Synergetic Effect of Plasmonic Gold Nanorods and MgO for Perovskite Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Gold Nanorods (Au NRs)
2.3. Device Fabrication
2.4. Characterization
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Huang, J.; Yuan, Y.; Shao, Y.; Yan, Y. Understanding the physical properties of hybrid perovskites for photovoltaic applications. Nat. Rev. Mater. 2017, 2, 1–19. [Google Scholar] [CrossRef]
- Green, M.A.; Ho-Baillie, A.; Snaith, H.J. The emergence of perovskite solar cells. Nat. Photonics 2014, 8, 506–514. [Google Scholar] [CrossRef]
- Yang, L.; Wang, X.; Mai, X.; Wang, T.; Wang, C.; Li, X.; Murugadoss, V.; Shao, Q.; Angaiah, S.; Guo, Z. Constructing efficient mixed-ion perovskite solar cells based on TiO2 nanorod array. J. Colloid Interface Sci. 2019, 534, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- NREL Best Research-Cell Efficiency Chart. Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 10 August 2020).
- Jeon, N.J.; Noh, J.H.; Kim, Y.C.; Yang, W.S.; Ryu, S.; Seok, S.I. Solvent engineering for high-performance inorganic–organic hybrid perovskite solar cells. Nat. Mater. 2014, 13, 897–903. [Google Scholar] [CrossRef]
- Zhang, C.; Luo, Y.; Chen, X.; Chen, Y.; Sun, Z.; Huang, S. Effective improvement of the photovoltaic performance of carbon-based perovskite solar cells by additional solvents. Nano-Micro Lett. 2016, 8, 347–357. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Q.; Zhao, Y.; Zhang, X.; Yang, X.; Chen, Y.; Chu, Z.; Ye, Q.; Li, X.; Yin, Z.; You, J. Surface passivation of perovskite film for efficient solar cells. Nat. Photonics 2019, 13, 460–466. [Google Scholar] [CrossRef]
