed to evaluate the photocatalytic activity of g-C3N4/TNTs [117] sheets-like and Pd-Bi2MoO6/g-C3N4 [118] flake-like heterostructures both following Z-scheme mechanisms. Pd–Bi2MoO6/g-C3N4 heterostructure is characterized by low energy consumption, being able to achieve 98.8% photocatalytic efficiency after 90 min of irradiation with 36 W Vis light source. In this heterostructure, Pd act as an electron mediator facilitating the charge migration. g-C3N4/TNTs heterostructure is able to induce total SMZ removal at high energy consumption, using a 450 W Vis source for 300 min irradiation period. The high photocatalytic efficiency is attributed to in situ transformations of titanate to anatase and rutile, leading to the formation of nanoscale “hot spots” and then subsequent charge transfer as well as to the large specific surface of TNTs skeleton. At higher SMZ (40 mg/L) concentration, the CuFe2O4/Ti3C2 [119] heterostructure with sheets-like morphology exhibits 70% photocatalytic efficiency after 60 min of irradiation with 300 W Vis light source. CuFe2O4/Ti3C2 follows a Z-scheme charges transport in which the Ti3C2 flakes serve as a shuttle and trap location for light-induced electrons.
To sum up, the most energy-efficient photocatalytic systems for PhACs removal correspond to Z-scheme ZnIn2S4/rGO/C3N4 heterostructure using 4 Wh to remove 48.5 mg/L of TCS and type II La(OH)3/BiOCl heterostructures requiring 5 Wh to remove 17 mg/L of TC. Higher energy consumption is attributed to p-n SnO2@ZnS heterostructures, which use 1000 Wh to remove 4.0 mg/L of TC.
4. Conclusions
The diversification of pollutant type and concentration in wastewater has underlined the importance of finding new alternatives to traditional treatment methods. AOPs, among others, are considered as a promising candidate to efficiently remove organic pollutants such as dyes of PhACs. The present minireview has considered several process parameters (radiation spectra, light intensity and irradiation period) and materials properties (crystallinity, morphology or charge transportation mechanism). However, there are also other parameters that were not the subject of this investigation but can have an important influence on the photocatalytic efficiency (specific surface, catalyst dosage, irradiance, pollutant type, etc.).
The presence of compatible crystalline structures between the heterostructure partners is a prerequisite to assure a good interfacial junction, which allows a facile charge carriers transport. The flower-like and sheets morphologies seem to host a larger number of active surface sites and, consequently, a higher photocatalytic activity. In order to obtain a balance between the energy consumption and photocatalytic efficiency, it is important to optimize the light spectra and intensity as well as the irradiance period according to the heterostructure type, pollutant molecule and concentrations. The total pollutant removal with the expense of high energy consumption will raise questions about the process sustainability and future large-scale implementation. It is possible to remove 85% of TC (20 mg/L) with low energy consumption (5 W Vis source, 60 min of irradiation) as well as 97.5% with higher energy consumption (300 W Vis, 50 min of irradiation). Sustainability is a key parameter to be considered when designing environmental treatment processes. AOPs based on photoactive heterostructures require chemically stable materials for long working periods and low energy consumption.
Dyes removal using photocatalytic heterostructures was evaluated for different molecules, and most of the results indicate high efficiencies. However, PhACs removal by photocatalytic processes still requires more experimental investigation on various molecules in order to have a detailed evaluation of the degradation mechanisms, including the by-products formation. The presence of by-products after the photocatalytic degradation of dyes and PhACs represents an important issue due to the hazard risk induced by their toxicity and environmental persistence. Consequently, the degradation mechanism of each pollutant molecule must be studied in relation to the photocatalytic parameters. The transfer from the laboratory investigations to large-scale applications will outline significant challenges in terms of economic costs and sustainability.
As perspectives, it will be recommended to use a uniform standardization regarding the photocatalytic activity experimental investigations. The scientific articles give several and often noncompatible parameters, which make the comparative investigation more difficult. For example, regarding the radiation parameters, most papers refer to light intensity (W), and other provides the irradiance (mW/cm2), which is more accurate. The photocatalytic efficiency improvement with few percentages is made by significant increase of the energy consumption instead of improving the intrinsic materials properties. Coupling photocatalysis with other techniques (adsorption, biodegradation, etc.) can be another pathway to follow for optimum energy consumption and organic pollutant removal.
Author Contributions
Conceptualization, A.E.; methodology, L.A.; validation, A.E. and L.A.; resources, L.A.; data curation, A.E.; writing—original draft preparation, A.E.; writing—review and editing, L.A.; visualization, L.A.; supervision, A.E.; project administration, A.E.; funding acquisition, A.E. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant of the Romanian National Authority for Scientific Research and Innovation, CCCDI-UEFISCDI, Project number 169/2020 ERANET-M.-3D-Photocat, within PNCDI III.
Conflicts of Interest
The authors declare no conflict of interest. The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript or in the decision to publish the results.
