Abatement of the Stimulatory Effect of Copper Nanoparticles Supported on Titania on Ovarian Cell Functions by Some Plants and Phytochemicals
Abstract
1. Introduction
2. Materials and Methods
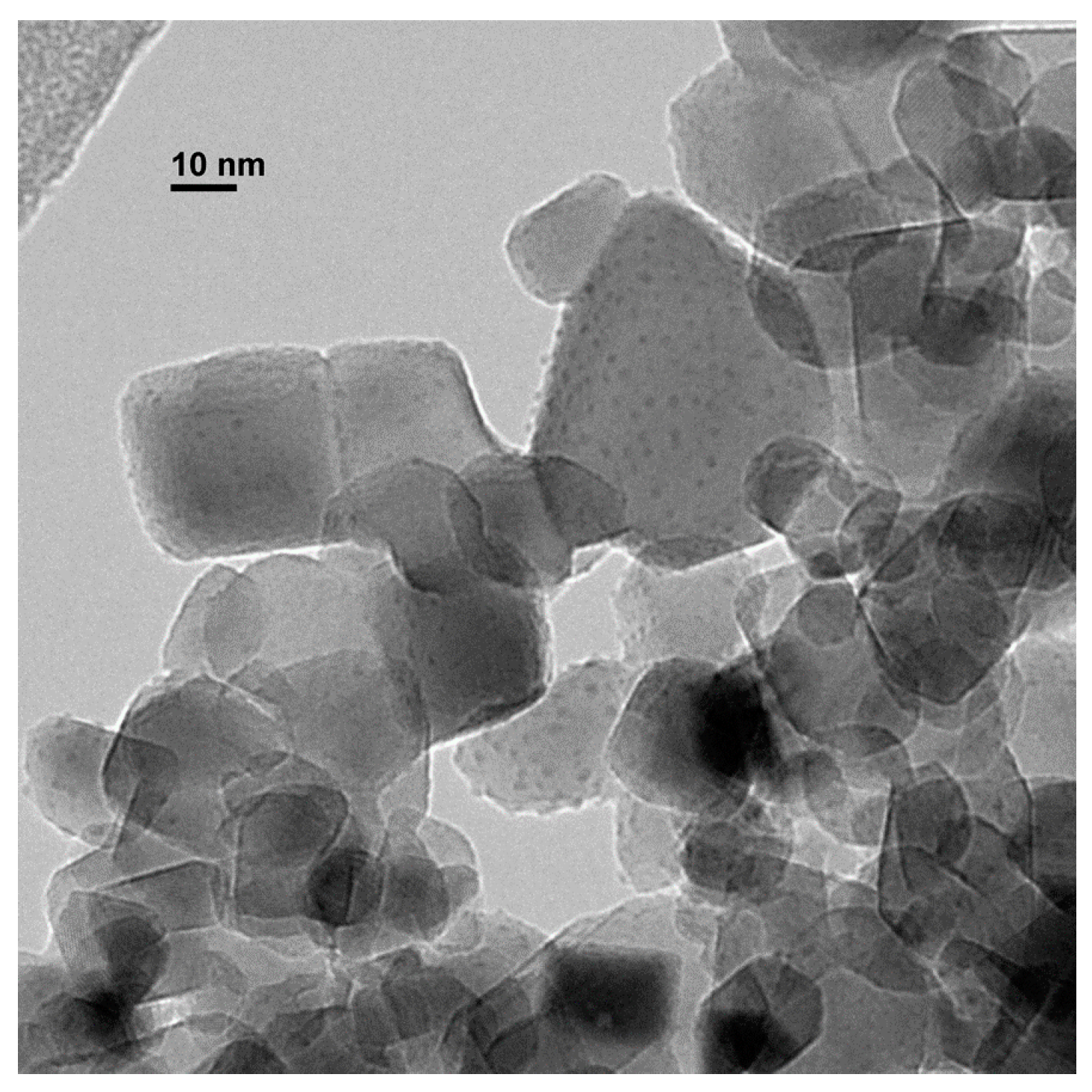
2.1. Preparation of Copper Nanoparticles Supported on Titania (CuNPs/TiO2)
2.2. Isolation and Culture of Granulosa Cells
2.3. Cell Viability Test
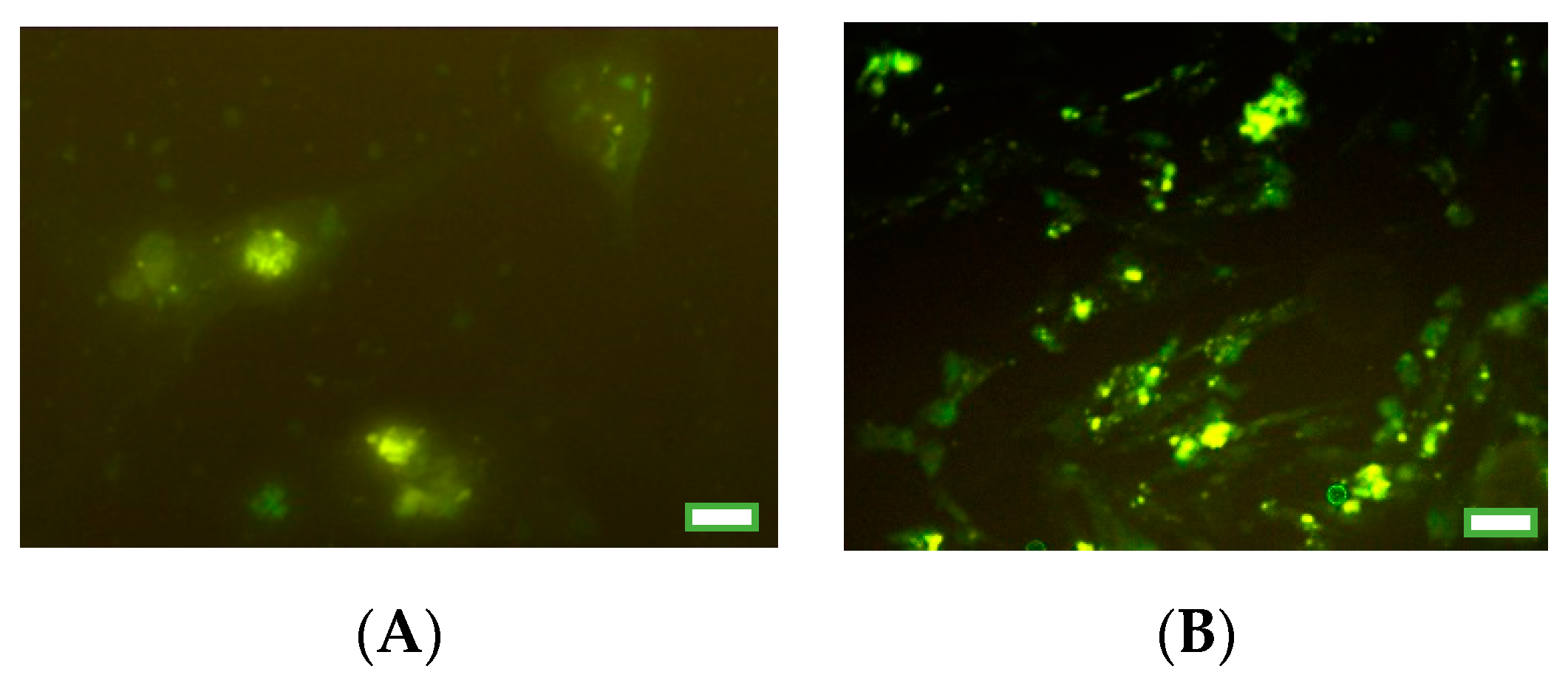
2.4. Immunocytochemical Analysis of Proliferation and Apoptosis Markers
2.5. Immunoassay of Hormones
2.6. Statistical Analysis
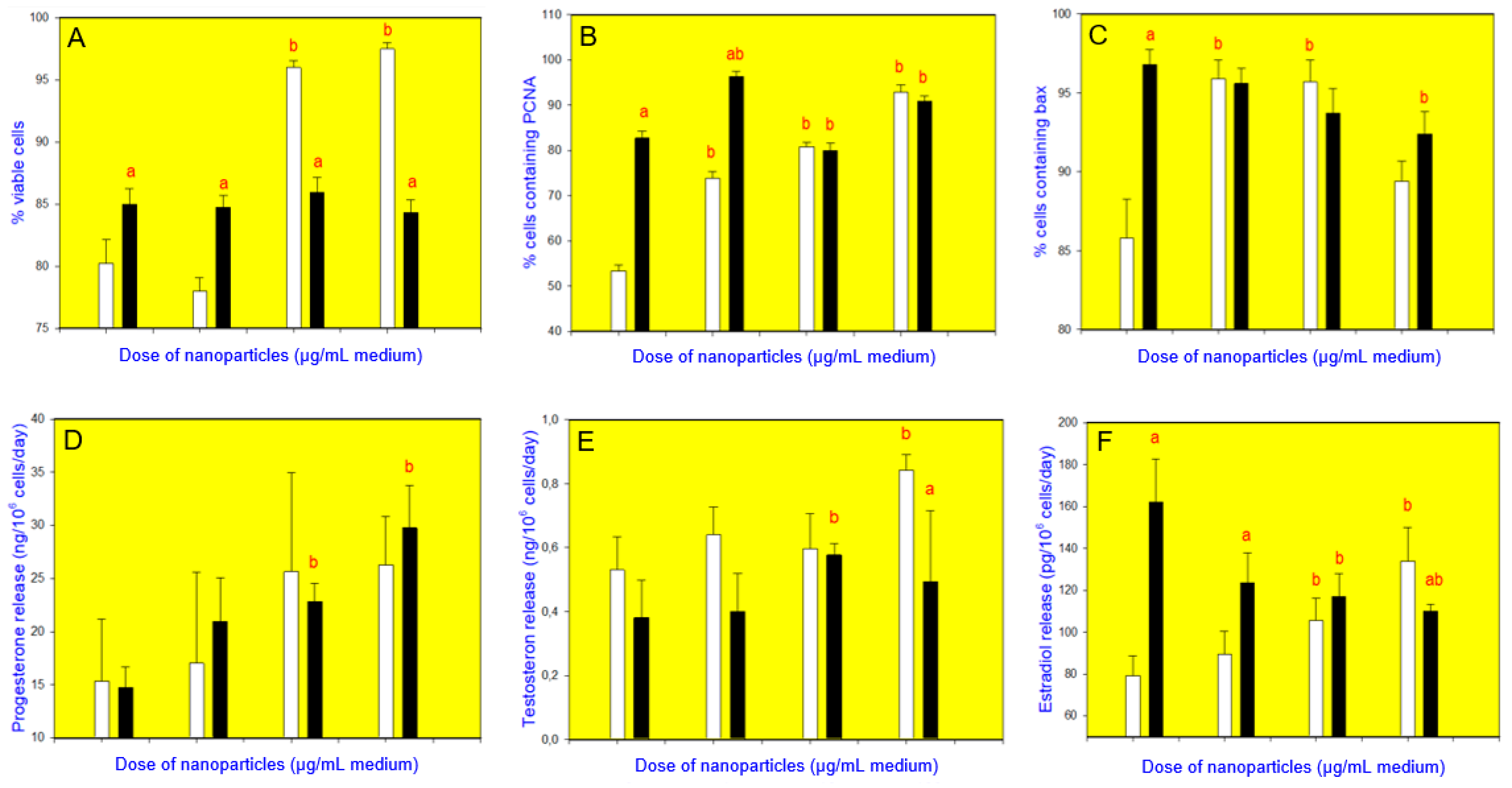
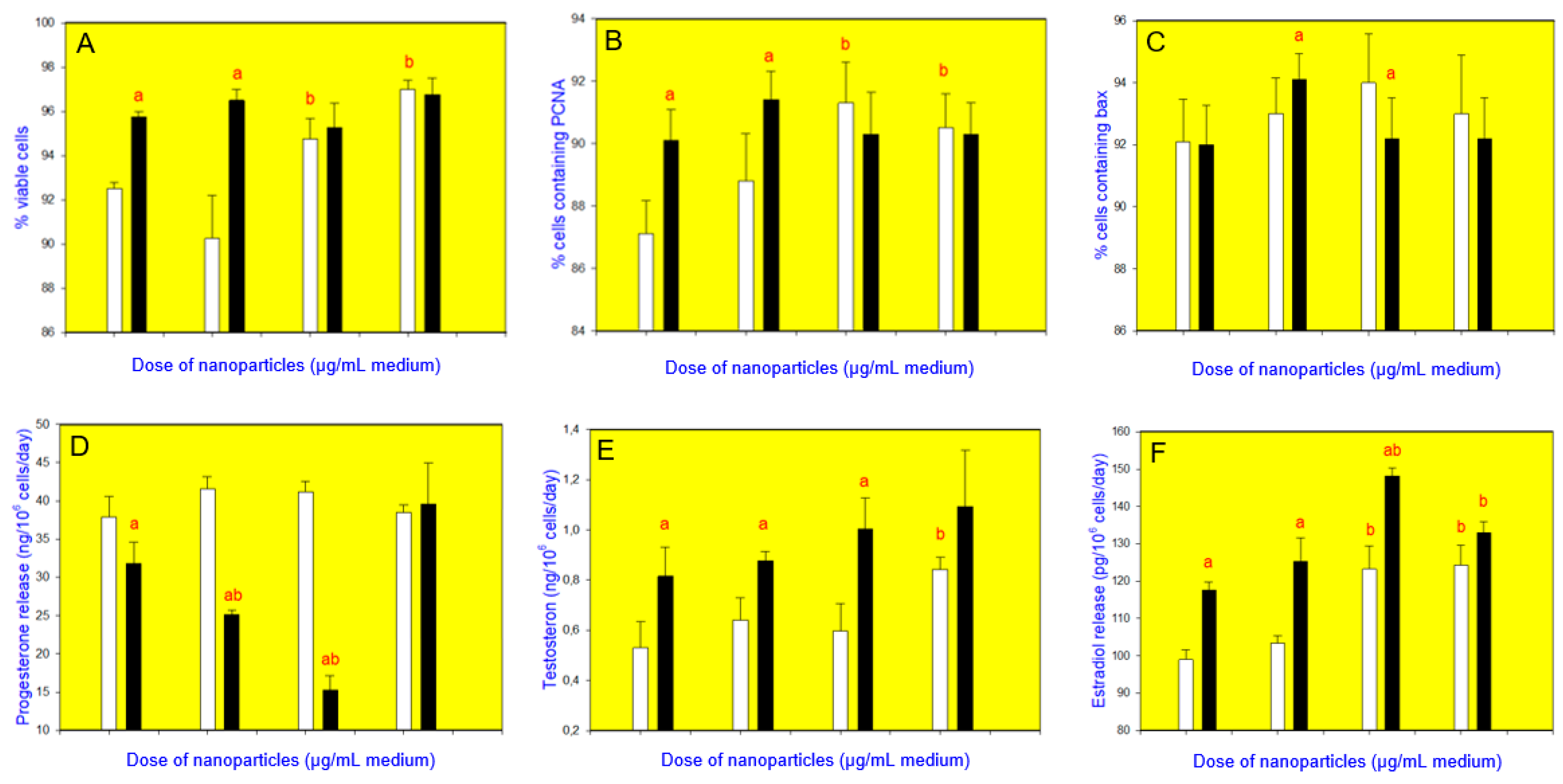
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kumar, C.S.S.R. (Ed.) Metallic Nanomaterials; Wiley-VCH: Weinheim, Germany, 2009; ISBN 9783527321513. [Google Scholar]
- Reddy, L.H.; Arias, J.L.; Nicolas, J.; Couvreur, P. Magnetic nanoparticles: Design and characterization, toxicity and biocompatibility, pharmaceutical and biomedical applications. Chem. Rev. 2012, 112, 5818–5878. [Google Scholar] [CrossRef]
- Stark, W.J.; Stoessel, P.R.; Wohlleben, W.; Hafner, A. Industrial applications of nanoparticles. Chem. Soc. Rev. 2015, 44, 5793–5805. [Google Scholar] [CrossRef]
- Bhagyaraj, S.M.; Oluwafemi, O.S.; Kalarikkal, N.; Thomas, S. (Eds.) Applications of Nanomaterials: Advances and Key Technologies; Woodhead Publishing: Cambridge, USA, 2018; ISBN 9780081019719. [Google Scholar]
- Gawande, M.B.; Goswami, A.; Felpin, F.X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-based nanoparticles: Synthesis and applications in catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef] [PubMed]
- Din, M.I.; Rehan, R. Synthesis, characterization, and applications of copper nanoparticles. Anal. Lett. 2017, 50, 50–62. [Google Scholar] [CrossRef]
- Rafique, M.; Shaikh, A.J.; Rasheed, R.; Tahir, M.B.; Bakhat, H.F.; Rafique, M.S.; Rabbani, F. A review on synthesis, characterization and applications of copper nanoparticles using green method. Nano 2017, 12, 1750043. [Google Scholar] [CrossRef]
- Zhou, M.; Tian, M.; Li, C. Copper-based nanomaterials for cancer imaging and therapy. Bioconjugate Chem. 2016, 27, 1188–1199. [Google Scholar] [CrossRef]
