Advances in Perovskite Light-Emitting Diodes Possessing Improved Lifetime
Abstract
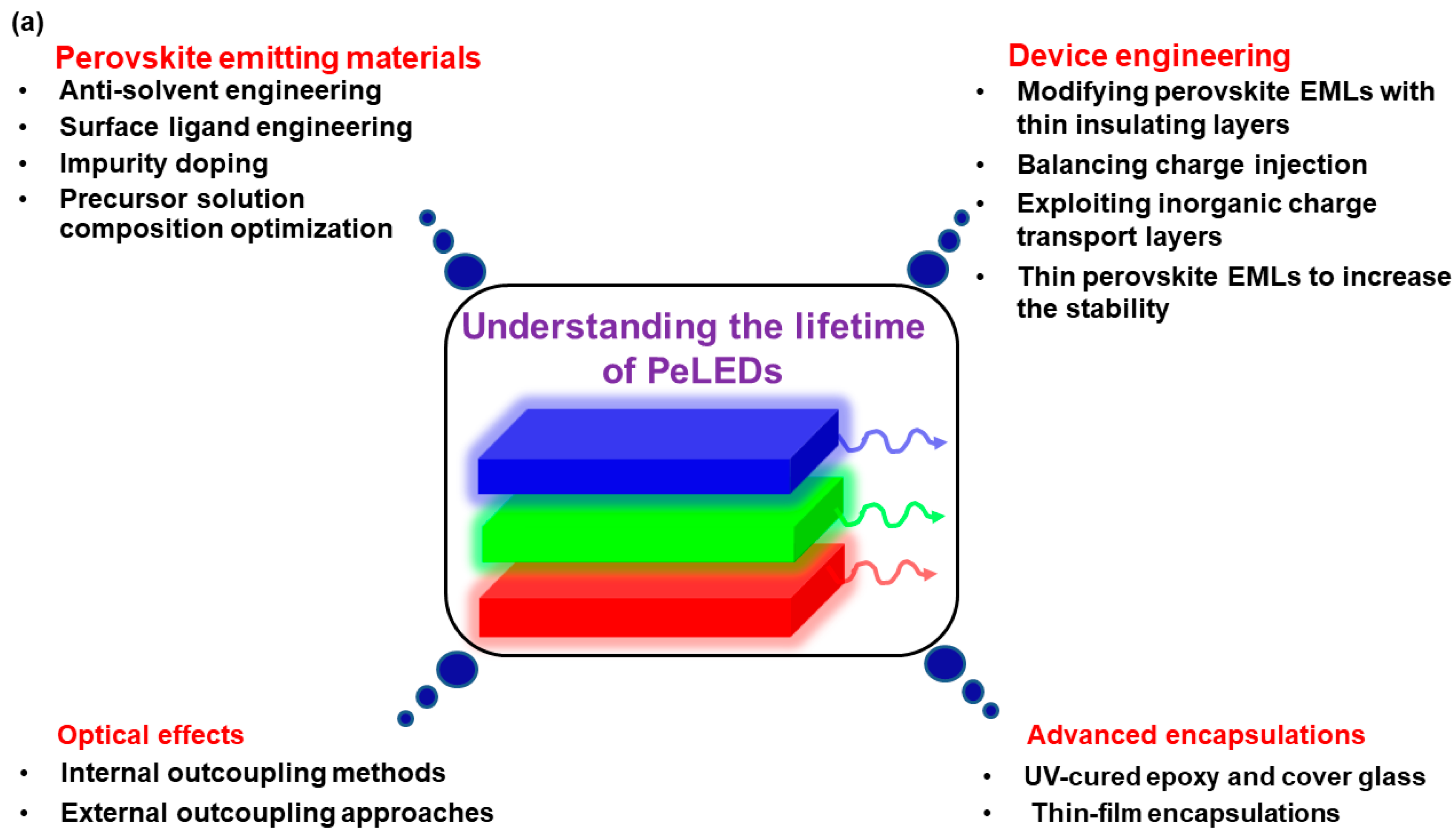
:1. Introduction
2. Fundamental Concepts Determining the Lifetime of PeLEDs
2.1. Perovskite Emitting Materials
2.2. Device Engineering
2.3. Optical Effects
2.4. Advanced Encapsulations
3. Strategies to Obtain PeLEDs with Improved Lifetime
3.1. Basic Aspects of the Lifetime of PeLEDs
3.2. The Design of Perovskite Emitting Materials
3.2.1. Anti-Solvent Engineering
3.2.2. Surface Ligand Engineering
3.2.3. Impurity Doping
3.2.4. Precursor Solution Composition Optimization
3.3. The Innovation of Device Engineering
3.3.1. Modifying Perovskite EMLs with Thin Insulating Layers
3.3.2. Balancing Charge Injection
3.3.3. Exploiting Inorganic Charge Transport Layers
3.3.4. Thin Perovskite EMLs to Increase the Stability
3.4. The Manipulation of Optical Effects
3.5. The Introduction of Advanced Encapsulations
4. Summary and Outlook
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, H.; Polavarapu, L.; Sichert, J.A.; Susha, A.S.; Urban, A.S.; Rogach, A.L. Colloidal Lead Halide Perovskite Nanocrystals: Synthesis, Optical Properties and Applications. NPG Asia Mater. 2016, 8, e328. [Google Scholar] [CrossRef] [Green Version]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Susha, A.S.; Kershaw, S.V.; Hung, T.F.; Rogach, A.L. Control of Emission Color of High Quantum Yield CH3NH3PbBr3 Perovskite Quantum Dots by Precipitation Temperature. Adv. Sci. 2015, 2, 1500194. [Google Scholar] [CrossRef]
- Cho, H.C.; Jeong, S.-H.; Park, M.-H.; Kim, Y.-H.; Wolf, C.; Lee, C.-L.; Heo, J.H.; Sadhanala, A.; Myoung, N.; Yoo, S. Overcoming the Electroluminescence Efficiency Limitations of Perovskite Light-emitting Diodes. Science 2015, 350, 1222–1225. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Cho, H.; Lee, T.-W. Metal Halide Perovskite Light Emitters. Proc. Natl. Acad. Sci. USA 2016, 113, 11694–11702. [Google Scholar] [CrossRef] [Green Version]
- Tan, Z.K.; Moghaddam, R.S.; Lai, M.L.; Docampo, P.; Higler, R.; Deschler, F.; Price, M.; Sadhanala, A.; Pazos, L.M.; Credgington, D. Bright Light-emitting Diodes Based on Organometal Halide Perovskite. Nat. Nanotechnol. 2014, 9, 687–692. [Google Scholar] [CrossRef]
- Song, J.; Li, J.; Li, X.M.; Xu, L.; Dong, Y.; Zeng, H. Quantum Dot Light-Emitting Diodes Based on Inorganic Perovskite Cesium Lead Halides (CsPbX3). Adv. Mater. 2015, 27, 7162–7167. [Google Scholar] [CrossRef]
- Perumal, A.; Shendre, S.; Li, M.J.; Tay, Y.K.E.; Sharma, V.K.; Chen, S.; Wei, Z.; Liu, Q.; Gao, Y.; Buenconsejo, P.J.S.; et al. High Brightness Formamidinium Lead Bromide Perovskite Nanocrystal Light Emitting Devices. Sci. Rep. 2016, 6, 36733. [Google Scholar] [CrossRef] [Green Version]
- Fang, Z.B.; Chen, W.J.; Shi, Y.L.; Zhao, J.; Chu, S.L.; Zhang, J.; Xiao, Z.G. Dual Passivation of Perovskite Defects for Light-Emitting Diodes with External Quantum Efficiency Exceeding 20%. Adv. Funct. Mater. 2020, 30, 1909754. [Google Scholar] [CrossRef]
- Shah, S.A.A.; Sayyad, M.H.; Sun, J.; Guo, Z. Hysteresis Analysis of Hole-Transport-Material-Free Monolithic Perovskite Solar Cells with Carbon Counter Electrode by Current Density–Voltage and Impedance Spectra Measurements. Nanomaterials 2021, 11, 48. [Google Scholar] [CrossRef]
- Chen, J.; Wang, J.; Xu, X.; Li, J.; Song, J.; Lan, S.; Liu, S.; Cai, B.; Han, B.; Precht, J.T.; et al. Efficient and Bright White Light-Emitting Diodes Based on Single-layer Heterophase Halide Perovskites. Nat. Photonics 2020, 1–7. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, N.N.; Tian, H.; Guo, J.S.; Wei, Y.Q.; Chen, H.; Miao, Y.F.; Zou, W.; Pan, K.; He, Y.R.; et al. Perovskite Light-emitting Diodes Based on Spontaneously Formed Submicrometre-scale Structures. Nature 2018, 562, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Chen, Q.; Qiu, Y.; Zhang, M.; Liu, B. Device Engineering for All-inorganic Perovskite Light-emitting Diodes. Nanomaterials 2019, 9, 1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiba, T.; Hayashi, Y.; Ebe, H.; Hoshi, K.; Sato, J.; Sato, S.; Pu, Y.-J.; Ohisa, S.; Kido, J. Anion-exchange Red Perovskite Quantum Dots with Ammonium Iodine Salts for Highly Efficient Light-emitting Devices. Nat. Photonics 2018, 12, 681–687. [Google Scholar] [CrossRef]
- Yang, X.L.; Zhou, G.J.; Wong, W.-Y. Functionalization of Phosphorescent Emitters and Their Host Materials by Main-group Elements for Phosphorescent Organic Light-emitting Devices. Chem. Soc. Rev. 2015, 44, 8484–8575. [Google Scholar] [CrossRef]
- Jou, J.-H.; Kumar, S.; Agrawal, A.; Li, T.-H.; Sahoo, S. Correction: Approaches for Fabricating High Efficiency Organic Light Emitting Diodes. J. Mater. Chem. C 2015, 3, 3500. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Li, X.; Tao, H.; Zou, J.; Xu, M.; Wang, L.; Peng, J.; Cao, Y. Manipulation of Excitons Distribution for High-performance Fluorescent/phosphorescent Hybrid White Organic Light-emitting Diodes. J. Mater. Chem. C 2017, 5, 7668–7683. [Google Scholar] [CrossRef]
- Wei, Z.; Xing, J. The Rise of Perovskite Light-Emitting Diodes. J. Phys. Chem. Lett. 2019, 10, 3035–3042. [Google Scholar] [CrossRef]
- Li, J.Q.; Shan, X.; Bade, S.G.R.; Geske, T.; Jiang, Q.L.; Yang, X.; Yu, Z.B. Single-Layer Halide Perovskite Light-Emitting Diodes with Sub-Band Gap Turn-On Voltage and High Brightness. J. Phys. Chem. Lett. 2016, 7, 4059–4066. [Google Scholar] [CrossRef]
- Shen, H.B.; Gao, Q.; Zhang, Y.B.; Lin, Y.; Lin, Q.L.; Li, Z.H.; Chen, L.; Zeng, Z.P.; Li, X.G.; Jia, Y.; et al. Visible Quantum Dot Light-emitting Diodes with Simultaneous High Brightness and Efficiency. Nat. Photonics 2019, 13, 192–197. [Google Scholar] [CrossRef]
- Luo, D.; Yang, Y.; Huang, L.; Liu, B.; Zhao, Y. High-performance Hybrid White Organic Light-emitting Diodes Exploiting Blue Thermally Activated Delayed Fluorescent Dyes. Dyes Pigments 2017, 147, 83–89. [Google Scholar] [CrossRef]
- Xiang, C.Y.; Koo, W.; So, F.; Sasabe, H.; Kido, J. A Systematic Study on Efficiency Enhancements in Phosphorescent Green, Red and Blue Microcavity Organic Light Emitting Devices. Light Sci. Appl. 2013, 2, e74. [Google Scholar] [CrossRef] [Green Version]
- Xiao, P.; Dong, T.; Xie, J.N.; Luo, D.X.; Yuan, J.; Liu, B. Emergence of White Organic Light-Emitting Diodes Based on Thermally Activated Delayed Fluorescence. Appl. Sci. 2018, 8, 299. [Google Scholar] [CrossRef] [Green Version]
- Lin, K.; Xing, J.; Quan, L.N.; Arquer, F.P.G.; Gong, X.; Lu, J.; Xie, L.; Zhao, W.; Zhang, D.; Yan, C.; et al. Perovskite Light-emitting Diodes with External Quantum Efficiency Exceeding 20 Percent. Nature 2018, 562, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.-H.; Zhu, B.-S.; Yao, J.-S.; Yao, H.-B. Chemical Regulation of Metal Halide Perovskite Nanomaterials for Efficient Light-Emitting Diodes. Sci. China. Chem. 2018, 61, 1047–1061. [Google Scholar] [CrossRef]
- Le, Q.V.; Jang, H.W.; Kim, S.Y. Recent Advances toward High-Efficiency Halide Perovskite Light-Emitting Diodes: Review and Perspective. Small Methods 2018, 2, 1700419. [Google Scholar]
- Zhao, X.F.; Ng, J.D.A.; Friend, R.H.; Tan, Z.-K. Efficient and Bright White Light-emitting Diodes Based on Single-layer Heterophase Halide Perovskites. ACS Photonics 2018, 5, 3866–3875. [Google Scholar] [CrossRef]
- Wang, H.R.; Zhang, X.Y.; Wu, Q.Q.; Cao, F.; Yang, D.W.; Shang, Y.Q.; Ning, Z.J.; Zhang, W.; Zheng, W.T.; Yan, Y.F.; et al. Trifluoroacetate Induced Small-Grained Cspbbr3 Perovskite Films Result in Efficient and Stable Light-Emitting Devices. Nat. Commun. 2019, 10, 665. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Li, X.; Luo, D.; Xiao, P.; Xiao, W.; Song, Y.; Ang, Q.; Liu, B. Strategies to Achieve High-Performance White Organic Light-Emitting Diodes. Materials 2017, 10, 1378. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Cheng, Z.Y.; Lin, J. An Overview on Enhancing the Stability of Lead Halide Perovskite Quantum Dots and Their Applications in Phos-phor-Converted Leds. Chem. Soc. Rev. 2019, 48, 310–350. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, D. Management of Charges and Excitons for High-Performance White Organic Light-Emitting Diodes. Chem. Soc. Rev. 2010, 39, 2387–2398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.T.; Yi, C.; Wang, N.N.; Sun, Y.; Zou, W.; Wei, Y.Q.; Cao, Y.; Miao, Y.F.; Li, R.Z.; Yin, Y.; et al. Efficient Red Perovskite Light-Emitting Diodes Based on Solution-Processed Multiple Quantum Wells. Adv. Mater. 2017, 29, 1606600. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Wang, N.N.; Kong, D.C.; Cao, Y.; He, Y.R.; Zhu, L.; Wang, Y.M.; Xue, C.; Peng, Q.M.; Gao, F.; et al. Defect Passivation for Red Perovskite Light-Emitting Diodes with Improved Brightness and Stability. J. Phys. Chem. Lett. 2018, 10, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.G.; Zhao, L.F.; Tran, N.L.; Lin, Y.H.L.S.; Silver, S.H.; Kerner, R.A.; Yao, N.; Kahn, A.; Scholes, G.D.; Rand, B.P. Mixed-Halide Perovskites with Stabilized Bandgaps. Nano Lett. 2017, 17, 6863–6869. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, S.H.; Nam, Y.S.; Yu, J.C.; Lee, S.; Kim, D.B.; Jung, E.D.; Woo, J.H.; Ahn, S.M.; Lee, S.; et al. Flexibility of Semitransparent Perovskite Light-Emitting Diodes Investigated by Tensile Properties of the Perovskite Layer. Nano Lett. 2019, 19, 971–976. [Google Scholar] [CrossRef]
