3.1. Physicochemical Properties
The X-ray diffraction pattern acquired at room temperature and presented in
Figure 1, confirms the amorphous state of the investigated material.
Some important physicochemical properties of Te-5 glass are presented in
Table 1, such as density (
ρglass), average molecular mass (
Mav), molar volume (
VM), oxygen packing density (
OPD), refractive index measured at 589 nm (
nD), refractive index measured from the dispersion graph (
nDD), molar refractivity (
Rm), electronic polarizability (
αm), reflection loss (
RL) and optical transmission (
T).
The density of glass,
ρglass, is an important physical property that estimates the compactness of the structure of a glassy system. It depends on the glass composition and it is mainly influenced by the glass network structure, preparation conditions and thermal history. The higher is the average molecular mass,
Mav, that is, the higher the molecular weights of the components are, the higher the density of the glass is. The density of Te-5 glass is lower by comparison with other high TeO
2 content glasses (over 60 mol. %) [
14,
16,
18].
In the case of the vitreous systems, the molar volume,
VM, reveals the network structure and the arrangement of the component units as it is directly correlated with the 3D structure/disposal of the oxygen atoms. Generally, the molar volume of the glasses increases due to the increase of the bond length or interatomic arrangement of metal ions/anions. Due to a lower density, the molar volume of the Te-5 glass is higher than that of recently investigated high TeO
2 content glasses [
15,
16,
17,
18], showing a less compact vitreous network.
Oxygen packing density,
OPD, may be associated with the number of oxygen atoms in a glass chemical composition unit (
C) and a glass volume unit. It is calculated based on the equation [
15,
17]:
where
C represents the number of oxygen atoms per glass mole and
VM is the molar volume. Thus, the oxide glass composition and the molar volume are decisive for the
OPD value. In the case of Te-5 glass,
OPD was relatively close to the values characteristic for the high TeO
2 content glasses [
15,
17].
The refractive index of glass,
nD, was influenced by the interaction of light with the electrons of the component atoms of the glass. The refractive index is an important feature of the optical glasses being closely connected with the electronic polarization of the ions and the local field inside the glass, mainly in connection with the electronic structure of the glasses. The non-bridging oxygen atoms are connected by cations (vitreous network modifiers) through ionic bonds whereas bridging oxygen atoms are connected by two phosphorous atoms (vitreous network formers) through covalent bonds. The ionic bonding results in a high polarizability of the non-bridging oxygen atoms (deformation of the outer electron shell in ligand fields) as compared to covalent bonding of the bridging oxygen atoms. Consequently, the decrease in the number of the non-bridging oxygen atoms determines the decrease of the refractive index value. In the present study, Te-5 glass, due to a low TeO
2 content, exhibits a refractive index value that is considerably lower than that of other high TeO
2 content glasses [
14,
15,
16,
17]. This can be explained by a decreased number of high polarizability Te atoms in the explored glass.
The dielectric constant (relative permeability),
ε, is a parameter that characterizes the electrical polarization state of the glass and is calculated using the following equation, under the implicit assumption of a relative magnetic permeability of 1 [
15,
16]:
In the case of Te-5 glass,
ε = 2.39, being lower than that of other high TeO
2 content glasses [
15,
16]. This is due to a reduced number of high polarizability Te atoms from the nanoclusters, these ones being responsible for the relative reduced electrical insulator character of the glass.
The molar refractivity (refraction) is the extent of the total polarizability per unit mole of glass. The Lorentz–Lorenz equation is used to correlate the molar refractivity,
Rm, with the refraction index,
n, and molar volume,
VM [
14,
15,
16,
17]. Actually, the molar refractivity provides the average molar refraction for substances that are isotropic in nature, such as glasses. The molar refractivity can also be calculated with dependence on the electronic polarizability,
αm, of a molecule, which measures the suitability of electrons to respond to an electric field [
14,
15]. The non-bridging oxygen atoms have a high tendency to polarize compared to bridging oxygen atoms. So, the higher the number of non-bridging oxygen atoms in the glass network is, the higher the molar refraction and electronic polarizability are. In the case of Te-5 glass, the molar refractivity and electronic polarizability have about the same values by comparison with the glasses containing about 60 mol. % TeO
2 [
16,
17] but lower values by comparison with other tellurite glasses containing more than 70 mol. % TeO
2 [
14,
15]. Thus, the investigated glass tends to be less polarized when an electric field is applied, comparatively to glasses containing more than 70% TeO
2.