- Chi, W.; Banerjee, S.K. Progress in materials development for the rapid efficiency advancement of perovskite solar cells. Small 2020, 1907531. [Google Scholar] [CrossRef]
- Kakavelakis, G.; Petridis, K.; Kymakis, E. Recent advances in plasmonic metal and rare-earth-element upconversion nanoparticle doped perovskite solar cells. J. Mater. Chem. A 2017, 5, 21604–21624. [Google Scholar] [CrossRef]
- Moakhar, R.S.; Gholipour, S.; Masudy-Panah, S.; Seza, A.; Mehdikhani, A.; Riahi-Noori, N.; Tafazoli, S.; Timasi, N.; Lim, Y.F.; Saliba, M. Recent advances in plasmonic perovskite solar cells. Adv. Sci. 2020, 7, 1902448. [Google Scholar] [CrossRef]
- Luo, Q.; Zhang, C.; Deng, X.; Zhu, H.; Li, Z.; Wang, Z.; Chen, X.; Huang, S. Plasmonic effects of metallic nanoparticles on enhancing performance of perovskite solar cells. ACS Appl. Mater. Interfaces 2017, 9, 34821–34832. [Google Scholar] [CrossRef]
- Xu, Q.; Zhao, Y.; Zhang, X. Light management in monolithic perovskite/silicon tandem solar cells. Sol. RRL 2020, 4, 1900206. [Google Scholar] [CrossRef]
- Sun, S.; Salim, T.; Mathews, N.; Duchamp, M.; Boothroyd, C.; Xing, G.; Sum, T.C.; Lam, Y.M. The origin of high efficiency in low-temperature solution-processable bilayer organometal halide hybrid solar cells. Energy Environ. Sci. 2014, 7, 399–407. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.-P.; Lien, D.-H.; Tsai, M.-L.; Lin, C.-A.; Chang, H.-C.; Lai, K.-Y.; He, J.-H. Photon management in nanostructured solar cells. J. Mater. Chem. C 2014, 2, 3144–3171. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, J.H.; Kim, J.S.; Lee, D.Y.; Cho, K. High efficiency polymer solar cells with wet deposited plasmonic gold nanodots. Org. Electron. 2009, 10, 416–420. [Google Scholar] [CrossRef]
