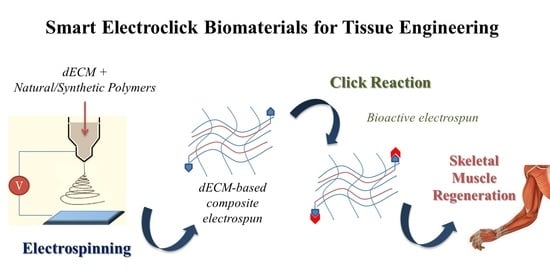
Smart ECM-Based Electrospun Biomaterials for Skeletal Muscle Regeneration
Abstract
1. Introduction
2. Biomaterials for Electrospinning in Tissue Engineering
2.1. Synthetic and Natural Polymers
2.2. Composite Polymeric Electrospun Fibers
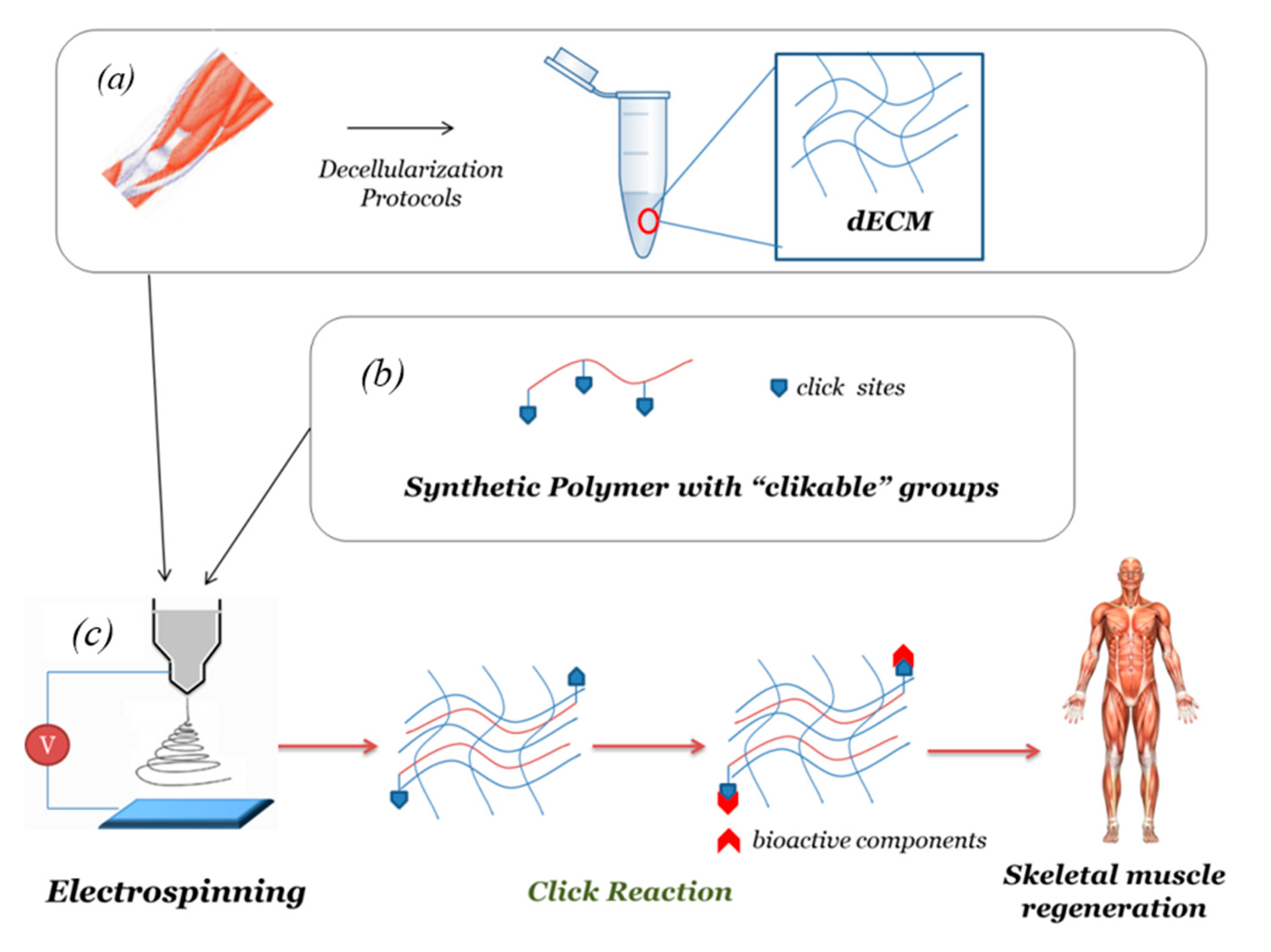
2.3. Decellularized Extracellular Matrix (dECM)-Based Electrospun Fibers
3. Bioactivity and Biofunctionalization of Electrospun Scaffolds
3.1. Bulk Biofunctionalization
3.2. Surface Biofunctionalization and Click Chemistry
- Physical adsorption is a simple approach that involves incubating the scaffold in a solution containing biomolecules. The biomolecules attach onto the scaffold surface owing to surface interactions, e.g., electrostatic forces, van der Waals forces, and hydrogen bonds.