Reflection loss,
RL, and optical transmission,
T, were calculated based on the refractive index,
nD, and for the Te-5 glass samples,
RL and optical transmission,
T show lower and higher values, respectively, by comparison with recently investigated tellurite glasses [
15,
16].
3.2. Optical Properties and Related Electron Structure Parameters
The absorption spectrum of Te-5 glass in the UV-Vis-NIR domain is presented in
Figure 2a. Two absorption bands at 420 and 532 nm were observed, specific to the electronic transitions of the Te
2 diatomic molecules that were formed as a result of TeO
2 decomposition at the melting temperature.
At 1100 °C, the decomposition of TeO
2 in metallic Te atoms takes place with the enhanced probability of giving rise to oxygen vacancies in the glass. As a result, Te atoms link to each other, resulting in Te
2 diatomic molecules that are responsible for absorption and luminescence characteristics. The absorption band from 432 nm is assigned to the
3Σ
g →
3Σ
u transition whereas the strong 532 nm absorption band is due to colloidal metallic Te [
15,
16,
17,
18,
19,
20,
21,
22]. Thus, according to these data, the final product is a composite material comprising of a non-crystalline vitreous phase and Te
2 nanoclusters, the latter giving the dark reddish color of Te-5 glass (see inset from
Figure 2a). Taking into consideration that diatomic tellurium molecule-based nanoclusters exhibit a green absorption range (see
Figure 2a), the complementary transmitted light is noticed in the red domain of the visible spectrum.
In
Figure 2b, the graphical determination of
Eg for Te-5 glass sample is presented [
14,
15,
16,
17]. The Mott and Davis/Tauc equation was applied to graphically determine the optical band gap,
Eg (
Figure 2b). Thus, the equation [
14,
15,
16,
17]:
allows one to determine the band gap,
Eg, where
α is the absorption coefficient,
h is the Planck constant,
ν is the light frequency and
n can be ½ for the allowed direct electron transitions and 2 for the allowed indirect electron transitions from the valence to the conduction band [
23]. For amorphous materials, in the case of Te-5 glass,
n takes the value 2. The optical band gap,
Eg value, was obtained by extrapolating the linear region of the curve to the zero absorption at which,
, and the result was
Eg = 3.18 eV (see
Table 2). The wavelength corresponding to the band gap value was
λg = 390 nm, which was different from the cut-off wavelength deduced from
Figure 2a, which was
λcutt-off = 346 nm.
In order to verify the validity of the graphical method to determine
Eg and, respectively,
λg, the absorption spectrum fitting (ASF) method was applied [
15,
16,
17] by means of which,
was determined and it was compared to the
Eg value determined by Mott and Davis/Tauc law
. Thus, the Tauc law is written as Equation (4) [
15,
16,
17]:
where,
α is the absorption coefficient,
h is the Planck constant and
c is the light speed in vacuum equal to 3 × 10
8 m/s,
λ is the wavelength and
Eg is the band gap energy
. Further on, it is graphically represented as
(
Figure 3a). The result is a curve and the tangent to the linear region of the curve will intersect the
x-axis in a point representing
1/λg(ASF), resulting in
λg(ASF). This value is compared to that determined from Tauc law. In our case,
1/λg(ASF) = 0.00259 and
λg(ASF) = 386 nm, which is relatively close to that determined from Tauc law,
λg = 390 nm. The Equation (5) [
15,
16,
17]:
is applied to calculate
, which, in our case is3.21 eV and is relatively close to 3.18 eV that is graphically determined by the Tauc law.
