1. Introduction
Research in advanced materials promotes development in societies [
1]. Metallic glassy alloys, shape memory alloys, nanostructured materials, nanoparticles, nanocomposites, nanocrystalline refractory materials, conducting polymers, carbon nanotubes, nanodiamonds, graphene, bio-nanomaterials, 2-D nanomaterials, and high-entropy alloys are some examples. Refractory metal nitrides, in particularly group IV transition metals (TM; Ti, Zr, and Hf), have received great attention due to their outstanding properties. The interest in this group of TM nitrides that possesses mixtures of ionic, covalent, and metallic bonding is due to their desirable properties such as high thermal and excellent chemical stability, superior oxidation resistance, excellent thermal conductivity, high hardness, prime wear resistance, and excellent strength [
2,
3]. These properties makes them desired materials for various applications, particularly as a surface protective coating and in cutting tools [
1,
2,
3].
For more than four decades, the cubic form of titanium nitride (TiN) has been the subject of research [
4,
5,
6,
7]. In contrast to TiN, the zirconium nitride (ZrN) binary system is relatively new and less studied. In addition to its applications in surface protective coatings and superhard cutting tools, ZrN has been implemented in photonics and plasmonics [
8]. The ZrN system is typically prepared by different approaches, such as reduction–nitridation of zirconium oxide [
3,
9,
10,
11], reactive sputtering [
12,
13,
14,
15,
16], chemical vapor deposition [
17,
18], self-propagating high-temperature [
19], benzene-thermal synthesis [
20,
21], and physical vapor deposition (PVD) [
22]. A downside of these methods is the fact that they require the application of high temperatures or pressures or both together. In the main process employed to produce industrial forms of ZrN, pure Zr metal and ZrCl
4 are directly nitrided with ammonia gas at elevated temperatures (~1500 °C) [
3]. The high cost of production alongside the presence of contaminants and the employment of environmentally unfriendly harmful gases are the main disadvantages of this method. The unfavorable aptness of the industrialized production of ZrN may lead to restriction of the mass production of such advanced material. Apart from the high-temperature/high-pressure approaches, the reactive ball milling (RBM) technique was introduced at the beginning of the 1990 s [
23,
24] and has been widely accepted for preparing metallic nitrides [
25,
26] and hydrides [
27] at ambient temperature through high-energy ball milling. This process is a simplistic method that has proven its viability in such works many times over.
The present work introduces the preparation of nanocrystalline ZrN powders through the RBM process under pressurized nitrogen (N2) gas. Variations of RBM time are also reported. The present work also investigates the effect of grain size on the microhardness of spark plasma sintered (SPS) ZrN. A paramount advantage of this work is related to the use of solid waste (SW) Zr metal as a precursor material. The described RBM process in this work can be adapted and economically employed to produce nano-crystalline of ZrN powders and bulk materials at a large scale.
2. Materials and Methods
2.1. Feedstock Materials
In the present study, a batch of 1 kg of pure (99.0 wt%) scrap Zr rods (100–105 mm in length and ~10 mm in thickness) were acquired from Shanghai Xinglu Chemical Technology Co. (Pudong Dist, Shanghai, China) and used as feedstock materials. The received rods were cut into shorter ones (~50 mm in length) and then sonicated in a cold-acetone bath for 15 min to ensure the removal of all machining oil coolants from their surfaces. Rinsing of the rods took place with alcohol (pure ethanol) (Sigma-Aldrich Chemie GmbH, Eschenstrasse 5, 82024 Taufkirchen, Germany) and then they were dried for 16 h (200 °C). This primer treatment was conducted to remove the undesired hydrocarbon contaminants from the surfaces. An amount of 250 g of oil-free rods was then placed in the water-cooled copper hearth of an arc melter (Edmund Bühler GmbH, Hechingen, Germany), where the melting of Zr scrap was conducted using titanium (Ti), under less than 0.8 bar of pure argon gas (Ar). The molten Zr button was turned over and remelted 5 times. The chemical analysis of the obtained Zr button showed that it possessed high purity of 98.7 wt%, with 0.8, 0.3, and 0.2 wt%, respectively, of hafnium (Hf), oxygen (O2), and carbon (C).