- Chemical immobilization of biomolecules to the surface fibers is realized by the creation of a chemical bonding between functional groups of the components and those of bioactive molecules. Compared to physical adsorption, the covalent surface immobilization of biomolecules results in a more efficient coating; moreover, the bioactive components are retained over a longer period of time, promoting tissue regeneration [75]. In particular, an appropriate choice of polymers—biodegradable or nondegradable—allows the release rate of bioactive components to be controlled.
4. Customized Functionalization by Click Chemistry of Composite dECM-Based Electrospun Scaffold for Skeletal Tissue Regeneration
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khan, F.; Tanaka, M. Designing smart biomaterials for tissue engineering. Int. J. Mol. Sci. 2018, 19, 17. [Google Scholar] [CrossRef] [PubMed]
- Pérez, R.A.; Won, J.E.; Knowles, J.C.; Kim, H.W. Naturally and synthetic smart composite biomaterials for tissue regeneration. Adv. Drug Deliv. Rev. 2013, 65, 471–496. [Google Scholar] [CrossRef] [PubMed]
- Smoak, M.M.; Mikos, A.G. Advances in biomaterials for skeletal muscle engineering and obstacles still to overcome. Mater. Today Bio 2020, 7, 100069. [Google Scholar] [CrossRef] [PubMed]
- Qazi, T.H.; Duda, G.N.; Ort, M.J.; Perka, C.; Geissler, S.; Winkler, T. Cell therapy to improve regeneration of skeletal muscle injuries. J. Cachexia. Sarcopenia Muscle 2019, 10, 501–516. [Google Scholar] [CrossRef]
- Shin, H.; Jo, S.; Mikos, A.G. Biomimetic materials for tissue engineering. Biomaterials 2003, 24, 4353–4364. [Google Scholar] [CrossRef]
- Carotenuto, F.; Teodori, L.; Maccari, A.M.; Delbono, L.; Orlando, G.; Di Nardo, P. Turning regenerative technologies into treatment to repair myocardial injuries. J. Cell. Mol. Med. 2020, 24, 2704–2716. [Google Scholar] [CrossRef]
- Ma, P.X. Biomimetic materials for tissue engineering. Adv. Drug Deliv. Rev. 2008, 60, 184–198. [Google Scholar] [CrossRef]
- Pina, S.; Ribeiro, V.P.; Marques, C.F.; Maia, F.R.; Silva, T.H.; Reis, R.L.; Oliveira, J.M. Scaffolding Strategies for Tissue Enginnering and Regenerative Medicine Applications. Materials (Basel) 2019, 12, 1824. [Google Scholar] [CrossRef]
- Badylak, S.F. The extracellular matrix as a scaffold for tissue reconstruction. Semin. Cell Dev. Biol. 2002, 13, 377–383. [Google Scholar] [CrossRef]
- Badylak, S.F.; Freytes, D.O.; Gilbert, T.W. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 2009, 5, 1–13. [Google Scholar] [CrossRef]
- Yao, T.; Baker, M.B.; Moroni, L. Strategies to improve nanofibrous scaffolds for vascular tissue engineering. Nanomaterials 2020, 10, 887. [Google Scholar] [CrossRef] [PubMed]
- Tebyetekerwa, M.; Ramakrishna, S. What Is Next for Electrospinning? Matter 2020, 2, 279–283. [Google Scholar] [CrossRef]
- Li, W.J.; Laurencin, C.T.; Caterson, E.J.; Tuan, R.S.; Ko, F.K. Electrospun nanofibrous structure: A novel scaffold for tissue engineering. J. Biomed. Mater. Res. 2002, 60, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, S.; Fujihara, K.; Teo, W.E.; Yong, T.; Ma, Z.; Ramaseshan, R. Electrospun nanofibers: Solving global issues. Mater. Today 2006, 9, 40–50. [Google Scholar] [CrossRef]
- Min, L.; Pan, H.; Chen, S.; Wang, C.; Wang, N.; Zhang, J.; Cao, Y.; Chen, X.; Hou, X. Recent progress in bio-inspired electrospun materials. Compos. Commun. 2019, 11, 12–20. [Google Scholar] [CrossRef]
- Ameer, J.M.; Anil Kumar, P.R.; Kasoju, N. Strategies to tune electrospun scaffold porosity for effective cell response in tissue engineering. J. Funct. Biomater. 2019, 10, 30. [Google Scholar] [CrossRef]
- Khorshidi, S.; Solouk, A.; Mirzadeh, H.; Mazinani, S.; Lagaron, J.M.; Sharifi, S.; Ramakrishna, S. A review of key challenges of electrospun scaffolds for tissue-engineering applications. J. Tissue Eng. Regen. Med. 2016, 10, 715–738. [Google Scholar] [CrossRef]