Generally, for amorphous materials, the allowed indirect electron transitions according to the Tauc equation are valid. In the case of optical absorption, for low photon energy ranged between 10
2 and 10
4 cm
−1, the absorption coefficient follows Urbach’s law. This is the width of the band tails of the localized states from the valence band. Urbach’s law is depicted by the following relationship (6) [
14,
16,
24]:
where,
α(ν) is the absorption coefficient dependent on the frequency,
hν is the energy and Δ
E is the Urbach energy. If the logarithm of Urbach’s relationship is applied, the following Equation (7) is obtained [
14,
16,
24]:
Hence, it is possible to deduce the ΔE value.
In the case of Te-5 glass, Urbach energy, Δ
E, was 0.390 eV (
Figure 3b). Urbach energy is a measure of the disorder degree of the vitreous materials and is correlated with an extension of the localized states within the band gap. Accordingly, the disorder degree of the investigated glass is comparable with that of high TeO
2- containing glasses [
14,
16].
The refractive index dependence on wavelength (optical dispersion) is presented in
Figure 4. It can be observed that the refractive index value measured at 589 nm (
nD, the yellow line of sodium) was about 1.550, close to 1.546, measured value by the Pulfrich refractometer, thus confirming the accuracy of those two measurement methods.
One should note that the measurement modes of the refractive index and optical absorption coefficient are different. The optical absorption is derived from conventional transmission data, in this case, the probing light interacting with the sample volume. On the other hand, the refractive index is inferred from the ellipsometry data, which, in this case, is giving information only from the sample surface and the raw data do not contain signatures of the absorption bands discussed above. Therefore, the presented optical properties are not Kramers–Kronig correlated.
Table 2, besides the
Eg value that has already been discussed, presents other optical properties of Te-5 glass such as refractive index-based oxide ion polarizability,
band gap-based oxide ion polarizability,
refractive index-based metallization criterion,
M(n), band gap-based-metallization criterion,
M(Eg), Duffy and Ingram theoretical optical basicity,
Λth, refractive index-based polarizability-based optical basicity,
Λ(, band gap-based polarizability-based optical basicity, Λ(
and Pauling optical basicity, Λ
P [
14,
15,
16,
17].
Dimitrov and Komatsu calculated the oxide ion polarizability,
, optical basicity,
Λ, and metallization criterion,
M, on the basis of two different properties: energy band gap,
Eg, and linear refractive index,
n. Thus, the calculation of the refractive index-based oxide ion polarizability,
is based on the molar refractivity,
Rm, total electronic polarizability of the compounds from the glass,
and the number of oxygen atoms from each oxide,
, reported in [
14,
17].
The estimation of the energy band gap-based oxide ion polarizability,
is based on the molar volume,
VM, band gap,
Eg, the total polarizability of cations,
and the number of oxygen atoms in the molar oxide glass composition,
, reported in [
14,
17]. In the case of Te-5 glass,
, and, based on the measured
nD value (see
Table 1), it was found
Refractive index-based oxide ion polarizability and energy band gap-based oxide ion polarizability values, in the case of Te-5 glass, are lower by comparison with high content TeO
2 glasses [
12,
15] (see
Table 2), due to the low tellurium oxide content from the vitreous material.
According to the metallization theory in condensed matter by Herzfeld [
25], the metallization parameter,
M, is theoretically calculated to estimate the tendency for metallization and to investigate the insulating behavior of a sample. This theory infers that the refractive index becomes infinite under the condition that
Rm/VM = 1, which is correlated with a covalent bonding. This ratio is related to the degree of electron localization, showing the tendency of a material to become metallic in nature [
14,
15,
16]. The case of
Rm/VM > 1 means a metallic nature (delocalized electrons) whereas
Rm/VM < 1 means a non-metallic nature (localized electrons). The large value of the metallization parameter
M, defined as
1- Rm/VM demonstrates a high energy band gap and insulating nature in the synthesized glasses, which is confirmed by the optical band gap energy,
Eg. A larger
M value indicates that the widths of both valence and conduction bands decrease, which results in a wider band gap [
16,
26].