- Rathore, K.; Sharma, K. Biological synthesis of copper nanoparticles and their antimicrobial properties: A review. World J. Pharm. Res. 2018, 7, 11–26. [Google Scholar] [CrossRef]
- Verma, N.; Kumar, N. Synthesis and biomedical applications of copper oxide nanoparticles: An expanding horizon. ACS Biomater. Sci. Eng. 2019, 5, 1170–1188. [Google Scholar] [CrossRef]
- Hejazy, M.; Koohy, M.K.; Pour, A.B.M.; Najafi, D. Toxicity of manufactured copper nanoparticles–a review. Nanomed. Res. J. 2018, 3, 1–9. [Google Scholar] [CrossRef]
- Pohanka, M. Copper and copper nanoparticles toxicity and their impact on basic functions in the body. Bratisl. Lek. Listy 2019, 120, 397–409. [Google Scholar] [CrossRef]
- Ameh, T.; Sayes, C.M. The potential exposure and hazards of copper nanoparticles: A review. Environ. Toxicol. Pharmacol. 2019, 71, 103220. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Wang, X.; Hayat, T.; Wang, X. Ecotoxicological effects and mechanism of CuO nanoparticles to individual organisms. Environ. Pollut. 2017, 221, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Roychoudhury, S.; Nath, S.; Massanyi, P.; Stawarz, R.; Kacaniova, M.; Kolesarova, A. Copper-induced changes in reproductive functions: In vivo and in vitro effects. Physiol. Res. 2016, 65, 11–22. [Google Scholar] [CrossRef]
- Yang, J.; Hu, S.; Rao, M.; Hu, L.; Lei, H.; Wu, Y.; Wang, Y.; Ke, D.; Xia, W.; Zhu, C. Copper nanoparticle-induced ovarian injury, follicular atresia, apoptosis, and gene expression alterations in female rats. Int. J. Nanomed. 2017, 12, 5959–5971. [Google Scholar] [CrossRef]
- Zhang, C.H.; Wang, Y.; Sun, Q.Q.; Xia, L.L.; Hu, J.J.; Cheng, K.; Wang, X.; Fu, X.X.; Gu, H. Copper nanoparticles show obvious in vitro and in vivo reproductive toxicity via erk mediated signaling pathway in female mice. Int. J. Biol. Sci. 2018, 14, 1834–1844. [Google Scholar] [CrossRef]
- Fevold, H.L.; Hisaw, F.L.; Greep, R. Augmentation of the gonad-stimulating action of pituitary extracts by inorganic substances, particularly copper salts. Am. J. Physiol. 1936, 117, 68–74. [Google Scholar] [CrossRef]