- Seyedmahmoud, R.; Rainer, A.; Mozetic, P.; Maria Giannitelli, S.; Trombetta, M.; Traversa, E.; Licoccia, S.; Rinaldi, A. A primer of statistical methods for correlating parameters and properties of electrospun poly(l-lactide) scaffolds for tissue engineering-PART 1: Design of experiments. J. Biomed. Mater. Res. Part A 2015, 103, 91–102. [Google Scholar] [CrossRef]
- Seyedmahmoud, R.; Mozetic, P.; Rainer, A.; Giannitelli, S.M.; Basoli, F.; Trombetta, M.; Traversa, E.; Licoccia, S.; Rinaldi, A. A primer of statistical methods for correlating parameters and properties of electrospun poly(l-lactide) scaffolds for tissue engineering-PART 2: Regression. J. Biomed. Mater. Res.-Part A 2015, 103, 103–114. [Google Scholar] [CrossRef]
- Ghasemi-Mobarakeh, L.; Prabhakaran, M.P.; Morshed, M.; Nasr-Esfahani, M.H.; Ramakrishna, S. Electrospun poly(ε-caprolactone)/gelatin nanofibrous scaffolds for nerve tissue engineering. Biomaterials 2008, 29, 4532–4539. [Google Scholar] [CrossRef]
- Liang, D.; Hsiao, B.S.; Chu, B. Functional electrospun nanofibrous scaffolds for biomedical applications. Adv. Drug Deliv. Rev. 2007, 59, 1392–1412. [Google Scholar] [CrossRef] [PubMed]
- Rim, N.G.; Shin, C.S.; Shin, H. Current Approaches to Electrospun Nanofibers for Tissue Engineering. Biomed. Mater. 2013, 8, 014102. [Google Scholar] [CrossRef] [PubMed]
- Hanumantharao, S.N.; Rao, S. Multi-functional electrospun nanofibers from polymer blends for scaffold tissue engineering. Fibers 2019, 7, 66. [Google Scholar] [CrossRef]
- Swinehart, I.T.; Badylak, S.F. Extracellular matrix bioscaffolds in tissue remodeling and morphogenesis. Dev. Dyn. 2016, 245, 351–360. [Google Scholar] [CrossRef]
- Porzionato, A.; Stocco, E.; Barbon, S.; Grandi, F.; Macchi, V.; De Caro, R. Tissue-engineered grafts from human decellularized extracellular matrices: A systematic review and future perspectives. Int. J. Mol. Sci. 2018, 19, 4117. [Google Scholar] [CrossRef]
- Krishtul, S.; Baruch, L.; Machluf, M. Processed Tissue–Derived Extracellular Matrices: Tailored Platforms Empowering Diverse Therapeutic Applications. Adv. Funct. Mater. 2020, 30, 1900386. [Google Scholar] [CrossRef]
- Santschi, M.; Vernengo, A.; Eglin, D.; D’Este, M.; Wuertz-Kozak, K. Decellularized matrix as a building block in bioprinting and electrospinning. Curr. Opin. Biomed. Eng. 2019, 10, 116–122. [Google Scholar] [CrossRef]
- Shute, J. Glycosaminoglycan and chemokine/growth factor interactions. Handb. Exp. Pharmacol. 2012, 207, 307–324. [Google Scholar] [CrossRef]
- Heath, D.E. A Review of Decellularized Extracellular Matrix Biomaterials for Regenerative Engineering Applications. Regen. Eng. Transl. Med. 2019, 5, 155–166. [Google Scholar] [CrossRef]
- Smoak, M.M.; Han, A.; Watson, E.; Kishan, A.; Grande-Allen, K.J.; Cosgriff-Hernandez, E.; Mikos, A.G. Fabrication and Characterization of Electrospun Decellularized Muscle-Derived Scaffolds. Tissue Eng. Part C Methods 2019, 25, 276–287. [Google Scholar] [CrossRef]
- Ji, W.; Sun, Y.; Yang, F.; Van Den Beucken, J.J.J.P.; Fan, M.; Chen, Z.; Jansen, J.A. Bioactive electrospun scaffolds delivering growth factors and genes for tissue engineering applications. Pharm. Res. 2011, 28, 1259–1272. [Google Scholar] [CrossRef]
- Yoo, H.S.; Kim, T.G.; Park, T.G. Surface-functionalized electrospun nanofibers for tissue engineering and drug delivery. Adv. Drug Deliv. Rev. 2009, 61, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Kalaoglu-Altan, O.I.; Sanyal, R.; Sanyal, A. Reactive and “clickable” electrospun polymeric nanofibers. Polym. Chem. 2015, 6, 3372–3381. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, L.; Yang, L.; Ding, M.; Lin, F.; Wang, Z.; Li, Y. “Click” chemistry in polymeric scaffolds: Bioactive materials for tissue engineering. J. Control. Release 2018, 273, 160–179. [Google Scholar] [CrossRef] [PubMed]