Metallization criterion,
M, is calculated based on the relationship that uses
Rm and
Vm and also based on the refractive index,
n and optical band gap,
Eg, respectively [
14,
15,
16]. In the case of Te-5 glass, metallization criterion,
M(
Rm/Vm) =
M(n) = 0.683 and
M(Eg) = 0.398, demonstrating a relative significant insulator behavior of the vitreous material, by comparison with high TeO
2 content glasses, which demonstrated a more conductive character [
14,
15,
16].
The understanding of glass optical basicity could be beneficial for the fabrication of novel useful materials with better optical performances. The degree of acidity or basicity of glass is related to the electron donor power of oxygen atom and it is influenced especially by the glass network formers. High optical basicity means high electron donor ability of the oxide ions to the cations [
16]. The optical basicity,
Λ, that is strongly influenced by the electronic polarizability,
αm, has been evidenced to be a determinant and a principal parameter for predicting the properties of a glass system before using the glass in various applications [
27].
The relationship proposed by Duffy and Ingram for the theoretical value of optical basicity of multi-component glasses is applied to obtain energy band gap based-optical basicity and refractive index based-optical basicity. Duffy and Ingram proposed a relationship to determine the theoretical optical basicity,
Λth, for multi-component glasses based on the optical basicity of each oxide component multiplied by the equivalent fraction of each component [
14]. Duffy has established an alternative approach of refractive index-based polarizability-based optical basicity, Λ(
and band gap-based polarizability-based optical basicity,
Λ( [
14]. Other equations are used to determine optical basicity based on elemental Pauling electronegativity [
16,
17]. In the case of Te-5 glass,
Λth = 0.702,
Λ( = Λ( = 1.13 and Λ
P = 0.4617. It is evident that the theoretical optical basicity value in the case of the Te-5 glass was close to the basicity calculated based on the oxide polarizability dependent on the refractive index and, respectively, on the energy band gap. It means that, in our case, the calculation models proposed by Duffy and Ingram and, respectively, Duffy, are valid and match Te-5 glass composition. Taking into consideration the optical basicity values, it is worth claiming that the Te-5 glass has an intermediate basicity value. Glass optical basicity is related to the electron donor power of oxygen atoms. The higher electron donor power of oxygen atoms means a reduced degree of covalence cation-oxygen that is an increase in the ionic character of the bond resulting in a reduced tendency to form the vitreous structure. The basicity of Te-5 glass is mainly influenced by ZnO (amphoteric character) and P