- Roychoudhury, S.; Bulla, J.; Sirotkin, A.V.; Kolesarova, A. In vitro changes in porcine ovarian granulosa cells induced by copper. J. Environ. Sci. Health A, Tox. Hazard. Subst. Environ. Eng. 2014, 49, 625–633. [Google Scholar] [CrossRef]
- Sirotkin, A.V.; Radosová, M.; Tarko, A.; Martín-García, I.; Alonso, F. Effect of morphology and support of copper nanoparticles on basic ovarian granulosa cell functions. Nanotoxicology 2020, 14, 683–695. [Google Scholar] [CrossRef]
- Alonso, F.; Yus, M. New synthetic methodologies based on active transition metals. Pure Appl. Chem. 2008, 80, 1005–1012. [Google Scholar] [CrossRef]
- Deka, P.; Borah, B.J.; Saikia, H.; Bharali, P. Cu-based nanoparticles as emerging environmental catalysts. Chem. Rec. 2019, 19, 462–473. [Google Scholar] [CrossRef]
- Abdulkin, P.; Moglie, Y.; Knappett, B.R.; Jefferson, D.A.; Yus, M.; Alonso, F.; Wheatley, A.E.H. New routes to Cu(I)/Cu nanocatalysts for the multicomponent click synthesis of 1,2,3-triazoles. Nanoscale 2013, 5, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Alonso, F.; Arroyo, A.; Martín-García, I.; Moglie, Y. Cross-dehydrogenative coupling of tertiary amines and terminal alkynes catalyzed by copper nanoparticles on zeolite. Adv. Synth. Catal. 2015, 357, 3549–3561. [Google Scholar] [CrossRef]
- Mitrofanov, A.Y.; Murashkina, A.V.; Martín-García, I.; Alonso, F.; Beletskaya, I.P. Formation of C-C, C-S and C-N bonds catalysed by supported copper nanoparticles. Catal. Sci. Technol. 2017, 7, 4401–4412. [Google Scholar] [CrossRef]
- Martín-García, I.; Alonso, F. Synthesis of dihydroindoloisoquinolines through the copper-catalyzed cross-dehydrogenative coupling of tetrahydroisoquinolines and nitroalkanes. Chem. Eur. J. 2018, 24, 18857–18862. [Google Scholar] [CrossRef] [PubMed]
- Kenneth, L.; Thanh, P.H.; Doan, D.L. Interaction of plant extracts with central nervous system receptors. Medicines 2017, 4, 12. [Google Scholar] [CrossRef]