- Seal, B.L.; Otero, T.C.; Panitch, A. Polymeric biomaterials for tissue and organ regeneration. Mater. Sci. Eng. R Rep. 2001, 34, 147–230. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, Z.; Zhou, T.; Liu, Y.; Leng, J. Shape Memory Polymer Nanofibers and Their Composites: Electrospinning, Structure, Performance, and Applications. Front. Mater. 2015, 2, 62. [Google Scholar] [CrossRef]
- Liu, X.; Holzwarth, J.M.; Ma, P.X. Functionalized Synthetic Biodegradable Polymer Scaffolds for Tissue Engineering. Macromol. Biosci. 2012, 12, 911–919. [Google Scholar] [CrossRef]
- Soares, R.M.D.; Siqueira, N.M.; Prabhakaram, M.P.; Ramakrishna, S. Electrospinning and electrospray of bio-based and natural polymers for biomaterials development. Mater. Sci. Eng. C 2018, 92, 969–982. [Google Scholar] [CrossRef]
- Sell, S.A.; Wolfe, P.S.; Garg, K.; McCool, J.M.; Rodriguez, I.A.; Bowlin, G.L. The use of natural polymers in tissue engineering: A focus on electrospun extracellular matrix analogues. Polymers 2010, 2, 522–553. [Google Scholar] [CrossRef]
- Hong, M.; Chen, E.Y.X. Chemically recyclable polymers: A circular economy approach to sustainability. Green Chem. 2017, 19, 3692–3706. [Google Scholar] [CrossRef]
- Zha, F.; Chen, W.; Zhang, L.; Yu, D. Electrospun natural polymer and its composite nanofibrous scaffolds for nerve tissue engineering. J. Biomater. Sci. Polym. Ed. 2020, 31, 519–548. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ouyang, H.; Chwee, T.L.; Ramakrishna, S.; Huang, Z.M. Electrospinning of gelatin fibers and gelatin/PCL composite fibrous scaffolds. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2005, 72, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Feng, B.; Zhang, W.; Yan, C.; Yao, Q.; Shao, C.; Yu, F.; Li, F.; Fu, Y. Electrospun gelatin/PCL and collagen/PCL scaffolds for modulating responses of bone marrow endothelial progenitor cells. Exp. Ther. Med. 2019, 17, 3717–3726. [Google Scholar] [CrossRef] [PubMed]
- Fadaie, M.; Mirzaei, E.; Geramizadeh, B.; Asvar, Z. Incorporation of nanofibrillated chitosan into electrospun PCL nanofibers makes scaffolds with enhanced mechanical and biological properties. Carbohydr. Polym. 2018, 199, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Mochane, M.J.; Motsoeneng, T.S.; Sadiku, E.R.; Mokhena, T.C.; Sefadi, J.S. Morphology and Properties of Electrospun PCL and Its Composites for Medical Applications: A Mini Review. Appl. Sci. 2019, 9, 2205. [Google Scholar] [CrossRef]
- Adeli-Sardou, M.; Yaghoobi, M.M.; Torkzadeh-Mahani, M.; Dodel, M. Controlled release of lawsone from polycaprolactone/gelatin electrospun nano fibers for skin tissue regeneration. Int. J. Biol. Macromol. 2019, 124, 478–491. [Google Scholar] [CrossRef]
- Choi, J.S.; Lee, S.J.; Christ, G.J.; Atala, A.; Yoo, J.J. The influence of electrospun aligned poly(ε-caprolactone)/collagen nanofiber meshes on the formation of self-aligned skeletal muscle myotubes. Biomaterials 2008, 29, 2899–2906. [Google Scholar] [CrossRef]
- Tillman, B.W.; Yazdani, S.K.; Lee, S.J.; Geary, R.L.; Atala, A.; Yoo, J.J. The in vivo stability of electrospun polycaprolactone-collagen scaffolds in vascular reconstruction. Biomaterials 2009, 30, 583–588. [Google Scholar] [CrossRef]
- Shrestha, B.K.; Mousa, H.M.; Tiwari, A.P.; Ko, S.W.; Park, C.H.; Kim, C.S. Development of polyamide-6,6/chitosan electrospun hybrid nanofibrous scaffolds for tissue engineering application. Carbohydr. Polym. 2016, 148, 107–114. [Google Scholar] [CrossRef]
- Aldana, A.A.; Abraham, G.A. Current advances in electrospun gelatin-based scaffolds for tissue engineering applications. Int. J. Pharm. 2017, 523, 441–453. [Google Scholar] [CrossRef]
- Kheradmandi, M.; Vasheghani-Farahani, E.; Ghiaseddin, A.; Ganji, F. Skeletal muscle regeneration via engineered tissue culture over electrospun nanofibrous chitosan/PVA scaffold. J. Biomed. Mater. Res.-Part A 2016, 104, 1720–1727. [Google Scholar] [CrossRef] [PubMed]
- Park, H.K.; Joo, W.; Gu, B.K.; Ha, M.Y.; You, S.J.; Chun, H.J. Collagen/poly(D,L-lactic-co-glycolic acid) composite fibrous scaffold prepared by independent nozzle control multi-electrospinning apparatus for dura repair. J. Ind. Eng. Chem. 2018, 66, 430–437. [Google Scholar] [CrossRef]