- Ding, B.; Lee, B.J.; Yang, M.; Jung, H.S.; Lee, J.K. Surface-plasmon assisted energy conversion in dye-sensitized solar cells. Adv. Energy Mater. 2011, 1, 415–421. [Google Scholar] [CrossRef]
- Wang, P.H.; Millard, M.; Brolo, A.G. Optimizing plasmonic silicon photovoltaics with Ag and Au nanoparticle mixtures. J. Phys. Chem. C. 2014, 118, 5889–5895. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Z.; Chen, X.; Liu, C.; Ye, X.; Wang, Z.; Sun, Z.; Huang, S. Improved performance of flexible amorphous silicon solar cells with silver nanowires. J. Appl. Phys. 2012, 112, 124320. [Google Scholar] [CrossRef]
- Zhang, W.; Saliba, M.; Stranks, S.D.; Sun, Y.; Shi, X.; Wiesner, U.; Snaith, H.J. Enhancement of perovskite-based solar cells employing core–shell metal nanoparticles. Nano Lett. 2013, 13, 4505–4510. [Google Scholar] [CrossRef] [Green Version]
- Saliba, M.; Zhang, W.; Burlakov, V.M.; Stranks, S.D.; Sun, Y.; Ball, J.M.; Johnston, M.B.; Goriely, A.; Wiesner, U.; Snaith, H.J. Plasmonic-induced photon recycling in metal halide perovskite solar cells. Adv. Funct. Mater. 2015, 25, 5038–5046. [Google Scholar] [CrossRef]
- Yuan, Z.; Wu, Z.; Bai, S.; Xia, Z.; Xu, W.; Song, T.; Wu, H.; Xu, L.; Si, J.; Jin, Y. Hot-electron injection in a sandwiched TiOx–Au–TiOx structure for high-performance planar perovskite solar cells. Adv. Energy Mater. 2015, 5, 1500038. [Google Scholar] [CrossRef]