2O
5 (acidic character), both of them being in a high amount by comparison with Al
2O
3 and TeO
2. It can be concluded that the present glass composition has an intermediate tendency to form the vitreous network.
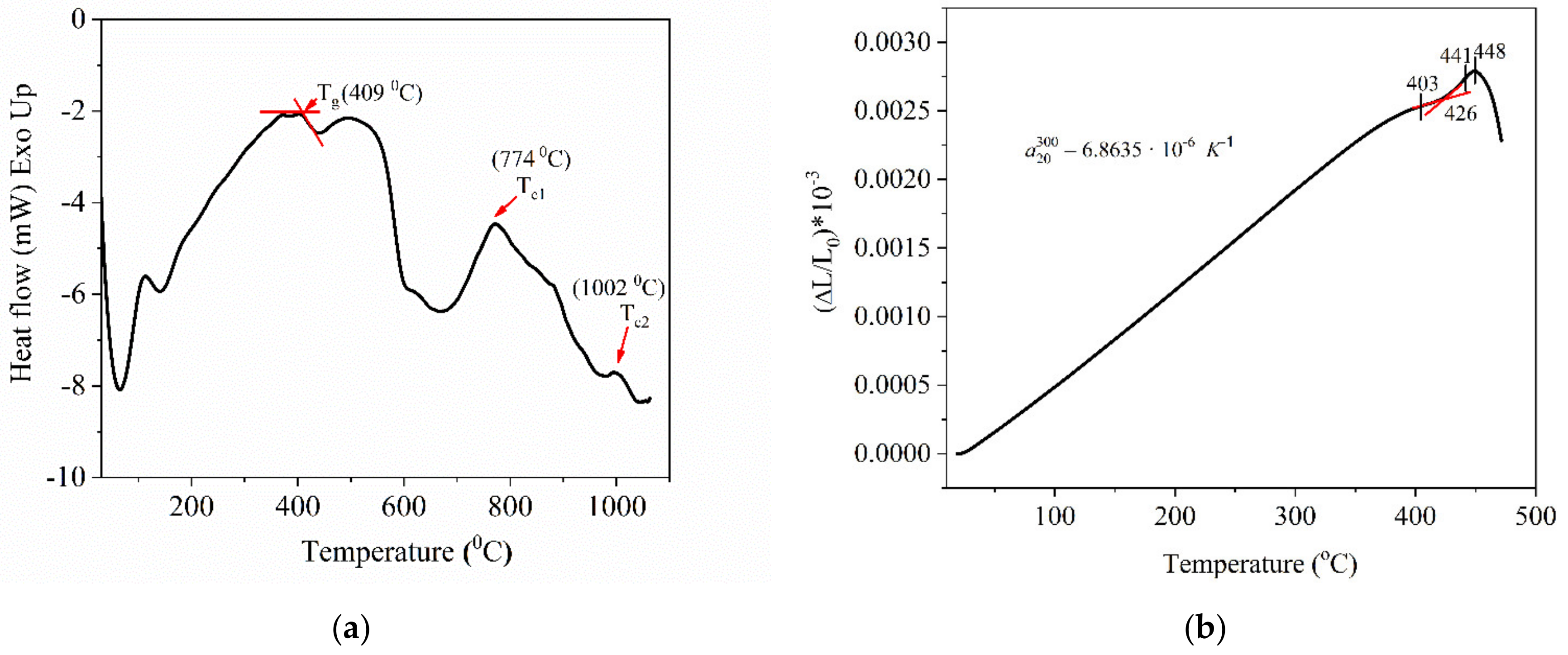
3.4. Structural Analysis
In
Figure 6, the FTIR absorption spectrum of Te-5 glass is presented in the range 600–1400 cm
−1. Absorption peaks are noticed, being specific to Te-O and P-O bonds from the phosphate network. Thus, the absorption maximum at 605 cm
−1 was assigned to the Te-O symmetrical stretching vibration mode from TeO
4, overlapping the P-O-P (bridging oxygen atoms, BOs) bending vibration mode [
16,
17,
29,
30,
31,
32,
33,
34]. The band from 665 cm
−1 is assigned to Te-O symmetrical stretching from TeO
3/TeO
4 [
16,
17,
30] and P-O-P symmetrical stretching for long chains in Q
2 units [
31,
32,
33]. The peaks found at 727 cm
−1 and 784 cm
−1, resulted by the deconvolution of a large envelope, are assigned to P-O-P symmetric stretching mode in between Q
1 and Q
2 units and, respectively, to the P-O-P symmetric stretching mode in Q
1 units [
31,
32,
33,
34]/Te-O symmetrical stretching vibration mode from TeO
3 [
16].
The peak from 934 cm
−1 is allotted to the P-O-P asymmetrical vibration mode [
29,
32,
33]/symmetric stretching of O-P-O units, NBOs (non-bridging oxygen atoms) in Q
0 tetrahedra [
30] and/or to the symmetrical stretching mode of (PO
4)
3− [
31,
34]. The peak from 1015 cm
−1 is assigned to the (PO
3)
− asymmetrical stretching mode in Q
1 tetrahedra [
29,
30]/(PO
4)
3− asymmetrical stretching mode [
32,
33,
34] and/or to symmetrical stretching mode of (P
2O
7)
4− [
31], the maximum at 1128 cm
−1 is ascribed to the O-P-O symmetrical stretching mode [
30,
31,
32,
33,
34] and that from 1208 cm
−1 is allotted to the O-P-O asymmetrical stretching mode in Q
2 tetrahedra [
29,
31,
32,
33,
34]. ZnO shows an absorption band from ZnO
4 at 300 cm
−1 that does not appear in the spectrum, being placed out of the spectrophotometer measurement range [
16].
