Production and Properties of Molybdenum Disulfide/Graphene Oxide Hybrid Nanostructures for Catalytic Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Graphene Oxide
2.2. Molybdenum Disulfide
2.3. MoS2/GO Hybrid Nanostructures
2.3.1. Synthesis in the Semi-Batch Reactor
2.3.2. Synthesis in the Impinging Jet Reactor
2.4. Purification
2.5. Structural Characterization
2.6. Electrochemical Measurements
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, X.; Zhu, H. Two-dimensional MoS2: Properties, preparation, and applications. J. Mater. 2015. [Google Scholar] [CrossRef]
- He, Z.; Que, W. Molybdenum disulfide nanomaterials: Structures, properties, synthesis and recent progress on hydrogen evolution reaction. Appl. Mater. Today 2016. [Google Scholar] [CrossRef]
- Wang, H.D.; Xu, B.-S.; Liu, J.-J.; Zhuang, D.-M. Characterization and anti-friction on the solid lubrication MoS2 film prepared by chemical reaction technique. Sci. Technol. Adv. Mater. 2005. [Google Scholar] [CrossRef]
- Boone, W.P.; Ekerdt, J.G. Hydrodesulfurization studies with a single-layer molybdenum disulfide catalyst. J. Catal. 2000. [Google Scholar] [CrossRef]
- Wang, T.; Chen, S.; Pang, H.; Xue, H.; Yu, Y. MoS2-based nanocomposites for electrochemical energy storage. Adv. Sci. 2017. [Google Scholar] [CrossRef]
- Shi, J. Single-atom co-doped MoS2 monolayers for highly active biomass hydrodeoxygenation. Chemical 2017. [Google Scholar] [CrossRef]
- Zhang, X.; Teng, S.Y.; Loy, A.C.M.; How, B.S.; Leong, W.D.; Tao, X. Transition metal dichalcogenides for the application of pollution reduction: A review. Nanomaterials 2020, 10, 1012. [Google Scholar] [CrossRef]
- De León, J.N.D.; Kumar, C.R.; Antúnez-García, J.; Fuentes-Moyado, S. Recent insights in transition metal sulfide hydrodesulfurization catalysts for the production of ultra low sulfur diesel: A short review. Catalysts 2019, 9, 87. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Xie, L.; Liang, Y.; Hong, G.; Dai, H. MoS2 nanoparticles grown on graphene: An advanced catalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 2011. [Google Scholar] [CrossRef]