- Jacak, W.A.; Jacak, J.E. New channel of plasmon photovoltaic effect in metalized perovskite solar cells. J. Phys. Chem. C. 2019, 123, 30633–30639. [Google Scholar] [CrossRef]
- Laska, M.; Krzemińska, Z.; Kluczyk-Korch, K.; Schaadt, D.; Popko, E.; Jacak, W.; Jacak, J. Metallization of solar cells, exciton channel of plasmon photovoltaic effect in perovskite cells. Nano Energy 2020, 104751. [Google Scholar] [CrossRef]
- Ball, J.M.; Stranks, S.D.; Hörantner, M.T.; Hüttner, S.; Zhang, W.; Crossland, E.J.; Ramirez, I.; Riede, M.; Johnston, M.B.; Friend, R.H. Optical properties and limiting photocurrent of thin-film perovskite solar cells. Energy Environ. Sci. 2015, 8, 602–609. [Google Scholar] [CrossRef]
- Tan, H.; Jain, A.; Voznyy, O.; Lan, X.; De Arquer, F.P.G.; Fan, J.Z.; Quintero-Bermudez, R.; Yuan, M.; Zhang, B.; Zhao, Y. Efficient and stable solution-processed planar perovskite solar cells via contact passivation. Science 2017, 355, 722–726. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, X.; Liang, C.; Li, P.; Hu, X.; Cai, Q.; Zhang, Y.; Li, F.; Li, M.; Song, Y. Enhanced efficiency of perovskite solar cells by using core–ultrathin shell structure Ag@ SiO2 nanowires as plasmonic antennas. Adv. Electron. Mater. 2017, 3, 1700169. [Google Scholar] [CrossRef] [Green Version]
- Gangadharan, D.T.; Xu, Z.; Liu, Y.; Izquierdo, R.; Ma, D. Recent advancements in plasmon-enhanced promising third-generation solar cells. Nanophotonics 2017, 6, 153–175. [Google Scholar] [CrossRef]
- Chan, K.; Wright, M.; Elumalai, N.; Uddin, A.; Pillai, S. Plasmonics in organic and perovskite solar cells: Optical and electrical effects. Adv. Opt. Mater. 2017, 5, 1600698. [Google Scholar] [CrossRef]
