The Influence of Photoactive Heterostructures on the Photocatalytic Removal of Dyes and Pharmaceutical Active Compounds: A Mini-Review
Abstract
:1. Introduction
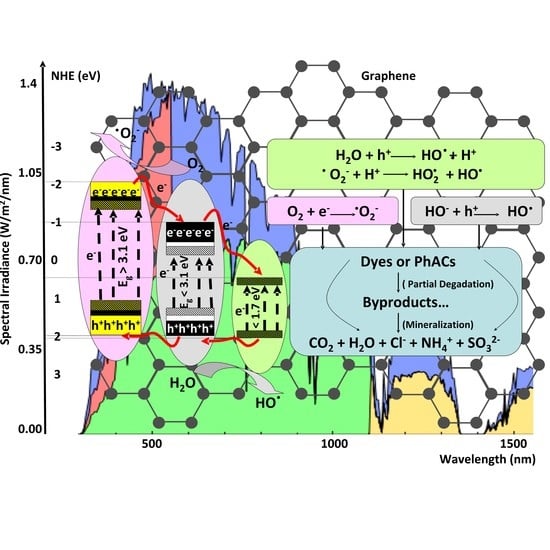
2. Heterostructure Mechanisms for Photocatalytic Application
3. Photocatalytic Organic Pollutants Removal by Heterostructures
3.1. Dyes
3.2. Pharmaceutical Active Compounds
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cheng, D.; Ngo, H.H.; Wei, D. A critical review on antibiotics and hormones in swine wastewater: Water pollution problems and control approaches. J. Hazard. Mater. 2020, 387, 121682. [Google Scholar] [CrossRef] [PubMed]
- Gautam, K.; Anbumani, S. Ecotoxicological effects of organic micro-pollutants on the environment. In Current Developments in Biotechnology and Bioengineering, 1st ed.; Varjani, S., Pandey, A., Tyagi, R.D., Ngo, H.H., Larroche, C., Eds.; Elsevier: New York, NY, USA, 2020; pp. 481–501. [Google Scholar]
- Duarte, R.M.; Matos, J.T.; Senesi, N. Organic pollutants in soils. In Soil Pollutions, 1st ed.; Duarte, A.C., Cachada, A., Rocha-Santos, T., Eds.; Elsevier: New York, NY, USA, 2018; pp. 103–126. [Google Scholar]
- Wood, D.; Shaw, S.; Cawte, T.; Shanen, E.; Heyst, B.V. An overview of photocatalyst immobilization methods for air pollution remedation. Chem. Eng. J. 2020, 391, 123490. [Google Scholar] [CrossRef]
- Hader, D.P.; Banaszak, A.T.; Helbling, E.W. Anthropogenic pollution of aquatic ecosystems: Emerging problems with global implications. Sci. Total Environ. 2020, 713, 136586. [Google Scholar] [CrossRef] [PubMed]
- Libralato, G.; Lofrano, G.; Siciliano, A.; Gambino, E.; Boccia, G.; Federica, C.; Francesco, A.; Galdiero, E.; Gesuele, R.; Guida, M. Toxicity assessment of wastewater after advanced oxidation processes for emerging contaminants’ degradation. In Visible Light Active Structured Photocatalysts for the Removal of Emerging Contaminants, 1st ed.; Sacco, O., Vaiano, V., Eds.; Elsevier: New York, NY, USA, 2020; pp. 195–211. [Google Scholar]
- Kaplan, A.; Mamane, H.; Lester, Y.; Avisar, D. Trace organic compound removal from wastewater reverse-osmosis concentrate by advanced oxidation processes with UV/O3/H2O2. Materials 2020, 13, 2785. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Zeng, L.; Qin, W.; Feng, J. Measures for reducing nitrate leaching in orchards: A review. Environ. Pollut. 2020, 263, 114553. [Google Scholar] [CrossRef]
- Serban, I.; Enesca, A. Metal Oxides-Based Semiconductors for Biosensors Applications. Front. Chem. 2020, 8, 354. [Google Scholar] [CrossRef]
- Tahreen, A.; Jami, M.S.; Ali, F. Role of electrocoagulation in wastewater treatment: A developmental review. J. Water Proc. Eng. 2020, 37, 101440. [Google Scholar] [CrossRef]
- Guo, W.; Umar, A.; Du, Y.; Wang, L.; Pei, M. Surface Modification of Bentonite with Polymer Brushes and Its Application as an Efficient Adsorbent for the Removal of Hazardous Dye Orange I. Nanomaterials 2020, 10, 1112. [Google Scholar] [CrossRef]
- Enesca, A.; Andronic, L.; Duta, A. The influence of surfactants on the crystalline structure, electrical and photocatalytic properties of hybrid multi-structured (SnO2, TiO2 and WO3) thin films. Appl. Surf. Sci. 2012, 258, 4339–4346. [Google Scholar] [CrossRef]
- Cornejo, O.M.; Murrieta, M.F.; Castañeda, L.F.; Nava, J.L. Characterization of the reaction environment in flow reactors fitted with BDD electrodes for use in electrochemical advanced oxidation processes: A critical review. Electrochim. Acta 2020, 33120, 135373. [Google Scholar] [CrossRef]
- Malakootian, M.; Shahesmaeili, A.; Faraji, M.; Amiri, H.; Martinez, S.S. Advanced oxidation processes for the removal of organophosphorus pesticides in aqueous matrices: A systematic review and meta-analysis. Process Saf. Environ. 2020, 134, 292–307. [Google Scholar] [CrossRef]
- Han, M.; Duan, X.; Cao, G.; Zhuc, S.; Ho, S.H. Graphitic nitride-catalyzed advanced oxidation processes (AOPs) for landfill leachate treatment: A mini review. Process Saf. Environ. 2020, 139, 230–240. [Google Scholar] [CrossRef]
- Yang, L.; He, L.; Xue, J.; Ma, Y.; Zhang, Z. UV/SO32− based advanced reduction processes of aqueous contaminants: Current status and prospects. Chem. Eng. J. 2020, 3971, 125412. [Google Scholar] [CrossRef]
- Bresolin, B.M.; Park, Y.; Bahnemann, D.W. Recent Progresses on Metal Halide Perovskite-Based Material as Potential Photocatalyst. Catalysts 2020, 10, 709. [Google Scholar] [CrossRef]
- Chen, L.; Tang, J.; Song, L.N.; Chen, P.; Yin, S.F. Heterogeneous photocatalysis for selective oxidation of alcohols and hydrocarbons. Appl. Catal. B 2019, 242, 379–388. [Google Scholar] [CrossRef]
- Chauhan, D.K.; Jain, S.; Battula, V.R.; Kailasam, K. Organic motif’s functionalization via covalent linkage in carbon nitride: An exemplification in photocatalysis. Carbon 2019, 152, 40–58. [Google Scholar] [CrossRef]
- Hu, X.; Ma, Q.; Wang, X.; Yang, Y.; Liu, N.; Zhang, C.; Kawazoe, N.; Chen, G.; Yang, Y. Layered Ag/Ag2O/BiPO4/Bi2WO6 heterostructures by two-step method for enhanced photocatalysis. J. Catal. 2020, 387, 28–38. [Google Scholar] [CrossRef]
