Metal Organic Framework Derived MnO2-Carbon Nanotubes for Efficient Oxygen Reduction Reaction and Arsenic Removal from Contaminated Water
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Methods
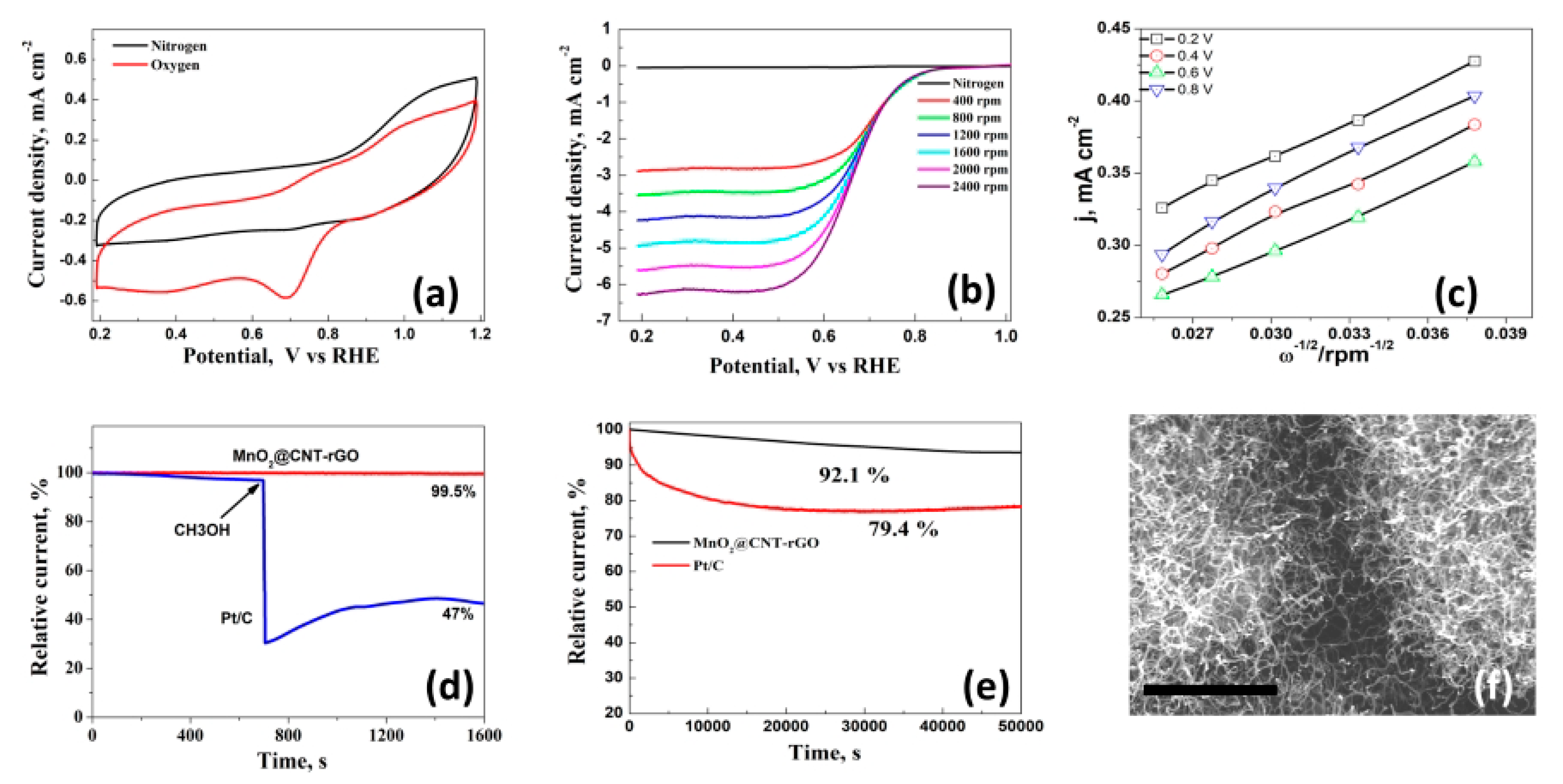
2.2. Electrochemical Measurements
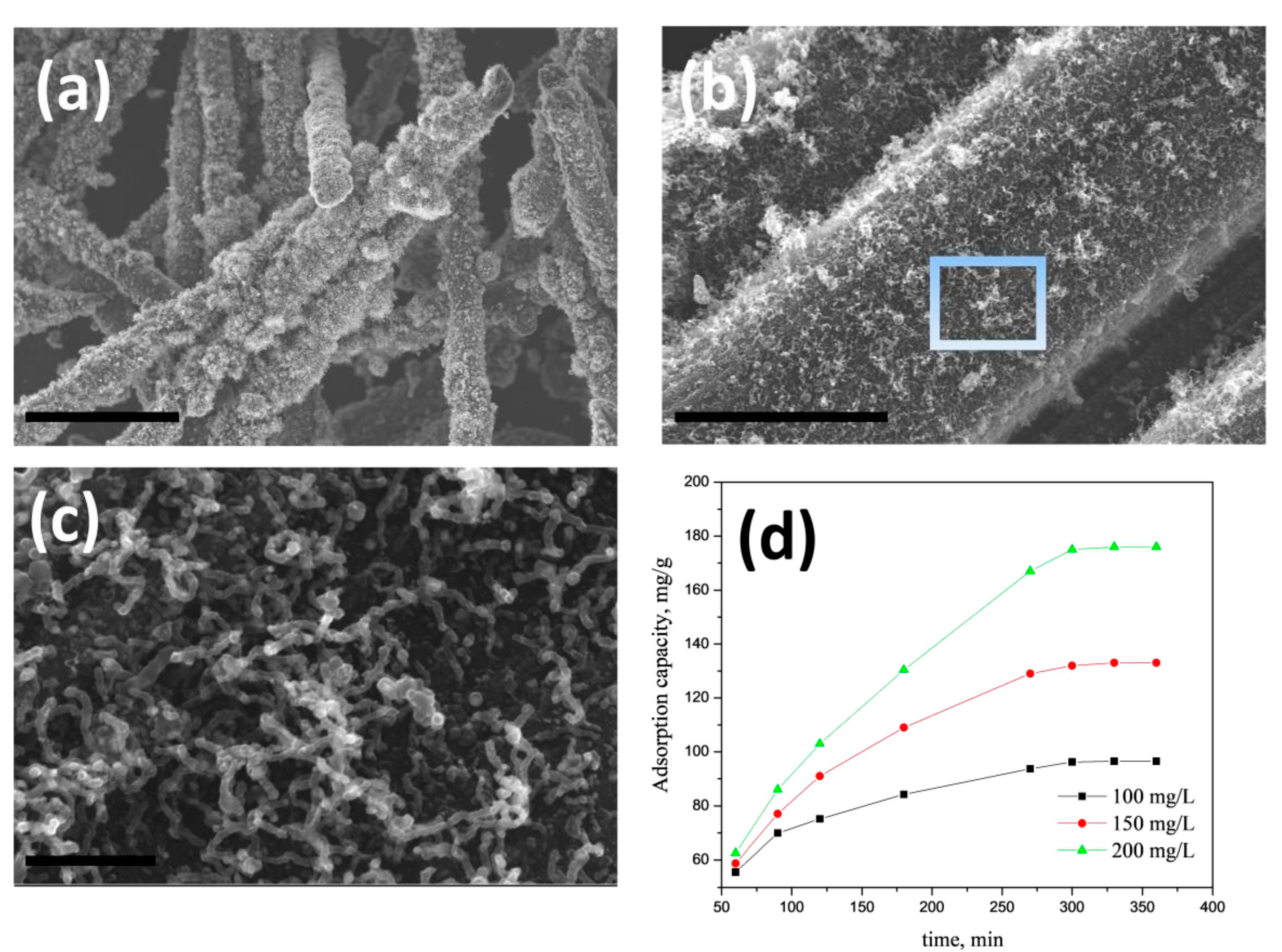
2.3. Arsenic Removal Tests
2.4. Synthesis of MnO2@CNT-rGO
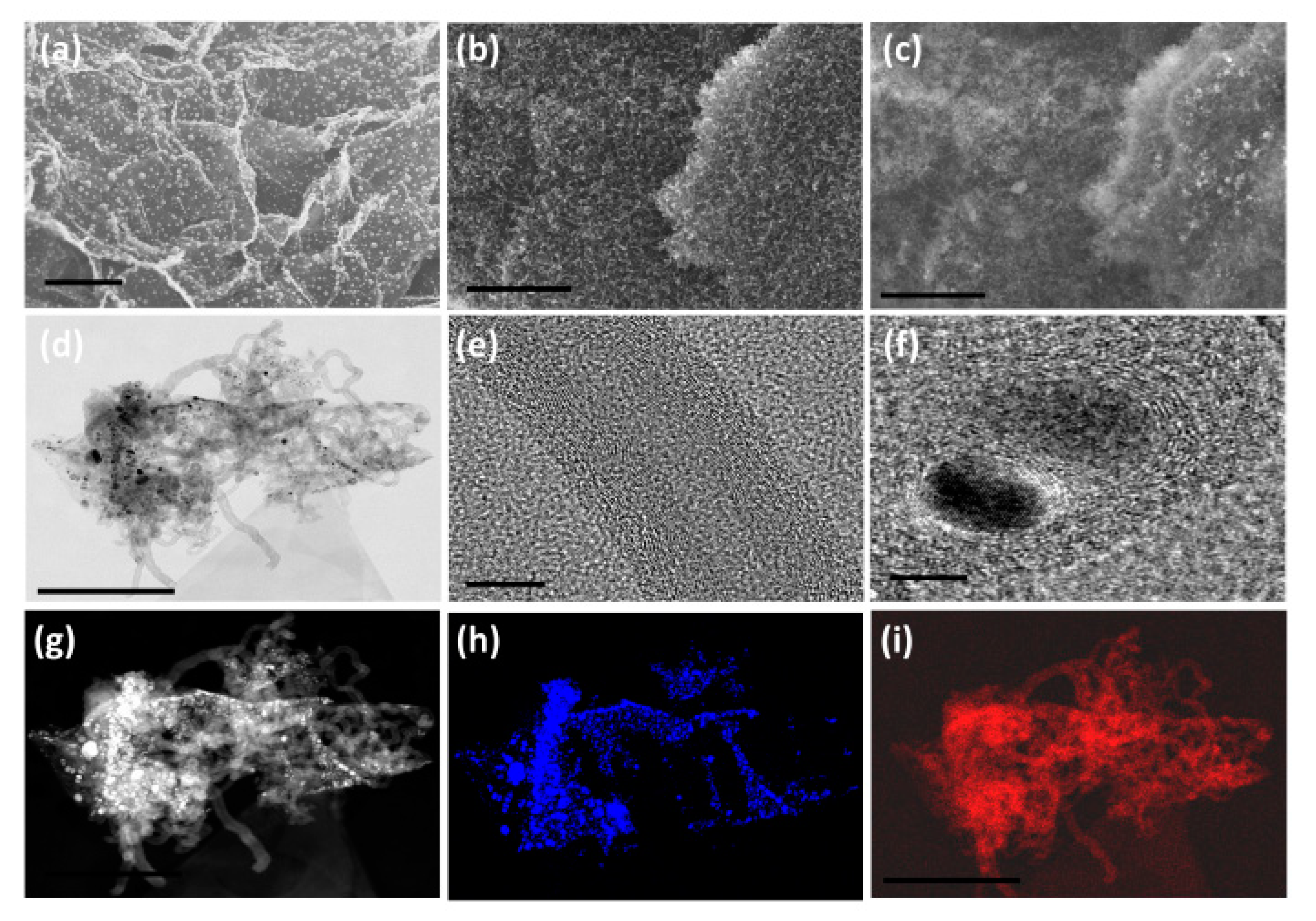
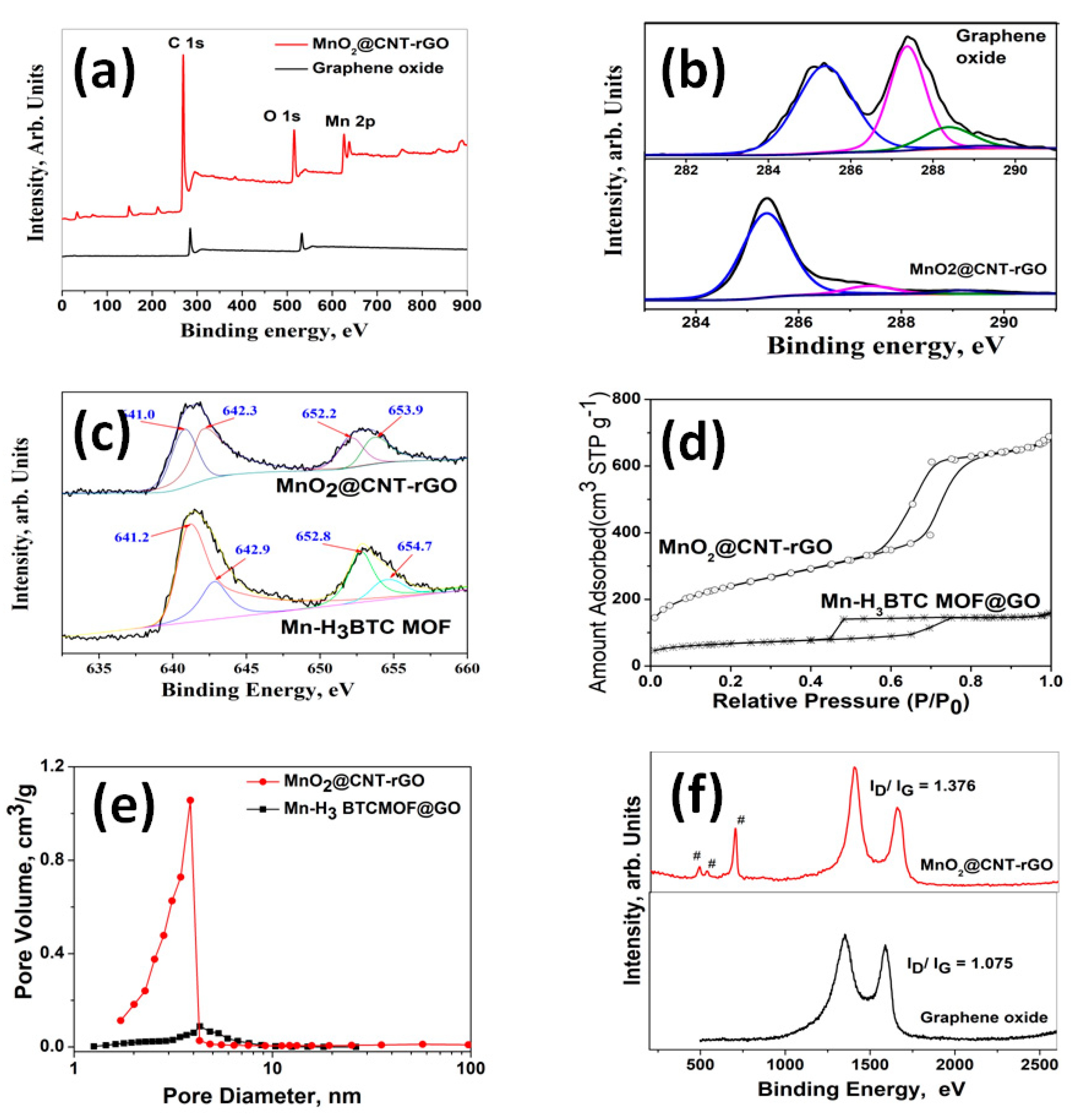
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kusano, S.; Matsumura, D.; Ishii, K.; Tanaka, H.; Mizuki, J. Electrochemical Adsorption on Pt Nanoparticles in Alkaline Solution Observed Using in Situ High Energy Resolution X-ray Absorption Spectroscopy. Nanomaterials 2019, 9, 642. [Google Scholar] [CrossRef] [Green Version]