- Dhama, K.; Karthik, K.; Khandia, R.; Munjal, A.; Tiwari, R.; Rana, R.; Khurana, S.K.; Ullah, S.; Khan, R.U.; Alagawany, M.; et al. Medicinal and therapeutic potential of herbs and plant metabolites/extracts countering viral pathogens—Current knowledge and future prospects. Curr. Drug Metab. 2018, 19, 236–263. [Google Scholar] [CrossRef]
- Pohl, F.; Lin, P.K.T. The potential use of plant natural products and plant extracts with antioxidant properties for the prevention/treatment of neurodegenerative diseases: In vitro, in vivo and clinical trials. Molecules 2018, 23, 3283. [Google Scholar] [CrossRef]
- Quero, J.; Marmol, I.; Rodriguez-Yoldi, M.J.; Cerrada, E. Insight into the potential application of polyphenol-rich dietary intervention in degenerative disease management. Food Funct. 2020, 11, 2805–2825. [Google Scholar] [CrossRef]
- Akour, A.; Kasabri, V.; Afifi, F.U.; Bulatova, N. The use of medicinal herbs in gynecological and pregnancy-related disorders by Jordanian women: A review of folkloric practice vs. evidence-based pharmacology. Pharm. Biol. 2016, 54, 1901–1918. [Google Scholar] [CrossRef]
- Sabourian, R.; Karimpour-Razkenari, E.; Saeedi, M.; Bagheri, M.S.; Khanavi, M.; Sadati, N.; Akbarzadeh, T.; Ardekani, M.R. Medicinal plants used in iranian traditional medicine (itm) as contraceptive agents. Curr. Pharm. Biotechnol. 2016, 17, 974–985. [Google Scholar] [CrossRef]
- Bruno, L.O.; Simoes, R.S.; de Jesus Simoes, M.; Girão, M.J.B.C.; Grundmann, O. Pregnancy and herbal medicines: An unnecessary risk for women’s health-A narrative review. Phytother Res. 2018, 32, 796–810. [Google Scholar] [CrossRef] [PubMed]
- Kam, P.C.; Barnett, D.W.; Douglas, I.D. Herbal medicines and pregnancy: A narrative review and anaesthetic considerations. Anaesth. Intensive Care. 2019, 47, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Bastida, J.A.; Zielinski, H.; Piskula, M.; Zielinska, D.; Szawara-Nowak, D. Buckwheat bioactive compounds, their derived phenolic metabolites and their health benefits. Mol. Nutr. Food Res. 2017, 61, 1600475. [Google Scholar] [CrossRef] [PubMed]