- Ojha, D.P.; Karki, H.P.; Song, J.H.; Kim, H.J. Amine-assisted synthesis of FeWO4 nanorodg-C3N4 for enhanced visible light-driven Z-scheme photocatalysis. Compos. Part B Eng. 2019, 1601, 277–284. [Google Scholar] [CrossRef]
- Wetchakun, K.; Wetchakun, N.; Sakulsermsuk, S. An overview of solar/visible light-driven heterogeneous photocatalysis for water purification: TiO2- and ZnO-based photocatalysts used in suspension photoreactors. J. Ind. Eng. Chem. 2019, 7125, 19–49. [Google Scholar] [CrossRef]
- Yao, S.; Wang, J.; Zhou, X.; Zhou, S.; Pu, X.; Li, W. One-pot low-temperature synthesis of BiOX/TiO2 hierarchic composites of adsorption coupled with photocatalysis for quick degradation of colored and colorless organic pollutants. Adv. Powder Technol. 2020, 31, 1924–1932. [Google Scholar] [CrossRef]
- Kant, S.; Pathania, D.; Singh, P.; Dhiman, P.; Kumar, A. Removal of malachite green and methylene blue by Fe0.01Ni0.01Zn0.98O/polyacrylamide nanocompositeusing coupled adsorption and photocatalysis. Appl. Catal. B 2014, 147, 340–352. [Google Scholar] [CrossRef]
- Ma, Y.; Xiong, H.; Zhao, Z.; Yu, Y.; Zhou, D.; Dong, S. Model-based evaluation of tetracycline hydrochloride removal and mineralization in an intimately coupled photocatalysis and biodegradation reactor. Chem. Eng. J. 2018, 351, 967–975. [Google Scholar] [CrossRef]
- Li, F.; Lan, X.; Wang, L.; Kong, X.; Xu, P.; Tai, Y.; Liu, G.; Shi, J. An efficient photocatalyst coating strategy for intimately coupled photocatalysis and biodegradation (ICPB): Powder spraying method. Chem. Eng. J. 2020, 383, 123092. [Google Scholar] [CrossRef]
- Ceretta, M.B.; Vieira, Y.; Wolski, E.A.; Foletto, E.L.; Silvestri, S. Biological degradation coupled to photocatalysis by ZnO/polypyrrole composite for the treatment of real textile wastewater. J. Water Proc. Eng. 2020, 35, 101230. [Google Scholar] [CrossRef]
- Grcic, I.; Vrsaljko, D.; Katancic, Z.; Papic, S. Purification of household grey water loaded with hair colorants by solar photocatalysis using TiO2-coated textile fibers coupled flocculation with chitosan. J. Water Proc. Eng. 2015, 5, 15–27. [Google Scholar] [CrossRef]
- Xu, T.; Wang, P.; Wang, D.; Zhao, K.; Wei, M.; Liu, X.; Liu, H.; Cao, J.; Fan, H.; Yang, L. Ultrasound-assisted synthesis of hyper-dispersed type-II tubular Fe3O4@SiO2@ZnO/ZnS core/shell heterostructure for improved visible-light photocatalysis. J. Alloy. Compd. 2020, 838, 155689. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Quan, X.; Wu, Y. Enhanced generation of oxidative species and phenol degradation in a discharge plasma system coupled with TiO2 photocatalysis. Appl. Catal. B 2008, 83, 72–77. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Ma, J.; Peng, Y.; Wang, A. A review on bidirectional analogies between the photocatalysis and antibacterial properties of ZnO. J. Alloy. Compd. 2019, 78330, 898–918. [Google Scholar] [CrossRef]
- Li, H.; Zhang, N.; Zhao, F.; Liu, T.; Wang, Y. Facile Fabrication of a Novel Au/Phosphorus-Doped g-C3N4 Photocatalyst with Excellent Visible Light Photocatalytic Activity. Catalysts 2020, 10, 701. [Google Scholar] [CrossRef]
- Enesca, A.; Yamaguchi, Y.; Terashima, C.; Fujishima, A.; Nakata, K.; Duta, A. Enhanced UV-Vis photocatalytic performance of the CuInS2/TiO2/SnO2 hetero-structure for air decontamination. J. Catal. 2017, 350, 174–181. [Google Scholar] [CrossRef]
- Roques-Carmes, T.; Alem, H.; Hamieh, T.; Toufaily, J.; Frochot, C.; Villieras, F. Different strategies of surface modification to improve the photocatalysis properties: Pollutant adsorption, visible activation, and catalyst recovery. In Handbook of Smart Photocatalytic Materials, 1st ed.; Hussain, C.M., Mishra, A.K., Eds.; Elsevier: New York, NY, USA, 2020; pp. 39–57. [Google Scholar]
- Zhang, X.; Teng, S.Y.; Loy, A.C.M.; How, B.S.; Leong, W.D.; Tao, X. Transition Metal Dichalcogenides for the Application of Pollution Reduction: A Review. Nanomaterials 2020, 10, 1012. [Google Scholar] [CrossRef] [PubMed]
- Mouchaal, Y.; Enesca, A.; Mihoreanu, C.; Khelil, A.; Duta, A. Tuning the opto-electrical properties of SnO2 thin films by Ag+1 and In+3 co-doping. Mater. Sci. Eng. B 2015, 199, 22–29. [Google Scholar] [CrossRef]
- Song, B.; Zeng, Z.; Zeng, G.; Gong, J.; Tang, X. Powerful combination of g-C3N4 and LDHs for enhanced photocatalytic performance: A review of strategy, synthesis, and applications. Adv. Colloid Interface Sci. 2019, 272, 101999. [Google Scholar] [CrossRef] [PubMed]
- Salgado, B.C.; Cardeal, R.A.; Valentini, A. Photocatalysis and Photodegradation of Pollutants. In Nanomaterials Applications for Environmental Matrices, 1st ed.; Nascimento, R.F., Ferreira, O.P., De Paula, A.J., Neto, V.O., Eds.; Elsevier: New York, NY, USA, 2019; pp. 449–488. [Google Scholar]