- Elmashhady, H.H.; Kraemer, B.A.; Patel, K.H.; Sell, S.A.; Garg, K. Decellularized extracellular matrices for tissue engineering applications. Electrospinning 2017, 1, 87–99. [Google Scholar] [CrossRef]
- Badylak, S.F.; Taylor, D.; Uygun, K. Whole-Organ Tissue Engineering: Decellularization and Recellularization of Three-Dimensional Matrix Scaffolds. Annu. Rev. Biomed. Eng. 2011, 13, 27–53. [Google Scholar] [CrossRef]
- Gilbert, T.W.; Sellaro, T.L.; Badylak, S.F. Decellularization of tissues and organs. Biomaterials 2006, 27, 3675–3683. [Google Scholar] [CrossRef]
- Taylor, D.A.; Sampaio, L.C.; Ferdous, Z.; Gobin, A.S.; Taite, L.J. Decellularized matrices in regenerative medicine. Acta Biomater. 2018, 74, 74–89. [Google Scholar] [CrossRef]
- Baiguera, S.; Del Gaudio, C.; Lucatelli, E.; Kuevda, E.; Boieri, M.; Mazzanti, B.; Bianco, A.; Macchiarini, P. Electrospun gelatin scaffolds incorporating rat decellularized brain extracellular matrix for neural tissue engineering. Biomaterials 2014, 35, 1205–1214. [Google Scholar] [CrossRef]
- Schoen, B.; Avrahami, R.; Baruch, L.; Efraim, Y.; Goldfracht, I.; Elul, O.; Davidov, T.; Gepstein, L.; Zussman, E.; Machluf, M. Electrospun Extracellular Matrix: Paving the Way to Tailor-Made Natural Scaffolds for Cardiac Tissue Regeneration. Adv. Funct. Mater. 2017, 27, 1700427. [Google Scholar] [CrossRef]
- Gao, S.; Guo, W.; Chen, M.; Yuan, Z.; Wang, M.; Zhang, Y.; Liu, S.; Xi, T.; Guo, Q. Fabrication and characterization of electrospun nanofibers composed of decellularized meniscus extracellular matrix and polycaprolactone for meniscus tissue engineering. J. Mater. Chem. B 2017, 5, 2273–2285. [Google Scholar] [CrossRef]
- Patel, K.H.; Dunn, A.J.; Talovic, M.; Haas, G.J.; Marcinczyk, M.; Elmashhady, H.; Kalaf, E.G.; Sell, S.A.; Garg, K. Aligned nanofibers of decellularized muscle ECM support myogenic activity in primary satellite cells in vitro. Biomed. Mater. 2019, 14, 035010. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, J.; Yu, G.; Cardenas, R.; Wei, S.; Wujcik, E.K.; Guo, Z. Coaxial electrospun fibers: Applications in drug delivery and tissue engineering. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 654–677. [Google Scholar] [CrossRef] [PubMed]
- McClellan, P.; Landis, W.J. Recent Applications of Coaxial and Emulsion Electrospinning Methods in the Field of Tissue Engineering. Biores. Open Access 2016, 5, 212–227. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Ang, L.T.; Goh, J.C.H.; Toh, S.L. Growth factor delivery through electrospun nanofibers in scaffolds for tissue engineering applications. J. Biomed. Mater. Res.-Part A 2010, 93, 1539–1550. [Google Scholar] [CrossRef] [PubMed]
- Mickova, A.; Buzgo, M.; Benada, O.; Rampichova, M.; Fisar, Z.; Filova, E.; Tesarova, M.; Lukas, D.; Amler, E. Core/shell nanofibers with embedded liposomes as a drug delivery system. Biomacromolecules 2012, 13, 952–962. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Prabhakaran, M.P.; Ding, X.; Kai, D.; Ramakrishna, S. Emulsion electrospun nanofibers as substrates for cardiomyogenic differentiation of mesenchymal stem cells. J. Mater. Sci. Mater. Med. 2013, 24, 2577–2587. [Google Scholar] [CrossRef]
- Zhang, H.; Jia, X.; Han, F.; Zhao, J.; Zhao, Y.; Fan, Y.; Yuan, X. Dual-delivery of VEGF and PDGF by double-layered electrospun membranes for blood vessel regeneration. Biomaterials 2013, 34, 2202–2212. [Google Scholar] [CrossRef]
- Park, K.E.; Kim, B.S.; Kim, M.H.; You, H.K.; Lee, J.; Park, W.H. Basic fibroblast growth factor-encapsulated PCL nano/microfibrous composite scaffolds for bone regeneration. Polymer 2015, 76, 8–16. [Google Scholar] [CrossRef]
- Jiang, Y.C.; Wang, X.F.; Xu, Y.Y.; Qiao, Y.H.; Guo, X.; Wang, D.F.; Li, Q.; Turng, L.S. Polycaprolactone Nanofibers Containing Vascular Endothelial Growth Factor-Encapsulated Gelatin Particles Enhance Mesenchymal Stem Cell Differentiation and Angiogenesis of Endothelial Cells. Biomacromolecules 2018, 19, 3747–3753. [Google Scholar] [CrossRef]