- Morozan, A.; Jousselme, B.; Palacin, S. Low-platinum and platinum-free catalysts for the oxygen reduction reaction at fuel cell cathodes. Energy Environ. Sci. 2011, 4, 1238–1254. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Z.; Liu, H.; Liao, M. Novel Porous Nitrogen Doped Graphene/Carbon Black Composites as Efficient Oxygen Reduction Reaction Electrocatalyst for Power Generation in Microbial Fuel Cell. Nanomaterials 2019, 9, 836. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Park, H.; Li, O.L. Cobalt Nanoparticles on Plasma-Controlled Nitrogen-Doped Carbon as High-Performance ORR Electrocatalyst for Primary Zn-Air Battery. Nanomaterials 2020, 10, 223. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Jeffery, A.A.; Min, J.; Jung, N. Modulating Catalytic Activity and Durability of PtFe Alloy Catalysts for Oxygen Reduction Reaction through Controlled Carbon Shell Formation. Nanomaterials 2019, 9, 1491. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Yu, F.; Ma, C.; Xue, X.; Fu, H.; Yuan, H.; Yang, S.; Wang, G.; Guo, X.; Zhang, L. Effective Oxygen Reduction Reaction Performance of FeCo Alloys in Situ Anchored on Nitrogen-Doped Carbon by the Microwave-Assistant Carbon Bath Method and Subsequent Plasma Etching. Nanomaterials 2019, 9, 1284. [Google Scholar] [CrossRef] [Green Version]
- Verma, A.; Jha, A.K.; Basu, S. Manganese dioxide as a cathode catalyst for a direct alcohol or sodium borohydride fuel cell with a flowing alkaline electrolyte. J. Power Sources 2005, 141, 30–34. [Google Scholar] [CrossRef]
- Roche, I.; Chaînet, E.; Chatenet, M.; Vondrák, J. Carbon-Supported Manganese Oxide Nanoparticles as Electrocatalysts for the Oxygen Reduction Reaction (ORR) in Alkaline Medium: Physical Characterizations and ORR Mechanism. J. Phys. Chem. C 2007, 111, 1434–1443. [Google Scholar] [CrossRef]
- Osgood, H.; Devaguptapu, S.V.; Xu, H.; Cho, J.P.; Wu, G. Transition metal (Fe, Co, Ni and Mn) oxides for oxygen reduction and evolution bifunctional catalysts in alkaline media. Nano. Today 2016, 11, 601–625. [Google Scholar] [CrossRef]
- Saito, Y.; Meguro, M.; Ashizawa, M.; Waki, K.; Yuksel, R.; Unalan, H.E.; Matsumoto, H. Manganese dioxide nanowires on carbon nanofiber frameworks for efficient electrochemical device electrodes. RSC Adv. 2017, 7, 12351–12358. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://en.wikipedia.org/wiki/Abundance_of_elements_in_Earth’s_crust (accessed on 12 July 2020).