References
- Cheng, D.; Ngo, H.H.; Wei, D. A critical review on antibiotics and hormones in swine wastewater: Water pollution problems and control approaches. J. Hazard. Mater. 2020, 387, 121682. [Google Scholar] [CrossRef] [PubMed]
- Gautam, K.; Anbumani, S. Ecotoxicological effects of organic micro-pollutants on the environment. In Current Developments in Biotechnology and Bioengineering, 1st ed.; Varjani, S., Pandey, A., Tyagi, R.D., Ngo, H.H., Larroche, C., Eds.; Elsevier: New York, NY, USA, 2020; pp. 481–501. [Google Scholar]
- Duarte, R.M.; Matos, J.T.; Senesi, N. Organic pollutants in soils. In Soil Pollutions, 1st ed.; Duarte, A.C., Cachada, A., Rocha-Santos, T., Eds.; Elsevier: New York, NY, USA, 2018; pp. 103–126. [Google Scholar]
- Wood, D.; Shaw, S.; Cawte, T.; Shanen, E.; Heyst, B.V. An overview of photocatalyst immobilization methods for air pollution remedation. Chem. Eng. J. 2020, 391, 123490. [Google Scholar] [CrossRef]
- Hader, D.P.; Banaszak, A.T.; Helbling, E.W. Anthropogenic pollution of aquatic ecosystems: Emerging problems with global implications. Sci. Total Environ. 2020, 713, 136586. [Google Scholar] [CrossRef] [PubMed]
- Libralato, G.; Lofrano, G.; Siciliano, A.; Gambino, E.; Boccia, G.; Federica, C.; Francesco, A.; Galdiero, E.; Gesuele, R.; Guida, M. Toxicity assessment of wastewater after advanced oxidation processes for emerging contaminants’ degradation. In Visible Light Active Structured Photocatalysts for the Removal of Emerging Contaminants, 1st ed.; Sacco, O., Vaiano, V., Eds.; Elsevier: New York, NY, USA, 2020; pp. 195–211. [Google Scholar]
- Kaplan, A.; Mamane, H.; Lester, Y.; Avisar, D. Trace organic compound removal from wastewater reverse-osmosis concentrate by advanced oxidation processes with UV/O3/H2O2. Materials 2020, 13, 2785. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Zeng, L.; Qin, W.; Feng, J. Measures for reducing nitrate leaching in orchards: A review. Environ. Pollut. 2020, 263, 114553. [Google Scholar] [CrossRef]
- Serban, I.; Enesca, A. Metal Oxides-Based Semiconductors for Biosensors Applications. Front. Chem. 2020, 8, 354. [Google Scholar] [CrossRef]
- Tahreen, A.; Jami, M.S.; Ali, F. Role of electrocoagulation in wastewater treatment: A developmental review. J. Water Proc. Eng. 2020, 37, 101440. [Google Scholar] [CrossRef]
- Guo, W.; Umar, A.; Du, Y.; Wang, L.; Pei, M. Surface Modification of Bentonite with Polymer Brushes and Its Application as an Efficient Adsorbent for the Removal of Hazardous Dye Orange I. Nanomaterials 2020, 10, 1112. [Google Scholar] [CrossRef]
- Enesca, A.; Andronic, L.; Duta, A. The influence of surfactants on the crystalline structure, electrical and photocatalytic properties of hybrid multi-structured (SnO2, TiO2 and WO3) thin films. Appl. Surf. Sci. 2012, 258, 4339–4346. [Google Scholar] [CrossRef]
- Cornejo, O.M.; Murrieta, M.F.; Castañeda, L.F.; Nava, J.L. Characterization of the reaction environment in flow reactors fitted with BDD electrodes for use in electrochemical advanced oxidation processes: A critical review. Electrochim. Acta 2020, 33120, 135373. [Google Scholar] [CrossRef]
- Malakootian, M.; Shahesmaeili, A.; Faraji, M.; Amiri, H.; Martinez, S.S. Advanced oxidation processes for the removal of organophosphorus pesticides in aqueous matrices: A systematic review and meta-analysis. Process Saf. Environ. 2020, 134, 292–307. [Google Scholar] [CrossRef]
- Han, M.; Duan, X.; Cao, G.; Zhuc, S.; Ho, S.H. Graphitic nitride-catalyzed advanced oxidation processes (AOPs) for landfill leachate treatment: A mini review. Process Saf. Environ. 2020, 139, 230–240. [Google Scholar] [CrossRef]
- Yang, L.; He, L.; Xue, J.; Ma, Y.; Zhang, Z. UV/SO32− based advanced reduction processes of aqueous contaminants: Current status and prospects. Chem. Eng. J. 2020, 3971, 125412. [Google Scholar] [CrossRef]
- Bresolin, B.M.; Park, Y.; Bahnemann, D.W. Recent Progresses on Metal Halide Perovskite-Based Material as Potential Photocatalyst. Catalysts 2020, 10, 709. [Google Scholar] [CrossRef]
- Chen, L.; Tang, J.; Song, L.N.; Chen, P.; Yin, S.F. Heterogeneous photocatalysis for selective oxidation of alcohols and hydrocarbons. Appl. Catal. B 2019, 242, 379–388. [Google Scholar] [CrossRef]
- Chauhan, D.K.; Jain, S.; Battula, V.R.; Kailasam, K. Organic motif’s functionalization via covalent linkage in carbon nitride: An exemplification in photocatalysis. Carbon 2019, 152, 40–58. [Google Scholar] [CrossRef]