- Augustine, R.; Zahid, A.A.; Hasan, A.; Wang, M.; Webster, T.J. Ctgf loaded electrospun dual porous core-shell membrane for diabetic wound healing. Int. J. Nanomed. 2019, 14, 8573–8588. [Google Scholar] [CrossRef]
- Baek, J.; Lee, E.; Lotz, M.K.; D’Lima, D.D. Bioactive proteins delivery through core-shell nanofibers for meniscal tissue regeneration. Nanomed. Nanotechnol. Biol. Med. 2020, 23, 102090. [Google Scholar] [CrossRef]
- Ferrand, A.; Eap, S.; Richert, L.; Lemoine, S.; Kalaskar, D.; Demoustier-Champagne, S.; Atmani, H.; Mély, Y.; Fioretti, F.; Schlatter, G.; et al. Osteogenetic Properties of Electrospun Nanofibrous PCL Scaffolds Equipped With Chitosan-Based Nanoreservoirs of Growth Factors. Macromol. Biosci. 2014, 14, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Decher, G.; Eckle, M.; Schmitt, J.; Struth, B. Layer-by-layer assembled multicomposite films. Curr. Opin. Colloid Interface Sci. 1998, 3, 32–39. [Google Scholar] [CrossRef]
- Jessel, N.; Atalar, F.; Lavalle, P.; Mutterer, J.; Decher, G.; Schaaf, P.; Voegel, J.C.; Ogier, J. Bioactive coatings based on a polyelectrolyte multilayer architecture functionalized by embedded proteins. Adv. Mater. 2003, 15, 692–695. [Google Scholar] [CrossRef]
- Asadian, M.; Rashidi, A.; Majidi, M.; Mehrjoo, M.; Emami, B.A.; Tavassoli, H.; Asl, M.P.; Bonakdar, S. Nanofiber protein adsorption affected by electrospinning physical processing parameters. J. Iran. Chem. Soc. 2015, 12, 1089–1097. [Google Scholar] [CrossRef]
- Koh, H.S.; Yong, T.; Chan, C.K.; Ramakrishna, S. Enhancement of neurite outgrowth using nano-structured scaffolds coupled with laminin. Biomaterials 2008, 29, 3574–3582. [Google Scholar] [CrossRef]
- Xi, W.; Scott, T.F.; Kloxin, C.J.; Bowman, C.N. Click chemistry in materials science. Adv. Funct. Mater. 2014, 24, 2572–2590. [Google Scholar] [CrossRef]
- Costa, A.; Walkowiak, B.; Campanella, L. Tissue Engineering Between Click Chemistry and Green Chemistry. An Int. J. Hist. Chem. 2019, 3, 29–38. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chemie Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Lancuški, A.; Fort, S.; Bossard, F. Electrospun azido-PCL nanofibers for enhanced surface functionalization by click chemistry. ACS Appl. Mater. Interfaces 2012, 4, 6499–6504. [Google Scholar] [CrossRef]
- Smith Callahan, L.A.; Xie, S.; Barker, I.A.; Zheng, J.; Reneker, D.H.; Dove, A.P.; Becker, M.L. Directed differentiation and neurite extension of mouse embryonic stem cell on aligned poly(lactide) nanofibers functionalized with YIGSR peptide. Biomaterials 2013, 34, 9089–9095. [Google Scholar] [CrossRef]
- Lin, F.; Yu, J.; Tang, W.; Zheng, J.; Xie, S.; Becker, M.L. Postelectrospinning “click” modification of degradable amino acid-based poly(ester urea) nanofibers. Macromolecules 2013, 46, 9515–9525. [Google Scholar] [CrossRef]
- Kalaoglu-Altan, O.I.; Kirac-Aydin, A.; Sumer Bolu, B.; Sanyal, R.; Sanyal, A. Diels-Alder “clickable” Biodegradable Nanofibers: Benign Tailoring of Scaffolds for Biomolecular Immobilization and Cell Growth. Bioconjug. Chem. 2017, 28, 2420–2428. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Kontoveros, D.; Lin, F.; Hua, G.; Reneker, D.H.; Becker, M.L.; Willits, R.K. Enhanced schwann cell attachment and alignment using one-pot “Dual Click” GRGDS and YIGSR derivatized nanofibers. Biomacromolecules 2015, 16, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Hua, G.; Yu, J.; Lin, F.; Wade, M.B.; Reneker, D.H.; Becker, M.L. Post-electrospinning “triclick” functionalization of degradable polymer nanofibers. ACS Macro Lett. 2015, 4, 207–213. [Google Scholar] [CrossRef]
- Csapo, R.; Gumpenberger, M.; Wessner, B. Skeletal Muscle Extracellular Matrix–What Do We Know About Its Composition, Regulation, and Physiological Roles? A Narrative Review. Front. Physiol. 2020, 11, 253. [Google Scholar] [CrossRef]