- Zhou, J.; Xiao, H.; Zhou, B.; Huang, F.; Zhou, S.; Xiao, W.; Wang, D. Hierarchical MoS2 -rGO nanosheets with high MoS2 loading with enhanced electro-catalytic performance. Appl. Surf. Sci. 2015. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Synergetic effect of MoS2 and graphene as cocatalysts for enhanced photocatalytic H 2 production activity of TiO2 nanoparticles. J. Am. Chem. Soc. 2012. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Zhou, Y.; Nie, W.; Chen, P. MoS2 -GO nanocomposites synthesized via a hydrothermal hydrogel method for solar light photocatalytic degradation of methylene blue. Appl. Surf. Sci. 2015. [Google Scholar] [CrossRef]
- Zhang, F.X.; Li, C.S.; Zhang, Y.; Tang, H. Tribological property of RGO/MoS2 and CNTs/MoS2 as lubricated oil additives. Rengong Jingti Xuebao/J. Synth. Cryst. 2015, 44, 801–807. [Google Scholar]
- Hou, K.; Wang, J.; Yang, Z.; Ma, L.; Wang, Z.; Yang, S. One-pot synthesis of reduced graphene oxide/molybdenum disulfide heterostructures with intrinsic incommensurateness for enhanced lubricating properties. Carbon 2017. [Google Scholar] [CrossRef]
- Bae, D.; Seger, B.; Vesborg, P.C.K.; Hansen, O.; Chorkendorff, I. Strategies for stable water splitting: Via protected photoelectrodes. Chem. Soc. Rev. 2017. [Google Scholar] [CrossRef]
- Owusu, P.A.; Asumadu-Sarkodie, S. A review of renewable energy sources, sustainability issues and climate change mitigation. Cogent Eng. 2016. [Google Scholar] [CrossRef]
- Liu, N.; Kim, J.; Oh, J.; Nguyen, T.Q.; Sahu, B.B.; Han, J.G.; Kim, S. Growth of multiorientated polycrystalline MoS2 using plasma-enhanced chemical vapor deposition for efficient hydrogen evolution reactions. Nanomaterials 2020, 10, 1465. [Google Scholar] [CrossRef]
- Yao, Y.; Ao, K.; Lv, P.; Wei, Q. MoS2 coexisting in 1T and 2H phases synthesized by common hydrothermal method for hydrogen evolution reaction. Nanomaterials 2019, 9, 844. [Google Scholar] [CrossRef]
- Eftekhari, A. Electrocatalysts for hydrogen evolution reaction. Int. J. Hydrogen Energy 2017. [Google Scholar] [CrossRef]