2.2. Sample Preparations
The Zr button was crushed down into small pieces of almost 2.5 cm in size and was charged into a Model RS 200 vibratory disc mill machine (Retsch GmbH Company, 42781 Haan, Germany) operated at a rotation speed of 1500 rpm for 2 min. Powders were sieved to obtain a product of less than 50 μm in diameter. Powders were placed in a helium UNILAB Pro Glove Box Workstation (M. BRAUN INERTGAS-SYSTEME GMBH, Garching, Germany) sealed with 50 balls of 11 mm (dia) tool steel balls coupled with an evico magnetics gas monitoring system (Mettler-Toledo GmbH, Ockerweg Giessen, Germany) (40:1 ball to powder). The process was continuously operated until selected times were reached to obtain 300 mg of powder using a Fritsch PULVERISETTE 6 mill (FRITSCH GmbH, Broyage et Granulométrie Idar-Oberstein, Germany).
2.3. Powder Consolidation
Powders which were as-ball milled with 11, 22, 43, 85, 175, 259, and 360 ks of continuous RBM were individually consolidated into dense buttons through spark plasma sintering (SPS) acquired from Dr. Sinter Lab. Instrument Co. Tokyo, Japan. The system consists of a press with vertical single-axis pressurization, electrodes incorporating a water cooler, a water-cooled vacuum chamber, a vacuum/air/argon-gas atmosphere control mechanism, a special direct current (DC) pulse sintering power generator, a cooling-water control unit, a Z-axis position measuring and control unit, temperature measuring and control units, an applied pressure display unit, and various safety interlock devices. In this study, powders obtained were placed into a die made of graphite. Sheets of graphite were also used to avoid reactions between surfaces. The die as a whole was wrapped with carbon felt held with a carbon yard in an effort to reduce heat transfer. An electric field was used to control the sintering. Rather than hot-pressing [
25], in this work we used sintering SPS conducted by internally heating samples by electric current passage. Heating and cooling rates of 580 and 280 K/min were used, respectively. An external pressure was applied during sintering in the range of 10–15 MPa (1673 °C). The process as a whole took about 6 min.
2.4. Sample Characterizations
2.4.1. Crystal Structure
CuKα radiation X-ray diffraction (XRD) analysis was conducted using 9 kW SmartLab-Rigaku XRD system (Applied Rigaku Technologies, Inc., Austin, TX 78717 USA). Field emission high resolution transmission electron microscopy (HRTEM) coupled with scanning transmission electron microscopy (STEM) was also used by operating a JEOL-2100F equipped Oxford Instruments EDS (Oxford Instruments NanoAnalysis & Asylum Research, UK). The objective lens spherical aberration coefficient (Cs) of this microscope is 0.5 mm, where the point resolution is 0.19 nm, and the lattice resolution is 0.12 nm. The maximum and minimum spot sizes for the nano beam diffraction (NBD) were 0.5 and 25 nm, respectively. A Cryo Ion IB-09060CIS slicer machine (JEOL, Musashino, Akishima, Tokyo, Japan) was also used to prepare the TEM specimens as a consolidated ZrN.
2.4.2. Morphology and Elemental Analysis
Field emission microscopy (FE-SEM) was used to study the samples with a 15 kV voltage (JSM-7800F JEOL Co., Musashino, Akishima, Tokyo, Japan), and elemental analysis (EA) was also conducted with an Oxford Co., UK, EDS interface (Oxford Instruments NanoAnalysis & Asylum Research, UK).
2.4.3. Density and Microhardness
The density was determined using toluene media. A 1 kg quantity of Vickers indenter was used to determine the microhardness of the compacted samples with an average reading of 10 indentations.
4. Discussion
Despite the traditional approaches used for preparations of metal nitrides under the applications of high temperature and pressure, the RBM technique has been utilized to synthesize different families of nanocrystalline metal nitrides. In the present study, a single phase of metastable fcc-ZrN nanopowders was prepared through high-energy RBM at room temperature. This process can be classified into three consequent stages, as elucidated in
Figure 8.
In the first stage (0 ks–22 ks) of the RBM, the starting Zr powders were subjected to GB defects (
Figure 2a), excessive plastic deformation (
Figure 2c), and lattice imperfections (e.g., dislocations, point defect, and vacancies). These structural-deformation defects, which were localized in the shear bands of Zr, led to the introduction of a highly dense network of dislocations, as can be noted in
Figure 2a. These localized severe defects stimulated the instabilities of the Zr lattice. Moreover, the atomic level strain was remarkably increased as a result of expanding the dislocation density in the overall hcp-Zr powders. Due to these successive accumulations of the dislocation density generated during the second stage of RBM (22 ks–86 ks), the large grains of starting Zr powders (~260 nm) were disintegrated into finer subgrains grains of ~173 nm after 22 ks of RBM time, as presented in
Figure 8. The defragmentation of microscaled-Zr grains (
Figure 3a) into nanosized grains (~90 nm) after 43 ks of RBM time (
Figure 3b) can be attributed to a gradual decrease in the atomic strain. The Zr powders were, therefore, disintegrated into smaller particles with large surface area, and very clean oxygen-free active surfaces. Accordingly, the reactive milling atmosphere of nitrogen was guttered and absorbed completely by the first atomically clean surfaces of Zr powders. This could be realized by the dramatic increase of nitrogen absorbed by Zr powders (
Figure 8a).