- Enesca, A.; Andronic, L.; Duta, A. Optimization of Opto-Electrical and Photocatalytic Properties of SnO2 Thin Films Using Zn2+ and W6+ Dopant Ions. Catal. Lett. 2012, 142, 224–230. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, C.; Xiong, W.; Zeng, G.; Huang, D.; Zhang, C.; Wang, W.; Song, B.; Tang, X.; Li, X.; et al. Recent advances in application of graphitic carbon nitride-based catalysts for degrading organic contaminants in water through advanced oxidation processes beyond photocatalysis: A critical review. Water Res. 2020, 184, 116200. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Wang, C.; Qi, Y.; Chen, Z.; Xu, Q. CO2-Induced Defect Engineering: A New Protocol by Doping Vacancies in 2D Heterostructures for Enhanced Visible-Light Photocatalysis. Appl. Surf. Sci. 2017, 419, 573–579. [Google Scholar] [CrossRef]
- Pedanekar, R.S.; Shaikh, S.K.; Rajpure, K.Y. Thin film photocatalysis for environmental remediation: A status review. Curr. Appl. Phys. 2020, 20, 931–952. [Google Scholar] [CrossRef]
- He, W.; Sun, Y.; Jiang, G.; Li, Y.; Dong, F. Defective Bi4MoO9/Bi metal core/shell heterostructure: Enhanced visible light photocatalysis and reaction mechanism. Appl. Catal. B 2018, 239, 619–627. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, J.; Xu, L.; Li, X.; Huang, S. A room-temperature methane sensor based on Pd-decorated ZnO/rGO hybrids enhanced by visible light photocatalysis. Sens. Actuat. B 2020, 3041, 127334. [Google Scholar] [CrossRef]
- Andronic, L.; Enesca, A.; Cazan, C.; Visa, M. TiO2-active carbon composites for wastewater photocatalysis. J. Sol-Gel Sci. Technol. 2014, 71, 399–405. [Google Scholar] [CrossRef]
- Irani, E.; Amoli-Diva, M. Hybrid adsorption–photocatalysis properties of quaternary magneto-plasmonic ZnO/MWCNTs nanocomposite for applying synergistic photocatalytic removal and membrane filtration in industrial wastewater treatment. J. Photochem. Photobiol. A Chem. 2020, 39115, 112359. [Google Scholar] [CrossRef]
- Kusmierek, E. Semiconductor Electrode Materials Applied in Photoelectrocatalytic Wastewater Treatment—An Overview. Catalysts 2020, 10, 439. [Google Scholar] [CrossRef] [Green Version]
- Xiao, W.Z.; Xu, L.; Rong, Q.Y.; Dai, X.Y.; Wang, L.L. Two-dimensional H-TiO2/MoS2(WS2) van der Waals heterostructures for visible-light photocatalysis and energy conversion. Appl. Surf. Sci. 2020, 504, 144425. [Google Scholar] [CrossRef]
- Kanakkillam, S.S.; Krishnan, B.; Avellaneda, D.A.; Shaji, S. Surfactant free stable cobalt oxide nanocolloid in water by pulsed laser fragmentation and its thin films for visible light photocatalysis. Colloid. Surface. A 2020, 5945, 124657. [Google Scholar] [CrossRef]
- Rafiq, U.; Majid, K. Mitigating the charge recombination by the targeted synthesis of Ag2WO4/Bi2Fe4O9 composite: The facile union of orthorhombic semiconductors towards efficient photocatalysis. J. Alloy. Compd. 2020, 84225, 155876. [Google Scholar] [CrossRef]
- Jia, S.; Xu, M.; Chen, S.; Yan, J.; Ma, X. A hierarchical sandwich-structured MoS2/SnO2/CC heterostructure for high photocatalysis performance. Mater. Lett. 2019, 236, 697–701. [Google Scholar] [CrossRef]
- Li, D.; Song, H.; Meng, X.; Shen, T.; Sun, J.; Han, W.; Wang, X. Effects of Particle Size on the Structure and Photocatalytic Performance by Alkali-Treated TiO2. Nanomaterials 2020, 10, 546. [Google Scholar] [CrossRef] [Green Version]
- Estahbanati, M.R.K.; Feilizadeh, M.; Babin, A.; Mei, B.; Iliuta, M.C. Selective photocatalytic oxidation of cyclohexanol to cyclohexanone: A spectroscopic and kinetic study. Chem. Eng. J. 2020, 38215, 122732. [Google Scholar] [CrossRef]
- Guo, X.; Zhou, X.; Li, X.; Shao, C.; Liu, Y. Bismuth oxychloride (BiOCl)/copper phthalocyanine (CuTNPc) heterostructures immobilized on electrospun polyacrylonitrile nanofibers with enhanced activity for floating photocatalysis. J. Colloid Interface Sci. 2018, 525, 187–195. [Google Scholar] [CrossRef]
- Li, X.; Chen, D.; Li, N.; Xu, Q.; Lu, J. Efficient reduction of Cr(VI) by a BMO/Bi2S3 heterojunction via synergistic adsorption and photocatalysis under visible light. J. Hazard. Mater. 2020, 4005, 123243. [Google Scholar] [CrossRef]
- Mintcheva, N.; Gicheva, G.; Panayotova, M.; Kulinich, S.A. Room-Temperature Synthesis of ZnS Nanoparticles Using Zinc Xanthates as Molecular Precursors. Materials 2020, 13, 171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Cai, C.; Gu, Y.; Cheng, W.; Zhao, C. Novel electronic properties of a new MoS2/TiO2 heterostructure and potential applications in solar cells and photocatalysis. Appl. Surf. Sci. 2017, 414, 34–40. [Google Scholar] [CrossRef]
- González-Burciaga, L.A.; Núñez-Núñez, C.M.; Morones-Esquivel, M.M.; Avila-Santos, M.; Lemus-Santana, A.; Proal-Nájera, J.B. Characterization and Comparative Performance of TiO2 Photocatalysts on 6-Mercaptopurine Degradation by Solar Heterogeneous Photocatalysis. Catalysts 2020, 10, 118. [Google Scholar] [CrossRef] [Green Version]
- Liu, N.; Lu, N.; Yu, H.T.; Chen, S.; Quan, X. Efficient day-night photocatalysis performance of 2D/2D Ti3C2/Porous g-C3N4 nanolayers composite and its application in the degradation of organic pollutants. Chemosphere 2020, 246, 125760. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, G.; Wang, J.; Hu, Z.; Su, Y. Step-scheme NiO/BiOI heterojunction photocatalyst for rhodamine photodegradation. App. Surf. Sci. 2020, 511, 145499. [Google Scholar] [CrossRef]