- She, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Nørskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017. [Google Scholar] [CrossRef]
- Han, B.; Hu, Y.H. MoS2 as a co-catalyst for photocatalytic hydrogen production from water. Energy Sci. Eng. 2016. [Google Scholar] [CrossRef]
- Christoforidis, K.C.; Fornasiero, P. Photocatalytic hydrogen production: A rift into the future energy supply. ChemCatChem 2017. [Google Scholar] [CrossRef]
- Barthelemy, H.; Weber, M.; Barbier, F. Hydrogen storage: Recent improvements and industrial perspectives. Int. J. Hydrogen Energy 2017. [Google Scholar] [CrossRef]
- Benck, J.D.; Hellstern, T.R.; Kibsgaard, J.; Chakthranont, P.; Jaramillo, T.F. Catalyzing the hydrogen evolution reaction (HER) with molybdenum sulfide nanomaterials. ACS Catal. 2014. [Google Scholar] [CrossRef]
- Vesborg, P.C.K.; Seger, B.; Chorkendorff, I. Recent development in hydrogen evolution reaction catalysts and their practical implementation. J. Phys. Chem. Lett. 2015. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, P.; Li, J.; Xiang, B. Two-dimensional material molybdenum disulfides as electrocatalysts for hydrogen evolution. Catalysts 2017, 7, 285. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, J.; Feng, C.; Xu, G.; Xie, C.; Yuan, X.; Xiang, B. MoS2 nanosheet/MoS2 flake homostructures for efficient electrocatalytic hydrogen evolution. Mater. Res. Express 2019. [Google Scholar] [CrossRef]
- Benson, E.E.; Zhang, H.; Schuman, A.; Nanayakkara, S.U.; Bronstein, N.D.; Ferrere, S.; Blackburn, J.L.; Miller, E.M. Balancing the hydrogen evolution reaction, surface energetics, and stability of metallic MoS2 nanosheets via covalent functionalization. J. Am. Chem. Soc. 2018. [Google Scholar] [CrossRef]
- Benck, J.D.; Chen, Z.; Kuritzky, L.Y.; Forman, A.J.; Jaramillo, T.F. Amorphous molybdenum sulfide catalysts for electrochemical hydrogen production: Insights into the origin of their catalytic activity. ACS Catal. 2012. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, X.; Yang, X.; Liu, C.; Qian, X.; Sun, T.; Chang, W.; Zhang, J.; Chen, Z. Synthesis of novel 1t/2h- MoS2 from moo3 nanowires with enhanced photocatalytic performance. Nanomaterials 2020, 10, 1124. [Google Scholar] [CrossRef]