- Hu, X.; Ma, Q.; Wang, X.; Yang, Y.; Liu, N.; Zhang, C.; Kawazoe, N.; Chen, G.; Yang, Y. Layered Ag/Ag2O/BiPO4/Bi2WO6 heterostructures by two-step method for enhanced photocatalysis. J. Catal. 2020, 387, 28–38. [Google Scholar] [CrossRef]
- Ojha, D.P.; Karki, H.P.; Song, J.H.; Kim, H.J. Amine-assisted synthesis of FeWO4 nanorodg-C3N4 for enhanced visible light-driven Z-scheme photocatalysis. Compos. Part B Eng. 2019, 1601, 277–284. [Google Scholar] [CrossRef]
- Wetchakun, K.; Wetchakun, N.; Sakulsermsuk, S. An overview of solar/visible light-driven heterogeneous photocatalysis for water purification: TiO2- and ZnO-based photocatalysts used in suspension photoreactors. J. Ind. Eng. Chem. 2019, 7125, 19–49. [Google Scholar] [CrossRef]
- Yao, S.; Wang, J.; Zhou, X.; Zhou, S.; Pu, X.; Li, W. One-pot low-temperature synthesis of BiOX/TiO2 hierarchic composites of adsorption coupled with photocatalysis for quick degradation of colored and colorless organic pollutants. Adv. Powder Technol. 2020, 31, 1924–1932. [Google Scholar] [CrossRef]
- Kant, S.; Pathania, D.; Singh, P.; Dhiman, P.; Kumar, A. Removal of malachite green and methylene blue by Fe0.01Ni0.01Zn0.98O/polyacrylamide nanocompositeusing coupled adsorption and photocatalysis. Appl. Catal. B 2014, 147, 340–352. [Google Scholar] [CrossRef]
- Ma, Y.; Xiong, H.; Zhao, Z.; Yu, Y.; Zhou, D.; Dong, S. Model-based evaluation of tetracycline hydrochloride removal and mineralization in an intimately coupled photocatalysis and biodegradation reactor. Chem. Eng. J. 2018, 351, 967–975. [Google Scholar] [CrossRef]
- Li, F.; Lan, X.; Wang, L.; Kong, X.; Xu, P.; Tai, Y.; Liu, G.; Shi, J. An efficient photocatalyst coating strategy for intimately coupled photocatalysis and biodegradation (ICPB): Powder spraying method. Chem. Eng. J. 2020, 383, 123092. [Google Scholar] [CrossRef]
- Ceretta, M.B.; Vieira, Y.; Wolski, E.A.; Foletto, E.L.; Silvestri, S. Biological degradation coupled to photocatalysis by ZnO/polypyrrole composite for the treatment of real textile wastewater. J. Water Proc. Eng. 2020, 35, 101230. [Google Scholar] [CrossRef]
- Grcic, I.; Vrsaljko, D.; Katancic, Z.; Papic, S. Purification of household grey water loaded with hair colorants by solar photocatalysis using TiO2-coated textile fibers coupled flocculation with chitosan. J. Water Proc. Eng. 2015, 5, 15–27. [Google Scholar] [CrossRef]
- Xu, T.; Wang, P.; Wang, D.; Zhao, K.; Wei, M.; Liu, X.; Liu, H.; Cao, J.; Fan, H.; Yang, L. Ultrasound-assisted synthesis of hyper-dispersed type-II tubular Fe3O4@SiO2@ZnO/ZnS core/shell heterostructure for improved visible-light photocatalysis. J. Alloy. Compd. 2020, 838, 155689. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Quan, X.; Wu, Y. Enhanced generation of oxidative species and phenol degradation in a discharge plasma system coupled with TiO2 photocatalysis. Appl. Catal. B 2008, 83, 72–77. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Ma, J.; Peng, Y.; Wang, A. A review on bidirectional analogies between the photocatalysis and antibacterial properties of ZnO. J. Alloy. Compd. 2019, 78330, 898–918. [Google Scholar] [CrossRef]
- Li, H.; Zhang, N.; Zhao, F.; Liu, T.; Wang, Y. Facile Fabrication of a Novel Au/Phosphorus-Doped g-C3N4 Photocatalyst with Excellent Visible Light Photocatalytic Activity. Catalysts 2020, 10, 701. [Google Scholar] [CrossRef]
- Enesca, A.; Yamaguchi, Y.; Terashima, C.; Fujishima, A.; Nakata, K.; Duta, A. Enhanced UV-Vis photocatalytic performance of the CuInS2/TiO2/SnO2 hetero-structure for air decontamination. J. Catal. 2017, 350, 174–181. [Google Scholar] [CrossRef]
- Roques-Carmes, T.; Alem, H.; Hamieh, T.; Toufaily, J.; Frochot, C.; Villieras, F. Different strategies of surface modification to improve the photocatalysis properties: Pollutant adsorption, visible activation, and catalyst recovery. In Handbook of Smart Photocatalytic Materials, 1st ed.; Hussain, C.M., Mishra, A.K., Eds.; Elsevier: New York, NY, USA, 2020; pp. 39–57. [Google Scholar]
- Zhang, X.; Teng, S.Y.; Loy, A.C.M.; How, B.S.; Leong, W.D.; Tao, X. Transition Metal Dichalcogenides for the Application of Pollution Reduction: A Review. Nanomaterials 2020, 10, 1012. [Google Scholar] [CrossRef] [PubMed]