- Kumar, R.S.; Min, K.S.; Lee, S.H.; Mergu, N.; Son, Y.A. Synthesis of novel panchromatic porphyrin-squaraine dye and application towards TiO2 combined photocatalysis. J. Photochem. Photobiol. A 2020, 397, 112595. [Google Scholar] [CrossRef]
- Tsai, C.G.; Tseng, W.J. Preparation of TiN–TiO2 composite nanoparticles for organic dye adsorption and photocatalysis. Ceram. Int. 2020, 46, 14529–14535. [Google Scholar] [CrossRef]
- Hernández-Carrillo, M.A.; Torres-Ricárdez, R.; García-Mendoza, M.F.; Ramírez-Morales, E.; Pérez-Hernández, G. Eu-modified ZnO nanoparticles for applications in photocatalysis. Catal. Today 2020, 3491, 191–197. [Google Scholar] [CrossRef]
- Cao, L.; Li, Y.F.; Tong, Y.; Yang, R.; Sun, L.; Cao, O.; Chen, R. A novel Bi12TiO20/g-C3N4 hybrid catalyst with a bionic granum configuration for enhanced photocatalytic degradation of organic pollutants. J. Hazard. Mater. 2019, 379, 120808. [Google Scholar] [CrossRef]
- Shende, A.G.; Tiwari, C.S.; Bhoyar, T.H.; Vidyasagar, D.; Umare, S.S. BWO nano-octahedron coupled with layered g-C3N4: An efficient visible light active photocatalyst for degradation of cationic/anionic dyes, and N2 reduction. J. Molec. Liq. 2019, 296, 111771. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Mei, J.; Sarina, S.; Wu, Z.; Liao, T.; Yan, C.; Sun, Z. Strongly interfacial-coupled 2D-2D TiO2/g-C3N4 heterostructure for enhanced visible-light induced synthesis and conversion. J. Hazard. Mater. 2020, 394, 122529. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Lan, H.; Zhang, M.; Zhu, H.; Bu, M. Preparation of heterostructure g-C3N4/ZnO nanorods for high photocatalytic activity on different pollutants (MB, RhB, Cr(VI) and eosin). Ceram. Int. 2020, 46, 12192–12199. [Google Scholar] [CrossRef]
- Fu, S.; Yuan, W.; Liu, X.; Yan, Y.; Liu, H.; Li, L.; Zhao, F.; Zhou, J. A novel 0D/2D WS2/BiOBr heterostructure with rich oxygen vacancies for enhanced broad-spectrum photocatalytic performance. J. Colloid. Interf. Sci. 2020, 569, 150–163. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Zhang, H.; Wu, Y.; Jia, Y.; Jin, R.; Gao, S. CTAB-assisted solvothermal construction of hierarchical Bi2MoO6/Bi5O7Br with improved photocatalytic performances. Sep. Purif. Technol. 2020, 242, 116775. [Google Scholar] [CrossRef]
- Zhao, K.; Zhang, Z.; Feng, Y.; Lin, S.; Li, H.; Gao, X. Surface oxygen vacancy modified Bi2MoO6/MIL-88B(Fe) heterostructure with enhanced spatial charge separation at the bulk & interface. Appl. Catal. B 2020, 268, 118740. [Google Scholar]
- Bao, L.; Yang, F.; Cheng, D.; Pan, X.; Zhang, H.; Zhao, F.; Zhao, S.; Tegus, O. Modified electronic structure of Ta2O5 via surface decorated with Ta3B2 nanodots for enhanced photocatalytic activity. App. Surf. Sci. 2020, 513, 145767. [Google Scholar] [CrossRef]
- Jin, J.; Xie, Y. Ultraviolet light induced oxygen vacancy-rich BiPO4−x/Bi2S3 nanorods with enhanced photocatalytic activity and mechanism. Res. Chem. Intermed. 2019, 45, 5609–5623. [Google Scholar] [CrossRef]
- Mandal, B.; Panda, J.; Paul, P.K.; Sarkar, R.; Tudu, B. MnFe2O4 decorated reduced graphene oxide heterostructures: Nanophotocatalyst for methylene blue dye degradation. Vacuum 2020, 173, 109150. [Google Scholar] [CrossRef]
- Alido, J.P.M.; Sari, F.N.I.; Ting, Y.M. Synthesis of Ag/hybridized 1T-2H MoS2/TiO2 heterostructure for enhanced visible-light photocatalytic activity. Ceram. Int. 2019, 45, 23651–23657. [Google Scholar] [CrossRef]
- Tian, Q.; Fang, G.; Ding, L.; Ran, M.; Zhang, H.; Pan, A.; Shen, K.; Deng, Y. ZnAl2O4/Bi2MoO6 heterostructures with enhanced photocatalytic activity for the treatment of organic pollutants and eucalyptus chemimechanical pulp wastewater. Mater. Chem. Phys. 2020, 241, 122299. [Google Scholar] [CrossRef]
- Çinar, B.; Kerimoglu, I.; Tonbül, B.; Demirbüken, A.; Dursun, S.; Kaya, I.C.; Kalem, V.; Akyildiz, H. Hydrothermal/electrospinning synthesis of CuO plate-like particles/TiO2 fibers heterostructures for high-efficiency photocatalytic degradation of organic dyes and phenolic pollutants. Mater. Sci. Semic. Proces. 2020, 109, 104919. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, J.; Geng, J.; Bao, R.; Wang, Z.; Xia, J.; Li, H. Perovskite LaNiO3/TiO2 step-scheme heterojunction with enhanced photocatalytic activity. Appl. Surf. Sci. 2020, 503, 144287. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, Z.; Kong, X.; He, F.; Zhao, R.; Wua, R.; Wei, T.; Wang, L.; Feng, J. A novel P-N heterojunction with staggered energy level based on ZnFe2O4 decorating SnS2 nanosheet for efficient photocatalytic degradation. App. Surf. Sci. 2020, 510, 145442. [Google Scholar] [CrossRef]
- Yan, H.; Zhu, Z.; Long, Y.; Li, W. Single-source-precursor-assisted synthesis of porous WO3/g-C3N4 with enhanced photocatalytic property. Colloid. Surfaces A 2019, 582, 123857. [Google Scholar] [CrossRef]