- Shen, X.Y.; Zhang, X.; Tang, C.Y.; Zhang, X.T.; Lu, P.; Shi, Z.F.; Xie, W.F.; Yu, W.W.; Zhang, Y. Silver–Bismuth Bilayer Anode for Perovskite Nanocrystal Light-Emitting Devices. J. Phys. Chem. Lett. 2020, 11, 3853–3859. [Google Scholar] [CrossRef]
- Xu, H.; Wang, X.C.; Li, Y.; Cai, L.; Tan, Y.S.; Zhang, G.H.; Wang, Y.S.; Li, R.Y.; Liang, D.; Song, T.; et al. Prominent Heat Dissipation in Perovskite Light-Emitting Diodes with Reduced Efficiency Droop for Silicon-Based Display. J. Phys. Chem. Lett. 2020, 11, 3689–3698. [Google Scholar] [CrossRef]
- Sasabe, H.; Kido, J. Development of High Performance OLEDs for General Lighting. J. Mater. Chem. C 2013, 1, 1699–1707. [Google Scholar] [CrossRef]
- Li, X.L.; Xie, G.Z.; Liu, M.; Chen, D.C.; Cai, X.Y.; Peng, J.B.; Cao, Y.; Su, S.J. High-Efficiency WOLEDs with High Color-Rendering Index based on a Chromaticity-Adjustable Yellow Thermally Activated Delayed Fluorescence Emitter. Adv. Mater 2016, 28, 4614–4619. [Google Scholar] [CrossRef]
- Xiao, P.; Huang, J.; Yu, Y.; Yuan, J.; Luo, D.; Liu, B.; Liang, D. Recent Advances of Exciplex-Based White Organic Light-Emitting Diodes. Appl. Sci. 2018, 8, 1449. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Wang, L.; Xu, M.; Tao, H.; Xia, X.; Zou, J.; Su, Y.; Gao, D.; Lan, L.; Peng, J. Simultaneous Achievement of Low Efficiency Roll-Off and Stable Color in Highly Efficient Single-Emitting-Layer Phosphorescent White Organic Light-Emitting Diodes. J. Mater. Chem. C 2014, 2, 5870–5877. [Google Scholar] [CrossRef]
- Shirasaki, Y.; Supran, G.J.; Bawendi, M.G.; Bulović, V. Emergence of Colloidal Quantum-Dot Light-Emitting Technologies. Nat. Photonics 2013, 7, 13–23. [Google Scholar] [CrossRef]
- Yang, Y.X.; Zheng, Y.; Cao, W.R.; Titov, A.; Hyvonen, J.; Manders, J.R.; Xue, J.G.; Holloway, P.H.; Qian, L. High-Efficiency Light-Emitting Devices Based on Quantum Dots with Tailored Nanostructures. Nat. Photonics 2015, 9, 259–266. [Google Scholar] [CrossRef]
- Hames, B.C.; Sanchez, R.S.; Fakharuddin, A.; Mora-Sero, I. A Comparative Study of Light-Emitting Diodes Based on All-Inorganic Perovskite Nanoparticles (CsPbBr3) Synthesized at Room Temperature and by a Hot-Injection Method. Chempluschem 2018, 83, 294–299. [Google Scholar] [CrossRef]
- Shen, X.Y.; Zhang, Y.; Kershaw, S.V.; Li, T.S.; Wang, C.C.; Zhang, X.Y.; Wang, W.Y.; Li, D.G.; Wang, Y.H.; Lu, M.; et al. Zn-Alloyed CsPbI 3 Nanocrystals for Highly Efficient Perovskite Light-Emitting Devices. Nano Lett. 2019, 19, 1552–1559. [Google Scholar] [CrossRef]
- Zhang, X.L.; Wang, W.G.; Xu, B.; Liu, S.; Dai, H.T.; Bian, D.; Chen, S.M.; Wang, K.; Sun, X.W. Thin Film Perovskite Light-Emitting Diode Based on Cspbbr3 Powders and Interfacial Engineering. Nano Energy 2017, 37, 40–45. [Google Scholar] [CrossRef]
- Le, Q.V.; Kim, J.B.; Kim, S.Y.; Lee, B.; Lee, D.R. Structural Investigation of Cesium Lead Halide Perovskites for High-Efficiency Quantum Dot Light-Emitting Diodes. J. Phys. Chem. Lett. 2017, 8, 4140–4147. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Lu, M.; Zhang, Y.; Wu, H.; Shen, X.Y.; Zhang, W.; Zheng, W.T.; Colvin, V.L.; Yu, W.W. PbS Capped CsPbI3 Nanocrystals for Efficient and Stable Light-Emitting Devices Using p–i–n Structures. ACS Cent. Sci. 2018, 4, 1352–1359. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.X.; Michael, W.; He, Q.Q.; Ma, B.W. Advances in light-emitting metal-halide perovskite nanocrystals. MRS Bull. 2020, 45, 458–466. [Google Scholar]
- Xiao, P.; Huang, J.; Yan, D.; Luo, D.; Yuan, J.; Liu, B.; Liang, D. Emergence of Nanoplatelet Light-Emitting Diodes. Materials 2018, 11, 1376. [Google Scholar] [CrossRef] [Green Version]
- Jeong, B.; Han, H.; Choi, Y.J.; Cho, S.H.; Kim, E.H.; Lee, S.W.; Kim, J.S.; Park, C.; Kim, D.; Park, C. All-Inorganic CsPbI3 Perovskite Phase-Stabilized by Poly(ethylene oxide) for Red-Light-Emitting Diodes. Adv. Funct. Mater. 2018, 28, 1706401. [Google Scholar] [CrossRef]
- Song, P.J.; Qiao, B.; Song, D.D.; Liang, Z.Q.; Gao, D.; Cao, J.Y.; Shen, Z.H.; Xu, Z.; Zhao, S.L. Colour-And Structure-Stable Cspbbr3-Cspb2Br5 Compounded Quantum Dots with Tuneable Blue and Green Light Emission. J. Alloys Compd. 2018, 767, 98–105. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, Y.; Lu, M.; Zhang, X.Y.; Sun, C.; Zhang, T.Q.; Colvin, V.L.; Yu, W.W. Surface Ligand Modification of Cesium Lead Bromide Nanocrystals for Improved Light-Emitting Performance. Nanoscale 2018, 10, 4173–4178. [Google Scholar] [CrossRef]
- Song, L.; Guo, X.Y.; Hu, Y.S.; Lv, Y.; Lin, J.; Fan, Y.; Zhang, N.; Liu, X.Y. Improved Performance of CsPbBr3 Perovskite Light-Emitting Devices by Both Boundary and Interface Defects Passivation. Nanoscale 2018, 10, 18315–18322. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.Q.; Zhao, S.L.; Xu, Z.; Song, D.D.; Qiao, B.; Yue, H.X.; Yang, J.; Zheng, X.G.; Wei, P. Highly Bright and Stable All-Inorganic Perovskite Light-Emitting Diodes with Methoxypolyethylene Glycols Modified Cspbbr3 Emission Layer. Appl. Phys. Lett. 2018, 113, 213501. [Google Scholar] [CrossRef]
- Seok, S.I.; Guo, T.F. Halide Perovskite Materials and Devices. MRS Bull. 2020, 45, 427–430. [Google Scholar] [CrossRef]
- Ng, Y.F.; Neo, W.J.; Jamaludin, N.F.; Yantara, N.; Mhaisalkar, S.; Mathews, N. Enhanced Coverage of All-Inorganic Perovskite CsPbBr3 through Sequential Deposition for Green Light-Emitting Diodes. Energy Technol. 2017, 5, 1859–1865. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.H.; Wei, J.; Sun, Y.; Wu, J.N.; Hou, Y.F.; Wang, P.; Wang, N.P.; Zhao, Z.F. Air-Stable All-Inorganic Perovskite Quantum Dot Inks for Multicolor Patterns and White Leds. J. Mater. Sci. 2019, 54, 6917–6929. [Google Scholar]
- Qu, J.; Rastogi, P.; Greboval, C.; Clément, L.; Lhuillier, E. Nanoplatelet-Based Light-Emitting Diode and Its Use in All-Nanocrystal LiFi-like Communication. ACS Appl. Mater. Interfaces 2020, 12, 22058–22065. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.-P.; Huang, J.-S.; Shen, Y.; Li, G.-P.; Liu, X.-K.; Li, W.; Wang, Y.-H.; Li, Y.-Q.; Jiang, Y.; Gao, F.; et al. Efficient CsPbBr3 Perovskite Light-Emitting Diodes Enabled by Synergetic Morphology Control. Adv. Opt. Mater. 2019, 7, 1801534. [Google Scholar] [CrossRef] [Green Version]
- Park, M.-H.; Jeong, S.-H.; Seo, H.-K.; Wolf, C.; Kim, Y.-H.; Kim, H.; Byun, J.; Kim, J.S.; Cho, H.; Lee, T.-W. Unravelling Additive-Based Nanocrystal Pinning for High Efficiency Organic-Inorganic Halide Perovskite Light-Emitting Diodes. Nano Energy 2017, 42, 157–165. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Zhao, Y.X. Chemical Stability and Instability of Inorganic Halide Perovskites. Energy Environ. Sci. 2019, 12, 1495–1511. [Google Scholar] [CrossRef]
- Butkus, J.; Vashishtha, P.; Chen, K.; Gallaher, J.K.; Prasad, S.K.K.; Metin, D.Z.; Laufersky, G.; Gaston, N.; Halpert, J.E.; Hodgkiss, J.M. The Evolution of Quantum Confinement in CsPbBr 3 Perovskite Nanocrystals. Chem. Mater. 2017, 29, 3644–3652. [Google Scholar] [CrossRef]
- Jiang, C.; Zhong, Z.M.; Liu, B.; He, Z.W.; Zou, J.; Wang, L.; Wang, J.; Peng, J.; Cao, Y. Coffee-Ring-Free Quantum Dot Thin Film Using Inkjet Printing from a Mixed-Solvent System on Modified Zno Transport Layer for Light-Emitting Devices. ACS Appl. Mater. Int. 2016, 8, 26162–26168. [Google Scholar] [CrossRef]
- Jiang, C.; Liu, H.; Liu, B.; Zhong, Z.; Zou, J.; Wang, J.; Wang, L.; Peng, J.; Cao, Y. Improved Performance of Inverted Quantum Dots Light Emitting Devices by Introducing Double Hole Transport Layers. Org. Electron. 2016, 31, 82–89. [Google Scholar] [CrossRef]
- Kim, M.; Park, B. Understanding Temporal Evolution of Electroluminescence Intensity in Lead Sulfide (PbS) Colloidal Quantum Dot Infrared Light-Emitting Diodes. Appl. Sci. 2020, 10, 7440. [Google Scholar] [CrossRef]
- Yao, E.-P.; Yang, Z.L.; Meng, L.; Sun, P.Y.; Dong, S.Q.; Yang, Y.; Yang, Y. High-Brightness Blue and White LEDs based on Inorganic Perovskite Nanocrystals and their Composites. Adv. Mater. 2017, 29, 1606859. [Google Scholar] [CrossRef]
- Li, G.R.; Rivarola, F.W.R.; Davis, N.J.L.K.; Bai, S.; Jellicoe, T.C.; de la Pena, F.; Hou, S.C.; Ducati, C.; Gao, F.; Friend, R.H.; et al. Highly Efficient Perovskite Nanocrystal Light-Emitting Diodes Enabled by a Universal Crosslinking Method. Adv. Mater. 2016, 28, 3528–3534. [Google Scholar] [CrossRef]
- Gangishetty, M.K.; Hou, S.C.; Quan, Q.M.; Congreve, D.N. Reducing Architecture Limitations for Efficient Blue Perovskite Light-Emitting Diodes. Adv. Mater. 2018, 30, 1706226. [Google Scholar] [CrossRef]
- Liu, B.; Gao, D.; Wang, J.; Wang, X.; Wang, L.; Zou, J.; Ning, H.; Peng, J. Progress of White Organic Light-Emitting Diodes. Acta Phys. Chim. Sin. 2015, 31, 1823–1852. [Google Scholar]
- Chen, B.; Liu, B.; Zeng, J.; Nie, H.; Xiong, Y.; Zou, J.; Ning, H.; Wang, Z.; Zhao, Z.; Tang, B. Efficient Bipolar Blue AlEgens for High-Performance Nondoped Blue OLEDs and Hybrid White OLEDs. Adv. Funct. Mater. 2018, 28, 1803369. [Google Scholar] [CrossRef]