- Chen, Z.; Jiao, Z.; Pan, D.; Li, Z.; Wu, M.; Shek, C.-H.; Wu, C.M.L.; Lai, J.K.L. Recent Advances in Manganese Oxide Nanocrystals: Fabrication, Characterization, and Microstructure. Chem. Rev. 2012, 112, 3833–3855. [Google Scholar] [CrossRef] [PubMed]
- Julien, C.M.; Mauger, A. Nanostructured MnO2 as Electrode Materials for Energy Storage. Nanomaterials 2017, 7, 396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sridhar, V.; Park, H. Manganese nitride stabilized on reduced graphene oxide substrate for high performance sodium ion batteries, super-capacitors and EMI shielding. J. Alloys Compd. 2019, 808, 151748. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, T.; Guo, W.; Chen, S.; Li, Y.; Song, J.; Chang, L.; Mu, S.; Zhao, Y.; Gao, F. Reduced graphene oxide supported MnS nanotubes hybrid as a novel non-precious metal electrocatalyst for oxygen reduction reaction with high performance. J. Power Sources 2017, 362, 1–9. [Google Scholar] [CrossRef]
- Zhan, Y.; Lu, M.; Yang, S.; Xu, C.; Liu, Z.; Lee, J.Y. Activity of Transition-Metal (Manganese, Iron, Cobalt, and Nickel) Phosphates for Oxygen Electrocatalysis in Alkaline Solution. ChemCatChem 2016, 8, 372–379. [Google Scholar] [CrossRef]
- Gangaraju, D.; Sridhar, V.; Lee, I.; Park, H. Graphene–carbon nanotube–Mn3O4 mesoporous nano-alloys as high capacity anodes for lithium-ion batteries. J. Alloys Compd. 2017, 699, 106–111. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Y.F.; Duan, Y.D.; Fu, N.Q.; Liu, Q.P.; Fang, Y.Y.; Sun, Q.W.; Lin, Y. Mn3O4/Graphene Composite as Counter Electrode in Dye-Sensitized Solar Cells. RSC Adv. 2014, 4, 15091–15097. [Google Scholar] [CrossRef]
- Dessie, Y.; Tadesse, S.; Eswaramoorthy, R.; Abebe, B. Recent developments in manganese oxide based nanomaterials with oxygen reduction reaction functionalities for energy conversion and storage applications: A review. J. Sci. Adv. Mater. Devices 2019, 4, 353–369. [Google Scholar] [CrossRef]
- Liu, X.; Chen, C.; Zhao, Y.; Jia, B. A Review on the Synthesis of Manganese Oxide Nanomaterials and Their Applications on Lithium-Ion Batteries. J. Nanomater. 2013, 2013, 736375. [Google Scholar] [CrossRef]
- Li, L.; Nan, C.; Lu, J.; Peng, Q.; Li, Y. α-MnO2 nanotubes: High surface area and enhanced lithium battery properties. Chem. Commun. 2012, 48, 6945–6947. [Google Scholar] [CrossRef]
- Ma, R.; Bando, Y.; Zhang, L.; Sasaki, T. Layered MnO2 Nanobelts: Hydrothermal Synthesis and Electrochemical Measurements. Adv. Mater. 2004, 16, 918–922. [Google Scholar] [CrossRef]
- Xu, K.; Li, S.; Yang, J.; Hu, J. Hierarchical hollow MnO2 nanofibers with enhanced supercapacitor performance. J. Colloid Interface Sci. 2018, 513, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Ranjith, K.S.; Raju, G.S.R.; Chodankar, N.R.; Ghoreishian, S.M.; Kwak, C.H.; Huh, Y.S.; Han, Y.-K. Electroactive Ultra-Thin rGO-Enriched FeMoO4 Nanotubes and MnO2 Nanorods as Electrodes for High-Performance All-Solid-State Asymmetric Supercapacitors. Nanomaterials 2020, 10, 289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elmacı, G.; Ertürk, A.S.; Sevim, M.; Metin, Ö. MnO2 nanowires anchored on mesoporous graphitic carbon nitride (MnO2@mpg-C3N4) as a highly efficient electrocatalyst for the oxygen evolution reaction. Int. J. Hydrogen Energy 2019, 44, 17995–18006. [Google Scholar] [CrossRef]
- Wan, X.; Yang, S.; Cai, Z.; He, Q.; Ye, Y.; Xia, Y.; Li, G.; Liu, J. Facile Synthesis of MnO2 Nanoflowers/N-Doped Reduced Graphene Oxide Composite and Its Application for Simultaneous Determination of Dopamine and Uric Acid. Nanomaterials 2019, 9, 847. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Rong, G.; Xie, Y.; Huang, L.; Feng, C. Low-Temperature Synthesis of A-MnO2 Hollow Urchins and Their Application in Rechargeable Li+ Batteries. Inorg. Chem. 2006, 45, 6404–6410. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.S.; Baik, H.; Kang, K.; Lee, K. Porous β-MnO2 nanoplates derived from MnCO3 nanoplates as highly efficient electrocatalysts toward oxygen evolution reaction. RSC Adv. 2016, 6, 26535–26539. [Google Scholar] [CrossRef]