We should emphasize that the diffusion of nitrogen in the Zr powders was enhanced upon the existence of crystal defects in the Zr lattice. In other words, increasing the volume fraction of the nanosized grains upon introduction of GB defects led to improvement in the solubility of nitrogen in Zr. For instance, when Zr grains were 173 nm, the powders were not able to absorb more than 4 wt% of nitrogen (
Figure 8a). The reduction of the grain size (93 nm) attained after 43 ks of the RBM time improved the uptake capability of Zr powders to absorb almost 30 wt% of nitrogen, as demonstrated in
Figure 8. It can be claimed, therefore, that GB defects may provide an excellent pathway for rapid diffusion of nitrogen. After 86 ks, a single phase of fcc-ZrN was obtained, as shown in
Figure 1d and
Figure 4b. This stoichiometric phase (~39 wt% nitrogen) could no longer withstand the lattice imperfections generated by the ball milling media, where it tended to transform into a metastable phase of fcc-ZrNi, as displayed in
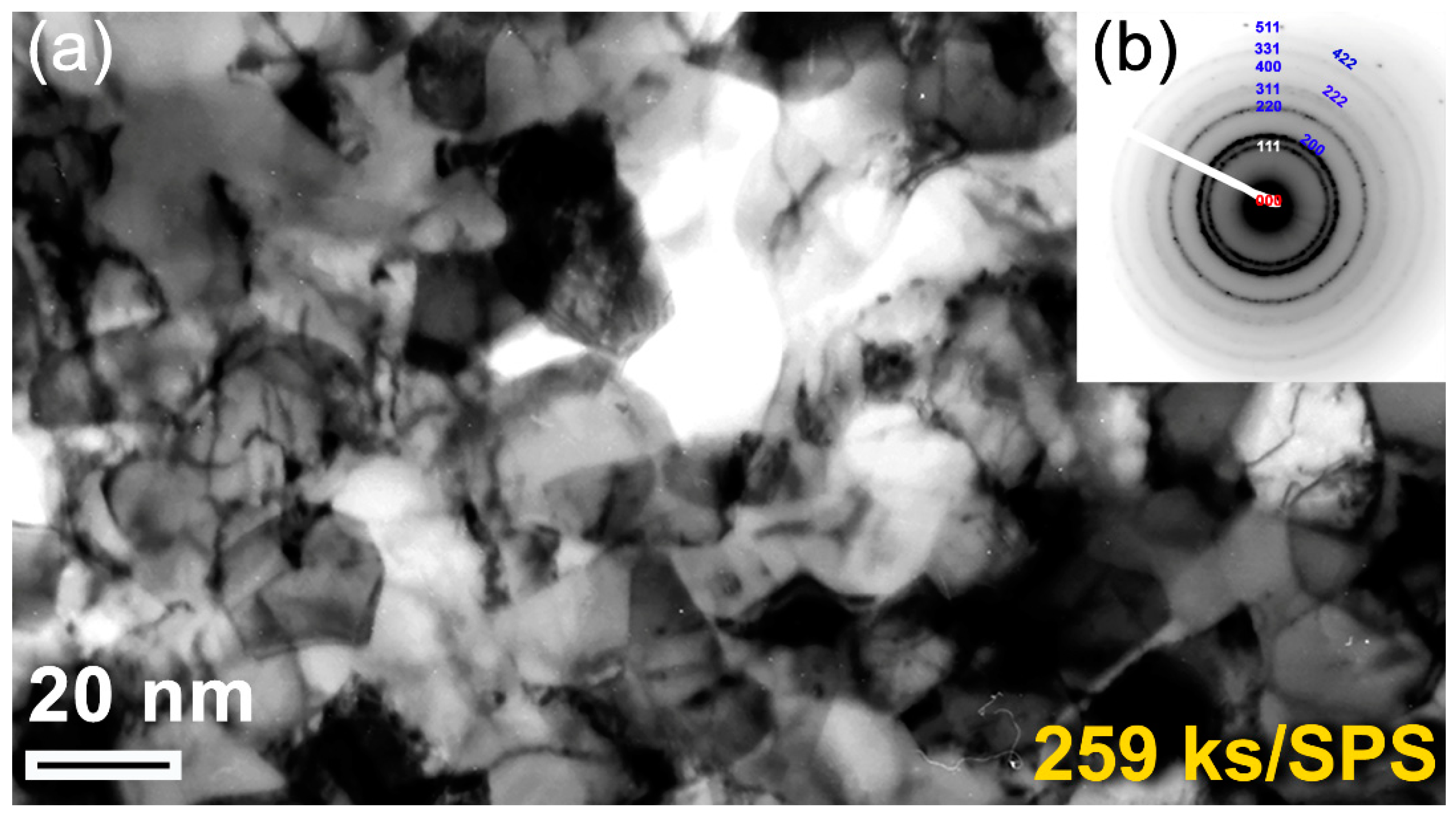
Figure 1e. During the last stage of the RBM (86 ks–360 ks), the brittle ZrN powders were dramatically disintegrated into ultrafine grains with sizes in the range from 3.5 nm to 5 nm, as presented in
Figure 4a. The corresponding increase of nitrogen content in this stage of RBM (
Figure 8a) may be attributed to a reaction conducted between ultrafine Zr powders that existed in the end product of the fcc-ZrN.
The present work reports sintering with SPS that is commonly known as the field-assisted sintering technique (FAST), as reported in the methods section. Pressure was externally applied (10–15 MPa). Consolidation was achieved by charging the intervals between the particles of the powders with electrical energy and effectively applying spark plasma. One merit of the SPS consolidation step is that it maintains the original nanocrystalline grains without dramatic growth (
Figure 8a). The variation of Vickers microhardness (VMH) with RBM time of as-SPS samples is displayed in
Figure 8b. The influence of RBM time on VMH values can be realized. This monotonical increase is attributed to the consequent decrease of the grain size upon increasing RBM time (