- Ahangarpour, A.; Najimi, S.A.; Farbood, Y. Effects of Vitex agnus-castus fruit on sex hormones and antioxidant indices in a D-galactose-induced aging female mouse model. J. Chin. Med. Assoc. 2016, 79, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Kakadia, N.; Patel, P.; Deshpande, S.; Shah, G. Effect of Vitex negundo L. seeds in letrozole induced polycystic ovarian syndrome. J. Tradit. Complement. Med. 2018, 9, 336–345. [Google Scholar] [CrossRef]
- Moini Jazani, A.; Nasimi Doost Azgomi, H.; Nasimi Doost Azgomi, A.; Nasimi Doost Azgomi, R. A comprehensive review of clinical studies with herbal medicine on polycystic ovary syndrome (PCOS). Daru 2019, 27, 863–877. [Google Scholar] [CrossRef]
- Rafieian-Kopaei, M.; Movahedi, M. Systematic review of premenstrual, postmenstrual and infertility disorders of vitex agnus castus. Electron. Physician. 2017, 9, 3685–3689. [Google Scholar] [CrossRef][Green Version]
- Csupor, D.; Lantos, T.; Hegyi, P.; Benkő, R.; Viola, R.; Gyöngyi, Z.; Csécsei, P.; Tóth, B.; Vasas, A.; Márta, K.; et al. Vitex agnus-castus in premenstrual syndrome: A meta-analysis of double-blind randomised controlled trials. Complement. Ther. Med. 2019, 47, 102190. [Google Scholar] [CrossRef]
- Ohyama, K.; Akaike, T.; Hirobe, C.; Yamakawa, T. Cytotoxicity and apoptotic inducibility of Vitex agnus-castus fruit extract in cultured human normal and cancer cells and effect on growth. Biol. Pharm. Bull. 2003, 26, 10–18. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Y.E.; Cao, J.; Zeng, G.; Shen, C.; Li, Y.; Zhou, M.; Chen, Y.; Pu, W.; Potters, L.; et al. Vitexins, nature-derived lignan compounds, induce apoptosis and suppress tumor growth. Clin. Cancer Res. 2009, 15, 5161–5169. [Google Scholar] [CrossRef]
- Abdallah, H.M.; El-Halawany, A.M. Rutin isolated from chrozophora tinctoria enhances bone cell proliferation and ossification markers. Oxid. Med. Cell. Longev. 2018, 5106469. [Google Scholar] [CrossRef]
- Wei, S.M.; Yan, Z.Z.; Zhou, J. Protective effect of rutin on testicular ischemia-reperfusion injury. J. Pediatric Surg. 2011, 46, 1419–1424. [Google Scholar] [CrossRef] [PubMed]