- Wang, N.; Cao, X.; Lin, G.; Shihe, Y. λ-MnO2 nanodisks and their magnetic properties. Nanotechnology 2007, 18, 475605. [Google Scholar] [CrossRef]
- Reddy, R.N.; Reddy, R.G. Sol–gel MnO2 as an electrode material for electrochemical capacitors. J. Power Sources 2003, 124, 330–337. [Google Scholar] [CrossRef]
- Abulizi, A.; Yang, G.H.; Okitsu, K.; Zhu, J.-J. Synthesis of MnO2 nanoparticles from sonochemical reduction of MnO4− in water under different pH conditions. Ultrason Sonochem. 2014, 21, 1629–1634. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, J.; Han, Q.; Zheng, Z.; Yang, Y.; Wang, X. Shape-Controlled Synthesis of One-Dimensional MnO2 via a Facile Quick-Precipitation Procedure and Its Electrochemical Properties. Cryst. Growth Des. 2009, 9, 4356–4361. [Google Scholar] [CrossRef]
- Walanda, D.K.; Lawrance, G.A.; Donne, S.W. Hydrothermal MnO2: Synthesis, structure, morphology and discharge performance. J. Power Sources 2005, 139, 325–341. [Google Scholar] [CrossRef]
- Shin, J.; Seo, J.K.; Yaylian, R.; Huang, A.; Meng, Y.S. A review on mechanistic understanding of MnO2 in aqueous electrolyte for electrical energy storage systems. Int. Mater. Rev. 2019, 1, 356–387. [Google Scholar] [CrossRef] [Green Version]
- Cheng, F.; Su, Y.; Liang, J.; Tao, Z.; Chen, J. MnO2-Based Nanostructures as Catalysts for Electrochemical Oxygen Reduction in Alkaline Media. Chem. Mater. 2010, 22, 898–905. [Google Scholar] [CrossRef]
- Manna, B.; Ghosh, U.C. Pilot-Scale Performance of Iron and Arsenic Removal from Contaminated Groundwater. Water Qual. Res. J. 2005, 40, 82–90. [Google Scholar] [CrossRef]
- Zhang, J.; Deng, P.-H.; Feng, Y.-L.; Kuang, Y.-F.; Yang, J.-J. Electrochemical Determination of Ascorbic Acid at β-MnO2 Modified Carbon Black Microelectrodes. Microchim. Acta 2004, 147, 279–282. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, J.; Wu, X.; Han, Q.; Wang, X. Graphene Oxide−MnO2 Nanocomposites for Supercapacitors. ACS Nano 2010, 4, 2822–2830. [Google Scholar] [CrossRef]
- Wang, H.; Peng, C.; Peng, F.; Yu, H.; Yang, J. Facile synthesis of MnO2/CNT nanocomposite and its electrochemical performance for supercapacitors. Mater. Sci. Eng. B. 2011, 176, 1073–1078. [Google Scholar] [CrossRef]
- El-Deen, A.G.; Barakat, N.A.M.; Kim, H.Y. Graphene wrapped MnO2-nanostructures as effective and stable electrode materials for capacitive deionization desalination technology. Desalination 2014, 344, 289–298. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, X.; Zhang, Q.; Yang, F.; Dong, H.; Sui, J.; Dong, L. One-step hydrothermal synthesis of MnO2/graphene composite for electrochemical energy storage. J. Electroanal. Chem. 2019, 837, 108–115. [Google Scholar] [CrossRef]
- Basirun, W.; Sookhakian, M.; Baradaran, S.; Endut, Z.; Mahmoudian, M.R.; Ebadi, M.; Yousefi, R.; Ghadimi, H.; Ahmed, S. Graphene oxide electrocatalyst on MnO2 air cathode as an efficient electron pump for enhanced oxygen reduction in alkaline solution. Sci. Rep. 2015, 5, 9108. [Google Scholar] [CrossRef] [PubMed]
- Llabrés i Xamena, F.; Corma, A.; Garcia, H. Applications for Metal–Organic Frameworks (MOFs) as Quantum Dot Semiconductors. J. Phys. Chem. C 2007, 111, 80–85. [Google Scholar] [CrossRef]
- Chen, L.Y.; Bai, J.F.; Wang, C.Z.; Pan, Y.; Scheer, M.; You, X.Z. One-step solid-state thermolysis of a metal–organic framework: A simple and facile route to large-scale of multiwalled carbon nanotubes. Chem. Commun. 2008, 13, 1581–1583. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, V.; Park, H. Zeolitic imidazolate frameworks as novel precursors for microwave synthesis of carbon nanotubes. J. Alloys Compd. 2019, 781, 166–173. [Google Scholar] [CrossRef]
- Arbulu, R.C.; Jiang, Y.-B.; Peterson, E.J.; Qin, Y. Metal–Organic Framework (MOF) Nanorods, Nanotubes, and Nanowires. Angew. Chem. Int. Ed. 2018, 57, 5813–5817. [Google Scholar] [CrossRef]