- Chang, K.; Pang, H.; Hai, X.; Zhao, G.; Zhang, H.; Shi, L.; Ichihara, F.; Ye, J. Ultra-small freestanding amorphous molybdenum sulfide colloidal nanodots for highly efficient photocatalytic hydrogen evolution reaction. Appl. Catal. B Environ. 2018. [Google Scholar] [CrossRef]
- Zhao, Q.; Guo, Y.; Zhou, Y.; Yan, X.; Xu, X. Solution-processable exfoliation and photoelectric properties of two-dimensional layered MoS2 photoelectrodes. J. Colloid Interface Sci. 2017. [Google Scholar] [CrossRef]
- Si, K.; Ma, J.; Guo, Y.; Zhou, Y.; Lu, C.; Xu, X.; Xu, X. Improving photoelectric performance of MoS2 photoelectrodes by annealing. Ceram. Int. 2018. [Google Scholar] [CrossRef]
- Meng, F.; Li, J.; Cushing, S.K.; Zhi, M.; Wu, N. Solar hydrogen generation by nanoscale p-n junction of p-type molybdenum disulfide/n-type nitrogen-doped reduced graphene oxide. J. Am. Chem. Soc. 2013. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, Y.; Zhang, L.; Li, H.; Liu, H.; Li, W. Transition metal-doped amorphous molybdenum sulfide/graphene ternary cocatalysts for excellent photocatalytic hydrogen evolution: Synergistic effect of transition metal and grapheme. J. Colloid Interface Sci. 2019. [Google Scholar] [CrossRef]
- Salarizadeh, P.; Askari, M.B.; Seifi, M.; Rozati, S.M. MoS2 coating on different carbonaceous materials: Comparison of electrochemical properties and hydrogen evolution reaction performance. J. Electroanal. Chem. 2019. [Google Scholar] [CrossRef]
- Shi, Y.; Zhou, W.; Lu, A.-Y.; Fang, W.; Lee, Y.-H.; Hsu, A.L.; Kim, S.M.; Kim, K.K.; Yang, H.Y.; Li, L.-J.; et al. Van der waals epitaxy of MoS2 layers using graphene as growth templates. Nano Lett. 2012. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Pan, L.; Qin, W.; Chen, T.; Sun, Z. MoS2-reduced graphene oxide composites synthesized via a microwave-assisted method for visible-light photocatalytic degradation of methylene blue. RSC Adv. 2014. [Google Scholar] [CrossRef]