- Gao, L.; Ma, L.; Yin, X.; Luo, Y.; Yang, H.; Zhang, B. Nano- and Microfabrication for Engineering Native-Like Muscle Tissues. Small Methods 2020, 4, 1900669. [Google Scholar] [CrossRef]
- Nakayama, K.H.; Shayan, M.; Huang, N.F. Engineering Biomimetic Materials for Skeletal Muscle Repair and Regeneration. Adv. Healthc. Mater. 2019, 8, 1801168. [Google Scholar] [CrossRef]
- Narayanan, N.; Jiang, C.; Uzunalli, G.; Thankappan, S.K.; Laurencin, C.T.; Deng, M. Polymeric Electrospinning for Musculoskeletal Regenerative Engineering. Regen. Eng. Transl. Med. 2016, 2, 69–84. [Google Scholar] [CrossRef]
- Wolf, M.T.; Daly, K.A.; Reing, J.E.; Badylak, S.F. Biologic scaffold composed of skeletal muscle extracellular matrix. Biomaterials 2012, 33, 2916–2925. [Google Scholar] [CrossRef]
- Teodori, L.; Costa, A.; Marzio, R.; Perniconi, B.; Coletti, D.; Adamo, S.; Gupta, B.; Tarnok, A. Native extracellular matrix: A new scaffolding platform for repair of damaged muscle. Front. Physiol. 2014, 5, 218. [Google Scholar] [CrossRef]
- Porzionato, A.; Sfriso, M.M.; Pontini, A.; Macchi, V.; Petrelli, L.; Pavan, P.G.; Natali, A.N.; Bassetto, F.; Vindigni, V.; De Caro, R. Decellularized human skeletal muscle as biologic scaffold for reconstructive surgery. Int. J. Mol. Sci. 2015, 16, 14808–14831. [Google Scholar] [CrossRef] [PubMed]
- Aviss, K.J.; Gough, J.E.; Downes, S. Aligned electrospun polymer fibres for skeletal muscle regeneration. Eur. Cells Mater. 2010, 19, 193–204. [Google Scholar] [CrossRef]
- Cooper, A.; Jana, S.; Bhattarai, N.; Zhang, M. Aligned chitosan-based nanofibers for enhanced myogenesis. J. Mater. Chem. 2010, 20, 8904–8911. [Google Scholar] [CrossRef]
- Narayanan, N.; Jiang, C.; Wang, C.; Uzunalli, G.; Whittern, N.; Chen, D.; Jones, O.G.; Kuang, S.; Deng, M. Harnessing Fiber Diameter-Dependent Effects of Myoblasts Toward Biomimetic Scaffold-Based Skeletal Muscle Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 203. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.H.; Talovic, M.; Dunn, A.J.; Patel, A.; Vendrell, S.; Schwartz, M.; Garg, K. Aligned nanofibers of decellularized muscle extracellular matrix for volumetric muscle loss. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2020, 108, 2528–2537. [Google Scholar] [CrossRef]
- Aubin, H.; Mas-Moruno, C.; Iijima, M.; Schütterle, N.; Steinbrink, M.; Assmann, A.; Gil, F.J.; Lichtenberg, A.; Pegueroles, M.; Akhyari, P. Customized Interface Biofunctionalization of Decellularized Extracellular Matrix: Toward Enhanced Endothelialization. Tissue Eng.-Part C Methods 2016, 22, 496–508. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, M.; Li, S.; Erasquin, U.J.; Wang, H.; Ren, L.; Chen, C.; Wang, Y.; Cai, C. “Click” immobilization of a VEGF-mimetic peptide on decellularized endothelial extracellular matrix to enhance angiogenesis. ACS Appl. Mater. Interfaces 2014, 6, 8401–8406. [Google Scholar] [CrossRef]
- Lee, H.; Ju, Y.M.; Kim, I.; Elsangeedy, E.; Lee, J.H.; Yoo, J.J.; Atala, A.; Lee, S.J. A novel decellularized skeletal muscle-derived ECM scaffolding system for in situ muscle regeneration. Methods 2020, 171, 77–85. [Google Scholar] [CrossRef]
- Jazkewitsch, O.; Ritter, H. Formation and characterization of inclusion complexes of alkyne functionalized poly(ε-caprolactone) with β-cyclodextrin. Pseudo-polyrotaxane-based supramolecular organogels. Macromolecules 2011, 44, 375–382. [Google Scholar] [CrossRef]
- Lee, H.J.; Fernandes-Cunha, G.M.; Putra, I.; Koh, W.G.; Myung, D. Tethering Growth Factors to Collagen Surfaces Using Copper-Free Click Chemistry: Surface Characterization and in Vitro Biological Response. ACS Appl. Mater. Interfaces 2017, 9, 23389–23399. [Google Scholar] [CrossRef]