- Volosskiy, B.; Niwa, K.; Chen, Y.; Zhao, Z.; Weiss, N.O.; Zhong, X.; Ding, M.; Lee, C.; Huang, Y.; Duan, X. Metal-organic framework templated synthesis of ultrathin, well-aligned metallic nanowires. ACS Nano 2015, 9, 3044–3049. [Google Scholar] [CrossRef]
- Sridhar, V.; Park, H. Microwave induced transformation of metal organic frameworks into defect rich carbon nanofibers. New J. Chem. 2020, 44, 5666–5672. [Google Scholar] [CrossRef]
- Mofarah, S.S.; Adabifiroozjaei, E.; Pardehkhorram, R.; Assadi, M.H.N.; Hinterstein, M.; Yao, Y.; Liu, X.; Ghasemian, M.B.; Kalantar-Zadeh, K.; Mehmood, R.; et al. Coordination Polymer to Atomically Thin, Holey, Metal-Oxide Nanosheets for Tuning Band Alignment. Adv. Mater. 2019, 31, 1905288. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Tseng, L.-T.; Lu, Y.; Fan, H.M.; Wang, Y.; Luo, X.; Liu, T.; Munroe, P.; Li, S.; Yi, J. Magnetic properties in α-MnO2 doped with alkaline elements. Sci. Rep. 2015, 5, 9094. [Google Scholar] [CrossRef] [Green Version]
- Elmas Kimyonok, A.B.; Ulutürk, M. Determination of the Thermal Decomposition Products of Terephthalic Acid by Using Curie-Point Pyrolyzer. J. Energetic Mater. 2016, 34, 113–122. [Google Scholar] [CrossRef]
- Montoya Sánchez, N.; de Klerk, A. Oxidative Ring-Opening of Aromatics: Decomposition of Biphenyl Carboxylic Acids and Zinc Biphenyl Carboxylates. Energy Fuels 2015, 29, 7910–7922. [Google Scholar] [CrossRef]
- Desimoni, E.; Brunetti, B. X-ray Photoelectron Spectroscopic Characterization of Chemically Modified Electrodes Used as Chemical Sensors and Biosensors: A Review. Chemosensors 2015, 3, 70–117. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Zheng, Y.; Podkolzin, S.G.; Lee, W. Band gap of reduced graphene oxide tuned by controlling functional groups. J. Mater. Chem. C 2020, 8, 4885–4894. [Google Scholar] [CrossRef]
- Ganguly, A.; Sharma, S.; Papakonstantinou, P.; Hamilton, J. Probing the Thermal Deoxygenation of Graphene Oxide Using High-Resolution in Situ X-ray-Based Spectroscopies. J. Phys. Chem. C 2011, 115, 17009–17019. [Google Scholar] [CrossRef] [Green Version]
- Valencia, S.; Calderón, M.J.; López-Mir, L.; Konstantinovic, Z.; Schierle, E.; Weschke, E.; Brey, L.; Martínez, B.; Balcells, L.I. Enhancement of spin-orbit coupling at manganite surfaces. Phys. Rev. B 2018, 98, 115142. [Google Scholar] [CrossRef] [Green Version]
- Şenocak, A.; Khataee, A.; Demirbas, E.; Doustkhah, E. Ultrasensitive detection of rutin antioxidant through a magnetic micro-mesoporous graphitized carbon wrapped Co nanoarchitecture. Sens. Actuators B Chem. 2020, 312, 127939. [Google Scholar] [CrossRef]
- Bernardini, S.; Bellatreccia, F.; Casanova Municchia, A.; Della Ventura, G.; Sodo, A. Raman spectra of natural manganese oxides. J. Raman Spectrosc. 2019, 50, 873–888. [Google Scholar]
- Rabe, M.; Toparli, C.; Chen, Y.-H.; Kasian, O.; Mayrhofer, K.J.J.; Erbe, A. Alkaline Manganese Electrochemistry Studied by in Situ and Operando Spectroscopic Methods-Metal Dissolution, Oxide Formation and Oxygen Evolution. Phys. Chem. Chem. Phys. 2019, 21, 10457–10469. [Google Scholar] [CrossRef] [Green Version]
- Begum, H.; Ahmed, M.S.; Jeon, S. δ-MnO2 nanoflowers on sulfonated graphene sheets for stable oxygen reduction and hydrogen evolution reaction. Electrochim. Acta 2019, 296, 235–242. [Google Scholar] [CrossRef]
- Li, Y.; Cao, S.; Fan, L.; Han, J.; Wang, M.; Guo, R. Hybrid shells of MnO2 nanosheets encapsulated by N-doped carbon towards nonprecious oxygen reduction reaction catalysts. J. Colloid Interface Sci. 2018, 527, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Dou, S.; Li, X.; Xu, J.; Wang, S.; Lai, L.; Liu, H.K.; Ma, J.; Dou, S.X. Hierarchical MnO2/rGO hybrid nanosheets as an efficient electrocatalyst for the oxygen reduction reaction. Int. J. Hydrogen Energy 2016, 41, 5260–5268. [Google Scholar] [CrossRef]