- Neto, N.F.A.; Lima, A.B.; Bomio, M.R.D.; Motta, F.V. Microwave-assisted hydrothermal synthesis of Ag2Mo1−xWxO4 (x = 0, 0.25, 0.50, 0.75 and 1 mol%) heterostructures for enhanced photocatalytic degradation of organic dyes. J. Alloy. Compd. 2020, 844, 156007. [Google Scholar]
- Wei, Y.; Huang, Y.; Fang, Y.; Zhao, Y.; Luo, D.; Guo, Q.; Fan, L.; Wu, J. Hollow mesoporous TiO2/WO3 sphere heterojunction with high visiblelight-driven photocatalytic activity. Mater. Res. Bull. 2019, 119, 110571. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, L.; Xin, A.; Yu, Y.; Wang, L.; Zhang, W. Visible light response and heterostructure of composite CdS@ZnSeZnO to enhance its photocatalytic activity. J. Alloys Compd. 2020, 813, 152190. [Google Scholar] [CrossRef]
- Yulizar, Y.; Apriandanua, D.O.B.; Ashna, R.I. La2CuO4-decorated ZnO nanoparticles with improved photocatalytic activity for malachite green degradation. Chem. Phys. Lett. 2020, 755, 137749. [Google Scholar] [CrossRef]
- Jia, J.; Du, X.; Zhang, Q.; Liu, E.; Fan, J. Z-scheme MgFe2O4/Bi2MoO6 heterojunction photocatalyst with enhanced visible light photocatalytic activity for malachite green removal. Appl. Surf. Sci. 2019, 492, 527–539. [Google Scholar] [CrossRef]
- Isari, A.A.; Hayati, F.; Kakavandi, B.; Rostami, M.; Dehghanifard, E. Cu co-doped TiO2@functionalized SWCNT photocatalyst coupled with ultrasound and visible-light: An effective sono-photocatalysis process for pharmaceutical wastewaters treatment. Chem. Eng. J. 2020, 392, 123685. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, X.; Zhang, W.; Liu, Z.; Chen, M. Advances in photocatalysis based on fullerene C60 and its derivatives: Properties, mechanism, synthesis, and applications. Appl. Catal. B 2020, 26515, 118579. [Google Scholar] [CrossRef]
- Ahmad, K.; Ghatak, H.R.; Ahuja, S.M. A review on photocatalytic remediation of environmental pollutants and H2 production through water splitting: A sustainable approach. Environ. Technol. Innov. 2020, 19, 100893. [Google Scholar] [CrossRef]
- Kovacic, M.; Papac, J.; Kusic, H.; Karamanis, P.; Bozic, A.L. Degradation of polar and non-polar pharmaceutical pollutants in water by solar assisted photocatalysis using hydrothermal TiO2-SnS2. Chem. Eng. J. 2020, 382, 122826. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, X.; Jiang, L.; Zhang, J.; Zeng, G. Powerful combination of 2D g-C3N4 and 2D nanomaterials for photocatalysis: Recent advances. Chem. Eng. J. 2020, 39015, 124475. [Google Scholar] [CrossRef]
- Gopinath, K.P.; Madhav, N.V.; Krishnan, A.; Malolan, R.; Rangarajan, G. Present applications of titanium dioxide for the photocatalytic removal of pollutants from water: A review. J. Environ. Manag. 2020, 27015, 110906. [Google Scholar] [CrossRef]
- Acharya, L.; Nayak, S.; Pattnaik, S.P.; Acharya, R.; Parida, K. Resurrection of boron nitride in p-n type-II boron nitride/B-doped-g-C3N4 nanocomposite during solid-state Z-scheme charge transfer path for the degradation of tetracycline hydrochloride. J. Colloid Interface Sci. 2020, 566, 211–223. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, W.; Zhu, J.; Fu, L.; Li, D.; Zhou, L. Enhanced visible light photocatalytic activity of g-C3N4 decorated ZrO2−x nanotubes heterostructure for degradation of tetracycline hydrochloride. J. Hazard. Mater. 2020, 384, 121275. [Google Scholar] [CrossRef]
- Shi, W.; Liu, C.; Li, M.; Lin, X.; Guo, F.; Shi, J. Fabrication of ternary Ag3PO4/Co3(PO4)2/g-C3N4 heterostructure with following Type II and Z-Scheme dual pathways for enhanced visible-light photocatalytic activity. J. Hazard. Mater. 2020, 389, 121907. [Google Scholar] [CrossRef]
- Kang, J.; Jin, C.; Li, Z.; Wang, M.; Chen, Z.; Wang, Y. Dual Z-scheme MoS2/g-C3N4/Bi24O31Cl10 ternary heterojunction photocatalysts for enhanced visible-light photodegradation of antibiotic. J. Alloys Compd. 2020, 825, 153975. [Google Scholar] [CrossRef]
- Wang, L.; Yang, G.; Wang, D.; Lu, C.; Guan, W.; Li, Y.; Deng, J.; Crittenden, J. Fabrication of the flower-flake-like CuBi2O4/Bi2WO6 heterostructure as efficient visible-light driven photocatalysts: Performance, kinetics and mechanism insight. Appl. Surf. Sci. 2019, 495, 143521. [Google Scholar] [CrossRef]
- Zhou, Z.; Xu, H.; Li, D.; Zou, Z.; Xia, Z. Microwave-assisted synthesis of La(OH)3/BiOCl n-n heterojunctions with high oxygen vacancies and its enhanced photocatalytic properties. Chem. Phys. Lett. 2019, 736, 136805. [Google Scholar] [CrossRef]
- Huang, J.; Chen, W.; Yu, X.; Fu, X.; Zhu, Y.; Zhang, Y. Fabrication of a ternary BiOCl/CQDs/rGO photocatalyst: The roles of CQDs and rGO in adsorption-photocatalytic removal of ciprofloxacin. Colloid. Surf. A 2020, 597, 124758. [Google Scholar] [CrossRef]
- Lu, C.; Guo, F.; Yan, Q.; Zhang, Z.; Li, D.; Wang, L.; Zhou, Y. Hydrothermal synthesis of type II ZnIn2S4/BiPO4 heterojunction photocatalyst with dandelion-like microflower structure for enhanced photocatalytic degradation of tetracycline under simulated solar light. J. Alloys Compd. 2019, 811, 151976. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, H.; Ma, X.; Zhang, X.; Zhu, Y.; Zhang, A.; Cao, Y.; Yang, P. Construction of AgI/Bi2MoO6/AgBi(MoO4)2 multi-heterostructure composite nanosheets for visible-light photocatalysis. Mater. Today Commun. 2020, 23, 100903. [Google Scholar] [CrossRef]