Figure 7 displays the Raman spectrum of the Te-5 glass in the range 125-1500 cm
−1, recorded by 514 nm laser excitation [
18]. It is worth mentioning the specific peak at 214 cm
−1 assigned to the vibration modes of Te
2 -based nanoclusters [
35].
The bands that appeared at 283 cm
−1, 320 cm
−1 and 529 cm
−1 are attributed to Te-O-Te bending and symmetrical stretching modes from TeO
4, TeO
3+1 and TeO
3 polyhedral [
16,
17,
29], (P
2O
7)
4− [
30], the P-O-P bending vibration mode [
32,
33,
34], the bending O-P-O vibration mode [
34] and the Zn-O vibration mode from ZnO
4 tetrahedra [
16]. The peaks from 351 cm
−1, 424 cm
−1 and 489 cm
−1 are ascribed to symmetric bending vibration of TeO
4 units [
16], stretching and bending of Te-O-Te bonds in TeO
4, TeO
3+1 and TeO
3 units [
16,
17], Zn-O from ZnO
4 units [
16], harmonics of the P-O-P bending vibrations mode [
33,
34] and the O-P-O bending vibration mode [
34], respectively. The band observed at 628 cm
−1 is allotted to the vibration modes of the TeO
4 continuous network [
16,
29] and the TeO
3+1, TeO
3 [
17] and P-O-P bending vibration mode [
34]. The maxima from 714 cm
−1 and 751 cm
−1 are due to symmetrical stretching of P-O-P groups in Q
2 and Q
1 structural units, respectively [
29,
30,
31,
32,
33,
34], overlapping the stretching vibration mode of non-bridged Te-O in TeO
3 and TeO
3+1 [
16,
17,
29]. The peak from 855 cm
−1 is assigned to the stretching vibration modes of TeO
3 [
16,
17,
29]. The peak from 941 cm
−1 is ascribed to the P-O-P asymmetric stretching vibration mode [
34]. Finally, two superposed spectral components are assigned as follows: (i) the most intense one at 1192 cm
−1 corresponds to the asymmetric stretching vibrations of PO
2 groups (Q
2 structural units) [
31], overlapping the (PO
3)
2− asymmetrical vibration mode [
32,
33] and to the symmetrical stretching mode of TeO
3 [
16] and (ii) the one at 1281cm
−1 corresponds to the asymmetrical stretching modes of PO
2 groups in Q
2 phosphate tetrahedral [
30,
31] and to the possible P=O stretching vibration mode for long phosphate chains [
32,
33].