- Luo, D.; Xiao, Y.; Hao, M.M.; Zhao, Y.; Yang, Y.; Gao, Y.; Liu, B. Doping-Free White Organic Light-Emitting Diodes without Blue Molecular Emitter: an Unexplored Approach to Achieve High Performance via Exciplex Emission. Appl. Phys. Lett. 2017, 110, 061105. [Google Scholar] [CrossRef]
- Luo, D.; Chen, Q.; Gao, Y.; Zhang, M.L.; Liu, B. Extremely Simplified, High-Performance, and Doping-Free White Organic Light-Emitting Diodes based on a Single Thermally Activated Delayed Fluorescent Emitter. ACS Energy Lett. 2018, 3, 1531–1538. [Google Scholar] [CrossRef]
- Yang, D.; Zou, Y.T.; Li, P.L.; Liu, Q.P.; Wu, L.Z.; Hu, H.C.; Xu, Y.; Sun, B.Q.; Zhang, Q.; Lee, S.-T. Large-Scale Synthesis of Ultrathin Cesium Lead Bromide Perovskite Nanoplates with Precisely Tunable Dimensions and Their Application in Blue Light-Emitting Diodes. Nano Energy 2018, 47, 235–242. [Google Scholar] [CrossRef]
- Wu, Y.; Wei, C.T.; Li, X.M.; Li, Y.L.; Qiu, S.C.; Shen, W.; Cai, B.; Sun, Z.G.; Yang, D.D.; Deng, Z.T.; et al. In Situ Passivation of PbBr64- Octahedra toward Blue Luminescent CsPbBr3 Nanoplatelets with Near 100% Absolute Quantum Yield. ACS Energy. Lett. 2018, 3, 2030–2037. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Xu, M.; Tao, H.; Ying, L.; Zou, J.H.; Wu, H.B.; Peng, J. Highly Efficient Red Phosphorescent Organic Light-Emitting Diodes Based on Solution Processed Emissive Layer. J. Lumin. 2013, 142, 35–39. [Google Scholar] [CrossRef]
- Mai, R.; Wu, X.; Jiang, Y.; Meng, Y.; Liu, B.; Hu, X.; Roncali, J.; Zhou, G.; Liu, J.; Kempa, K.; et al. An Efficient Multi-Functional Material based on Polyether-Substituted Indolocarbazole for Perovskite Solar Cells And Solution-Processed Non-Doped Oleds. J. Mater. Chem. A 2019, 7, 1539–1547. [Google Scholar] [CrossRef]
- Diroll, B.T. Colloidal quantum wells for optoelectronic devices. J. Mater. Chem. C 2020, 8, 10628–10640. [Google Scholar] [CrossRef]
- Zhu, L.P.; Zhao, Y.B.; Zhang, H.M.; Chen, J.S.; Ma, D.G. Colloidal Quantum Wells for Optoelectronic Devices. J. Appl. Phys. 2014, 115, 244512. [Google Scholar] [CrossRef]
- Liu, B.; Zou, J.H.; Zhou, Z.W.; Wang, L.; Xu, M.; Tao, H.; Gao, D.Y.; Lan, L.F.; Ning, H.L.; Peng, J. Efficient Single-Emitting Layer Hybrid white Organic Light-Emitting Diodes with Low Efficiency Roll-Off, Stable Color and Extremely High Luminance. J. Ind. Eng. Chem. 2015, 30, 85–91. [Google Scholar] [CrossRef]
- Liu, B.; Wang, L.; Xu, M.; Tao, H.; Zou, J.H.; Gao, D.Y.; Lan, L.F.; Ning, H.L.; Peng, J.; Cao, Y. Efficient Hybrid White Organic Light-emitting Diodes with Extremely Long Lifetime: the Effect of n-type Interlayer. Sci. Rep. 2014, 4, 7198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honda, Y.; Matsushima, T.; Murata, H. Enhanced Performance of Organic Light-Emitting Diodes by Inserting Wide-Energy-Gap Interlayer between Hole-Transport Layer and Light-Emitting Layer. Thin Solid Films 2009, 518, 545–547. [Google Scholar] [CrossRef]
- Liu, B.; Altintas, Y.; Wang, L.; Shendre, S.; Sharma, M.; Sun, H.; Mutlugun, E.; Demir, H.V. Record High External Quantum Efficiency of 19.2% Achieved in Light-Emitting Diodes of Colloidal Quantum Wells Enabled by Hot-Injection Shell Growth. Adv. Mater. 2020, 32, 1905824. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Zhao, Y.B.; Zhao, F.C.; Chen, Y.H.; Yang, D.Z.; Chen, J.S.; Ma, D.G. A White Organic Light-Emitting Diode with Ultra-High Color Rendering Index, High Efficiency, and Extremely Low Efficiency Roll-Off. Appl. Phys. Lett. 2014, 105, 013303. [Google Scholar] [CrossRef]
- Liu, B.; Luo, D.; Zou, J.; Gao, D.; Ning, H.; Wang, L.; Peng, J.; Cao, Y. A Host-Guest System Comprising High Guest Concentration to Achieve Simplified and High-Performance Hybrid White Organic Light-Emitting Diodes. J. Mater. Chem. C 2015, 3, 6359–6366. [Google Scholar] [CrossRef]
- Ying, L.; Ho, C.L.; Wu, H.B.; Cao, Y.; Wong, W.Y. White Polymer Light-Emitting Devices for Solid-State Lighting: Materials, Devices, and Recent Progress. Adv. Mater. 2014, 26, 2459–2473. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.M.; Yang, Z.; Zhou, N.H.; Deng, Y.H.; Zhao, J.J.; Xiao, X.; Rudd, P.; Moran, A.; Yan, Y.F.; et al. Efficient Sky-Blue Perovskite Light-Emitting Diodes via Photoluminescence Enhancement. Nat. Commun. 2019, 10, 5633. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Delikanli, S.; Gao, Y.; Gungor, K.; Demir, H.V. Nanocrystal Light-Emitting Diodes based on Type Ii Nanoplatelets. Nano Energy 2018, 47, 115–122. [Google Scholar] [CrossRef]
- Luo, D.; Yang, Y.; Xiao, Y.; Zhao, Y.; Yang, Y.; Liu, B. Regulating Charge and Exciton Distribution in High-Performance Hybrid White Organic Light-Emitting Diodes with n-Type Interlayer Switch. Nano-Micro Lett. 2017, 9, 37. [Google Scholar] [CrossRef] [Green Version]
- Jin, F.; Zhao, B.; Chu, B.; Zhao, H.; Su, Z.; Li, W.; Zhu, F. Morphology Control Towards Bright and Stable Inorganic Halide Perovskite Light-Emitting Diodes. J. Mater. Chem. C 2018, 6, 1573–1578. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Cheng, L.P.; Li, Y.Q.; Li, W.; Chen, J.D.; Lee, S.T.; Tang, J.-X. High-Efficiency Perovskite Light-Emitting Diodes with Synergetic Outcoupling Enhancement. Adv. Mater. 2019, 31, 1901517. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.-Y.; Li, Y.-Q.; Meng, S.-S.; Lee, C.-S.; Chen, L.-S.; Tang, J.-X. Extremely Efficient Transparent Flexible Organic Light-Emitting Diodes with Nanostructured Composite Electrodes. Adv. Opt. Mater. 2018, 6, 1800831. [Google Scholar] [CrossRef]
- Liu, B.; Wang, L.; Xu, M.; Tao, H.; Gao, D.Y.; Zou, J.H.; Lan, L.F.; Ning, H.L.; Peng, J.; Cao, Y. Extremely Stable-color Flexible White Organic Light-emitting Diodes with Efficiency Exceeding 100 lm W-1. J. Mater. Chem. C 2014, 2, 9836–9841. [Google Scholar] [CrossRef]
- Luo, D.; Chen, Q.; Liu, B.; Qiu, Y. Emergence of Flexible White Organic Light-Emitting Diodes. Polymers 2019, 11, 384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Xiao, W.P.; Wu, W.J.; Liu, B. Research Progress on Flexible Oxide-Based Thin Film Transistors. Appl. Sci. 2019, 9, 773. [Google Scholar] [CrossRef] [Green Version]
- Hiragond, C.B.; Powar, N.S.; In, S. Recent Developments in Lead and Lead-Free Halide Perovskite Nanostructures towards Photocatalytic CO2 Reduction. Nanomaterials 2020, 10, 2569. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.-H.; Ou, Q.-D.; Li, Y.-Q.; Zhang, Y.-B.; Zhao, X.-D.; Xiang, H.-Y.; Chen, J.-D.; Zhou, L.; Lee, S.-T.; Tang, J.-X. Microcavity-Free Broadband Light Outcoupling Enhancement in Flexible Organic Light-Emitting Diodes with Nanostructured Transparent Metal-Dielectric Composite Electrodes. ACS Nano 2016, 10, 1625–1632. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.-R.; Lee, S.J.; Lee, H.W.; Lee, D.H.; Yang, H.J.; Kim, W.Y.; Kim, Y.K. Flexible Bottom-Emitting White Organic Light-Emitting Diodes with Semitransparent Ni/Ag/Ni Anode. Opt. Express 2013, 21, 11086–11094. [Google Scholar] [CrossRef]
- Ou, Q.D.; Zhou, L.; Li, Y.-Q.; Chen, S.; Chen, J.-D.; Li, C.; Wang, Q.-K.; Lee, S.-T.; Tang, J.-X. Light-Emitting Diodes: Extremely Efficient White Organic Light-Emitting Diodes for General Lighting. Adv. Funct. Mater. 2014, 24, 7249–7256. [Google Scholar] [CrossRef]
- Wierer, J.J.; David, A.; Megens, M.M. III-nitride Photonic-crystal Light-emitting Diodes with High Extraction Efficiency. Nat. Photonics 2009, 3, 163–169. [Google Scholar] [CrossRef]
- Bai, P.; Hu, A.; Liu, Y.; Jin, Y.Z.; Gao, Y.N. Print and In-Situ Assemble CdSe/CdS Nanoplatelets to Uniform Films with Unity In-Plane Transition Dipole Moment. J. Phys. Chem Lett. 2020, 11, 4524–4529. [Google Scholar]
- Lu, M.; Zhang, Y.; Wang, S.X.; Guo, J.; Yu, W.W.; Rogach, A.L. Metal Halide Perovskite Light-Emitting Devices: Promising Technology for Next-Generation Displays. Adv. Funct. Mater. 2019, 29, 1902008. [Google Scholar] [CrossRef]
- Yang, D.; Cao, M.; Zhong, Q.X.; Li, P.L.; Zhang, X.H.; Zhang, Q. All-Inorganic Cesium Lead Halide Perovskite Nanocrystals: Synthesis, Surface Engineering and Applications. J. Mater. Chem. C 2019, 7, 757–789. [Google Scholar] [CrossRef]
- Park, J.-S.; Chae, H.; Chung, H.K.; Lee, S.I. Thin Film Encapsulation for Flexible AM-OLED: A Review. Semicond. Sci. Tech. 2011, 26, 034001. [Google Scholar] [CrossRef]
- Jeon, Y.; Choi, H.-R.; Park, K.C.; Choi, K.C. Flexible Organic Light-Emitting-Diode-Based Photonic Skin for Attachable Phototherapeutics. J. Soc. Inf. Display 2020, 28, 324–332. [Google Scholar] [CrossRef]
- Mizukami, M.; Cho, S.-I.; Watanabe, K.; Abiko, M.; Suzuri, Y.; Tokito, S.; Kido, J. Flexible Organic Light-Emitting Diode Displays Driven by Inkjet-Printed High-Mobility Organic Thin-Film Transistors. IEEE Electron. Device Lett. 2017, 39, 39–42. [Google Scholar] [CrossRef]
- Kim, E.; Han, Y.; Kim, W.; Choi, K.C.; Im, H.G.; Bae, B.S. Thin Film Encapsulation for Organic Light Emitting Diodes Using a Multi-Barrier Composed of Mgo Prepared by Atomic Layer Deposition and Hybrid Materials. Org. Electron. 2013, 14, 1737–1743. [Google Scholar] [CrossRef]
- Xiao, P.; Huang, J.; Yu, Y.; Liu, B. Recent Developments in Tandem White Organic Light-Emitting Diodes. Molecules 2019, 24, 151. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.J.; Wang, Y.A.; Li, L.S.; Wang, D.Y.; Zhu, T.; Xu, J.; Yang, C.H.; Li, Y.F. Multicoloured Light-emitting Diodes Based on Quantum Dots. Nat. Photonics 2007, 1, 717–722. [Google Scholar] [CrossRef]