- Chen, M.; Dai, Y.; Guo, J.; Yang, H.; Liu, D.; Zhai, Y. Solvothermal synthesis of biochar@ZnFe2O4/BiOBr Z-scheme heterojunction for efficient photocatalytic ciprofloxacin degradation under visible light. Appl. Surf. Sci. 2019, 493, 1361–1367. [Google Scholar] [CrossRef]
- Bariki, R.; Majhi, D.; Das, K.; Behera, A.; Mishra, B.G. Facile synthesis and photocatalytic efficacy of UiO-66/CdIn2S4 nanocomposites with flowerlike 3D-microspheres towards aqueous phase decontamination of triclosan and H2 evolution. Appl. Catal. B 2020, 270, 118882. [Google Scholar] [CrossRef]
- Hojamberdiev, M.; Czech, B.; Goktaş, A.C.; Yubuta, K.; Kadirova, Z.C. SnO2@ZnS photocatalyst with enhanced photocatalytic activity for the degradation of selected pharmaceuticals and personal care products in model wastewater. J. Alloy. Compd. 2020, 827, 154339. [Google Scholar] [CrossRef]
- Li, M.; Xu, G.; Guan, Z.; Wang, Y.; Yu, H.; Yu, Y. Synthesis of Ag/BiVO4/rGO composite with enhanced photocatalytic degradation of triclosan. Sci. Total Environ. 2019, 664, 230–239. [Google Scholar] [CrossRef]
- Chang, C.; Yang, H.; Mu, W.; Cai, Y.; Wang, L.; Yang, L.; Qin, H. In situ fabrication of bismuth oxyiodide (Bi7O9I3/Bi5O7I) n-n heterojunction for enhanced degradation of triclosan (TCS) under simulated solar light irradiation. Appl. Catal. B. 2019, 254, 647–658. [Google Scholar] [CrossRef]
- Yu, T.; Wu, W.W.; Liu, L.; Gao, C.; Yang, T. Novel ternary p-ZnIn2S4/rGO/n-g-C3N4 Z-scheme nanocatalyst with enhanced antibiotic degradation in a dark self-biased fuel cell. Ceram. Int. 2020, 46, 9567–9574. [Google Scholar] [CrossRef]
- Yentür, G.; Dükkanci, M. Synthesis of Visible-Light Heterostructured Photocatalyst of Ag/AgCl Deposited on (040) Facet of Monoclinic BiVO4 for Efficient Carbamazepine Photocatalytic Removal. Appl. Surf. Sci. 2020, in press. [Google Scholar] [CrossRef]
- Yentür, G.; Dükkancı, M. Fabrication of magnetically separable plasmonic composite photocatalyst of Ag/AgBr/ZnFe2O4 for visible light photocatalytic oxidation of carbamazepine. Appl. Surf. Sci. 2020, 510, 145374. [Google Scholar] [CrossRef]
- Hu, Z.; Cai, X.; Wang, Z.; Li, S.; Wang, Z.; Xie, X. Construction of carbon-doped supramolecule-based g-C3N4/TiO2 composites for removal of diclofenac and carbamazepine: A comparative study of operating parameters, mechanisms, degradation pathways. J. Hazard. Mater. 2019, 380, 120812. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, Y.; Li, L. Facet-engineered surface and interface design of WO3/Bi2WO6 photocatalyst with direct Z-scheme heterojunction for efficient salicylic acid removal. Appl. Surf. Sci. 2020, 508, 144796. [Google Scholar] [CrossRef]
- Plodinec, M.; Grcic, I.; Willinger, M.G.; Hammud, A.; Huang, X.; Panzic, I.; Gajovi, A. Black TiO2 nanotube arrays decorated with Ag nanoparticles for enhanced visible-light photocatalytic oxidation of salicylic acid. J. Alloy. Compd. 2019, 776, 883–896. [Google Scholar] [CrossRef]
- Malefane, M.E.; Feleni, U.; Mafa, P.J.; Kuvarega, A.T. Fabrication of direct Z-scheme Co3O4/BiOI for ibuprofen and trimethoprim degradation under visible light irradiation. Appl. Surf. Sci. 2020, 514, 145940. [Google Scholar] [CrossRef]
- Deng, Y.; Feng, C.; Tang, L.; Zhou, Y.; Chen, Z.; Feng, H.; Wang, J.; Yu, J.; Liu, Y. Ultrathin low dimensional heterostructure composites with superior photocatalytic activity: Insight into the multichannel charge transfer mechanism. Chem. Eng. J. 2020, 393, 124718. [Google Scholar] [CrossRef]
- Liu, N.; Wang, J.; Wu, J.; Li, Z.; Huang, W.; Zheng, Y.; Lei, J.; Zhang, X.; Tang, L. Magnetic Fe3O4@MIL-53(Fe) nanocomposites derived from MIL-53(Fe) for the photocatalytic degradation of ibuprofen under visible light irradiation. Mater. Res. Bull. 2020, 132, 111000. [Google Scholar] [CrossRef]
- Wang, N.; Li, X.; Yang, Y.; Zhou, Z.; Shang, Y.; Zhuang, X.; Zhang, T. Two-stage calcination composite of Bi2O3-TiO2 supported on powdered activated carbon for enhanced degradation of sulfamethazine under solar irradiation. J. Water Proc. Eng. 2020, 35, 101220. [Google Scholar] [CrossRef]
- Liang, M.; Yu, Y.; Wang, Y.; Yu, Y. Remarkably efficient charge transfer through a double heterojunction mechanism by a CdS-SnS-SnS2/rGO composite with excellent photocatalytic performance under visible light. J. Hazard. Mater. 2020, 391, 121016. [Google Scholar] [CrossRef]
- Kumar, A.; Khan, M.; Zeng, X.; Lo, I. Development of g-C3N4/TiO2/Fe3O4@SiO2 heterojunction via sol-gel route: A magnetically recyclable direct contact Z-scheme nanophotocatalyst for enhanced photocatalytic removal of ibuprofen from real sewage effluent under visible light. Chem. Eng. J. 2018, 353, 645–656. [Google Scholar] [CrossRef]
- Ji, H.; Du, P.; Zhao, D.; Li, S.; Sun, F.; Duin, E.C.; Liu, W. 2D/1D graphitic carbon nitride/titanate nanotubes heterostructure for efficient photocatalysis of sulfamethazine under solar light: Catalytic “hot spots” at the rutile–anatase–titanate interfaces. Appl. Catal. B 2020, 263, 118357. [Google Scholar] [CrossRef]