- Nikoobakht, B.; El-Sayed, M.A. Preparation and growth mechanism of gold nanorods (NRs) using seed-mediated growth method. Chem. Mater. 2003, 15, 1957–1962. [Google Scholar] [CrossRef]
- Jiang, Z.; Chen, X.; Lin, X.; Jia, X.; Zhuo, S. Amazing stable open-circuit voltage in perovskite solar cells using AgAl alloy electrode. Sol. Energy Mater. Sol. Cells 2016, 146, 35–43. [Google Scholar] [CrossRef]
- Subramanian, V.; Wolf, E.E.; Kamat, P.V. Catalysis with TiO2/gold nanocomposites. Effect of metal particle size on the Fermi level equilibration. J. Am. Chem. Soc. 2004, 126, 4943–4950. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Luo, Q.; Shi, J.; Yue, L.; Wang, Z.; Chen, X.; Huang, S. Efficient perovskite solar cells by combination use of Au nanoparticles and insulating metal oxide. Nanoscale 2017, 9, 2852–2864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domanski, K.; Correa-Baena, J.P.; Mine, N.; Nazeeruddin, M.K.; Abate, A.; Saliba, M.; Tress, W.; Hagfeldt, A.; Grätzel, M. Not all that glitters is gold: Metal-migration-induced degradation in perovskite solar cells. Acs Nano 2016, 10, 6306–6314. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qin, M.; Tao, H.; Ke, W.; Chen, Z.; Wan, J.; Qin, P.; Xiong, L.; Lei, H.; Yu, H.; et al. Performance enhancement of perovskite solar cells with Mg-doped TiO2 compact film as the hole-blocking layer. Appl. Phys. Lett. 2015, 106, 121104. [Google Scholar] [CrossRef]
- Guo, X.; Dong, H.; Li, W.; Li, N.; Wang, L. Multifunctional MgO layer in perovskite solar cells. ChemPhysChem 2015, 16, 1727–1732. [Google Scholar] [CrossRef]