- Mouchaal, Y.; Enesca, A.; Mihoreanu, C.; Khelil, A.; Duta, A. Tuning the opto-electrical properties of SnO2 thin films by Ag+1 and In+3 co-doping. Mater. Sci. Eng. B 2015, 199, 22–29. [Google Scholar] [CrossRef]
- Song, B.; Zeng, Z.; Zeng, G.; Gong, J.; Tang, X. Powerful combination of g-C3N4 and LDHs for enhanced photocatalytic performance: A review of strategy, synthesis, and applications. Adv. Colloid Interface Sci. 2019, 272, 101999. [Google Scholar] [CrossRef] [PubMed]
- Salgado, B.C.; Cardeal, R.A.; Valentini, A. Photocatalysis and Photodegradation of Pollutants. In Nanomaterials Applications for Environmental Matrices, 1st ed.; Nascimento, R.F., Ferreira, O.P., De Paula, A.J., Neto, V.O., Eds.; Elsevier: New York, NY, USA, 2019; pp. 449–488. [Google Scholar]
- Enesca, A.; Andronic, L.; Duta, A. Optimization of Opto-Electrical and Photocatalytic Properties of SnO2 Thin Films Using Zn2+ and W6+ Dopant Ions. Catal. Lett. 2012, 142, 224–230. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, C.; Xiong, W.; Zeng, G.; Huang, D.; Zhang, C.; Wang, W.; Song, B.; Tang, X.; Li, X.; et al. Recent advances in application of graphitic carbon nitride-based catalysts for degrading organic contaminants in water through advanced oxidation processes beyond photocatalysis: A critical review. Water Res. 2020, 184, 116200. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Wang, C.; Qi, Y.; Chen, Z.; Xu, Q. CO2-Induced Defect Engineering: A New Protocol by Doping Vacancies in 2D Heterostructures for Enhanced Visible-Light Photocatalysis. Appl. Surf. Sci. 2017, 419, 573–579. [Google Scholar] [CrossRef]
- Pedanekar, R.S.; Shaikh, S.K.; Rajpure, K.Y. Thin film photocatalysis for environmental remediation: A status review. Curr. Appl. Phys. 2020, 20, 931–952. [Google Scholar] [CrossRef]
- He, W.; Sun, Y.; Jiang, G.; Li, Y.; Dong, F. Defective Bi4MoO9/Bi metal core/shell heterostructure: Enhanced visible light photocatalysis and reaction mechanism. Appl. Catal. B 2018, 239, 619–627. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, J.; Xu, L.; Li, X.; Huang, S. A room-temperature methane sensor based on Pd-decorated ZnO/rGO hybrids enhanced by visible light photocatalysis. Sens. Actuat. B 2020, 3041, 127334. [Google Scholar] [CrossRef]
- Andronic, L.; Enesca, A.; Cazan, C.; Visa, M. TiO2-active carbon composites for wastewater photocatalysis. J. Sol-Gel Sci. Technol. 2014, 71, 399–405. [Google Scholar] [CrossRef]
- Irani, E.; Amoli-Diva, M. Hybrid adsorption–photocatalysis properties of quaternary magneto-plasmonic ZnO/MWCNTs nanocomposite for applying synergistic photocatalytic removal and membrane filtration in industrial wastewater treatment. J. Photochem. Photobiol. A Chem. 2020, 39115, 112359. [Google Scholar] [CrossRef]
- Kusmierek, E. Semiconductor Electrode Materials Applied in Photoelectrocatalytic Wastewater Treatment—An Overview. Catalysts 2020, 10, 439. [Google Scholar] [CrossRef]
- Xiao, W.Z.; Xu, L.; Rong, Q.Y.; Dai, X.Y.; Wang, L.L. Two-dimensional H-TiO2/MoS2(WS2) van der Waals heterostructures for visible-light photocatalysis and energy conversion. Appl. Surf. Sci. 2020, 504, 144425. [Google Scholar] [CrossRef]
- Kanakkillam, S.S.; Krishnan, B.; Avellaneda, D.A.; Shaji, S. Surfactant free stable cobalt oxide nanocolloid in water by pulsed laser fragmentation and its thin films for visible light photocatalysis. Colloid. Surface. A 2020, 5945, 124657. [Google Scholar] [CrossRef]
- Rafiq, U.; Majid, K. Mitigating the charge recombination by the targeted synthesis of Ag2WO4/Bi2Fe4O9 composite: The facile union of orthorhombic semiconductors towards efficient photocatalysis. J. Alloy. Compd. 2020, 84225, 155876. [Google Scholar] [CrossRef]
- Jia, S.; Xu, M.; Chen, S.; Yan, J.; Ma, X. A hierarchical sandwich-structured MoS2/SnO2/CC heterostructure for high photocatalysis performance. Mater. Lett. 2019, 236, 697–701. [Google Scholar] [CrossRef]
- Li, D.; Song, H.; Meng, X.; Shen, T.; Sun, J.; Han, W.; Wang, X. Effects of Particle Size on the Structure and Photocatalytic Performance by Alkali-Treated TiO2. Nanomaterials 2020, 10, 546. [Google Scholar] [CrossRef]
- Estahbanati, M.R.K.; Feilizadeh, M.; Babin, A.; Mei, B.; Iliuta, M.C. Selective photocatalytic oxidation of cyclohexanol to cyclohexanone: A spectroscopic and kinetic study. Chem. Eng. J. 2020, 38215, 122732. [Google Scholar] [CrossRef]