- Song, H.; Tang, A.; Xu, G.; Liu, L.; Yin, M.; Pan, Y. One-step convenient hydrothermal synthesis of MoS2/RGO as a high-performance anode for sodium-ion batteries. Int. J. Electrochem. Sci. 2018. [Google Scholar] [CrossRef]
- Murugan, M.; Kumar, R.M.; Alsalme, A.; Alghamdi, A.; Jayavel, R. Synthesis and property studies of molybdenum disulfide modified reduced graphene oxide (MoS2-rGO) Nanocomposites for Supercapacitor Applications. J. Nanosci. Nanotechnol. 2017. [Google Scholar] [CrossRef]
- Kumar, S.; Sahoo, P.K.; Satpati, A.K. Electrochemical and SECM Investigation of MoS2/GO and MoS2/rGO Nanocomposite Materials for HER Electrocatalysis. ACS Omega 2017. [Google Scholar] [CrossRef] [PubMed]
- Wojtalik, M.; Bojarska, Z.; Makowski, Ł. Experimental studies on the chemical wet synthesis for obtaining high-quality MoS2 nanoparticles using impinging jet reactor. J. Solid State Chem. 2020. [Google Scholar] [CrossRef]
- Wojtalik, M.; Przybyt, P.; Zubanska, K.; Zuchowska, M.; Bojarska, Z.; Arasimowicz-Andres, M.; Rozen, A.; Makowski, L. Production of synthetic particles of MoS2 using wet chemical synthesis. Przem. Chem. 2019. [Google Scholar] [CrossRef]
- Wojtalik, M.; Orciuch, W.; Makowski, Ł. Nucleation and growth kinetics of MoS2 nanoparticles obtained by chemical wet synthesis in a jet reactor. Chem. Eng. Sci. 2020. [Google Scholar] [CrossRef]
- Wojtas, K.; Makowski, Ł.; Orciuch, W. Barium sulfate precipitation in jet reactors: Large eddy simulations, kinetics study and design considerations. Chem. Eng. Res. Des. 2020. [Google Scholar] [CrossRef]
- Makowski, Ł.; Bałdyga, J. Large Eddy Simulation of mixing effects on the course of parallel chemical reactions and comparison with k-e{open} modeling. Chem. Eng. Process. Process Intensif. 2011. [Google Scholar] [CrossRef]
- Wojtas, K.; Orciuch, W.; Wysocki, Ł.; Makowski, Ł. Modeling and experimental validation of subgrid scale scalar variance at high Schmidt numbers. Chem. Eng. Res. Des. 2017. [Google Scholar] [CrossRef]