- Syverud, B.C.; VanDusen, K.W.; Larkin, L.M. Growth factors for skeletal muscle tissue engineering. Cells Tissues Organs 2016, 202, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, L.; Elsangeedy, E.; Lee, H.; Atala, A.; Yoo, J.; Lee, S.; YM, J. In Vitro Evaluation of Functionalized Decellularized Muscle Scaffold for in Situ Skeletal Muscle Regeneration. Biomed. Mater. 2019, 14. [Google Scholar] [CrossRef] [PubMed]
- Bishop, C.J.; Kim, J.; Green, J.J. Biomolecule delivery to engineer the cellular microenvironment for regenerative medicine. Ann. Biomed. Eng. 2014, 42, 1557–1572. [Google Scholar] [CrossRef] [PubMed]


| Polymeric Component | Loaded Biomolecules | Method of Preparation | Reference |
|---|---|---|---|
| PLGA | bFGF | Coaxial electrospinning | [63] |
| PVA core PCL shell | GF loaded liposomes | Coaxial electrospinning | [64] |
| PCLC | VEGF | Emulsion electrospinning | [65] |
| PELCL core PELCL shell | VEGF PDGF | Coaxial electrospinning | [66] |
| PCL | bFGF | Emulsion electrospinning | [67] |
| PCL | VEGF | Blend electrospinning | [68] |
| PVA core PLA shell | CTGF | Coaxial electrospinning | [69] |
| PLA | PDGF | Coaxial electrospinning | [70] |
| Electrospun Biomaterials | Experimental Model | Outcomes | Reference |
|---|---|---|---|
| PCL/collagen I | In vitro: Human skeletal muscle cells (hSkMCs) | Aligned PCL/collagen nanofibers significantly induced muscle cell alignment and myotube formation as compared to randomly oriented nanofibers | [47] |
| PLGA | In vitro: Murine myoblast cells (C2C12) | Aligned PLGA fibers control the myoblast elongation and alignment and encourage myoblast differentiation. | [92] |
| Chitosan/PCL | In vitro: Murine myoblast cells (C2C12) | Aligned chitosan-PCL nanofibrous scaffolds exhibited superior tensile strength compared to randomly oriented nanofibers and promoted muscle cell proliferation. | [93] |
| Chitosan/PVA | In vitro: Rabbit’s bone marrow (MSCs) In vivo: Adult New Zealand rabbit | Good cell viability, adhesion growth, and significant proliferation with less immune responses when the scaffold was implanted into the leg muscle of rabbit. | [51] |
| dECM from rabbit skeletal muscle | In vivo: Rabbit | The decellularization protocol of skeletal muscle tissue retains important ECM components. Electrospun scaffold derived completely from skeletal muscle dECM. | [30] |
| PLGA | In vitro: Murine myoblast cells (C2C12) In vivo: Mdx mice | Aligned PLGA fiber with larger diameter support enhanced alignment, growth, and differentiation of myoblasts. In vivo the optimized scaffolds seeded with primary myoblasts result in the formation of dystrophin-positive myofibers network. | [94] |
| PCL/dECM from bovine skeletal muscle | In vitro: Rat muscle precursor cells In vivo: C57/BL6 adult mice | Aligned nanofibers support satellite cell growth, myogenic protein expression, and myokine production. In vivo: myofiber regeneration was observed. | [60,95] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Politi, S.; Carotenuto, F.; Rinaldi, A.; Di Nardo, P.; Manzari, V.; Albertini, M.C.; Araneo, R.; Ramakrishna, S.; Teodori, L. Smart ECM-Based Electrospun Biomaterials for Skeletal Muscle Regeneration. Nanomaterials 2020, 10, 1781. https://doi.org/10.3390/nano10091781
Politi S, Carotenuto F, Rinaldi A, Di Nardo P, Manzari V, Albertini MC, Araneo R, Ramakrishna S, Teodori L. Smart ECM-Based Electrospun Biomaterials for Skeletal Muscle Regeneration. Nanomaterials. 2020; 10(9):1781. https://doi.org/10.3390/nano10091781
Chicago/Turabian StylePoliti, Sara, Felicia Carotenuto, Antonio Rinaldi, Paolo Di Nardo, Vittorio Manzari, Maria Cristina Albertini, Rodolfo Araneo, Seeram Ramakrishna, and Laura Teodori. 2020. "Smart ECM-Based Electrospun Biomaterials for Skeletal Muscle Regeneration" Nanomaterials 10, no. 9: 1781. https://doi.org/10.3390/nano10091781
APA StylePoliti, S., Carotenuto, F., Rinaldi, A., Di Nardo, P., Manzari, V., Albertini, M. C., Araneo, R., Ramakrishna, S., & Teodori, L. (2020). Smart ECM-Based Electrospun Biomaterials for Skeletal Muscle Regeneration. Nanomaterials, 10(9), 1781. https://doi.org/10.3390/nano10091781