- Guo, X.; Zhou, X.; Li, X.; Shao, C.; Liu, Y. Bismuth oxychloride (BiOCl)/copper phthalocyanine (CuTNPc) heterostructures immobilized on electrospun polyacrylonitrile nanofibers with enhanced activity for floating photocatalysis. J. Colloid Interface Sci. 2018, 525, 187–195. [Google Scholar] [CrossRef]
- Li, X.; Chen, D.; Li, N.; Xu, Q.; Lu, J. Efficient reduction of Cr(VI) by a BMO/Bi2S3 heterojunction via synergistic adsorption and photocatalysis under visible light. J. Hazard. Mater. 2020, 4005, 123243. [Google Scholar] [CrossRef]
- Mintcheva, N.; Gicheva, G.; Panayotova, M.; Kulinich, S.A. Room-Temperature Synthesis of ZnS Nanoparticles Using Zinc Xanthates as Molecular Precursors. Materials 2020, 13, 171. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cai, C.; Gu, Y.; Cheng, W.; Zhao, C. Novel electronic properties of a new MoS2/TiO2 heterostructure and potential applications in solar cells and photocatalysis. Appl. Surf. Sci. 2017, 414, 34–40. [Google Scholar] [CrossRef]
- González-Burciaga, L.A.; Núñez-Núñez, C.M.; Morones-Esquivel, M.M.; Avila-Santos, M.; Lemus-Santana, A.; Proal-Nájera, J.B. Characterization and Comparative Performance of TiO2 Photocatalysts on 6-Mercaptopurine Degradation by Solar Heterogeneous Photocatalysis. Catalysts 2020, 10, 118. [Google Scholar] [CrossRef]
- Liu, N.; Lu, N.; Yu, H.T.; Chen, S.; Quan, X. Efficient day-night photocatalysis performance of 2D/2D Ti3C2/Porous g-C3N4 nanolayers composite and its application in the degradation of organic pollutants. Chemosphere 2020, 246, 125760. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, G.; Wang, J.; Hu, Z.; Su, Y. Step-scheme NiO/BiOI heterojunction photocatalyst for rhodamine photodegradation. App. Surf. Sci. 2020, 511, 145499. [Google Scholar] [CrossRef]
- Kumar, R.S.; Min, K.S.; Lee, S.H.; Mergu, N.; Son, Y.A. Synthesis of novel panchromatic porphyrin-squaraine dye and application towards TiO2 combined photocatalysis. J. Photochem. Photobiol. A 2020, 397, 112595. [Google Scholar] [CrossRef]
- Tsai, C.G.; Tseng, W.J. Preparation of TiN–TiO2 composite nanoparticles for organic dye adsorption and photocatalysis. Ceram. Int. 2020, 46, 14529–14535. [Google Scholar] [CrossRef]
- Hernández-Carrillo, M.A.; Torres-Ricárdez, R.; García-Mendoza, M.F.; Ramírez-Morales, E.; Pérez-Hernández, G. Eu-modified ZnO nanoparticles for applications in photocatalysis. Catal. Today 2020, 3491, 191–197. [Google Scholar] [CrossRef]
- Cao, L.; Li, Y.F.; Tong, Y.; Yang, R.; Sun, L.; Cao, O.; Chen, R. A novel Bi12TiO20/g-C3N4 hybrid catalyst with a bionic granum configuration for enhanced photocatalytic degradation of organic pollutants. J. Hazard. Mater. 2019, 379, 120808. [Google Scholar] [CrossRef]
- Shende, A.G.; Tiwari, C.S.; Bhoyar, T.H.; Vidyasagar, D.; Umare, S.S. BWO nano-octahedron coupled with layered g-C3N4: An efficient visible light active photocatalyst for degradation of cationic/anionic dyes, and N2 reduction. J. Molec. Liq. 2019, 296, 111771. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Mei, J.; Sarina, S.; Wu, Z.; Liao, T.; Yan, C.; Sun, Z. Strongly interfacial-coupled 2D-2D TiO2/g-C3N4 heterostructure for enhanced visible-light induced synthesis and conversion. J. Hazard. Mater. 2020, 394, 122529. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Lan, H.; Zhang, M.; Zhu, H.; Bu, M. Preparation of heterostructure g-C3N4/ZnO nanorods for high photocatalytic activity on different pollutants (MB, RhB, Cr(VI) and eosin). Ceram. Int. 2020, 46, 12192–12199. [Google Scholar] [CrossRef]
- Fu, S.; Yuan, W.; Liu, X.; Yan, Y.; Liu, H.; Li, L.; Zhao, F.; Zhou, J. A novel 0D/2D WS2/BiOBr heterostructure with rich oxygen vacancies for enhanced broad-spectrum photocatalytic performance. J. Colloid. Interf. Sci. 2020, 569, 150–163. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Zhang, H.; Wu, Y.; Jia, Y.; Jin, R.; Gao, S. CTAB-assisted solvothermal construction of hierarchical Bi2MoO6/Bi5O7Br with improved photocatalytic performances. Sep. Purif. Technol. 2020, 242, 116775. [Google Scholar] [CrossRef]
- Zhao, K.; Zhang, Z.; Feng, Y.; Lin, S.; Li, H.; Gao, X. Surface oxygen vacancy modified Bi2MoO6/MIL-88B(Fe) heterostructure with enhanced spatial charge separation at the bulk & interface. Appl. Catal. B 2020, 268, 118740. [Google Scholar]