- Hang, Y.; Zhang, C.; Luo, X.; Xie, Y.; Xin, S.; Li, Y.; Zhang, D.; Goodenough, J.B. α-MnO2 nanorods supported on porous graphitic carbon nitride as efficient electrocatalysts for lithium-air batteries. J. Power Sources 2018, 392, 15–22. [Google Scholar] [CrossRef]
- Chen, R.; Yan, J.; Liu, Y.; Li, J. Three-dimensional nitrogen-doped graphene/MnO nanoparticle hybrids as a high-performance catalyst for oxygen reduction reaction. J. Phys. Chem. C 2015, 119, 8032–8037. [Google Scholar] [CrossRef]
- Yu, Q.; Xu, J.; Wu, C.; Zhang, J.; Guan, L. MnO2 nanofilms on nitrogen-doped hollow graphene spheres as a high-performance electrocatalyst for oxygen reduction reaction. ACS Appl. Mater. Interfaces 2016, 8, 35264–35269. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Li, C.; Chen, X.; Cheng, F.; Chen, J. Spinel cobalt–manganese oxide supported on non-oxidized carbon nanotubes as a highly efficient oxygen reduction/evolution electrocatalyst. Inorg. Chem. Front. 2017, 4, 1628–1633. [Google Scholar] [CrossRef]
- Marsudi, M.A.; Ma, Y.; Prakoso, B.; Hutani, J.J.; Wibowo, A.; Zong, Y.; Liu, Z.; Sumboja, A. Manganese Oxide Nanorods Decorated Table Sugar Derived Carbon as Efficient Bifunctional Catalyst in Rechargeable Zn-Air Batteries. Catalysts 2020, 10, 64. [Google Scholar] [CrossRef] [Green Version]
- Mathumba, P.; Fernandes, D.M.; Matos, R.; Iwuoha, E.I.; Freire, C. Metal Oxide (Co3O4 and Mn3O4) Impregnation into S, N-doped Graphene for Oxygen Reduction Reaction (ORR). Materials 2020, 13, 1562. [Google Scholar] [CrossRef] [Green Version]
- Sridhar, V.; Jung, K.H.; Park, H. Vitamin Derived Nitrogen Doped Carbon Nanotubes for Efficient Oxygen Reduction Reaction and Arsenic Removal from Contaminated Water. Materials 2020, 13, 1686. [Google Scholar] [CrossRef] [Green Version]

| Serial Number | Type of Mn Based Catalyst | Electrolyte | Eonset (V) | Epeak (V) | Electron Transfer Number, n | Reference |
|---|---|---|---|---|---|---|
| 1 | MnO2 nano-particles anchored on sulphur doped graphene | 0.1 M KOH | 0.91 | 0.79 | 3.95 | [61] |
| 2 | MnO2 nanosheets on nitrogen doped carbon | 0.1 M KOH | 0.918 | 0.78 | 3.9 | [62] |
| 3 | MnO2 nano-particles anchored on reduced graphene oxide | 0.1 M KOH | 0.847 | 0.76 | 3.85 | [63] |
| 4 | MnO2 nano-rods anchored on carbon nitride | 0.1 M KOH | 0.8 | 0.74 | 3.8 | [64] |
| 5 | MnO nano-particles on nitrogen doped graphene | 0.1 M KOH | 0.83 | 0.72 | 3.03 | [65] |
| 6 | MnO2 nano-films anchored on hollow graphene spheres | 0.1 M KOH | 0.94 | 0.73 | 3.85 | [66] |
| 7 | MnxCoyO4 nano-particles anchored on carbon nanotubes | 1 M KOH | 0.81 | 0.89 | Not reported | [67] |
| 8 | MnO2 nano-rods anchored on sugar derived carbon nanosheets | 0.1 M KOH | 0.914 | Not reported | 3.12 | [68] |
| 9 | MnO2 nano-particles on nitrogen doped graphene | 0.1 M KOH | 0.91 | 0.8 | 3.9 | [69] |
| 10 | MnO2 nano-particles in three-dimensional graphene–CNT hybrids | 0.1 M KOH | 0.89 | 0.72 | 3.92 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sridhar, V.; Lee, I.; Jung, K.H.; Park, H. Metal Organic Framework Derived MnO2-Carbon Nanotubes for Efficient Oxygen Reduction Reaction and Arsenic Removal from Contaminated Water. Nanomaterials 2020, 10, 1895. https://doi.org/10.3390/nano10091895
Sridhar V, Lee I, Jung KH, Park H. Metal Organic Framework Derived MnO2-Carbon Nanotubes for Efficient Oxygen Reduction Reaction and Arsenic Removal from Contaminated Water. Nanomaterials. 2020; 10(9):1895. https://doi.org/10.3390/nano10091895
Chicago/Turabian StyleSridhar, Vadahanambi, Inwon Lee, Kwang Hyo Jung, and Hyun Park. 2020. "Metal Organic Framework Derived MnO2-Carbon Nanotubes for Efficient Oxygen Reduction Reaction and Arsenic Removal from Contaminated Water" Nanomaterials 10, no. 9: 1895. https://doi.org/10.3390/nano10091895
APA StyleSridhar, V., Lee, I., Jung, K. H., & Park, H. (2020). Metal Organic Framework Derived MnO2-Carbon Nanotubes for Efficient Oxygen Reduction Reaction and Arsenic Removal from Contaminated Water. Nanomaterials, 10(9), 1895. https://doi.org/10.3390/nano10091895