Figure 8a). After 11 ks of RBM time, VMH revealed a wide distribution in the range from 0.15 GPa to 2 GPa, as shown in
Figure 8b. The VMH of the as-SPS sample of the powders milled for 22 ks was varied from 3 GPa to 4.6 GPa. This significant broad variation decreased with increasing RBM time (43 ks) to be in the range of 8 GPa to 9.4 GPa, as presented in
Figure 8b. This significant broad variation in VMH is attributed to the existence of a large volume fraction of unprocessed Zr in the original feedstock of RBM powders, as evidenced by the XRD analysis (
Figure 1b,c). The VMH of the SPS sample obtained of the powders milled for 86 ks was 15.14 GPa, as shown in
Figure 8b. The dramatic increase in VMH of this sample suggested the absence of unprocessed Zr metal, where a single fcc-ZrN phase existed (
Figure 1d).
Further RBM time (86 ks–360 ks) led to the refining of the ZrN powders, which was necessary to obtain ultrafine powders of ZrN nanograins, as demonstrated in
Figure 8a. During this final stage of RBM, the VMH increased significantly to attain higher values of 17.84 GPa and 19.8 GPa after RBM times of 173 ks and 259 ks, respectively (
Figure 8b). As the grain size of the consolidated 360 ks-ZrN sample did not show a remarkable change (
Figure 8a), the VMH tended to be saturated at 20.2 GPa, as displayed in
Figure 8b. The grain size softening effect concerning the hardness (inverse Hall–Petch) is an accepted phenomenon used to interpret the increase of hardness upon grain size reduction for nanocrystalline materials. This beneficial effect is attributed to the changes in the plastic deformation mechanisms, as pointed out by several authors. In general, low-temperature plastic deformation during the Vickers hardness test is principally controlled by the dynamics of dislocations in the well-ordered structure of the grain interiors. The dislocation slip taking place within the deformation is blocked by the grain boundaries, which act as deformation barriers. As a result, the hardness increases with decreasing grain size, as described in the Hall–Petch relationship, over a wide range of grain size, as presented in
Figure 8a,b. It should be noted that the VMH value of SPS-fcc-ZrN nanocrystalline metastable phase (~20 GPa) of this work is far above that of the regular polycrystalline ZrN (15.8 GPa).