- Bao, L.; Yang, F.; Cheng, D.; Pan, X.; Zhang, H.; Zhao, F.; Zhao, S.; Tegus, O. Modified electronic structure of Ta2O5 via surface decorated with Ta3B2 nanodots for enhanced photocatalytic activity. App. Surf. Sci. 2020, 513, 145767. [Google Scholar] [CrossRef]
- Jin, J.; Xie, Y. Ultraviolet light induced oxygen vacancy-rich BiPO4−x/Bi2S3 nanorods with enhanced photocatalytic activity and mechanism. Res. Chem. Intermed. 2019, 45, 5609–5623. [Google Scholar] [CrossRef]
- Mandal, B.; Panda, J.; Paul, P.K.; Sarkar, R.; Tudu, B. MnFe2O4 decorated reduced graphene oxide heterostructures: Nanophotocatalyst for methylene blue dye degradation. Vacuum 2020, 173, 109150. [Google Scholar] [CrossRef]
- Alido, J.P.M.; Sari, F.N.I.; Ting, Y.M. Synthesis of Ag/hybridized 1T-2H MoS2/TiO2 heterostructure for enhanced visible-light photocatalytic activity. Ceram. Int. 2019, 45, 23651–23657. [Google Scholar] [CrossRef]
- Tian, Q.; Fang, G.; Ding, L.; Ran, M.; Zhang, H.; Pan, A.; Shen, K.; Deng, Y. ZnAl2O4/Bi2MoO6 heterostructures with enhanced photocatalytic activity for the treatment of organic pollutants and eucalyptus chemimechanical pulp wastewater. Mater. Chem. Phys. 2020, 241, 122299. [Google Scholar] [CrossRef]
- Çinar, B.; Kerimoglu, I.; Tonbül, B.; Demirbüken, A.; Dursun, S.; Kaya, I.C.; Kalem, V.; Akyildiz, H. Hydrothermal/electrospinning synthesis of CuO plate-like particles/TiO2 fibers heterostructures for high-efficiency photocatalytic degradation of organic dyes and phenolic pollutants. Mater. Sci. Semic. Proces. 2020, 109, 104919. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, J.; Geng, J.; Bao, R.; Wang, Z.; Xia, J.; Li, H. Perovskite LaNiO3/TiO2 step-scheme heterojunction with enhanced photocatalytic activity. Appl. Surf. Sci. 2020, 503, 144287. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, Z.; Kong, X.; He, F.; Zhao, R.; Wua, R.; Wei, T.; Wang, L.; Feng, J. A novel P-N heterojunction with staggered energy level based on ZnFe2O4 decorating SnS2 nanosheet for efficient photocatalytic degradation. App. Surf. Sci. 2020, 510, 145442. [Google Scholar] [CrossRef]
- Yan, H.; Zhu, Z.; Long, Y.; Li, W. Single-source-precursor-assisted synthesis of porous WO3/g-C3N4 with enhanced photocatalytic property. Colloid. Surfaces A 2019, 582, 123857. [Google Scholar] [CrossRef]
- Neto, N.F.A.; Lima, A.B.; Bomio, M.R.D.; Motta, F.V. Microwave-assisted hydrothermal synthesis of Ag2Mo1−xWxO4 (x = 0, 0.25, 0.50, 0.75 and 1 mol%) heterostructures for enhanced photocatalytic degradation of organic dyes. J. Alloy. Compd. 2020, 844, 156007. [Google Scholar]
- Wei, Y.; Huang, Y.; Fang, Y.; Zhao, Y.; Luo, D.; Guo, Q.; Fan, L.; Wu, J. Hollow mesoporous TiO2/WO3 sphere heterojunction with high visiblelight-driven photocatalytic activity. Mater. Res. Bull. 2019, 119, 110571. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, L.; Xin, A.; Yu, Y.; Wang, L.; Zhang, W. Visible light response and heterostructure of composite CdS@ZnSeZnO to enhance its photocatalytic activity. J. Alloys Compd. 2020, 813, 152190. [Google Scholar] [CrossRef]
- Yulizar, Y.; Apriandanua, D.O.B.; Ashna, R.I. La2CuO4-decorated ZnO nanoparticles with improved photocatalytic activity for malachite green degradation. Chem. Phys. Lett. 2020, 755, 137749. [Google Scholar] [CrossRef]
- Jia, J.; Du, X.; Zhang, Q.; Liu, E.; Fan, J. Z-scheme MgFe2O4/Bi2MoO6 heterojunction photocatalyst with enhanced visible light photocatalytic activity for malachite green removal. Appl. Surf. Sci. 2019, 492, 527–539. [Google Scholar] [CrossRef]
- Isari, A.A.; Hayati, F.; Kakavandi, B.; Rostami, M.; Dehghanifard, E. Cu co-doped TiO2@functionalized SWCNT photocatalyst coupled with ultrasound and visible-light: An effective sono-photocatalysis process for pharmaceutical wastewaters treatment. Chem. Eng. J. 2020, 392, 123685. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, X.; Zhang, W.; Liu, Z.; Chen, M. Advances in photocatalysis based on fullerene C60 and its derivatives: Properties, mechanism, synthesis, and applications. Appl. Catal. B 2020, 26515, 118579. [Google Scholar] [CrossRef]
- Ahmad, K.; Ghatak, H.R.; Ahuja, S.M. A review on photocatalytic remediation of environmental pollutants and H2 production through water splitting: A sustainable approach. Environ. Technol. Innov. 2020, 19, 100893. [Google Scholar] [CrossRef]