- Kim, J.H.; Han, S.H.; Lee, J.Y. Concentration Quenching Resistant Donor-Acceptor Molecular Structure for High Efficiency and Long Lifetime Thermally Activated Delayed Fluorescent Organic Light-Emitting Diodes via Suppressed Non-Radiative Channel. Chem. Eng. J. 2020, 395, 125159. [Google Scholar] [CrossRef]
- VanSlyke, S.A.; Chen, C.H.; Tang, C.W. Organic Electroluminescent Devices with Improved Stability. Appl. Phys. Lett. 1996, 69, 2160–2162. [Google Scholar] [CrossRef]
- Fery, C.; Racine, B.; Vaufrey, D.; Doyeux, H.; Cina, S. Physical Mechanism Responsible for the Stretched Exponential Decay Behavior of Aging Organic Light-emitting Diodes. Appl. Phys. Lett. 2005, 87, 213502. [Google Scholar] [CrossRef]
- Chu, T.Y.; Chen, J.F.; Chen, S.Y.; Chen, C.H. Comparative Study of Single and Multiemissive Layers in Inverted White Organic Light-Emitting Devices. Appl. Phys. Lett. 2006, 89, 113502. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.N.; Zhang, M.Y.; Li, C.; Shang, Y.Z.; Lu, Y.F.; Wei, B.; Huang, W. Fine-Tuning the Thicknesses of Organic Layers to Realize High-Efficiency and Long-Lifetime Blue Organic Light-Emitting Diodes. Chin. Phys. B 2012, 21, 083303. [Google Scholar] [CrossRef] [Green Version]
- Chua, T.-Y.; Chen, J.-F.; Chen, S.-Y.; Chen, C.-J.; Chen, C.H. Highly Efficient and Stable Inverted Bottom-Emission Organic Light Emitting Devices. Appl. Phys. Lett. 2006, 89, 053503. [Google Scholar] [CrossRef] [Green Version]
- Dutta, A.; Medda, A.; Patra, A. Recent Advances and Perspectives on Colloidal Semiconductor Nanoplatelets for Optoelectronic Applications. J. Phys. Chem. C 2020. [Google Scholar] [CrossRef]
- Lindla, F.; Boesing, M.; van Gemmern, P.; Bertram, D.; Keiper, D.; Heuken, M.; Kalisch, H.; Jansen, R.H. Employing Exciton Transfer Molecules to Increase the Lifetime of Phosphorescent Red Organic Light Emitting Diodes. App. Phys. Lett. 2011, 98, 173304. [Google Scholar] [CrossRef]
- So, F.; Kondakov, D. Mechanisms in Small-Molecule and Polymer Organic Light-Emitting Diodes. Adv. Mater. 2010, 22, 3762–3777. [Google Scholar] [CrossRef]
- Seifert, R.; de Moraes, I.R.; Scholz, S.; Gather, M.C.; Lussem, B.; Leo, K. Chemical Degradation Mechanisms of Highly Efficient Blue Phosphorescent Emitters Used for Organic Light Emitting Diodes. Org. Electron. 2013, 14, 115–123. [Google Scholar] [CrossRef]
- Yang, W.S.; Noh, J.H.; Jeon, N.J.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. High-Performance Photovoltaic Perovskite Layers Fabricated Through Intramolecular Exchange. Science 2015, 348, 1234–1237. [Google Scholar] [CrossRef]
- Jeon, N.J.; Noh, J.H.; Yang, W.S.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. Compositional Engineeringof Perovskite Materials for High-Performance Solar Cells. Nature 2015, 517, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Bi, D.Q.; Tress, W.; Dar, M.I.; Gao, P.; Luo, J.S.; Renevier, C.; Schenk, K.; Abate, A.; Giordano, F.; Baena, J.-P.C.; et al. Efficient Luminescent Solar Cells Based on Tailored Mixed-Cation Perovskites. Sci. Adv. 2016, 2, e1501170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.X.; Trinh, M.T.; Niesner, D.; Zhu, H.M.; Norman, Z.; Owen, J.S.; Yaffe, O.; Kudisch, B.J.; Zhu, X.Y. Trap States Lead Iodide Perovskites. J. Am. Chem. Soc. 2015, 137, 2089–2096. [Google Scholar] [CrossRef] [PubMed]
- Porotnikov, D.; Zamkov, M. Progress and Prospects of Solution-Processed Two-Dimensional Semiconductor Nanocrystals. J. Phys. Chem. C 2020, 124, 21895–21908. [Google Scholar] [CrossRef]
- Shin, Y.S.; Yoon, Y.J.; Lee, K.T.; Lee, W.; Kim, H.S.; Kim, J.W.; Jang, H.; Kim, M.; Kim, D.S.; Kim, G.H. High-Performance Perovskite Light-Emitting Diodes with Surface Passivation of CsPbBrxI3-x Nanocrystals via Antisolvent-Triggered Ion-Exchange. ACS Appl. Mater. Interfaces 2020, 12, 31582–31590. [Google Scholar] [CrossRef]
- Bu, T.L.; Wu, L.; Liu, X.; Yang, X.P.; Zhou, P.; Yu, X.X.; Qin, T.S.; Shi, J.J.; Wang, S.; Li, S.S.; et al. Synergic Interface Optimization with Green Solvent Engineering in Mixed Perovskite Solar Cells. Adv. Energy. Mater. 2017, 7, 1700576. [Google Scholar] [CrossRef]
- Dong, C.; Han, X.X.; Zhao, Y.; Li, J.J.; Chang, L.; Zhao, W.N. Green Anti-Solvent Process for High Performance Carbon-Based CsPbI2 Br All-Inorganic Perovskite Solar Cell. Sol. RRL 2018, 2, 1800139. [Google Scholar] [CrossRef]
- Zhu, Y.Z.; Zhao, X.F.; Zhang, B.H.; Yao, B.; Li, Z.G.; Qu, Y.; Xie, Z.Y. Very Efficient Green Light-Emitting Diodes Based on Polycrystalline Ch(Nh3)2Pbbr3 Film Achieved by Regulating Precursor Concentration and Employing Novel Anti-Solvent. Org. Electron. 2018, 55, 35–41. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Z.H.; Zhou, B.; Jia, X.G.; Ma, Q.S.; Yuan, N.Y.; Zheng, X.J.; Ding, J.N.; Zhang, W.-H. Green Anti-Solvent Processed Planar Perovskite Solar Cells with Efficiency Beyond 19%. Sol. RRL 2018, 2, 1700213. [Google Scholar] [CrossRef]
- Wang, Z.J.; Huai, B.X.; Yang, G.J.; Wu, M.G.; Yu, J.S. High Performance Perovskite Light-Emitting Diodes Realized by Isopropyl Alcohol as Green Anti-Solvent. J. Lumin. 2018, 204, 110–115. [Google Scholar] [CrossRef]
- Xu, L.B.; Che, S.Y.; Huang, J.Y.; Xie, D.Y.; Yao, Y.X.; Wang, P.; Lin, P.; Piao, H.J.; Hu, H.J.; Cui, C.; et al. Towards Green Antisolvent For Efficient Ch3Nh3Pbbr3 Perovskite Light Emitting Diodes: A Comparison of Toluene, Chlorobenzene, and Ethyl Acetate. Appl. Phys. Lett. 2019, 115, 033101. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, A.Y.; Yu, J.C.; Nam, Y.S.; Choi, Y.; Park, J.; Song, M.H. Surface Ligand Engineering For Efficient Perovskite Nanocrystal-Based Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2019, 11, 8428–8435. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, K.; Chiba, T.; Sato, J.; Hayashi, Y.; Takahashi, Y.; Ebe, H.; Kido, J. Purification of Perovskite Quantum Dots Using Low-Dielectric-Constant Washing Solvent “Diglyme” for Highly Efficient Light-Emitting Devices. ACS Appl. Mater. Interfaces 2018, 10, 24607–24612. [Google Scholar] [CrossRef]
- Li, J.H.; Xu, L.M.; Wang, T.; Song, J.Z.; Chen, J.W.; Xue, J.; Dong, Y.H.; Cai, B.; Shan, Q.S.; Han, B.N.; et al. 50-Fold EQE Improvement up to 6.27% of Solution-Processed All-Inorganic Perovskite CsPbBr3 QLEDs via Surface Ligand Density Control. Adv. Mater. 2017, 29, 1603885. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Wang, Z.; Wang, Y.; Wang, W.; Feng, L.; Xia, S.; Zeng, H. Halide Perovskite Lateral Heterostructures for Energy Routing Based Photonic Applications. Adv. Opt. Mater. 2020, 8, 2001347. [Google Scholar] [CrossRef]
- Pan, J.; Shang, Y.Q.; Yin, J.; de Bastiani, M.; Peng, W.; Dursun, I.; Sinatra, L.; El-Zohry, A.M.; Hedhili, M.N.; Emwas, A.H.; et al. Bidentate Ligand-Passivated CsPbI3 Perovskite Nanocrystals for Stable Near-Unity Photoluminescence Quantum Yield and Efficient Red Light-Emitting Diodes. J. Am. Chem. Soc. 2017, 140, 562–565. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y.S.; Zou, Y.T.; Wu, L.Z.; Huang, Q.; Yang, D.; Chen, M.; Ban, M.Y.; Wu, C.; Wu, T.; Bai, S.; et al. Highly Luminescent and Stable Perovskite Nanocrystals with Octylphosphonic Acid as a Ligand for Efficient Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2018, 10, 3784–3792. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Wan, Q.; Wang, B.; Zheng, W.L.; Liu, M.M.; Zhang, Q.G.; Kong, L.; Li, L. Surface Ligand Engineering toward Brightly Luminescent and Stable Cesium Lead Halide Perovskite Nanoplatelets for Efficient Blue-Light-Emitting Diodes. J. Phys. Chem. C 2019, 123, 26161–26169. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, Z.K.; Yuan, S.; Wang, Q.; Qin, C.C.; Wang, K.L.; Dong, C.; Li, M.; Liu, Y.F.; Liao, L.S. Synergistic Effect of Dual Ligands on Stable Blue Quasi-2D Perovskite Light-Emitting Diodes. Adv. Funct. Mater. 2020, 30, 1908339. [Google Scholar] [CrossRef]
- Wu, Y.A.; Liu, L.H.; Wang, W.; Zhang, W.Z.; Yu, H.T.; Qian, J.; Chen, Y.F.; Shen, W.; Sui, S.Q.; Deng, Z.T.; et al. Enhanced Stability and Performance of Light-Emitting Diodes Based on In Situ Fabricated Fapbbr3 Nanocrystals via Ligand Compensation with n-Octylphosphonic Acid. J. Mater. Chem. C 2020, 8, 9936–9944. [Google Scholar] [CrossRef]
- Erwin, S.C.; Zu, L.J.; Haftel, M.I.; Efros, A.L.; Kennedy, T.A.; Norris, D.J. Doping Semiconductor Nanocrystals. Nature 2005, 436, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Norris, D.J.; Efros, A.L.; Erwin, S.C. ChemInform Abstract: Doped Nanocrystals. Science 2008, 319, 1776–1779. [Google Scholar] [CrossRef] [PubMed]
- Altintas, Y.; Liu, B.; Gheshlaghi, N.; Shabani, F.; Sharma, M.; Wang, L.; Sun, H.D.; Mutlugun, E.; Demir, H.V. Spectrally Wide-Range-Tunable, Efficient, and Bright Colloidal Light-Emitting Diodes of Quasi-2D Nanoplatelets Enabled by Engineered Alloyed Heterostructures. Chem. Mater. 2020, 32, 7874–7883. [Google Scholar] [CrossRef]