- Dagar, J.; Castro-Hermosa, S.; Lucarelli, G.; Cacialli, F.; Brown, T.M. Highly efficient perovskite solar cells for light harvesting under indoor illumination via solution-processed SnO2/MgO composite electron transport layers. Nano Energy. 2018, 49, 290–299. [Google Scholar] [CrossRef] [Green Version]
- Stratakis, E.; Kymakis, E. Nanoparticle-based plasmonic organic photovoltaic devices. Mater. Today 2013, 16, 133–146. [Google Scholar] [CrossRef]
- Wang, D.H.; Kim, D.Y.; Choi, K.W.; Seo, J.H.; Im, S.H.; Park, J.H.; Park, O.O.; Heeger, A.J. Rücktitelbild: Enhancement of donor–acceptor polymer bulk heterojunction solar cell power conversion efficiencies by addition of Au nanoparticles. Angew. Chem. Int. Ed. 2011, 50, 5519–5523. [Google Scholar] [CrossRef]
- Jang, Y.H.; Jang, Y.J.; Kim, S.; Quan, L.N.; Chung, K.; Kim, D.H. Plasmonic solar cells: From rational design to mechanism overview. Chem. Rev. 2016, 116, 14982–15034. [Google Scholar] [CrossRef]
- Zhu, G.; Lin, T.; Lü, X.; Zhao, W.; Yang, C.; Wang, Z.; Yin, H.; Liu, Z.; Huang, F.; Lin, J. Black brookite titania with high solar absorption and excellent photocatalytic performance. J. Mater. Chem. A 2013, 1, 9650–9653. [Google Scholar] [CrossRef]
- Boix, P.P.; Larramona, G.; Jacob, A.; Delatouche, B.; Mora-Seró, I.; Bisquert, J. Hole transport and recombination in all-solid Sb2S3-sensitized TiO2 solar cells using CuSCN as hole transporter. J. Phys. Chem. C 2012, 116, 1579–1587. [Google Scholar] [CrossRef] [Green Version]