- Kovacic, M.; Papac, J.; Kusic, H.; Karamanis, P.; Bozic, A.L. Degradation of polar and non-polar pharmaceutical pollutants in water by solar assisted photocatalysis using hydrothermal TiO2-SnS2. Chem. Eng. J. 2020, 382, 122826. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, X.; Jiang, L.; Zhang, J.; Zeng, G. Powerful combination of 2D g-C3N4 and 2D nanomaterials for photocatalysis: Recent advances. Chem. Eng. J. 2020, 39015, 124475. [Google Scholar] [CrossRef]
- Gopinath, K.P.; Madhav, N.V.; Krishnan, A.; Malolan, R.; Rangarajan, G. Present applications of titanium dioxide for the photocatalytic removal of pollutants from water: A review. J. Environ. Manag. 2020, 27015, 110906. [Google Scholar] [CrossRef]
- Acharya, L.; Nayak, S.; Pattnaik, S.P.; Acharya, R.; Parida, K. Resurrection of boron nitride in p-n type-II boron nitride/B-doped-g-C3N4 nanocomposite during solid-state Z-scheme charge transfer path for the degradation of tetracycline hydrochloride. J. Colloid Interface Sci. 2020, 566, 211–223. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, W.; Zhu, J.; Fu, L.; Li, D.; Zhou, L. Enhanced visible light photocatalytic activity of g-C3N4 decorated ZrO2−x nanotubes heterostructure for degradation of tetracycline hydrochloride. J. Hazard. Mater. 2020, 384, 121275. [Google Scholar] [CrossRef]
- Shi, W.; Liu, C.; Li, M.; Lin, X.; Guo, F.; Shi, J. Fabrication of ternary Ag3PO4/Co3(PO4)2/g-C3N4 heterostructure with following Type II and Z-Scheme dual pathways for enhanced visible-light photocatalytic activity. J. Hazard. Mater. 2020, 389, 121907. [Google Scholar] [CrossRef]
- Kang, J.; Jin, C.; Li, Z.; Wang, M.; Chen, Z.; Wang, Y. Dual Z-scheme MoS2/g-C3N4/Bi24O31Cl10 ternary heterojunction photocatalysts for enhanced visible-light photodegradation of antibiotic. J. Alloys Compd. 2020, 825, 153975. [Google Scholar] [CrossRef]
- Wang, L.; Yang, G.; Wang, D.; Lu, C.; Guan, W.; Li, Y.; Deng, J.; Crittenden, J. Fabrication of the flower-flake-like CuBi2O4/Bi2WO6 heterostructure as efficient visible-light driven photocatalysts: Performance, kinetics and mechanism insight. Appl. Surf. Sci. 2019, 495, 143521. [Google Scholar] [CrossRef]
- Zhou, Z.; Xu, H.; Li, D.; Zou, Z.; Xia, Z. Microwave-assisted synthesis of La(OH)3/BiOCl n-n heterojunctions with high oxygen vacancies and its enhanced photocatalytic properties. Chem. Phys. Lett. 2019, 736, 136805. [Google Scholar] [CrossRef]
- Huang, J.; Chen, W.; Yu, X.; Fu, X.; Zhu, Y.; Zhang, Y. Fabrication of a ternary BiOCl/CQDs/rGO photocatalyst: The roles of CQDs and rGO in adsorption-photocatalytic removal of ciprofloxacin. Colloid. Surf. A 2020, 597, 124758. [Google Scholar] [CrossRef]
- Lu, C.; Guo, F.; Yan, Q.; Zhang, Z.; Li, D.; Wang, L.; Zhou, Y. Hydrothermal synthesis of type II ZnIn2S4/BiPO4 heterojunction photocatalyst with dandelion-like microflower structure for enhanced photocatalytic degradation of tetracycline under simulated solar light. J. Alloys Compd. 2019, 811, 151976. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, H.; Ma, X.; Zhang, X.; Zhu, Y.; Zhang, A.; Cao, Y.; Yang, P. Construction of AgI/Bi2MoO6/AgBi(MoO4)2 multi-heterostructure composite nanosheets for visible-light photocatalysis. Mater. Today Commun. 2020, 23, 100903. [Google Scholar] [CrossRef]
- Chen, M.; Dai, Y.; Guo, J.; Yang, H.; Liu, D.; Zhai, Y. Solvothermal synthesis of biochar@ZnFe2O4/BiOBr Z-scheme heterojunction for efficient photocatalytic ciprofloxacin degradation under visible light. Appl. Surf. Sci. 2019, 493, 1361–1367. [Google Scholar] [CrossRef]
- Bariki, R.; Majhi, D.; Das, K.; Behera, A.; Mishra, B.G. Facile synthesis and photocatalytic efficacy of UiO-66/CdIn2S4 nanocomposites with flowerlike 3D-microspheres towards aqueous phase decontamination of triclosan and H2 evolution. Appl. Catal. B 2020, 270, 118882. [Google Scholar] [CrossRef]
- Hojamberdiev, M.; Czech, B.; Goktaş, A.C.; Yubuta, K.; Kadirova, Z.C. SnO2@ZnS photocatalyst with enhanced photocatalytic activity for the degradation of selected pharmaceuticals and personal care products in model wastewater. J. Alloy. Compd. 2020, 827, 154339. [Google Scholar] [CrossRef]
- Li, M.; Xu, G.; Guan, Z.; Wang, Y.; Yu, H.; Yu, Y. Synthesis of Ag/BiVO4/rGO composite with enhanced photocatalytic degradation of triclosan. Sci. Total Environ. 2019, 664, 230–239. [Google Scholar] [CrossRef]
- Chang, C.; Yang, H.; Mu, W.; Cai, Y.; Wang, L.; Yang, L.; Qin, H. In situ fabrication of bismuth oxyiodide (Bi7O9I3/Bi5O7I) n-n heterojunction for enhanced degradation of triclosan (TCS) under simulated solar light irradiation. Appl. Catal. B. 2019, 254, 647–658. [Google Scholar] [CrossRef]