- Liu, B.; Sharma, M.; Yu, J.H.; Shendre, S.; Hettiarachchi, C.; Sharma, A.; Yeltik, A.; Wang, L.; Sun, H.D.; Dang, C.; et al. Light-Emitting Diodes with Cu-Doped Colloidal Quantum Wells: From Ultrapure Green, Tunable Dual-Emission to White Light. Small 2019, 15, 1901983. [Google Scholar] [CrossRef]
- Luo, D.; Wang, L.; Qiu, Y.; Huang, R.; Liu, B. Emergence of Impurity-Doped Nanocrystal Light-Emitting Diodes. Nanomaterials 2020, 10, 1226. [Google Scholar] [CrossRef]
- Wang, F.; Han, Y.; Lim, C.S.; Lu, Y.; Wang, J.; Xu, J.; Chen, H.; Zhang, C.; Hong, M.; Liu, X. Simultaneous Phase and Size Control of Upconversion Nanocrystals through Lanthanide Doping. Nature 2010, 463, 1061–1065. [Google Scholar] [CrossRef]
- Liu, W.; Lin, Q.; Li, H.; Wu, K.; Robel, I.; Pietryga, J.; Klimov, V.I. Mn2+ -Doped Lead Halide Perovskite Nanocrystals with Dual-Color Emission Controlled by Halide Content. J. Am. Chem. Soc. 2016, 138, 14954–14961. [Google Scholar] [CrossRef]
- Sharma, M.; Olutas, M.; Yeltik, A.; Kelestemur, Y.; Sharma, A.; Delikanli, S.; Guzelturk, B.; Güngör, K.; McBride, J.R.; Demir, H.V. Understanding the Journey of Dopant Copper Ions in Atomically Flat Colloidal Nanocrystals of Cdse Nanoplatelets Using Partial Cation Exchange Reactions. Chem. Mater. 2018, 30, 3265–3275. [Google Scholar] [CrossRef] [Green Version]
- Dufour, M.; Izquierdo, E.; Livache, C.; Martinez, B.; Silly, M.G.; Pons, T.; Lhuillier, E.; Delerue, C.; Ithurria, S. Doping as a Strategy to Tune Color of 2D Colloidal Nanoplatelets. ACS Appl. Mater. Interfaces 2019, 11, 10128–10134. [Google Scholar] [CrossRef]
- Khan, A.H.; Pinchetti, V.; Tanghe, I.; Dang, Z.; Martín-García, B.; Hens, Z.; van Thourhout, D.; Geiregat, P.; Brovelli, S.; Moreels, I. Tunable and Efficient Red to Near-Infrared Photoluminescence by Synergistic Exploitation of Core and Surface Silver Doping of CdSe Nanoplatelets. Chem. Mater. 2019, 31, 1450–1459. [Google Scholar] [CrossRef]
- van der Stam, W.; Geuchies, J.J.; Altantzis, T.; Bos, K.H.W.V.D.; Meeldijk, J.D.; van Aert, S.; Bals, S.; Vanmaekelbergh, D.; Donega, C.D.M. Highly Emissive Divalent-Ion-Doped Colloidal CsPb1-xMxBr3 Perovskite Nanocrystals through Cation Exchange. J. Am. Chem. Soc. 2017, 139, 4087–4097. [Google Scholar] [CrossRef] [PubMed]
- Akkerman, Q.A.; Meggiolaro, D.; Dang, Z.; Angelis, F.D.; Manna, L. Fluorescent Alloy CsPbxMn1−xI3 Perovskite Nanocrystals with High Structural and Optical Stability. ACS Energy Lett. 2017, 2, 2183–2186. [Google Scholar] [CrossRef] [PubMed]
- Jena, A.K.; Kulkarni, A.; Sanehira, Y.; Ikegami, M.; Miyasaka, T. Stabilization of α-CsPbI3 in Ambient Room Temperature Conditions by Incorporating Eu into CsPbI3. Chem. Mater. 2018, 30, 6668–6674. [Google Scholar] [CrossRef]
- Zhang, C.; Kuang, D.-B.; Wu, W. A Review of Diverse Halide Perovskite Morphologies for Efficient Optoelectronic Applications. Small Methods 2020, 4, 1900662. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Q.; Shi, Y.-L.; Li, M.; Zhang, L.; Wang, Z.-K.; Liao, L.-S. Vacuum-Evaporated All-Inorganic Cesium Lead Bromine Perovskites for High-Performance Light-Emitting Diodes. J. Mater. Chem. C 2017, 5, 8144–8149. [Google Scholar] [CrossRef]
- Li, Z.; Dong, J.; Liu, C.; Guo, J.; Shen, L.; Guo, W. Surface Passivation of Perovskite Solar Cells Toward Improved Efficiency and Stability. Nano-Micro Lett. 2019, 11, 50. [Google Scholar] [CrossRef] [Green Version]
- Yi, C.; Meloni, S.; Boziki, A.; Astani, N.A.; Grätzel, C.; Luo, J.; Zakeeruddin, S.M.; Rothlisberger, U. Entropic Stabilization of Mixed A-Cation ABX3 Metal Halide Perovskites for High Performance Perovskite Solar Cells. Energy Environ. Sci. 2016, 9, 656–662. [Google Scholar] [CrossRef]
- Zhang, X.L.; Liu, H.; Wang, W.G.; Zhang, J.B.; Xu, B.; Karen, K.L.; Zheng, Y.J.; Liu, S.; Chen, S.M.; Wang, K. Hybrid Perovskite Light-Emitting Diodes Based on Perovskite Nanocrystals with Organic-Inorganic Mixed Cations. Adv. Mater. 2017, 29, 1606405. [Google Scholar] [CrossRef]
- Shi, Y.F.; Xi, J.; Lei, T.; Yuan, F.; Dai, J.F.; Ran, C.X.; Dong, H.; Jiao, B.; Hou, X.; Wu, Z.X. Rubidium Doping for Enhanced Performance of Highly Efficient Forma Midinium-Based Perovskite Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2018, 10, 9849–9857. [Google Scholar] [CrossRef]
- Xu, B.; Wang, W.; Zhang, X.; Cao, W.; Wu, D.; Liu, S.; Dai, H.; Chen, S.; Wang, K.; Sun, X.W. Bright and Efficient Light-Emitting Diodes Based on Ma/Cs Double Cation Perovskite Nanocrystals. J. Mater. Chem. C. 2017, 5, 6123–6128. [Google Scholar] [CrossRef]
- Song, J.; Li, J.; Xu, L.; Li, J.; Zhang, F.; Han, B.; Shan, Q.; Zeng, H. Room-Temperature Triple-Ligand Surface Engineering Synergistically Boosts Ink Stability, Recombination Dynamics, and Charge Injection toward EQE-11.6% Perovskite QLEDs. Adv. Mater. 2018, 30, 180076. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.A.; Mhaisalkar, S.G.; Mathews, N.; Boix, P.P. Perovskite Nanoparticles: Synthesis, Properties, and Novel Applications in Photovoltaics and LEDs. Small Methods 2019, 3, 1800231. [Google Scholar] [CrossRef] [Green Version]
- Xing, G.; Kumar, M.H.; Chong, W.K.; Liu, X.; Cai, Y.; Ding, H.; Asta, M.; Grätzel, M.; Mhaisalkar, S.G.; Mathews, N. Solution-Processed Tin-Based Perovskite for Near-Infrared Lasing. Adv. Mater. 2016, 28, 8191–8196. [Google Scholar] [CrossRef] [PubMed]
- Jellicoe, T.C.; Richter, J.M.; Glass, H.F.J.; Tabachnyk, M.; Brady, R.; Dutton, S.E.; Rao, A.; Friend, R.H.; Credgington, D.; Greenham, N.C. Synthesis and Optical Properties of Lead-Free Cesium Tin Halide Perovskite Nanocrystals. J. Am. Chem. Soc. 2016, 138, 2941–2944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.-C.; Wang, W.; Tang, A.; Tsai, H.-Y.; Bao, Z.; Ihara, T.; Yarita, N.; Tahara, H.; Kanemitsu, Y.; Chen, S. High-Performance CsPb1-xSnxBr3 Perovskite Quantum Dots for Light-Emitting Diodes. Angew. Chem. Int. Ed. 2017, 56, 13650–13654. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Liu, Y.; Li, J.; Liu, C.; Feng, R.; Jiang, F.; Li, Y.; Song, J.; Zeng, H.; Hong, M. Stabilizing Cesium Lead Halide Perovskite Lattice through Mn(II) Substitution for Air-Stable Light-Emitting Diodes. J. Am. Chem. Soc. 2017, 139, 11443–11450. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Zou, Y.; Bourelle, S.A.; Zhai, T.; Wu, T.; Tan, Y.; Li, Y.; Li, J.; Duhm, S.; Song, T. Suppressing Defect States in Cspbbr 3 Perovskite via Magnesium Substitution for Efficient All-Inorganic Light-Emitting Diodes. Nanoscale Horiz. 2019, 4, 924–932. [Google Scholar] [CrossRef]
- Luo, D.; Xiao, P.; Liu, B. Doping-Free White Organic Light-Emitting Diodes. Chem. Rec. 2019, 19, 1596–1610. [Google Scholar] [CrossRef]
- Schwartz, G.; Fehse, K.; Pfeiffer, M.; Walzer, K.; Leo, K. Highly Efficient White Organic Light Emitting Diodes Comprising An Interlayer to Separate Fluorescent and Phosphorescent Regions. Appl. Phys. Lett. 2006, 89, 083509. [Google Scholar] [CrossRef]
- Liu, B.; Xu, Z.P.; Zou, J.H.; Tao, H.; Xu, M.; Gao, D.Y.; Lan, L.F.; Wang, L.; Ning, H.L.; Peng, J. High-Performance Hybrid White Organic Light-Emitting Diodes Employing p-Type Interlayers. J. Ind. Eng. Chem. 2015, 27, 240–244. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, X.; Zhang, Y.; Guo, J.; Shen, X.; Yu, W.W.; Rogach, A.L. Simultaneous Strontium Doping and Chlorine Surface Passivation Improve Luminescence Intensity and Stability of CsPbI3 Nanocrystals Enabling Efficient Light-Emitting Devices. Adv. Mater. 2018, 30, 1804691. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.-S.; Ge, J.; Wang, K.-H.; Zhang, G.; Zhu, B.-S.; Chen, C.; Zhang, Q.; Luo, Y.; Yu, S.-H.; Yao, H.-B. Few-Nanometer-Sized α-CsPbI3 Quantum Dots Enabled by Strontium Substitution and Iodide Passivation for Efficient Red-Light Emitting Diodes. J. Am. Chem. Soc. 2019, 141, 2069–2079. [Google Scholar] [CrossRef] [PubMed]
- Himchan, C.; Chritoph, W.; Kim, J.S.; Yun, H.J.; Bae, J.S.; Kim, H.; Heo, J.M.; Ahn, S.; Lee, T.-W. High-Efficiency Solution-Processed Inorganic Metal Halide Perovskite Light-Emitting Diodes. Adv. Mater. 2017, 29, 1700579. [Google Scholar]
- Wei, Z.H.; Perumal, A.; Su, R.; Sunshant, S.; Xing, J.; Zhang, Q.; Tian, S.T.; Demir, H.V.; Xiong, Q.H. Solution-Processed Highly Bright and Durable Cesium Lead Halide Perovskite Light-Emitting Diodes. Nanoscale 2016, 8, 18021–18026. [Google Scholar] [CrossRef]
- Ling, Y.C.; Tian, Y.; Wang, X.; Wang, J.C.; Knox, J.M.; Fernando, P.-O.; Du, Y.J.; Tan, L.; Hanson, K.; Ma, B.W.; et al. Enhanced Optical and Electrical Properties of Polymer-Assisted All-Inorganic Perovskites for Light-Emitting Diodes. Adv. Mater. 2016, 28, 8983–8989. [Google Scholar] [CrossRef]
- Pan, J.; Quan, L.N.; Zhao, Y.B.; Peng, W.; Murali, B.; Sarmah, S.P.; Yuan, M.J.; Sinatra, L.; Alyami, M.N.; Liu, J.K.; et al. Highly Efficient Perovskite-Quantum-Dot Light-Emitting Diodes by Surface Engineering. Adv. Mater. 2016, 28, 8718–8725. [Google Scholar] [CrossRef]
- Zhang, L.Q.; Yang, X.L.; Jiang, Q.; Wang, P.Y.; Yin, Z.G.; Zhang, X.W.; Tan, H.R.; Yang, Y.; Wei, M.Y.; Sutherland, B.R.; et al. Ultra-Bright and Highly Efficient Inorganic Based Perovskite Light-Emitting Diodes. Nat. Commun. 2017, 8, 15640. [Google Scholar] [CrossRef]