- Barnes, P.R.; Anderson, A.Y.; Koops, S.E.; Durrant, J.R.; O’Regan, B.C. Electron injection efficiency and diffusion length in dye-sensitized solar cells derived from incident photon conversion efficiency measurements. J. Phys. Chem. C 2009, 113, 1126–1136. [Google Scholar] [CrossRef]
- Morawiec, S.; Mendes, M.J.; Mirabella, S.; Simone, F.; Priolo, F.; Crupi, I. Self-assembled silver nanoparticles for plasmon-enhanced solar cell back reflectors: Correlation between structural and optical properties. Nanotechnology 2013, 24, 265601. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Yan, B.; Wang, Z.; Wu, L. Three-dimensional all-dielectric metamaterial solid immersion lens for subwavelength imaging at visible frequencies. Sci. Adv. 2016, 2, e1600901. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Zhong, H.; Bai, Z.; Zhang, T.; Fu, W.; Shi, L.; Xie, H.; Deng, L.; Zou, B. Controllable transformation from rhombohedral Cu1.8S nanocrystals to hexagonal CuS clusters: Phase-and composition-dependent plasmonic properties. Chem. Mater. 2013, 25, 4828–4834. [Google Scholar] [CrossRef]
- Johnson, P.B.; Christy, R.-W. Optical constants of the noble metals. Phys. Rev. B 1972, 6, 4370. [Google Scholar] [CrossRef]
- Wintzheimer, S.; Granath, T.; Oppmann, M.; Kister, T.; Thai, T.; Kraus, T.; Vogel, N.; Mandel, K. Supraparticles: Functionality from uniform structural motifs. ACS Nano 2018, 12, 5093–5120. [Google Scholar] [CrossRef]
- Li, J.; Cushing, S.K.; Meng, F.; Senty, T.R.; Bristow, A.D.; Wu, N. Plasmon-induced resonance energy transfer for solar energy conversion. Nat. Photonics 2015, 9, 601–607. [Google Scholar] [CrossRef]
- Sönnichsen, C.; Franzl, T.; Wilk, T.; Plessen, G.; Feldmann, J.; Wilson, O.; Mulvaney, P. Drastic reduction of plasmon damping in gold nanorods. Phys. Rev. Lett. 2002, 88, 077402. [Google Scholar]