- Di, G.; Zhu, Z.; Zhang, H.; Qiu, Y.; Yin, D.; Crittenden, J. Simultaneous sulfamethazine oxidation and bromate reduction by Pd mediated Z-scheme Bi2MoO6/g-C3N4 photocatalysts: Synergetic mechanism and degradative pathway. Chem. Eng. J. 2020, 401, 126061. [Google Scholar] [CrossRef]
- Cao, Y.; Fang, Y.; Lei, X.; Tan, B.; Hu, X.; Liu, B.; Chen, Q. Fabrication of novel CuFe2O4/MXene hierarchical heterostructures for enhanced photocatalytic degradation of sulfonamides under visible light. J. Hazard. Mater. 2020, 387, 122021. [Google Scholar] [CrossRef] [PubMed]






| Heterostructure Composition | Synthesis Method | Morphology/Crystallinity | Pollutant and Concentration (mg/L) | Radiation Type, Intensity (I), Irradiation Time (t) | Efficiency and Rate Constant (min−1) | Ref. |
|---|---|---|---|---|---|---|
| Bi12TiO20/g-C3N4 | Ultrasonication | Multilayer structure/cubic Bi12TiO20 | RhB = 10 MO = 20 | Vis I = 500 W t = 50 min | 97% (RhB), 90% (MO)/0.0537 (RhB), 0.0328 (MO) | [64] |
| Bi3.84W0.16O6.24 (BWO)/g-C3N4 | Ultrasonication | Sheets/cubic BWO | RhB = 10 | Vis I = 100 W t = 50 min | 99.8%/ 0.0562 | [65] |
| Bi2MoO6/Bi5O7Br/TiO2 | Solvothermal | Tube arrays/anatase TiO2, orthorhombic Bi2MoO6 | RhB = 10 MO = 16 MB = 6.5 | Vis I = 500 W t = 180 min | 73.43% (RhB) 47.77% (MO) 93.81% (MB)/0.00742 (RhB) 0.00354 (MO) 0.00225 (MB) | [69] |
| Bi2MoO6/Fe3O | Solvothermal | Flower/orthorhombic Bi2MoO6 | RhB = 20 | Vis I = 350 W t = 120 min | 99.5%/ 0.0364 | [70] |
| NiO/BiOI | Solvothermal | Foam/crystalline NiO | RhB = 4.8 | Vis I = 300 W t = 60 min | 90%/ 0.0572 | [60] |
| TiO2/g-C3N4 | Hydrothermal | 2D sheet/pristine 2D-TiO2 | RhB = 10 | Vis I = 500 W t = 60 min | 85%/ 0.03 | [66] |
| WS2/BiOBr | Hydrothermal | Plates/tetragonal BiOBr | RhB = 20 | Vis I = 500 W t = 100 min | 95%/ np * | [68] |
| g-C3N4/ZnO | Hydrothermal | Rod/hexagonal wurtzite ZnO | RhB = 10 MB = 10 | Vis I = 300 W t = 70 min | 98% (MB), 98.5% (RhB)/ np | [67] |
| BiPO4−x/B2S3 | Hydrothermal | Sheet/monoclinic BiPO4 and orthorhombic Bi2S3 | MB = 5 | Vis I = 300 W t = 360 min | 98%/ 0.0222 | [72] |
| MnFe2O4/rGO | Coprecipitation | Spherical/cubic MnFe2O4 | MB = 10 | UV I = 40 W t = 60 min | 97%/ 0.0589 | [73] |
| ZnAl2O4/Bi2MoO6 | Coprecipitation | Sheet/koechlinite Bi2MoO6 and gahnite ZnAl2O4 | MB = 30 | UV I = 100 W t = 180 min | 86.36%/ 0.638 | [75] |
| Ag/hybridized 1T-2H MoS2/TiO2 | Chemical reduction | Flower/anatase TiO2 | MB = 20 | UV I = 235 W t = 60 min | 96.8%/ 0.0539 | [74] |
| Ta3B2@Ta2O5 | In situ | Powder/crystalline Ta3B2 and Ta2O5 | MB = 50 | Vis I = 500 W t = 180 min | 80%/ np | [71] |
| CuO–TiO2 | Ultrasonication | Fiber/anatase TiO2 and monoclinic CuO | MB = 1 | UVc, Vis IUVc = 96 W IVis = 250 W tUVc = 30 min tVis = 240 min | 99% (UVc) 98% (Vis)/ 0.135 (UVc) 0.015 (Vis) | [76] |
| WO3/g-C3N4 | Polymerization | Sheet/crystalline WO3/g-C3N4 | MO = 10 | Vis I = 300 W t = 120 min | 93%/ 0.0213 | [79] |
| LaNiO3/TiO2 | Sol–gel | Particles/perovskite LaNiO3, anatase and rutile TiO2 | MO = 10 MO = 20 | Vis I = 300 W t = 150 min | 100% (10 mg/L) 92% (20 mg/L)/ np | [77] |
| ZnFe2O4/SnS2 | Solvothermal | Particles/crystalline ZnFe2O4 and SnS2 | MO = 50 | Vis I = 300 W t = 20 min | 99%/ 0.214 | [78] |
| Ag2Mo1−xWxO4 | Microwave-assisted hydrothermal | Rod/cubic Ag2MoO4, orthorhombic Ag2WO4 | MO = 5 | UVc I = 90 W t = 140 min | 45%/ 0.0058 | [80] |
| TiO2/WO3 | One pot | Hollow sphere/anatase TiO2 and monoclinic WO3 | MG = 50 | Vis I = 300 W t = 60 min | 98%/ 0.0746 | [81] |
| La2CuO4-decorated ZnO | In situ extraction | Particles/crystalline ZnO, orthorhombic La2CuO4 | MG = 25 | Vis I = 125 W t = 120 min | 91%/ 0.063 | [83] |
| MgFe2O4/Bi2MoO6 | Hydrothermal | Plates/crystalline Bi2MoO6 and MgFe2O4 | MG = 20 | Vis I = 300 W t = 120 min | 97%/ 0.0113 | [84] |
| CdS@ZnS@ZnO | Hydrothermal | Spherical/cubic ZnS, hexagonal CdS and ZnO | MG = 50 | UV, Vis IUV = 125 W IVis = 400 W tUV = 30 min tVis = 180 min | 95% (UV) 65% (Vis)/ np | [82] |
| Heterostructure Composition | Synthesis Method | Morphology/Crystallinity | Pollutant and Concentration (mg/L) | Radiation Type, Intensity (I), Irradiation Time (t) | Efficiency and Rate Constant (min−1) | Ref. |
|---|---|---|---|---|---|---|
| BN/B-doped-g-C3N4 | In situ growth | Sheet/hexagonal BN | TC = 10 | Vis I = 300 W t = 60 min | 88.1%/ 0.034 | [91] |
| WO3/g-C3N4 | Polymerization | Sheet/crystalline WO3/g-C3N4 | TC = 10 | Vis I = 300 W t = 180 min | 97%/ np * | [79] |
| Ag3PO4/Co3(PO4)2/g-C3N4 | Precipitation | 3D flower/crystalline Co3(PO4)2, g-C3N4 and Ag3PO4 | TC = 10 | Vis I = 300 W t = 120 min | 88%/ 0.0159 | [93] |
| g-C3N4-decorated ZrO2−x | Anodic oxidation and PVD | Tube/tetragonal and monoclinic zirconia | TC = 10 | Vis I = 300 W t = 60 min | 90.6%/ 0.0474 | [92] |
| ZnIn2S4/BiPO4 | Hydrothermal | Flower/monoclinic BiPO4 | TC = 40 | Vis I = 300 W t = 90 min | 84%/ 0.0201 | [98] |