- Chen, Q.; Zhou, H.P.; Song, T.B.; Luo, S.; Hong, Z.R.; Duan, H.S.; Dou, L.T.; Liu, Y.S.; Yang, Y. Controllable Self-Induced Passivation of Hybrid Lead Iodide Perovskites Toward High Performance Solar Cells. Nano Lett. 2014, 14, 4158–4163. [Google Scholar] [CrossRef]
- Ban, M.Y.; Zou, Y.T.; Rivett, J.P.H.; Yang, Y.G.; Thomas, T.H.; Tan, Y.S.; Song, T.; Gao, X.Y.; Credgington, D.; Deschler, F.; et al. Solution-Processed Perovskite Light Emitting Diodes with Efficiency Exceeding 15% through Additive-Controlled Nanostructure Tailoring. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Yuan, S.; Wang, Z.K.; Zhuo, M.P.; Tian, Q.S.; Jin, Y.; Liao, L.S. Self-Assembled High Quality Cspbbr3 Quantum Dot Films Toward Highly Efficient Light-Emitting Diodes. ACS Nano 2018, 12, 9541–9548. [Google Scholar] [CrossRef]
- Han, D.B.; Imran, M.; Zhang, M.J.; Chang, S.; Wu, X.-G.; Zhang, X.; Tang, J.L.; Wang, M.S.; Ali, S.; Li, X.G.; et al. Efficient Light-Emitting Diodes Based on in Situ Fabricated Fapbbr3 Nanocrystals: the Enhancing Role of The Ligand-Assisted Reprecipitation Process. ACS Nano 2018, 12, 8808–8816. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zou, Y.T.; Wu, T.; Ban, M.Y.; Pecunia, V.; Han, Y.J.; Liu, Q.P.; Song, T.; Duhm, S.; Sun, B.Q. Improved Performance and Stability of All-Inorganic Perovskite Light-Emitting Diodes by Antisolvent Vapor Treatment. Adv. Funct. Mater. 2017, 27, 1700338. [Google Scholar] [CrossRef]
- Shao, Y.C.; Fang, Y.J.; Li, T.; Wang, Q.; Dong, Q.F.; Deng, Y.H.; Yuan, Y.B.; Wei, H.T.; Wang, M.Y.; Gruverman, A.; et al. Grain Boundary Dominated Ion Migration in Polycrystalline Organic-Inorganic Halide Perovskite Films. Energy Environ. Sci. 2016, 9, 1752–1759. [Google Scholar] [CrossRef]
- Xiao, Z.G.; Kerner, R.A.; Zhao, L.F.; Tran, N.L.; Lee, K.M.; Koh, T.-W.; Scholes, G.D.; Rand, B.P. Efficient Perovskite Light-Emitting Diodes Featuring Nanometresized Crystallites. Nat. Photonics 2017, 11, 108. [Google Scholar] [CrossRef]
- Liu, B.; Zou, J.H.; Su, Y.J.; Gao, D.Y.; Lan, L.F.; Tao, H.; Peng, J. Hybrid White Organic Light Emitting Diodes with Low Efficiency Roll-Off, Stable Color and Extreme Brightness. J. Lumin. 2014, 151, 161–164. [Google Scholar] [CrossRef]
- Liu, B.; Xu, M.; Wang, L.; Tao, H.; Su, Y.; Gao, D.; Zou, J.; Lan, L.; Peng, J. omprehensive Study on the Electron Transport Layer in Blue Flourescent Organic Light-Emitting Diodes. ECS J. Solid State Sci. Technol. 2015, 2, R258–R261. [Google Scholar] [CrossRef]
- Liu, B.; Wang, L.; Zou, J.H.; Tao, H.; Su, Y.J.; Gao, D.Y.; Xu, M.; Lan, L.F.; Peng, J.B. Investigation on Spacers and Structures: A Simple but Effective Approach Toward High-Performance Hybrid White Organic Light Emitting Diodes. Sythetic Met. 2013, 184, 5–9. [Google Scholar] [CrossRef]
- Luo, D.; Li, X.-L.; Zhao, Y.; Gao, Y.; Liu, B. High-Performance Blue Molecular Emitter-Free and Doping-Free Hybrid White Organic Light-Emitting Diodes: an Alternative Concept to Manipulate Charges and Excitons Based on Exciplex and Electroplex Emission. ACS Photonics 2017, 4, 1566–1575. [Google Scholar] [CrossRef]
- Schwartz, G.; Reineke, S.; Rosenow, T.C.; Walzer, K.; Leo, K. Triplet Harvesting in Hybrid White Organic Light-Emitting Diodes. Adv. Funct. Mater. 2009, 19, 1319–1333. [Google Scholar] [CrossRef]
- Wu, B.; Zhou, Y.; Xing, G.; Xu, Q.; Garces, H.F.; Solanki, A.; Goh, T.W.; Padture, N.P.; Sum, T.C. Long Minority-Carrier Diffusion Length and Low Surface-Recombination Velocity in Inorganic Lead-Free CsSnI3Perovskite Crystal for Solar Cells. Adv. Funct. Mater. 2017, 27, 1604818. [Google Scholar] [CrossRef]
- Yao, J.-S.; Ge, J.; Han, B.-N.; Wang, K.-H.; Yao, H.-B.; Yu, H.-L.; Li, J.-H.; Zhu, B.-S.; Song, J.; Chen, C. Ce3+-Doping to Modulate Photoluminescence Kinetics for Efficient CsPbBr3 Nanocrystals Based Light-Emitting Diodes. J. Am. Chem. Soc. 2018, 140, 3626–3634. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cui, J.Y.; Du, K.; Tian, H.; He, Z.F.; Zhou, Q.H.; Yang, Z.L.; Deng, Y.Z.; Chen, D.; Zuo, X.B.; et al. Efficient Blue Light-Emitting Diodes Based on Quantum-Confined Bromide Perovskite Nanostructures. Nat. Photonics 2019, 13, 760. [Google Scholar] [CrossRef]
- Huang, H.; Lin, H.; Kershaw, S.V.; Susha, A.S.; Choy, W.C.H.; Rogach, A.L. Polyhedral Oligomeric Silsesquioxane Enhances The Brightness of Perovskite Nanocrystal-Based Green Light-Emitting Devices. J. Phys. Chem. Lett. 2016, 7, 4398–4404. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Zhang, Z.; Jin, Y.; Niu, Y.; Cao, H.; Liang, X.; Chen, L.; Wang, J.; Peng, X. Solution-Processed, High Performance Light-Emitting Diodes Based on Quantum Dots. Nature 2014, 515, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chen, B.; Wang, Z.; Hung, T.F.; Susha, A.S.; Zhong, H.; Rogach, A.L. Water Resistant CsPbX3 Nanocrystals Coated with Polyhedral Oligomeric Silsesquioxane and Their Use as Solid State Luminophores in All-Perovskite White Light-emitting Devices. Chem. Sci. 2016, 7, 5699–5703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Z.; Zheng, Y.F.; Wang, Z.J.; Yu, J.S. Solvent-Assisted Surface Engineering for High Performance All-Inorganic Perovskite Nanocrystals Light-Emitting Diodes. J. Lumin. 2018, 201, 359–363. [Google Scholar] [CrossRef]
- Wang, L.; Liu, B.; Zhao, X.; Demir, H.V.; Gu, H.; Sun, H. Solvent-Assisted Surface Engineering for High-Performance All-Inorganic Perovskite Nanocrystal Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2018, 10, 19828–19835. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.R.; Giebink, N.C.; Kanno, H.; Ma, B.W.; Thompson, M.E.; Forrest, S.R. Management of Singlet and Triplet Excitons for Efficient White Organic Light-Emitting Devices. Nature 2006, 440, 908–912. [Google Scholar] [CrossRef] [Green Version]
- Shangguan, Z.; Zheng, X.; Zhang, J.; Lin, W.; Guo, W.; Li, C.; Wu, T.; Lin, Y.; Chen, Z. The Stability of Metal Halide Perovskite Nanocrystals—A Key Issue for the Application on Quantum-Dot-Based Micro Light-Emitting Diodes Display. Nanomaterials 2020, 10, 1375. [Google Scholar] [CrossRef]
- Liu, B.; Nie, H.; Zhou, X.B.; Hu, S.B.; Luo, D.X.; Gao, D.Y.; Zou, J.H.; Xu, M.; Wang, L.; Zhao, Z.J.; et al. Manipulation of Charge and Exciton Distribution Based on Blue Aggregation-Induced Emission Fluorophors: A Novel Concept to Achieve High-Performance Hybrid White Organic Light-Emitting Diodes. Adv. Funct. Mater. 2016, 26, 776–783. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, H.; Huang, H.; Reckmeier, C.; Zhang, Y.; Choy, W.C.H.; Rogach, A.L. Enhancing the Brightnessof Cesium Lead Halide Perovskite Nanocrystal Based Green Light-Emitting Devices through the Interface Engineering with Perfluorinated Ionomer. Nano Lett. 2016, 16, 1415–1420. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, L.; Gu, H.; Sun, H.; Demir, H.V. Highly Efficient Green Light-Emitting Diodes from All-Inorganic Perovskite Nanocrystals Enabled by a New Electron Transport Layer. Adv. Opt. Mater. 2018, 5, 1800220. [Google Scholar] [CrossRef] [Green Version]
- Qaid, S.M.H.; Ghaithan, H.M.; Al-Asbahi, B.A.; Aldwayyan, A.S. Ultra-Stable Polycrystalline CsPbBr3 Perovskite–Polymer Composite Thin Disk for Light-Emitting Applications. Nanomaterials 2020, 10, 2382. [Google Scholar] [CrossRef] [PubMed]
- Wu, I.-W.; Wang, P.-S.; Tseng, W.-H.; Chang, J.-H.; Wu, C.-I. Correlations of Impedance–Voltage Characteristics and Carrier Mobility in Organic Light Emitting Diodes. Org. Electron. 2012, 13, 13. [Google Scholar] [CrossRef]
- Mashford, B.S.; Stevenson, M.; Popovic, Z.; Hamilton, C.; Zhou, Z.; Breen, C.; Steckel, J.; Bulovic, V.; Bawendi, M.; Coe-Sullivan, S.; et al. High-efficiency Quantum-dot Light-emitting Devices with Enhanced Charge Injection. Nat. Photonics 2013, 7, 407–412. [Google Scholar] [CrossRef]
- Yang, X.; Ma, Y.; Mutlugun, E.; Zhao, Y.; Leck, K.S.; Tan, S.T.; Demir, H.V.; Zhang, Q.; Du, H.; Sun, X.W. Stable, Efficient, and All-solution-processed Quantum Dot Light-emitting Diodes with Double-sided Metal Oxide Nanoparticle Charge Transport Layers. ACS Appl. Mater. Interfaces 2014, 6, 495–499. [Google Scholar] [CrossRef]
- Kinner, L.; Nau, S.; Popovic, K.; Sax, S.; Burgués-Ceballos, I.; Hermerschmidt, F.; Lange, A.; Boeffel, C.; Choulis, S.A.; List-Kratochvil, E.J.W. Inkjet-printed Embedded Ag-PEDOT: PSS Electrodes with Improved Light Out Coupling Effects for Highly Efficient ITO-freeBlue Polymer Light Emitting Diodes. Appl. Phys. Lett. 2017, 110, 101107. [Google Scholar] [CrossRef]
- Shen, P.Y.; Li, X.M.; Cao, F.; Ding, X.W.; Yang, X.Y. Highly Efficient, All-solution-processed, Flexible White Quantum Dot Light-emitting Diodes. J. Mater. Chem. C 2018, 6, 9642–9648. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, D.; Li, C.; Xu, F.; Lei, W.; Sun, L.; Nathan, A.; Sun, X.W. All Solution-processed Stable White Quantum Dot Light-emitting Diodes with Hybrid ZnO@TiO2 as Blue Emitters. Sci. Rep. 2014, 4, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Shi, Z.; Li, Y.; Li, S.; Li, X.J.; Wu, D.; Xu, T.T.; Tian, Y.T.; Chen, Y.S.; Zhang, Y.T.; Zhang, B.L.; et al. Localized Surface Plasmon Enhanced All-Inorganic Perovskite Quantum Dot Light-Emitting Diodes Based onCoaxial Core/Shell Heterojunction Architecture. Adv. Funct. Mater. 2018, 28, 1707031. [Google Scholar] [CrossRef]