3.5. Magnetic and Magneto-Optical Properties
The dependence of the magnetization versus the applied field at two different temperatures is shown in
Figure 8a. It is observed in both cases that after an initial increment of the magnetization at lower fields of up to about 5000 Oe, the specific diamagnetic behavior consisting of the linear decrease of the magnetization was evidenced at higher fields. The negative slope was clearly lower in absolute value at 10 K, giving an indirect indication for an additional paramagnetic contribution of positive slope, which compensates partially for the decrease of magnetization at this temperature. The direct indication for the paramagnetic contribution can be observed from the strong increase of the magnetization at temperatures lower than 20 K (see inset of
Figure 8a) resembling closely to the typical paramagnetic
C/T type dependence with
C the Curie constant and
T the temperature [
36]. The diamagnetic susceptibility of the Te-5 glass can be calculated from the slope of the linear decrease of the magnetization at 300 K, where the paramagnetic contribution was negligible. A value of −72(2) × 10
−6 emu/(mol·Oe) = −81(2) × 10
−10 m
3/kg of the diamagnetic susceptibility
Χdia was calculated from the slope of the linear decrease of magnetization at 300 K in
Figure 8a. Considering also the slope of the linear decrease of the magnetization at 10 K of only -0.22(2) × 10
−6 emu/(mol·Oe) from the same figure, a paramagnetic contribution
Χpara = +50(2) × 10
−6 emu/(mol·Oe) = +56(2) × 10
−10 m
3/kg was straightforwardly deduced. Accordingly, the equation
Χpara =
C/T with
T=10 K provides a value of 5.0(2) × 10
−4 (emu·K)/(mol·Oe) for the Curie constant. The later one can be expressed as
C =
NA*μ2/3kB, where
kB is the Boltzmann constant,
μ is the magnetic moment of the magnetic center and
NA is the Avogadro number [
36]. A value of 2.6(1) × 10
−20 emu or equivalently of 2.7 µ
B (µB is the Bohr magneton) was obtained for the paramagnetic moment per each glass molecule (formula unit). On the other hand, the initial increase of the magnetization at lower fields, with a much higher positive slope than the paramagnetic one, had to be related to a ferromagnetic signal, which might be fully investigated via the hysteresis loops taken at various temperatures. Indeed, by cycling the magnetic field, the magnetization always opens finite loops, even at 300 K, as observed from
Figure 8b,c. Such ferromagnetic signals persisting up to room temperature in materials that do not contain magnetic elements (as the present glass is) has to be related to the so called diluted magnetic oxide behavior being presently related mainly to oxygen vacancies [
36,
37,
38]. One of the most reliable models for the long-range ferromagnetism in such oxides is the model of the magnetic polarons, which consists of compensating electronic charge distributed over nm range volumes around a specific type of oxygen vacancies [
37]. While the magnetic centers can be always related to the oxygen defects around transition metals, only a part of such magnetic centers remain in the exchange interaction as overlapping polarons, leading to the long-range ferromagnetic signal. The rest of the non-interacting magnetic centers gave rise to the paramagnetic signal, which had significant contribution to only the magnetization (and not to the coercivity) only at lower temperatures. The paramagnetic contribution superposed over the diamagnetic one is the already discussed reason for collecting less rotated magnetic hysteresis loops at low temperatures. By removing the two contributions, saturated loops were obtained with saturation magnetizations decreasing from 0.98(2) emu/mol at 10 K to 0.75(3) emu/mol at 300 K. While the saturation magnetization at 10 K represents the spontaneous magnetic moments of
NA glass molecules, each glass molecule has to contribute to the spontaneous ferromagnetic signal with a magnetic moment of 1.8 × 10
−4 µ
B. However, the ferromagnetic contribution to the magnetization of the Te-5 glass was almost four orders of magnitude lower than of the paramagnetic one, the last one becoming however negligible over the diamagnetic contribution at 300 K mainly in high applied magnetic fields. To note the ferromagnetically coupled magnetic moments can rotate much easier under the magnetic field excitation as compared to the paramagnetic moments and, therefore, under low magnetic fields (e.g., lower than 2,000 Oe) the overall susceptibility was positive and mainly due to the ferromagnetic component. Therefore, of interest for the magnetic response of such a glass under low magnetic fields is the full magnetization reversal process, which was also characterized by the associated coercive field. At a glance of
Figure 8b, it can be observed the complex nature of the hysteresis loops at low temperatures, consisting mainly of two components. The most intense one had an impressive coercive field of about 10,000 Oe, decreasing very slightly with the temperature up to 50 K and much faster above 150 K, which has never been reported up to now in any glassy structure.