- Makowski, Ł.; Orciuch, W.; Bałdyga, J. Large eddy simulations of mixing effects on the course of precipitation process. Chem. Eng. Sci. 2012. [Google Scholar] [CrossRef]
- Gavi, E.; Rivautella, L.; Marchisio, D.L.; Vanni, M.; Barresi, A.A.; Baldi, G. CFD modelling of nano-particle precipitation in confined impinging jet reactors. Chem. Eng. Res. Des. 2007. [Google Scholar] [CrossRef]
- Stobinski, L.; Lesiak, B.; Malolepszy, A.; Mazurkiewicz, M.; Mierzwa, B.; Zemek, J.; Jiricek, P.; Bieloshapka, I. Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J. Electron Spectrosc. Relat. Phenom. 2014. [Google Scholar] [CrossRef]
- Mazurkiewicz-Pawlicka, M.; Nowak, M.; Malolepszy, A.; Witowski, A.; Wasik, D.; Hu, Y.; Stobinski, L. Graphene oxide with controlled content of oxygen groups as a filler for polymer composites used for infrared radiation shielding. Nanomaterials 2020, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Longo, A.; Dzade, N.; Sharma, A.; Hendrix, M.M.R.M.; Bol, A.A.; de Leeuw, N.H.; Hensen, E.J.M.; Hofmann, J.P. The origin of high activity of amorphous MoS2 in the hydrogen evolution reaction. ChemSusChem 2019. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Yu, B.-B.; Yin, X.-L.; Jiang, W.-J.; Jiang, Y.; Hu, J.-S.; Wan, L.-J. Physical vapor deposition of amorphous MoS2 nanosheet arrays on carbon cloth for highly reproducible large-area electrocatalysts for the hydrogen evolution reaction. J. Mater. Chem. A 2015. [Google Scholar] [CrossRef]
- Greenwood, N.N.; Earnshaw, A. Chemistry of Elements. Cryst. Res. Technol. 1968. [Google Scholar] [CrossRef]
- Zou, L.; Qu, R.; Gao, H.; Guan, X.; Qi, X.; Liu, C.; Zhang, Z.; Lei, X. MoS2/RGO hybrids prepared by a hydrothermal route as a highly efficient catalytic for sonocatalytic degradation of methylene blue. Results Phys. 2019. [Google Scholar] [CrossRef]
- Akbarzadeh, E.; Setayesh, S.R.; Gholami, M.R. Investigating the role of MoS2/reduced graphene oxide as cocatalyst on Cu2O activity in catalytic and photocatalytic reactions. New J. Chem. 2017. [Google Scholar] [CrossRef]