- Yu, T.; Wu, W.W.; Liu, L.; Gao, C.; Yang, T. Novel ternary p-ZnIn2S4/rGO/n-g-C3N4 Z-scheme nanocatalyst with enhanced antibiotic degradation in a dark self-biased fuel cell. Ceram. Int. 2020, 46, 9567–9574. [Google Scholar] [CrossRef]
- Yentür, G.; Dükkanci, M. Synthesis of Visible-Light Heterostructured Photocatalyst of Ag/AgCl Deposited on (040) Facet of Monoclinic BiVO4 for Efficient Carbamazepine Photocatalytic Removal. Appl. Surf. Sci. 2020, in press. [Google Scholar] [CrossRef]
- Yentür, G.; Dükkancı, M. Fabrication of magnetically separable plasmonic composite photocatalyst of Ag/AgBr/ZnFe2O4 for visible light photocatalytic oxidation of carbamazepine. Appl. Surf. Sci. 2020, 510, 145374. [Google Scholar] [CrossRef]
- Hu, Z.; Cai, X.; Wang, Z.; Li, S.; Wang, Z.; Xie, X. Construction of carbon-doped supramolecule-based g-C3N4/TiO2 composites for removal of diclofenac and carbamazepine: A comparative study of operating parameters, mechanisms, degradation pathways. J. Hazard. Mater. 2019, 380, 120812. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, Y.; Li, L. Facet-engineered surface and interface design of WO3/Bi2WO6 photocatalyst with direct Z-scheme heterojunction for efficient salicylic acid removal. Appl. Surf. Sci. 2020, 508, 144796. [Google Scholar] [CrossRef]
- Plodinec, M.; Grcic, I.; Willinger, M.G.; Hammud, A.; Huang, X.; Panzic, I.; Gajovi, A. Black TiO2 nanotube arrays decorated with Ag nanoparticles for enhanced visible-light photocatalytic oxidation of salicylic acid. J. Alloy. Compd. 2019, 776, 883–896. [Google Scholar] [CrossRef]
- Malefane, M.E.; Feleni, U.; Mafa, P.J.; Kuvarega, A.T. Fabrication of direct Z-scheme Co3O4/BiOI for ibuprofen and trimethoprim degradation under visible light irradiation. Appl. Surf. Sci. 2020, 514, 145940. [Google Scholar] [CrossRef]
- Deng, Y.; Feng, C.; Tang, L.; Zhou, Y.; Chen, Z.; Feng, H.; Wang, J.; Yu, J.; Liu, Y. Ultrathin low dimensional heterostructure composites with superior photocatalytic activity: Insight into the multichannel charge transfer mechanism. Chem. Eng. J. 2020, 393, 124718. [Google Scholar] [CrossRef]
- Liu, N.; Wang, J.; Wu, J.; Li, Z.; Huang, W.; Zheng, Y.; Lei, J.; Zhang, X.; Tang, L. Magnetic Fe3O4@MIL-53(Fe) nanocomposites derived from MIL-53(Fe) for the photocatalytic degradation of ibuprofen under visible light irradiation. Mater. Res. Bull. 2020, 132, 111000. [Google Scholar] [CrossRef]
- Wang, N.; Li, X.; Yang, Y.; Zhou, Z.; Shang, Y.; Zhuang, X.; Zhang, T. Two-stage calcination composite of Bi2O3-TiO2 supported on powdered activated carbon for enhanced degradation of sulfamethazine under solar irradiation. J. Water Proc. Eng. 2020, 35, 101220. [Google Scholar] [CrossRef]
- Liang, M.; Yu, Y.; Wang, Y.; Yu, Y. Remarkably efficient charge transfer through a double heterojunction mechanism by a CdS-SnS-SnS2/rGO composite with excellent photocatalytic performance under visible light. J. Hazard. Mater. 2020, 391, 121016. [Google Scholar] [CrossRef]
- Kumar, A.; Khan, M.; Zeng, X.; Lo, I. Development of g-C3N4/TiO2/Fe3O4@SiO2 heterojunction via sol-gel route: A magnetically recyclable direct contact Z-scheme nanophotocatalyst for enhanced photocatalytic removal of ibuprofen from real sewage effluent under visible light. Chem. Eng. J. 2018, 353, 645–656. [Google Scholar] [CrossRef]
- Ji, H.; Du, P.; Zhao, D.; Li, S.; Sun, F.; Duin, E.C.; Liu, W. 2D/1D graphitic carbon nitride/titanate nanotubes heterostructure for efficient photocatalysis of sulfamethazine under solar light: Catalytic “hot spots” at the rutile–anatase–titanate interfaces. Appl. Catal. B 2020, 263, 118357. [Google Scholar] [CrossRef]
- Di, G.; Zhu, Z.; Zhang, H.; Qiu, Y.; Yin, D.; Crittenden, J. Simultaneous sulfamethazine oxidation and bromate reduction by Pd mediated Z-scheme Bi2MoO6/g-C3N4 photocatalysts: Synergetic mechanism and degradative pathway. Chem. Eng. J. 2020, 401, 126061. [Google Scholar] [CrossRef]
- Cao, Y.; Fang, Y.; Lei, X.; Tan, B.; Hu, X.; Liu, B.; Chen, Q. Fabrication of novel CuFe2O4/MXene hierarchical heterostructures for enhanced photocatalytic degradation of sulfonamides under visible light. J. Hazard. Mater. 2020, 387, 122021. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).