- Mackey, M.A.; Ali, M.R.; Austin, L.A.; Near, R.D.; El-Sayed, M.A. The most effective gold nanorod size for plasmonic photothermal therapy: Theory and in vitro experiments. J. Phys. Chem. B 2014, 118, 1319–1326. [Google Scholar] [CrossRef]
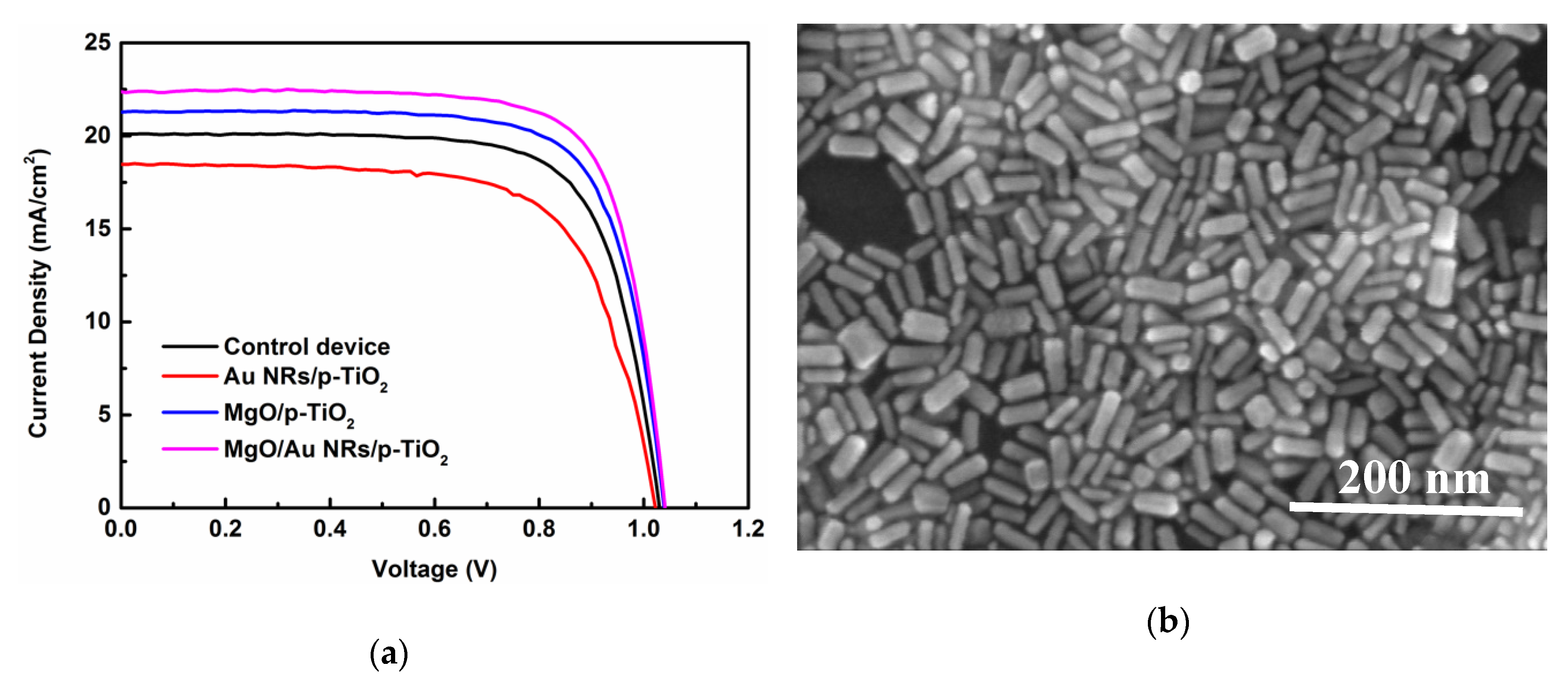
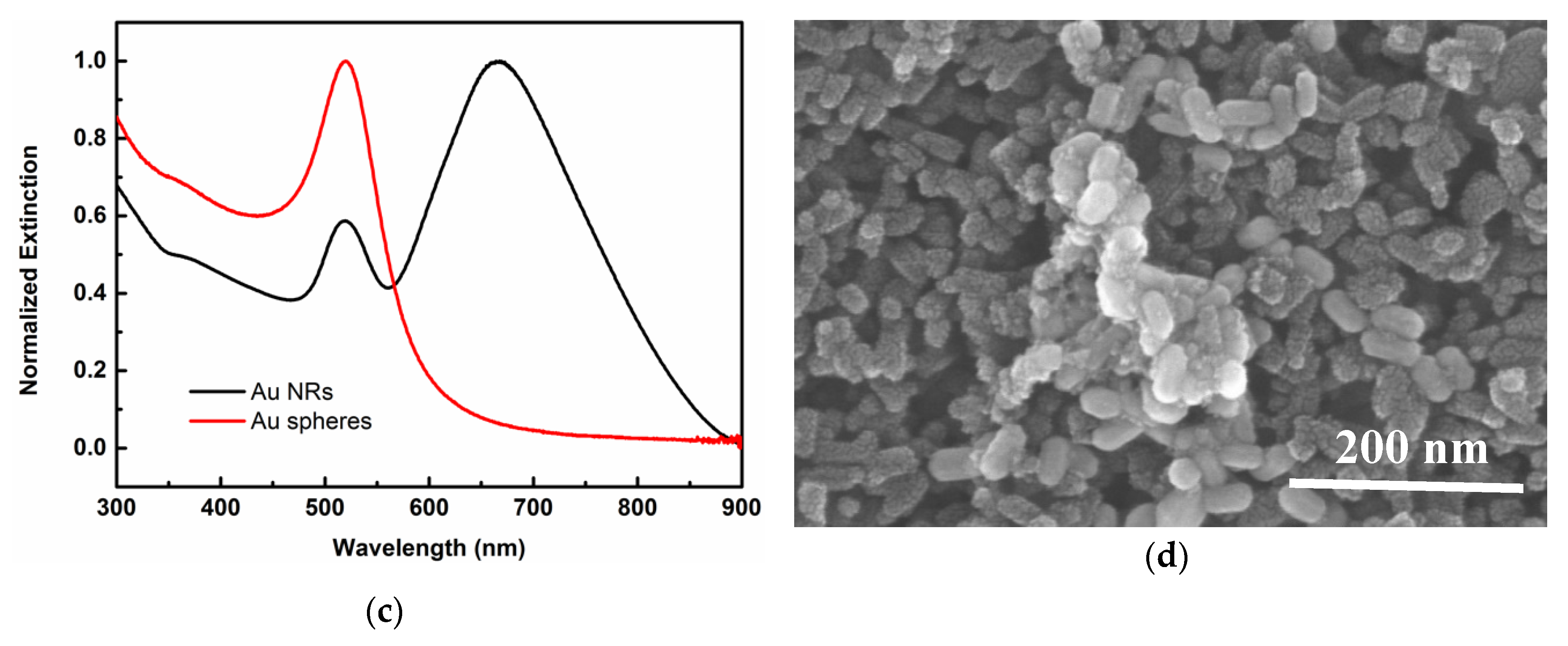
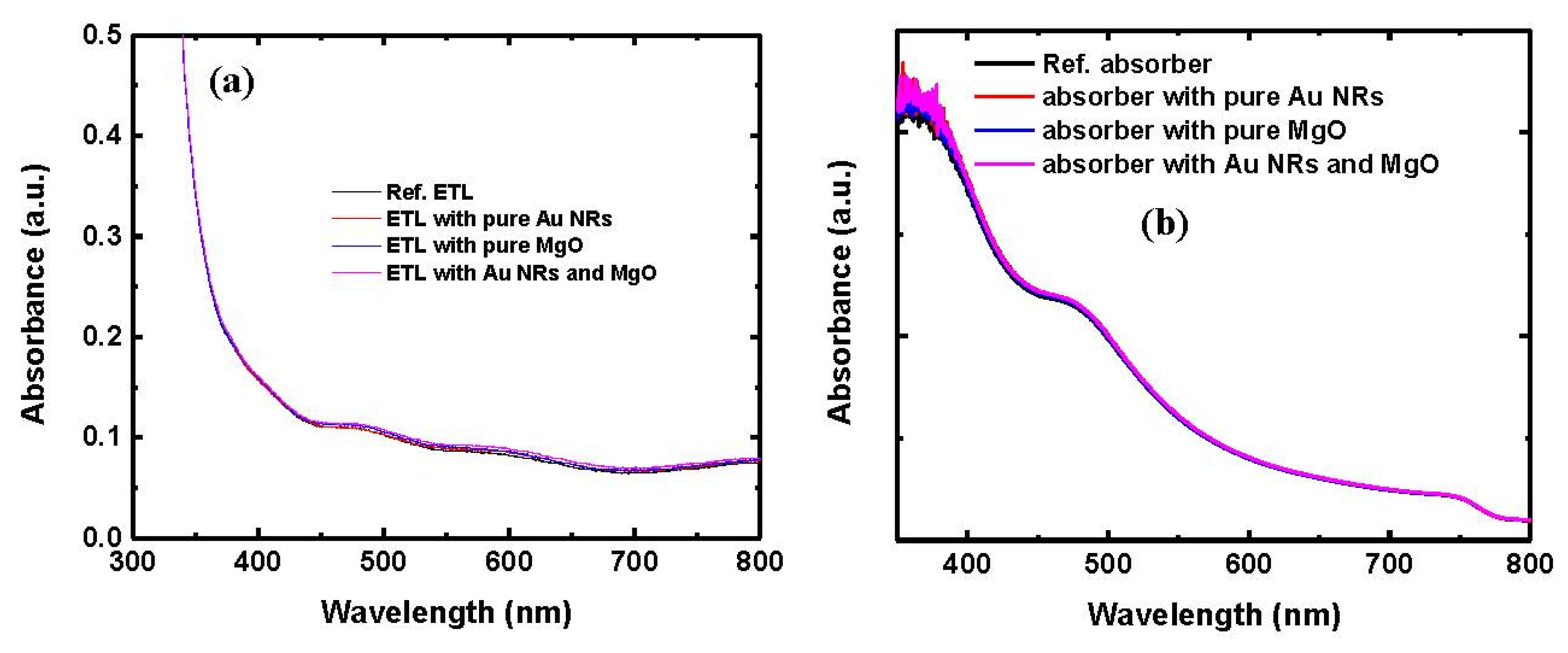
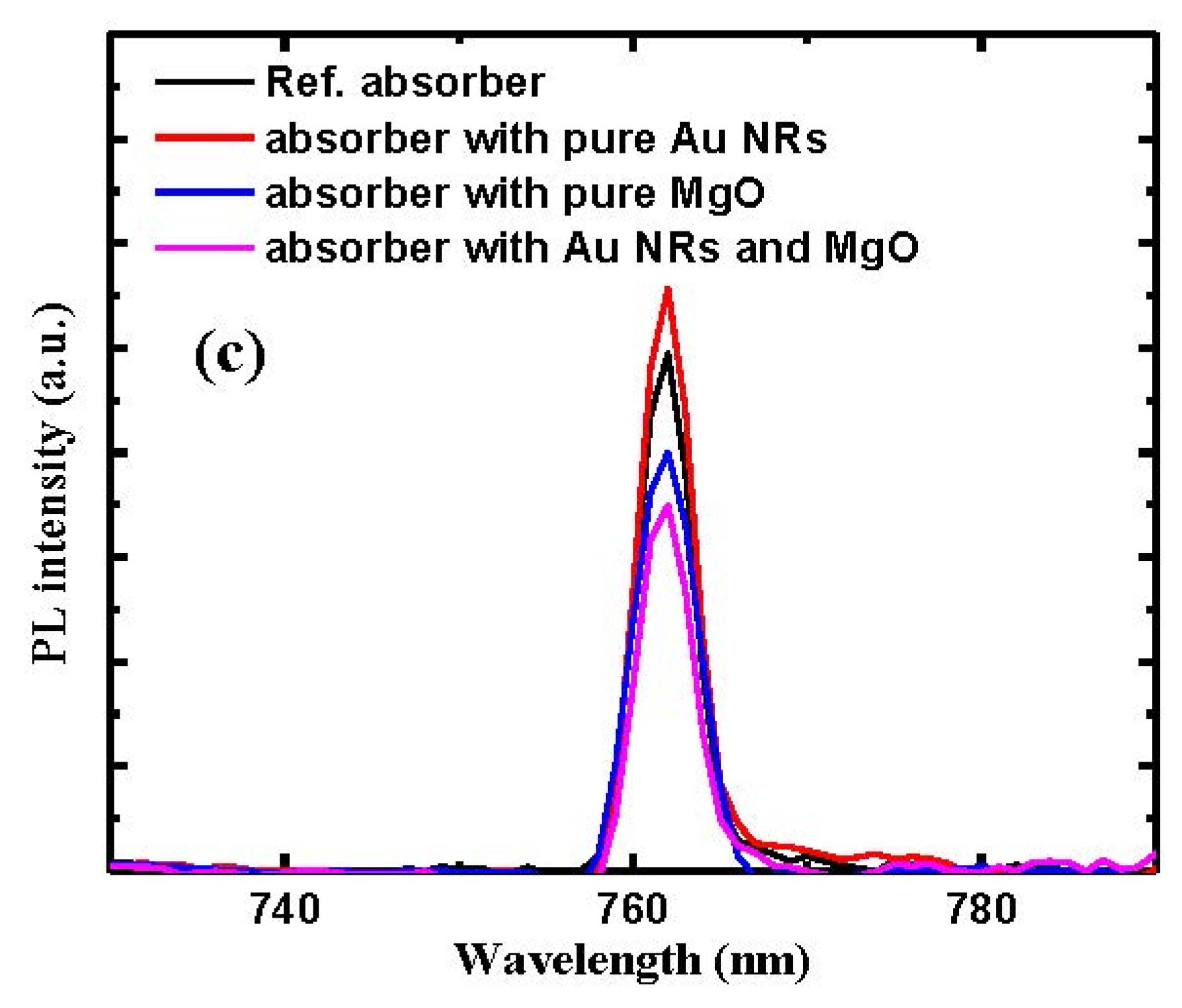
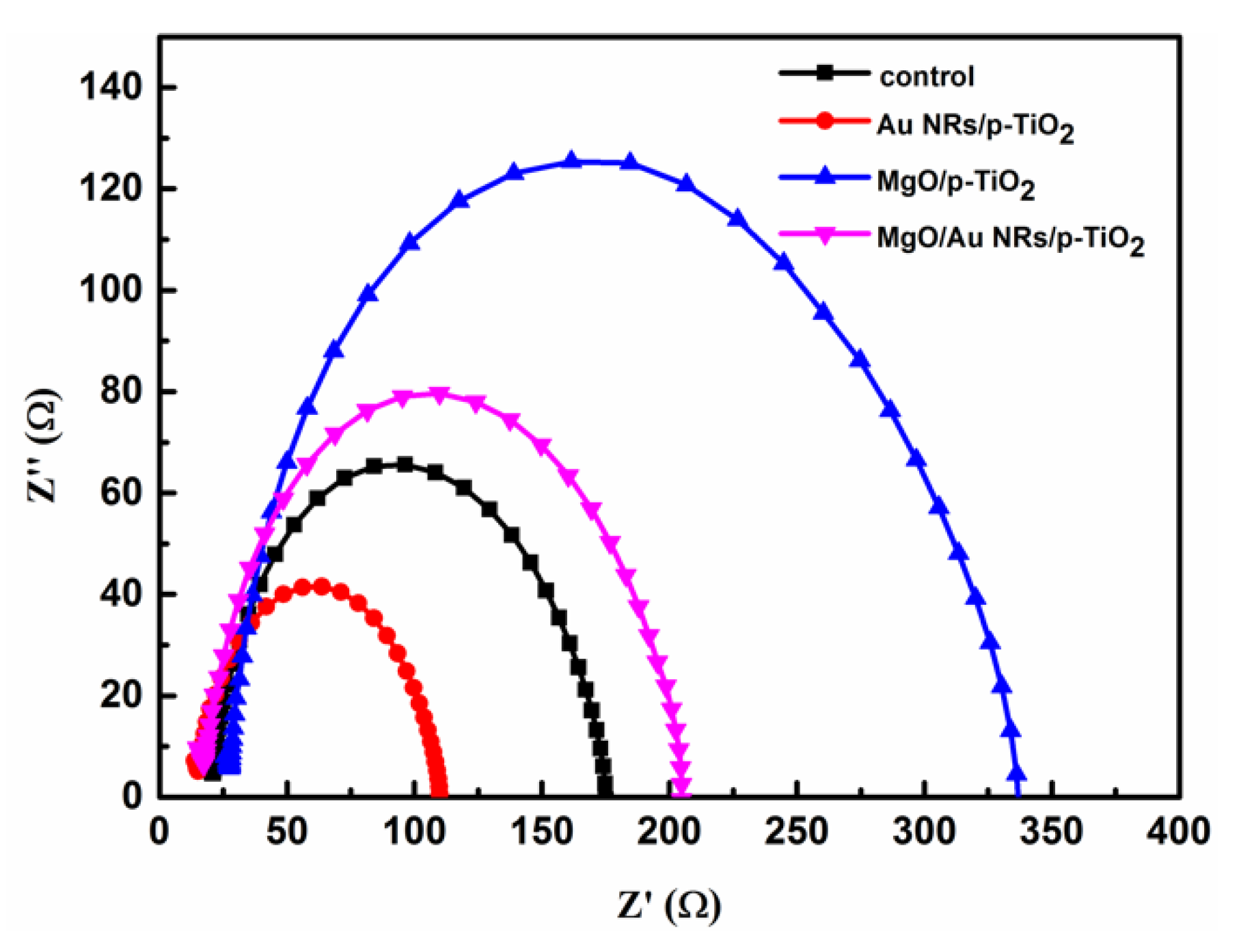
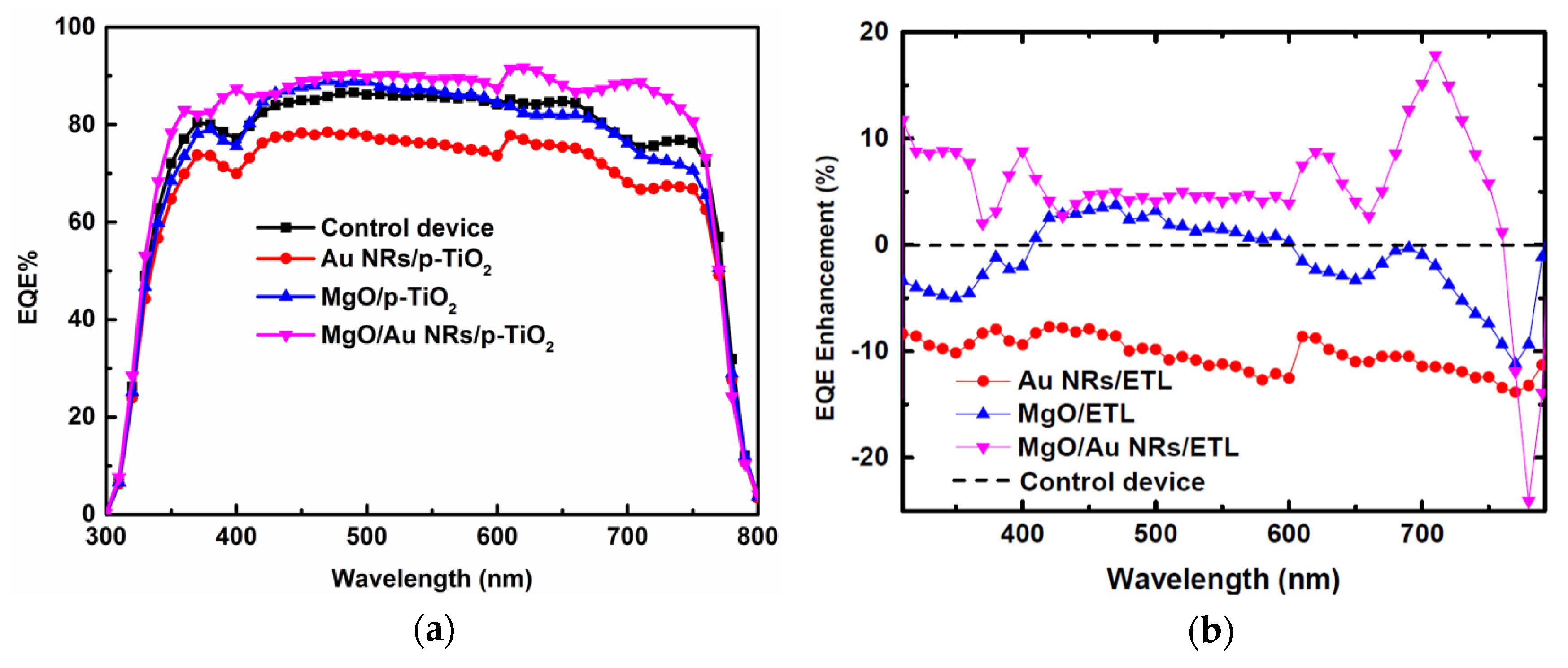

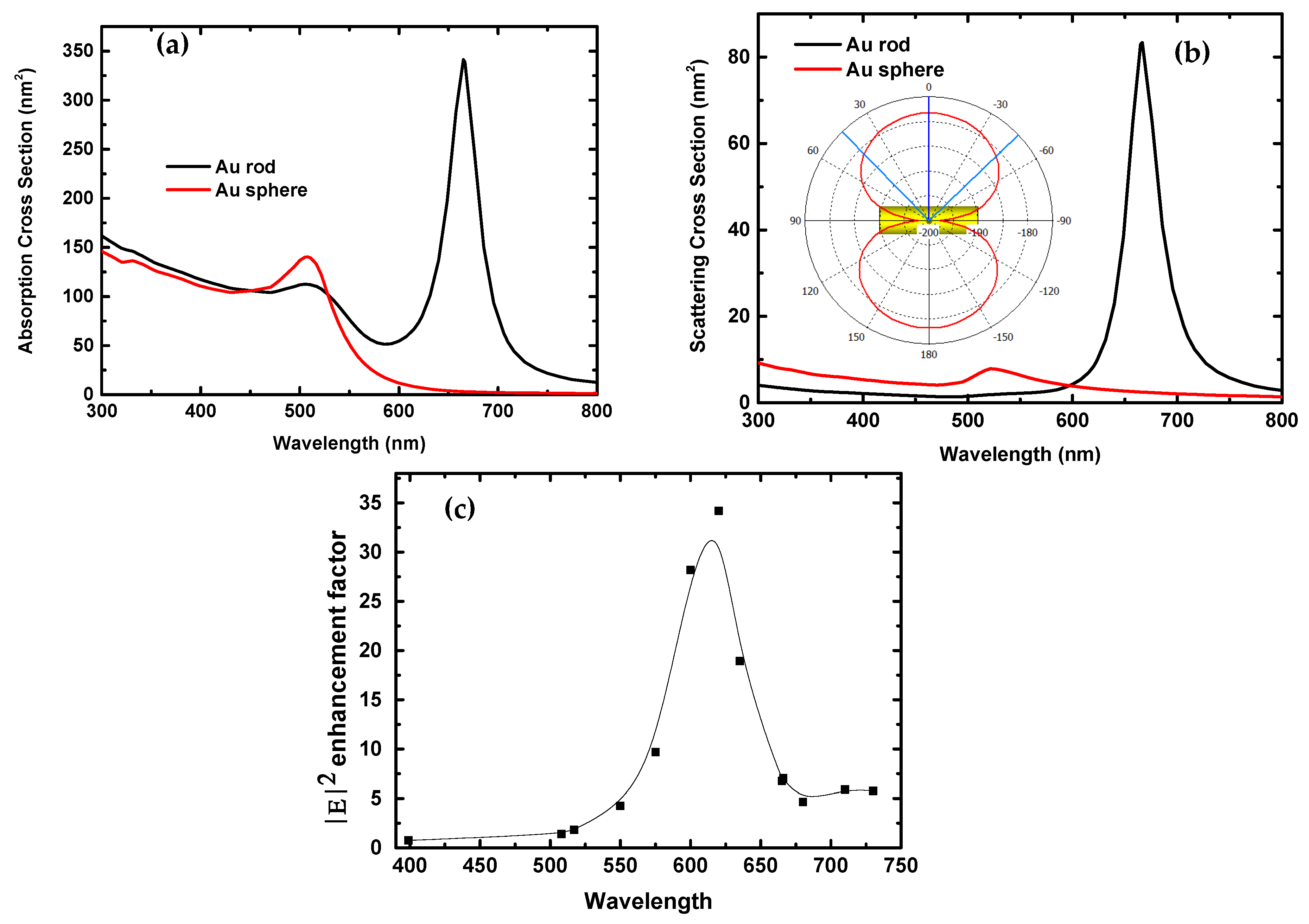
| Parameters | VOC (V) | JSC (mA/cm2) | FF | PCE (%) |
|---|---|---|---|---|
| Control | 1.02 | 20.10 | 0.72 | 14.7 |
| With pure Au NRs | 1.01 | 18.51 | 0.68 | 12.7 |
| With pure MgO | 1.04 | 21.30 | 0.74 | 16.4 |
| With Au NRs/MgO | 1.04 | 22.35 | 0.75 | 17.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, Z.; Zhang, C.; Feng, Z.; Wu, Z.; Wang, Z.; Chen, X.; Huang, S. Synergetic Effect of Plasmonic Gold Nanorods and MgO for Perovskite Solar Cells. Nanomaterials 2020, 10, 1830. https://doi.org/10.3390/nano10091830
Xia Z, Zhang C, Feng Z, Wu Z, Wang Z, Chen X, Huang S. Synergetic Effect of Plasmonic Gold Nanorods and MgO for Perovskite Solar Cells. Nanomaterials. 2020; 10(9):1830. https://doi.org/10.3390/nano10091830
Chicago/Turabian StyleXia, Zhetao, Chenxi Zhang, Zhiying Feng, Zhixing Wu, Zengbo Wang, Xiaohong Chen, and Sumei Huang. 2020. "Synergetic Effect of Plasmonic Gold Nanorods and MgO for Perovskite Solar Cells" Nanomaterials 10, no. 9: 1830. https://doi.org/10.3390/nano10091830