- Bojarska, Z.; Mazurkiewicz-Pawlicka, M.; Makowski, Ł. Graphene oxide-based nanomaterials as catalysts for oxygen reduction reaction. Chem. Process. Eng. Inz. Chem. Proces. 2019. [Google Scholar] [CrossRef]
- Vinoth, R.; Patil, I.M.; Pandikumar, A.; Kakade, B.A.; Huang, N.M.; Dionysios, D.D.; Neppolian, B. Synergistically enhanced electrocatalytic performance of an n-doped graphene quantum dot-decorated 3d MoS2-graphene nanohybrid for oxygen reduction reaction. ACS Omega 2016. [Google Scholar] [CrossRef]


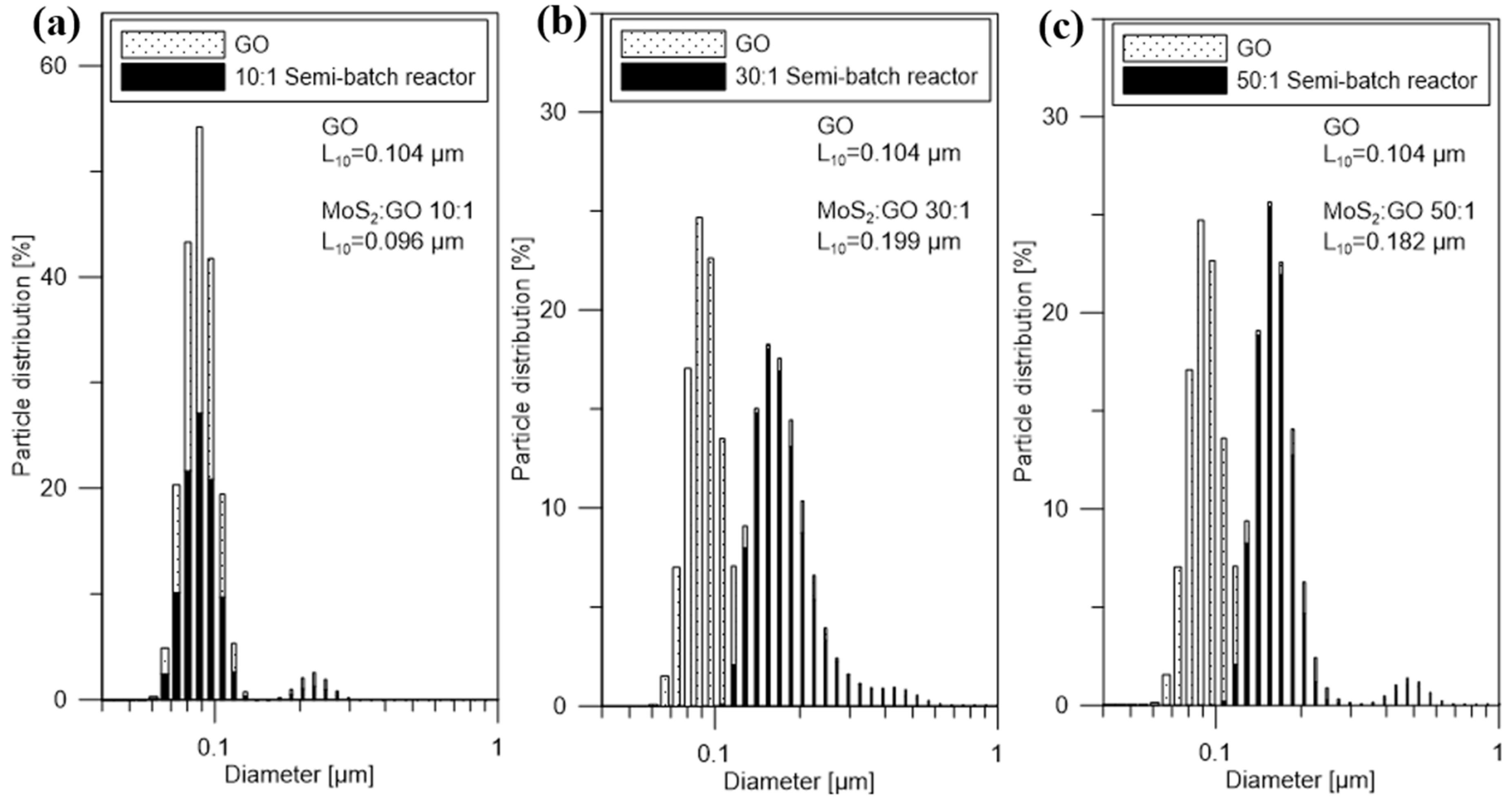

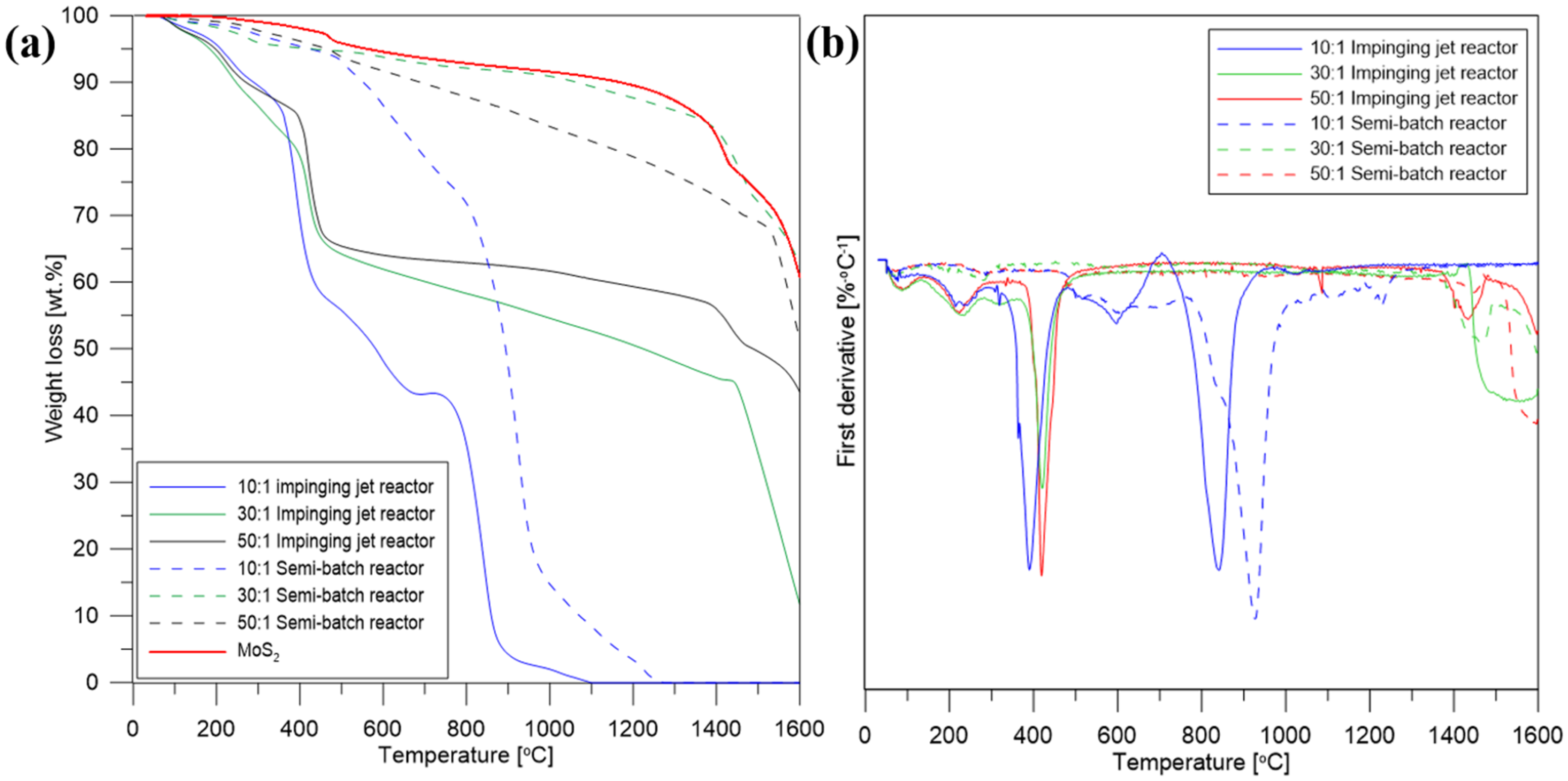
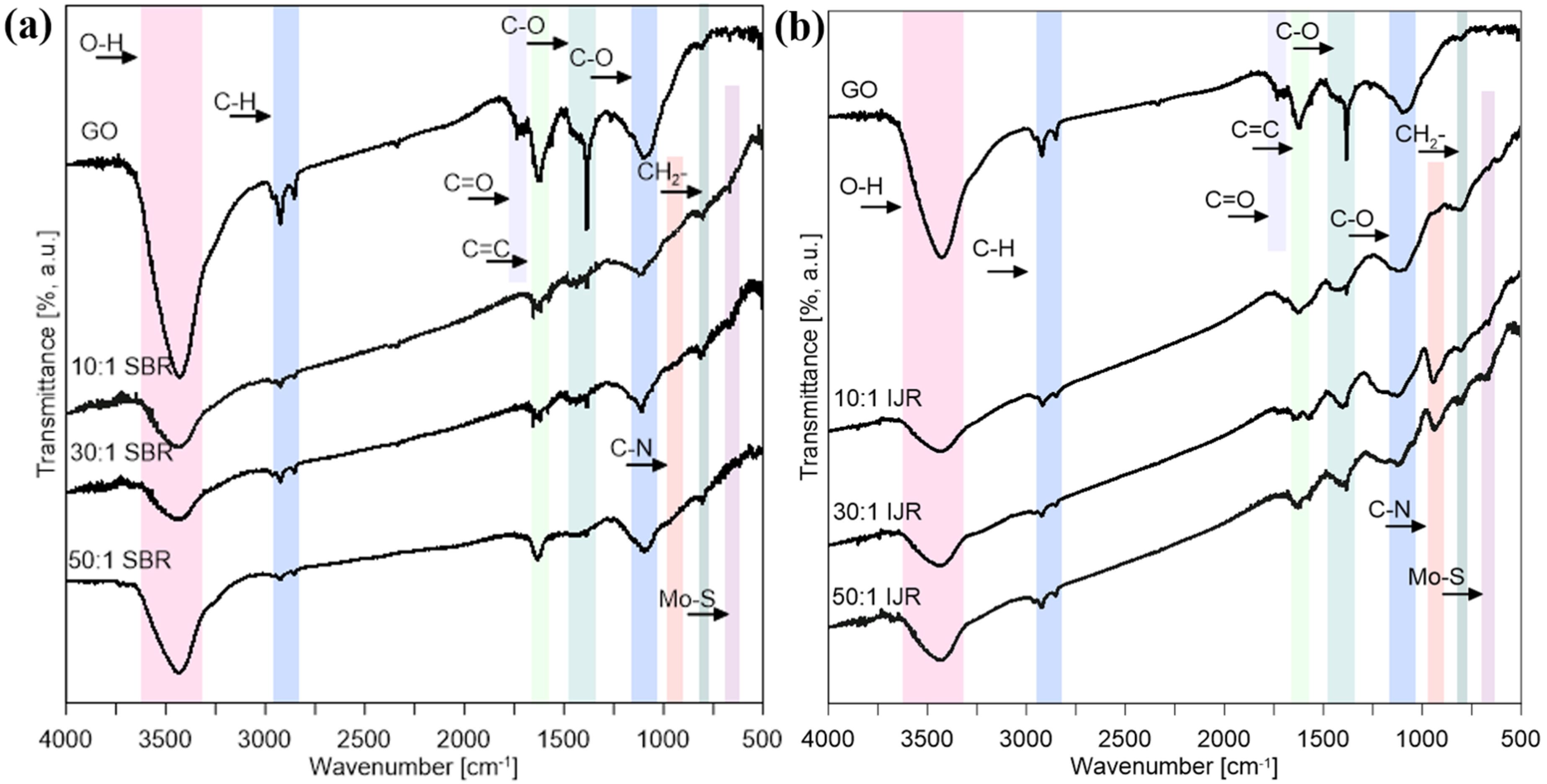





| MoS2/GO Ratio | 10:1 | 30:1 | 50:1 |
|---|---|---|---|
| Volume of reagents’ solution [mL] | 20 | ||
| 20 wt.% AS mass [mL] | 2.734 | ||
| HMA mass [g] | 0.706 | ||
| CA mass [g] | 1.537 | ||
| 0.34 wt.% GO mass [g] | 12.556 | 4.186 | 2.512 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bojarska, Z.; Mazurkiewicz-Pawlicka, M.; Gierlotka, S.; Makowski, Ł. Production and Properties of Molybdenum Disulfide/Graphene Oxide Hybrid Nanostructures for Catalytic Applications. Nanomaterials 2020, 10, 1865. https://doi.org/10.3390/nano10091865
Bojarska Z, Mazurkiewicz-Pawlicka M, Gierlotka S, Makowski Ł. Production and Properties of Molybdenum Disulfide/Graphene Oxide Hybrid Nanostructures for Catalytic Applications. Nanomaterials. 2020; 10(9):1865. https://doi.org/10.3390/nano10091865
Chicago/Turabian StyleBojarska, Zuzanna, Marta Mazurkiewicz-Pawlicka, Stanisław Gierlotka, and Łukasz Makowski. 2020. "Production and Properties of Molybdenum Disulfide/Graphene Oxide Hybrid Nanostructures for Catalytic Applications" Nanomaterials 10, no. 9: 1865. https://doi.org/10.3390/nano10091865
APA StyleBojarska, Z., Mazurkiewicz-Pawlicka, M., Gierlotka, S., & Makowski, Ł. (2020). Production and Properties of Molybdenum Disulfide/Graphene Oxide Hybrid Nanostructures for Catalytic Applications. Nanomaterials, 10(9), 1865. https://doi.org/10.3390/nano10091865