- Shi, Z.; Li, Y.; Zhang, Y.T.; Chen, Y.S.; Li, X.J.; Wu, D.; Xu, T.T.; Shan, C.X.; Du, G.T. High-Efficiency and Air-Stable Perovskite Quantum Dots Light-Emitting Diodes with an All-Inorganic Heterostructure. Nano Lett. 2017, 17, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Li, S.; Li, Y.; Ji, H.; Li, X.; Wu, D.; Xu, T.; Chen, Y.; Tian, Y.; Zhang, Y.; et al. Strategy of solution-processed all-inorganic Heterostructure for Humidity/temperature-stable Perovskite Quantum Dot light-emitting Diodes. ACS Nano 2018, 12, 1462–1472. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, Z.; Sun, W.; Zhang, J.; Hu, S.; Hayat, T.; Alsaedi, A.; Tan, Z. All-solution-processed Perovskite Light-emitting diodes with All Metal Oxide Transport Layers. Chem. Commun. 2018, 54, 13283–13286. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Pan, Z.; Zhang, Z.; Ahmad, I.; Chen, J.; Liu, M.; Cheng, S.; Xu, Y.; Wu, J.; Lei, W.; et al. Interfacial Energy-Level Alignment for High-Performance All-Inorganic Perovskite CsPbBr3Quantum Dot-Based Inverted Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2018, 10, 13236–13243. [Google Scholar] [CrossRef] [PubMed]
- Khan, Q.; Subramanian, A.; Yu, G.; Maaz, K.; Li, D.; Sagar, R.U.R.; Chen, K.; Lei, W.; Shabbir, B.; Zhang, Y. Structure Optimization of Perovskite Quantum Dot Light-emitting Diodes. Nanoscale 2019, 11, 5021–5029. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.B.; Luo, Z.; Zhao, C.Y.; Guo, Q.; Wang, Y.P.; Wang, F.Z.; Bian, X.M.; Alsaedi, A.; Hayat, T.; Tan, Z.A. Efficient and Stable Pure Green All-Inorganic Perovskite CsPbBr3 Light-Emitting Diodes with a Solution-Processed NiOx Interlayer. J. Phys. Chem. C 2017, 121, 28132–28138. [Google Scholar] [CrossRef]
- Chih, Y.K.; Wang, J.C.; Yang, R.T.; Liu, C.C.; Chang, Y.C.; Fu, Y.S.; Lai, W.C.; Chen, P.; Wen, T.C.; Huang, Y.C.; et al. NiOx Electrode Interlayer and CH3NH2/CH3NH3PbBr3 Interface Treatment to Markedly Advance Hybrid Perovskite-Based Light-Emitting Diodes. Adv. Mater. 2016, 28, 8687–8694. [Google Scholar] [CrossRef]
- Zhuang, S.W.; He, J.; Ma, X.; Zhao, Y.; Wang, H.; Zhang, B.L. Fabrication and optimization of hole transport layer NiO for all inorganic perovskite light emitting diodes. Mat. Sci. Semicon. Proc. 2020, 109, 104924. [Google Scholar] [CrossRef]
- Liu, B.; Tao, H.; Wang, L.; Gao, D.Y.; Liu, W.C.; Zou, J.H.; Xu, M.; Ning, H.L.; Peng, J.B.; Cao, Y. High-performance Doping-free Hybrid White Organic Light-emitting Diodes: The Exploitation of Ultrathin Emitting Nanolayers (<1 nm). Nano Energy 2016, 26, 26–36. [Google Scholar] [CrossRef]
- Liu, B.; Xu, M.; Wang, L.; Yan, X.; Tao, H.; Su, Y.; Gao, D.; Lan, L.; Zou, J.; Peng, J. Regulating charges and excitons in simplified hybrid white organic light-emitting diodes: The key role of concentration in single dopant host–guest systems. Org. Electron. 2014, 15, 926–936. [Google Scholar] [CrossRef]
- Liu, B.; Wang, L.; Gao, D.Y.; Zou, J.H.; Ning, H.L.; Peng, J.B.; Cao, Y. Extremely High-efficiency and Ultrasimplified Hybrid White Organic Light-emitting Diodes Exploiting Double Multifunctional Blue Emitting Layers. Light Sci. Appl. 2016, 5, e16137. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zou, J.; Dai, C.; Zhang, Y.; Luo, X.; Liu, B. High-Efficiency and High-Luminance Three-Color White Organic Light-Emitting Diodes with Low Efficiency Roll-Off. ECS J. Solid State Sci. Technol. 2018, 7, R99. [Google Scholar] [CrossRef]
- Liu, B.; Xu, M.; Tao, H.; Su, Y.J.; Gao, D.Y.; Zou, J.H.; Lan, L.F.; Peng, J.B. The Effect of Spacer in Hybrid White Organic Light Emitting Diodes. Chinese Sci. Bull. 2014, 59, 3090–3097. [Google Scholar] [CrossRef]
- Liu, B.; Tao, H.; Su, Y.J.; Gao, D.Y.; Lan, L.F.; Zou, J.H.; Peng, J.B. Color-stable, Reduced Efficiency Roll-off hybrid White Organic Light Emitting Diodes with Ultra High Brightness. Chinese Phys. B 2013, 22, 077303. [Google Scholar] [CrossRef]
- Liu, B.; Wang, L.; Gao, D.Y.; Xu, M.; Zhu, X.H.; Zou, J.H.; Lan, L.F.; Ning, H.L.; Peng, J.B.; Cao, Y. Harnessing Charge and Exciton Distribution Towards Extremely High Performance: The Critical Role of Guests in Single-emitting-layer White OLEDs. Mater. Horiz. 2015, 2, 536–544. [Google Scholar] [CrossRef]
- Sun, Y.Z.; Jiang, Y.B.; Sun, X.W.; Zhang, S.D.; Chen, S.M. Beyond OLED: Efficient Quantum Dot Light-Emitting Diodes for Display and Lighting Application. Chem. Rec. 2019, 19, 1729–1752. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, X.; Deng, J.; Chu, Z.; Jiang, Q.; Meng, J.; Wang, P.; Zhang, L.; Yin, Z.; You, J. Efficient Green Light-emitting diodes Based on Quasi-two-dimensional Composition and Phase Engineered Perovskite with Surface Passivation. Nat. Commun. 2018, 9, 570–577. [Google Scholar] [CrossRef]
- Zhao, L.; Rolston, N.; Lee, K.M.; Zhao, X.; Reyes-Martinez, M.A.; Tran, N.L.; Yeh, Y.-W.; Yao, N.; Scholes, G.D.; Loo, Y.-L.; et al. Influence of Bulky Organo-Ammonium Halide Additive Choice on the Flexibility and Efficiency of Perovskite Light-Emitting Devices. Adv. Funct. Mater. 2018, 28, 1802060. [Google Scholar] [CrossRef]
- Zhao, L.; Lee, K.M.; Roh, K.; Khan, S.U.Z.; Rand, B.P. Improved Outcoupling Efficiency and Stability of Perovskite Light-Emitting Diodes using Thin Emitting Layers. Adv. Mater. 2019, 31, 1805836. [Google Scholar] [CrossRef]
- Zhao, L.; Gao, J.; Lin, Y.L.; Yeh, Y.-W.; Lee, K.M.; Yao, N.; Loo, Y.-L.; Rand, B.P. Electrical Stress Influences the Efficiency of CH3NH3PbI3 Perovskite Light Emitting Devices. Adv. Mater. 2017, 29, 1605317. [Google Scholar] [CrossRef]
- Skurlov, I.; Sokolova, A.; Galle, T.; Cherevkov, S.; Ushakova, E.; Baranov, A.; Lesnyak, V.; Fedorov, A.; Litvin, A. Temperature-Dependent Photoluminescent Properties of PbSe Nanoplatelets. Nanomaterials 2020, 10, 2570. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.F.; Wu, X.Y.; Ji, X.X.; Ren, J.; Wang, Q.; Li, G.Q.; Yang, X.H. Modified Conducting Polymer Hole Injection Layer for High-Efficiency Perovskite Light-Emitting Devices: Enhanced Hole Injection and Reduced Luminescence Quenching. J. Phys. Chem. Lett. 2017, 8, 4691–4697. [Google Scholar] [CrossRef]
- Reineke, S.; Lindner, F.; Schwartz, G.; Seidler, N.; Walzer, K.; Lüssem, B.; Leo, K. White Organic Light-Emitting Diodes with Fluorescent Tube Efficiency. Nature 2009, 459, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.-S.; Li, Y.-Q.; Tang, J.-X. Theoretical Perspective to Light Outcoupling and Management in Perovskite Light-Emitting Diodes. Org. Electron. 2018, 61, 351. [Google Scholar] [CrossRef]
- Shi, X.-B.; Liu, Y.; Yuan, Z.; Liu, X.-K.; Miao, Y.; Wang, J.; Lenk, S.; Reineke, S.; Gao, F. Optical Energy Losses in Organic–Inorganic Hybrid Perovskite Light-Emitting Diodes. Adv. Opt. Mater. 2018, 6, 1800667. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.-W.; Yuan, S.; Sun, S.-Q.; Luo, W.; Zhang, Y.-J.; Liao, L.-S.; Fung, M.-K. Interfacial Engineering for Highly Efficient Quasi-Two Dimensional Organic–Inorganic Hybrid Perovskite Light-Emitting Diodes. J. Mater. Chem. C 2019, 7, 4344–4349. [Google Scholar] [CrossRef]
- Jeon, S.; Zhao, L.F.; Jung, Y.J.; Kim, J.W.; Kim, S.Y.; Kang, H.; Jeong, J.H.; Rand, B.P.; Lee, J.H. Perovskite Light-Emitting Diodes with Improved Outcoupling Using a High-Index Contrast Nanoarray. Small 2019, 15, 1900135. [Google Scholar] [CrossRef]
- Purcell, E.M. Spontaneous Emission Probabilities at Radio Frequencies. Phys. Rev. 1946, 69, 681. [Google Scholar]
- Vahala, K.J. Optical Microcavities. Nature 2003, 424, 839–846. [Google Scholar] [CrossRef]
- Miao, Y.F.; Cheng, L.; Zou, W.; Gu, L.H.; Zhang, J.; Guo, Q.; Peng, Q.M.; Xu, M.M.; He, Y.R.; Zhang, S.T.; et al. Microcavity Top-Emission Perovskite Light-Emitting Diodes. Light Sci. Appl. 2020, 9, 89. [Google Scholar] [CrossRef]
- Wu, J.B.; Agrawal, M.; Becerril, H.A.; Bao, Z.A.; Liu, Z.F.; Chen, Y.S.; Peumans, P. Organic Light-Emitting Diodes on Solution Processed Graphene Transparent Electrodes. ACS Nano 2010, 4, 43–48. [Google Scholar] [CrossRef]
- Sun, T.; Wang, Z.L.; Shi, Z.J.; Ran, G.Z.; Xu, W.J.; Wang, Z.Y.; Li, Y.Z.; Dai, L.; Qin, G.G. Multilayered Graphene Used as Anode of Organic Light Emitting Devices. Appl. Phys. Lett. 2010, 96, 133301. [Google Scholar] [CrossRef]
- Liu, B.; Xu, M.; Wang, L.; Tao, H.; Su, Y.J.; Gao, D.Y.; Lan, L.F.; Zou, J.H.; Peng, J.B. Simplified Hybrid White Organic Light-Emitting Diodes with Efficiency/Efficiency Roll-Off/Color Rendering Index/Color-Stability Trade-Off. Phys. Status Solidi RRL 2014, 8, 719–723. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Li, Y.-H.; Lei, I.-A.; Kuo, C.-Y.; Lee, C.-F.; Chiu, W.-Y.; Don, T.-M. Enhanced Reliability of Leds Encapsulated with Surface-Modified Zirconia/Silicone Hybrids under Thermal Shock. Mater. Chem. Phys. 2018, 206, 136–143. [Google Scholar] [CrossRef]
- He, X.; Wang, Z.; Pu, Y.; Wan, D.; Tang, R.; Cui, S.; Wang, J.-X.; Chen, J.-F. High-Gravityassisted Scalable Synthesis of Zirconia Nanodispersion for Light Emitting Diodes Encapsulation with Enhanced Light Extraction Efficiency. Chem. Eng. Sci. 2019, 195, 1–10. [Google Scholar] [CrossRef]