| AgI/Bi2MoO6/AgBi(MoO4)2 | Hydrothermal | Sheets/crystalline AgI, Bi2MoO6 and AgBi(MoO4)2 | TC = 5 | Vis I = 400 W t = 90 min | 91.9%/ 0.0097 | [99] |
| MoS2/g-C3N4/Bi24O31Cl10 | Calcination | Sheet/monoclinic Bi24O31Cl10 and MoS2 | TC = 20 | Vis I = 300 W t = 50 min | 97.5%/ 0.0642 | [94] |
| CuBi2O4/Bi2WO6 | Hydrothermal | Pseudo-sphere/tetragonal CuBi2O4 and orthorhombic Bi2WO6 | TC = 20 | Vis I = 300 W t = 120 min | 93%/ 0.0286 | [95] |
| La(OH)3/BiOCl | Microwave | Sheet/crystalline BiOCl | TC = 20 | Vis I = 5 W t = 60 min | 85%/ 0.037 | [96] |
| WS2/BiOBr | Hydrothermal | Plates/tetragonal BiOBr | TC = 20 CIP = 20 | Vis I = 500 W t = 100 min | 96% (TC) 92% (CIP)/ np (TC) 0.01708 (CIP) | [68] |
| BiOCl/CQDs/rGO | Hydrothermal | Sheet/tetragonal BiOCl | CIP = 20 | Vis I = 300 W t = 100 min | 87%/ 0.0146 | [97] |
| LaNiO3/TiO2 | In situ sol–gel | Granular/anatase TiO2 and perovskite LaNiO3 | CIP = 10 | UV, Vis IUV, Vis = 300 W t = 180 min | 90% (UV) 55% (Vis)/ np | [77] |
| PVPbiochar@ZnF2O4/BiOBr | Solvothermal | Sheet/tetragonal BiOBr, spinel ZnFe2O4 | CIP = 15 | Vis I = 300 W t = 60 min | 84%/ np | [100] |
| UiO-66/CdIn2S4 | Solvothermal | 3D flower/pristine CIS | TCS = 10 | Vis I = 150 W t = 180 min | 92%/ 0.0094 | [101] |
| Ag/BiVO4/rGO | Hydrothermal | Irregular Particles/monoclinic BiVO4 | TCS = 10 | Vis I = 300 W t = 120 min | 100%/ np | [103] |
| SnO2@ZnS | Hydrothermal | Sheet/cubic ZnS, tetragonal SnO2 | TCS = 10 | Vis I = 500 W t = 120 min | 40%/ 0.0033 | [102] |
| Bi7O9I3/Bi5O7I | Calcination | Bone-stick/crystalline Bi7O9I3 and Bi5O7I | TCS = 20 | Vis I = 500 W t = 180 min | 89.28%/ 0.0168 | [104] |
| p-ZnIn2S4/rGO/n-g-C3N4 | Hydrothermal | Sheet/crystalline ZnIn2S4 | TCS = 50 | UV, Vis IUV = 20 W IVis = 2 W t = 120 min | 100% (UV) 97% (Vis)/ np | [105] |
| Ag/AgCl/BiVO4 | Ultrasonication | Octahedral particle/monoclinic BiVO4, crystalline AgCl and Ag | CBZ = 10 | Vis I = 93.38 W t = 240 min | 70.6%/ np | [106] |
| g-C3N4/TiO2 | Calcination | Sheet/crystalline g-C3N4, anatase TiO2 | CBZ = 10 | Vis I = 50 W t = 360 min | 99.77%/ 0.1796 | [108] |
| Ag/AgBr/ZnFe2O4 | Ultrasonication | Spherical/cubic AgBr and ZnFe2O4 | CBZ = 10 | Vis I = 93.38 W t = 240 min | 22.7%/ np | [107] |
| Bi12TiO20/g-C3N4 | Ultrasonication | Spherical/cubic Bi12TiO20 | SA = 10 | Vis I = 500 W t = 50 min | 50%/ np | [64] |
| WO3/Bi2WO6 | Hydrothermal | Flower/orthorhombic Bi2WO6 | SA = 5 | Vis I = 300 W t = 360 min | 74.5%/ 0.00435 | [109] |
| TiO2-NT’s@Ag-HA | Photoreduction | Tubes/anatase TiO2 | SA = 28 | Full Spectrum (FS), Vis IFS = 120 W IVis = 100 W t = 240 min | 75% (FS) 30% (Vis)/ 0.00581 (FS) 0.00129 (Vis) | [110] |
| TiO2/WO3 | One pot | Hollow sphere/anatase TiO2 and monoclinic WO3 | SA = 50 | UV I = 300 W t = 60 min | 42%/ np | [81] |
| CdS-SnS-SnS2/rGO | Solvothermal | Sheet/hexagonal CdS, SnS2 and orthorhombic SnS | IBF = 100 | Vis I = 300 W t = 60 min | 84.4%/ 0.0257 | [115] |
| Bi2O3-TiO2/carbon | Calcination | Particle/anatase and rutile TiO2 | IBF = 20 | Vis I = 300 W t = 120 min | 100%/ 0.0290 | [114] |
| W18O49/g-C3N4 | Hydrothermal | Sheet/monoclinic W18O49 | IBF = 10 | Vis, NIR IVis, NIR = 300 W tVis = 60 min tNIR = 120 min | 96.3% (Vis) 39.2% (NIR)/0.0464 (Vis) 0.0027 (NIR) | [112] |
| Fe3O4@MIL-53(Fe) | Calcination | Particles with polyhedron structure/crystalline Fe3O4 | IBF = 10 | Vis I = 500 W t = 60 min | 99%/ 0.0471 | [113] |
| Co3O4/BiOI | Solvothermal | Plates/crystalline Co3O4, Tetragonal BiOI | IBF = 10 | Vis I = 60 W t = 60 min | 93.87%/ 0.0945 | [111] |
| g-C3N4/TiO2/Fe3O4@SiO2 | Sol–gel | Sheet/standard magnetite, anatase TiO2 | IBF = 2 | Vis I = 64 W t = 15 min | 97%/ np | [116] |
| g-C3N4/TNTs | Hydrothermal | Sheet/anatase and rutile TiO2 | SMZ = 5 | Vis I = 450 W t = 300 min | 100%/ 0.0193 | [117] |
| Pd-Bi2MoO6/g-C3N4 | Precipitation | Flake/crystalline Bi2MoO6 and g-C3N4 | SMZ = 5 | Vis I = 36 W t = 90 min | 98.8%/ 0.0440 | [118] |
| CuFe2O4/Ti3C2 | Hydrothermal | Sheet/spinel CuFe2O4 | SMZ = 40 | Vis I = 300 W t = 60 min | 70%/ 0.0128 | [119] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enesca, A.; Andronic, L. The Influence of Photoactive Heterostructures on the Photocatalytic Removal of Dyes and Pharmaceutical Active Compounds: A Mini-Review. Nanomaterials 2020, 10, 1766. https://doi.org/10.3390/nano10091766
Enesca A, Andronic L. The Influence of Photoactive Heterostructures on the Photocatalytic Removal of Dyes and Pharmaceutical Active Compounds: A Mini-Review. Nanomaterials. 2020; 10(9):1766. https://doi.org/10.3390/nano10091766
Chicago/Turabian StyleEnesca, Alexandru, and Luminita Andronic. 2020. "The Influence of Photoactive Heterostructures on the Photocatalytic Removal of Dyes and Pharmaceutical Active Compounds: A Mini-Review" Nanomaterials 10, no. 9: 1766. https://doi.org/10.3390/nano10091766
APA StyleEnesca, A., & Andronic, L. (2020). The Influence of Photoactive Heterostructures on the Photocatalytic Removal of Dyes and Pharmaceutical Active Compounds: A Mini-Review. Nanomaterials, 10(9), 1766. https://doi.org/10.3390/nano10091766