- Mehfooz, A.; Wei, Q.; Zheng, K.; Fadlalla, M.B.; Maltasic, G.; Shi, F. Protective roles of Rutin against restraint stress on spermatogenesis in testes of adult mice. Tissue Cell 2018, 50, 133–143. [Google Scholar] [CrossRef]
- Aksu, E.H.; Kandemir, F.M.; Özkaraca, M.; Ömür, A.D.; Küçükler, S.; Çomaklı, S. Rutin ameliorates cisplatin-induced reproductive damage via suppression of oxidative stress and apoptosis in adult male rats. Andrologia 2017, 49, e12593. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Yuan, X.; Ye, R.; Zhou, H.; Lin, J.; Zhang, C.; Zhang, H.; Wei, G.; Dong, M.; Huang, Y.; et al. Brown adipose tissue activation by rutin ameliorates polycystic ovary syndrome in rat. J. Nutr. Biochem. 2017, 47, 21–28. [Google Scholar] [CrossRef]
- Yan, X.; Qi, M.; Li, P.; Zhan, Y.; Shao, H. Apigenin in cancer therapy: Anti-cancer effects and mechanisms of action. Cell Biosci. 2017, 7, 50. [Google Scholar] [CrossRef]
- Ozbey, U.; Attar, R.; Romero, M.A.; Alhewairini, S.S.; Afshar, B.; Sabitaliyevich, U.Y.; Hanna-Wakim, L.; Ozcelik, B.; Farooqi, A.A. Apigenin as an effective anticancer natural product: Spotlight on TRAIL, WNT/β-catenin, JAK-STAT pathways, and microRNAs. J. Cell. Biochem. 2018, 120, 1060–1067. [Google Scholar] [CrossRef]
- Tavsan, Z.; Kayali, H.A. Flavonoids showed anticancer effects on the ovarian cancer cells: Involvement of reactive oxygen species, apoptosis, cell cycle and invasion. Biomed. Pharmacother. 2019, 116, 109004. [Google Scholar] [CrossRef] [PubMed]
- Darabi, P.; Khazali, H.; Mehrabani Natanzi, M. Therapeutic potentials of the natural plant flavonoid apigenin in polycystic ovary syndrome in rat model: Via modulation of pro-inflammatory cytokines and antioxidant activity. Gynecol. Endocrinol. 2019. [Google Scholar] [CrossRef]
- Soyman, Z.; Kelekçi, S.; Sal, V.; Şevket, O.; Bayındır, N.; Uzun, H. Effects of apigenin on experimental ischemia/reperfusion injury in the rat ovary. Balkan Med. J. 2017, 34, 444–449. [Google Scholar] [CrossRef]
- Peña-Blanco, A.; García-Sáez, A.J. Bax, Bak and beyond – mitochondrial performance in apoptosis. FEBS J. 2018, 285, 416–431. [Google Scholar] [CrossRef]