The second component had a lower contribution and was characterized by a coercive field, which decreased slightly from hundreds of Oe at 10 K to tenths of Oe at 300 K. This is often reported for diluted magnetic oxides [
36,
37] and even in Te based glasses [
38]. To note that an increased coercivity at low temperature can be associated to size dispersed magnetic clusters in the compound, either if they are due to clustering magnetic defects related to oxygen vacancies or to clustering magnetic elements [
36]. In the present case, the only sources of interacting magnetic defects are the oxygen vacancies (possible transition metal magnetic impurities can contribute to only the paramagnetic signal) and most probably they are directly related to the formation of the Te
2 molecules under different clustering degrees. Such metallic-like Te-based colloids may consist of a source of oxygen vacancies around the Te centers, giving rise in turn to nanoclusters of magnetic defects, which can be either isolated (with superparamagnetic behavior at room temperature but of high coercive field at low temperatures) or in magnetic interaction, providing the weak ferromagnetic signal that persists up to room temperature. Given this specific magnetic behavior of the Te-5 glass, the associated magneto-optical behavior will be further investigated.
The Faraday angle (rotation of the polarization plane under an applied field of induction B) was calculated using Equation (8) [
9]:
where,
is the ellipsometric angle measured with the applied magnetic field (+0.2 T) and
is the ellipsometric angle without an applied magnetic field. Faraday ellipticity would be the difference between ellipsometric parameters Δ (the phase difference), recorded with and without the magnetic field. One should note that no difference between the recorded Δ
(B+) and Δ
(B=0) was observed within the equipment and measurement (probing with linear polarized light) accuracy, inferring that the Faraday ellipticity was absent or negligible. It is worth noticing that in the present case of linearly polarized light (for both incoming and transmitted radiations, Δ = 0°), the ellipsometric parameter
was equal with the azimuthal angle,
, and thus the Faraday rotation was given by Equation (8).
In
Figure 9a the dispersion of the Faraday rotation angle,
θF, on the wavelength under the magnetic induction
B = 0.2 T for the Te-5 glass sample is presented. One should note the decrease of the rotation angle,
θF, with increasing wavelength, and that the Faraday rotation angle was not affected by the light absorption of the glass in the 450–600 nm range. The reference values were 0.43° at 400 nm and 0.15° at 650 nm.
The Faraday rotation angle,
θF, is directly proportional with the applied magnetic field,
B, the geometrical pathway of the light through the glass (e.g., the thickness of the glass),
d, and a proportional constant,
V, named Verdet constant that is a characteristic for each material. Verdet constant was calculated using Equation (9) [
9,
10]:
Figure 9b presents the wavelength dependence of the Verdet constant as obtained from the Faraday rotation angle values expressed above and via Equation (1) (with
B = 0.2 T and
d = 0.275 cm). From
Figure 9b a decrease of the Verdet constant with wavelength is noticed, the reference values being 0.047 min·Oe
−1·cm
−1 at 400 nm and 0.016 min·Oe
−·cm
−1 at 650 nm. Verdet constant values were consistent with the low TeO
2 content of the investigated glass.
By comparison with alumina-phosphate glasses containing a high amount of Bi
2O
3 and PbO, reported by the authors in a previous work related to diamagnetic materials [
19], Te-5 glass had twice lower Verdet constant values, measured at 400 nm and 600 nm reference wavelength, due to a reduced amount of TeO
2 component. It is worth noticing that the Verdet constant of the Te-5 diamagnetic glass was about 5 rad/(T·m) at 650 nm, being one order of magnitude lower than Verdet constant values of currently applied paramagnetic Faraday glasses [
39], containing most probably rare-earth ions. These currently applied Faraday glasses were paramagnetic/ferromagnetic materials characterized by a positive susceptibility, usually higher than the negative susceptibility of a diamagnetic material. Hence, the Faraday rotation angle and Verdet constant were higher than those of the diamagnetic Faraday glasses. However, the authors considered that the study of the present phosphate-tellurite glass was justified taking into consideration the low cost of heavy ions-doped glasses compared to rare-earth-doped glasses. Future works will be focused on the investigation of magnetic and magneto-optical properties of high TeO
2 content glasses as reported in [
1].