- Sharma, A.; Boise, L.H.; Shanmugam, M. Cancer metabolism and the evasion of apoptotic cell death. Cancers 2019, 11, 1144. [Google Scholar] [CrossRef]
- Wang, S.C. PCNA: A silent housekeeper or a potential therapeutic target? Trends Pharmacol. Sci. 2014, 35, 178–186. [Google Scholar] [CrossRef]
- Sirotkin, A.V. Regulators of Ovarian Functions; Nova Publishers Inc.: New York, NY, USA, 2014; p. 194. [Google Scholar]
- Pavlová, S.; Klucska, K.; Vašíček, D.; Kotwica, J.; Sirotkin, A.V. Transcription factor NF-κB (p50/p50, p65/p65) controls porcine ovarian cells functions. Anim. Reprod. Sci. 2011, 128, 73–84. [Google Scholar] [CrossRef]
- Sirotkin, A.V.; Hrabovszká, S.; Štochmaľová, A.; Grossmann, R.; Alwasel, S.; Halim Harrath, A. Effect of quercetin on ovarian cells of pigs and cattle. Anim. Reprod. Sci. 2019, 205, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Sirotkin, A.V.; Štochmaľová, A.; Alexa, R.; Kádasi, A.; Bauer, M.; Grossmann, R.; Alrezaki, A.; Alwasel, S.; Harrath, A.H. Quercetin directly inhibits basal ovarian cell functions and their response to the stimulatory action of FSH. Eur. J. Pharmacol. 2019, 860, 172560. [Google Scholar] [CrossRef]
- Tarko, A.; Štochmalova, A.; Hrabovszka, S.; Vachanova, A.; Harrath, A.H.; Alwasel, S.; Grossman, R.; Sirotkin, A.V. Can xylene and quercetin directly affect basic ovarian cell functions? Res. Vet. Sci. 2018, 119, 308–312. [Google Scholar] [CrossRef]
- Tarko, A.; Štochmaľová, A.; Jedličková, K.; Hrabovszká, S.; Vachanová, A.; Harrath, A.H.; Alwasel, S.; Alrezaki, A.; Kotwica, J.; Baláži, A.; et al. Effects of benzene, quercetin, and their combination on porcine ovarian cell proliferation, apoptosis, and hormone release. Arch. Anim. Breed. 2019, 62, 345–351. [Google Scholar] [CrossRef]
- Strober, W. Trypan blue exclusion test of cell viability. Curr. Protoc. Immunol. 2001. Appendix 3B. [Google Scholar] [CrossRef]
- Cissen, M.; van Wely, M.V.; Scholten, I.; Mansell, S.; de Bruin, J.P.; Mol, B.W.; Braat, D.; Repping, S.; Hamer, G. measuring sperm DNA fragmentation and clinical outcomes of medically assisted reproduction: A systematic review and meta-analysis. PLoS ONE 2016, 11, e0165125. [Google Scholar] [CrossRef]
- Lee, J.M.; Park, J.H.; Kim, B.Y.; Kim, I.-H. Terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL) assay to characterize histopathologic changes following thermal injury. Ann. Dermatol. 2018, 30, 41–46. [Google Scholar] [CrossRef]


| Additive b | Cell Viability | Proliferation (PCNA) | Apoptosis (Bax) | Release of Steroid Hormones | ||
|---|---|---|---|---|---|---|
| Progesterone | Testosterone | Estradiol | ||||
| CuNPs/TiO2 | + | + | + | 0 | + | + |
| Buckwheat | + | 0 | + | 0 | – | 0 |
| Buckwheat and CuNPs/TiO2 | – | – | – | – | – | – |
| Vitex | + | + | + | 0 | 0 | + |
| Vitex and CuNPs/TiO2 | – | – | – | 0 | – | – |
| Rutin | + | 0 | 0 | 0 | + | + |
| Rutin and CuNPs/TiO2 | – | 0 | 0 | – | – | – |
| Apigenin | + | + | 0 | – | + | + |
| Apigenin and CuNPs/TiO2 | – | – | 0 | – | – | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sirotkin, A.V.; Radosová, M.; Tarko, A.; Fabova, Z.; Martín-García, I.; Alonso, F. Abatement of the Stimulatory Effect of Copper Nanoparticles Supported on Titania on Ovarian Cell Functions by Some Plants and Phytochemicals. Nanomaterials 2020, 10, 1859. https://doi.org/10.3390/nano10091859
Sirotkin AV, Radosová M, Tarko A, Fabova Z, Martín-García I, Alonso F. Abatement of the Stimulatory Effect of Copper Nanoparticles Supported on Titania on Ovarian Cell Functions by Some Plants and Phytochemicals. Nanomaterials. 2020; 10(9):1859. https://doi.org/10.3390/nano10091859
Chicago/Turabian StyleSirotkin, Alexander V., Monika Radosová, Adam Tarko, Zuzana Fabova, Iris Martín-García, and Francisco Alonso. 2020. "Abatement of the Stimulatory Effect of Copper Nanoparticles Supported on Titania on Ovarian Cell Functions by Some Plants and Phytochemicals" Nanomaterials 10, no. 9: 1859. https://doi.org/10.3390/nano10091859
APA StyleSirotkin, A. V., Radosová, M., Tarko, A., Fabova, Z., Martín-García, I., & Alonso, F. (2020). Abatement of the Stimulatory Effect of Copper Nanoparticles Supported on Titania on Ovarian Cell Functions by Some Plants and Phytochemicals. Nanomaterials, 10(9), 1859. https://doi.org/10.3390/nano10091859