- Zhang, M.; Hofle, S.; Czolk, J.; Mertens, A.; Colsmann, A. All-Solution Processed Transparent Organic Light Emitting Diodes. Nanoscale 2015, 7, 20009–20014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; Xiang, H.-Y.; Shen, S.; Li, Y.-Q.; Chen, J.-D.; Xie, H.-J.; Goldthorpe, I.A.; Chen, L.-S.; Lee, S.-T.; Tang, J.-X. High-Performance Flexible Organic Light-Emitting Diodes Using Embedded Silver Network Transparent Electrodes. ACS Nano 2014, 8, 12796–12805. [Google Scholar] [CrossRef]
- He, X.L.; Tang, R.J.; Pu, Y.; Wang, J.X.; Wang, Z.; Wang, D.; Chen, J.F. High-Gravity-Hydrolysis Approach to Transparent Nanozirconia/Silicone Encapsulation Materials of Light Emitting Diodes Devices for Healthy Lighting. Nano Energy 2019, 62, 1–10. [Google Scholar] [CrossRef]
- Chwang, A.B.; Rothman, M.A.; Mao, S.Y.; Hewitt, R.H.; Weaver, M.S.; Silvernail, J.A.; Rajan, K.; Hack, M.; Brown, J.J.; Chu, X. Thin Film Encapsulated Flexible Organic Electroluminescent Displays. Appl. Phys. Lett. 2003, 83, 413–415. [Google Scholar] [CrossRef]
- Seo, S.W.; Chae, H.; Seo, S.J.; Chung, H.K.; Cho, S.M. Extremely Bendable Thin-Film Encapsulation of Organic Light-Emitting Diodes. Appl. Phys. Lett. 2013, 102, 161908. [Google Scholar] [CrossRef]
- Seo, S.W.; Jung, E.; Seo, S.J.; Chae, H.; Chung, H.K.; Cho, S.M. Toward Fully Flexible Multilayer Moisture-Barriers for Organic Light-Emitting Diodes. J. Appl. Phys. 2013, 114, 143505. [Google Scholar] [CrossRef]
- Meyer, J.; Schneidenbach, D.; Winkler, T.; Hamwi, S.; Weimann, T.; Hinze, P.; Ammermann, S.; Johannes, H.H.; Riedl, T.; Kowalsky, W. Reliable Thin Film Encapsulation for Organic Light Emitting Diodes Grown by Low-Temperature Atomic Layer Deposition. Appl. Phys. Lett. 2009, 94, 233305. [Google Scholar] [CrossRef]
- Kenuning, W.; van de Weijer, P.; Lifka, H.; Kessels, W.M.M.; Creatore, M. Cathode Encapsulation of Organic Light Emitting Diodes by Atomic Layer Deposited Al2O3 Films and Al2O3/a-SiNx:H Stacks. J. Vac. Sci. Technol. A 2012, 30, 01A131. [Google Scholar] [CrossRef]
- Kim, Y.H.; Cho, H.; Heo, J.H.; Kim, T.S.; Myoung, N.; Lee, C.L.; Im, S.H.; Lee, T.W. Multicolored Organic/Inorganic Hybrid Perovskite Light-Emitting Diodes. Adv. Mater. 2015, 27, 1403751. [Google Scholar] [CrossRef]
- Bade, S.G.R.; Li, J.Q.; Shan, X.; Ling, Y.C.; Tian, Y.; Dilbeck, T.; Besara, T.; Geske, T.; Gao, H.W.; Ma, B.W. Fully Printed Halide Perovskite Light-Emitting Diodes with Silver Nanowire Electrodes. ACS Nano 2016, 10, 195–1801. [Google Scholar] [CrossRef]
- Tong, Y.; Yao, E.P.; Manzi, A.; Bladt, E.; Wang, K.; Doblinger, M.; Bals, S.; Uran, A.S.; Polavarapu, L. Spontaneous Self-Assembly of Perovskite Nanocrystals into Electronically Coupled Supercrystals: Toward Filling the Green Gap. Adv. Mater. 2018, 30, 1801117. [Google Scholar] [CrossRef]
- Lu, M.; Wu, H.; Zhang, X.Y.; Wang, H.; Hu, Y.; Colvin, V.L.; Zhang, Y.; Yu, W.W. Highly Flexible CsPbI3 Perovskite Nanocrystal Light-Emitting Diodes. Chemnanomat 2018, 5, 313–317. [Google Scholar] [CrossRef]
- Duan, L.; Zhang, D.Q.; Wu, K.W.; Huang, X.Q.; Wang, L.D.; Qiu, Y. Controlling the Recombination Zone of White Organic Light-Emitting Diodes with Extremely Long Lifetimes. Adv. Funct. Mater. 2011, 21, 3540–3545. [Google Scholar] [CrossRef]
- Jou, J.-H.; Wu, R.-Z.; Yu, H.-H.; Li, C.-J.; Jou, Y.-C.; Peng, S.-H.; Chen, Y.-L.; Chen, C.-T.; Shen, S.-M.; Joers, P.; et al. Artificial Dusk-Light Based on Organic Light Emitting Diodes. ACS Photonics 2014, 1, 27–31. [Google Scholar] [CrossRef]
- Liu, B.; Xu, M.; Wang, L.; Tao, H.; Su, Y.; Gao, D.; Lan, L.; Zou, J.; Peng, J. Very-High Color Rendering Index Hybrid White Organic Light-Emitting Diodes with Double Emitting Nanolayers. Nano-Micro Lett. 2014, 6, 335–339. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Luo, D.; Gao, D.; Wang, X.; Xu, M.; Zou, J.; Ning, H.; Wang, L.; Peng, J.; Cao, Y. An Ideal Host-Guest System to Accomplish High-Performance Greenishyellow and Hybrid White Organic Light-Emitting Diodes. Org. Electron. 2015, 27, 29–34. [Google Scholar] [CrossRef]
- Yantara, N.; Bhaumik, S.; Yan, F.; Sabba, D.; Dewi, H.A.; Mathews, N.; Boix, P.P.; Demir, H.V.; Mhaisalkar, S. Inorganic Halide Perovskites for Efficient Light-Emitting Diodes. J. Phys. Chem. Lett. 2015, 6, 4360–4364. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Song, J.; Han, B.; Fang, T.; Li, J.; Zeng, H. High-Effciency Pure-Color Inorganic Halide Perovskite Emitters for Ultrahigh-Defnition Displays: Progressfor Backlighting Displays and Electrically Driven Devices. Small Methods 2018, 2, 1700382. [Google Scholar] [CrossRef]
- Zhang, F.; Cai, B.; Song, J.Z.; Han, B.; Zhang, B.; Zeng, H.B. Efficient Blue Perovskite Light-Emitting Diodes Boosted by 2D/3D Energy Cascade Channels. Adv. Funct. Mater. 2020, 30, 2001732. [Google Scholar] [CrossRef]
- Pust, P.; Schmidt, P.J.; Schnick, W. A Revolution in Lighting. Nat. Mater. 2015, 14, 454–458. [Google Scholar] [CrossRef]
- Zhu, P.; Tansu, N. Effect of Packing Density and Packing Geometry on Light Extraction of III-Nitride Light-Emitting Diodes with Microsphere Arrays. Photonics Res. 2015, 3, 184–191. [Google Scholar] [CrossRef]
- Zhu, P.; Tansu, N. Resonant Cavity Effect Optimization of iii-nitride Thin Film Flip-Chip Light-Emitting Diodes with Microsphere Arrays. Appl. Opt. 2019, 54, 6305–6312. [Google Scholar] [CrossRef]
- Yang, B.; Chen, J.S.; Yang, S.Q.; Hong, F.; Sun, L.; Han, P.G.; Pullerits, T.; Deng, W.Q.; Han, K.L. Lead-Free Sliver-Bismuch Halide Double Perovskite Nanocrystals. Angew. Chem. Int. Ed. 2018, 57, 5359–5363. [Google Scholar] [CrossRef]
- Ahmed, G.H.; Yin, J.; Bark, O.M.; Mohammed, O.F. Near-Unity Photoluminescence Quantum Yield in Inorganic Perovskite Nanocrystals by Metal-Ion Doping. J. Chem. Phys. 2020, 152, 020902. [Google Scholar] [CrossRef]
- Liu, Y.; Jing, Y.Y.; Zhao, J.; Liu, Q.L.; Xia, Z.G. Design optimization of lead-free perovskite Cs2AgInCl6: Bi nanocrystals with 11.4% photoluminescence quantum yield. Chem. Mater. 2019, 31, 3333–3339. [Google Scholar] [CrossRef]
- Liu, B.; Nie, H.; Lin, G.; Hu, S.; Gao, D.; Zou, J.; Xu, M.; Wang, L.; Zhao, Z.; Ning, H.; et al. High-Performance Doping-Free Hybrid White OLEDs Based on Blue Aggregation-Induced Emission Luminogens. ACS Appl. Mater. Interfaces 2017, 9, 34162–34171. [Google Scholar] [CrossRef] [PubMed]
- Castan, A.; Kim, H.-M.; Jang, J. All-Solution-Processed Inverted Quantum-Dot Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2014, 6, 2508–2515. [Google Scholar] [CrossRef] [PubMed]
- Jou, J.H.; Wu, M.H.; Shen, S.M.; Wang, H.C.; Chen, S.Z.; Chen, S.H.; Lin, C.R.; Hsieh, Y.L. Sunlight-Style Color-Temperature Tunable Organic Light-Emitting Diode. Appl. Phys. Lett. 2009, 95, 013307. [Google Scholar] [CrossRef]
- Liu, B.; Wang, L.; Tao, H.; Xu, M.; Zou, J.; Ning, H.; Peng, J.; Cao, Y. Doping-Free Tandem White Organic Light-Emitting Diodes. Sci. Bull. 2017, 62, 1193–1200. [Google Scholar] [CrossRef] [Green Version]
- Chiba, T.; Pu, Y.J.; Kido, J. Solution-Processed White Phosphorescent Tandem Organic Light-Emitting Devices. Adv. Mater. 2015, 27, 4681–4687. [Google Scholar] [CrossRef]
- Chen, Y.H.; Chen, J.S.; Ma, D.G.; Yan, D.H.; Wang, L.X.; Zhu, F.R. High Power Efficiency Tandem Organic Light-Emitting Diodes Based on Bulk Heterojunction Organic Bipolar Charge Generatio Layer. Appl. Phys. Lett. 2011, 98, 243309. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, B.; Chu, B.; Li, W.; Su, Z.; Yan, X.; Liu, C.; Wu, H.; Gao, Y.; Jin, F.; et al. Simple Structured Hybrid WOLEDs Based on Incomplete Energy Transfer Mechanism: From Blue Exciplex to Orange Dopant. Sci. Rep. 2015, 5, 10234. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Xu, M.; Wang, L.; Su, Y.J.; Gao, D.Y.; Tao, H.; Lan, L.F.; Zou, J.H.; Peng, J.B. High-Performance Hybrid White Organic Light-Emitting Diodes Comprising Ultrathin Blue and Orange Emissive Layers. Appl. Phys. Express 2013, 6, 122101. [Google Scholar] [CrossRef]
- Liu, B.; Lan, L.; Zou, J.; Peng, J. A Novel Organic Light-Emitting Diode by Utilizing Double Hole Injection Layer. Acta Phys. Sin. 2013, 62, 087302. [Google Scholar]
- Saraf, R.; Pu, L.; Maheshwari, V. A Light Harvesting, Self-Powered Monolith Tactile Sensor Based on Electric Field Induced Effects in MAPbI3 Perovskite. Adv. Mater. 2018, 30, 1705778. [Google Scholar] [CrossRef]
- Weng, Z.; Qin, J.; Umar, A.A.; Wang, J.; Zhang, X.; Wang, H.; Cui, X.; Li, X.; Zheng, L.; Zhang, Y. Lead-Free Cs2BiAgBr6 Double Perovskite-Based Humidity Sensor with Superfast Recovery Time. Adv. Funct. Mater. 2019, 29, 1902234. [Google Scholar] [CrossRef]
- Al-Asbahi, B.A.; Jumali, M.H.H.; AlSalhi, M.S. Enhanced Optoelectronic Properties of PFO/Fluorol 7GA Hybrid Light Emitting Diodes via Additions of TiO2 Nanoparticles. Polymers 2016, 8, 334. [Google Scholar] [CrossRef] [PubMed]
- Zaumseil, J.; Donley, C.L.; Kim, J.S.; Friend, R.H.; Sirringhaus, H. Efficient Top-Gate, Ambipolar, Light-Emitting Field-Effect Transistors Based on A Green-Light-Emitting Polyfluorene. Advanced Materials. Adv. Mater. 2006, 18, 2708. [Google Scholar] [CrossRef]
- Xiao, P.; Huang, J.; Dong, T.; Xie, J.; Yuan, J.; Luo, D.; Liu, B. Room-Temperature Fabricated Thin-Film Transistors Based on Compounds with Lanthanum and Main Family Element Boron. Molecules 2018, 23, 1373. [Google Scholar] [CrossRef] [PubMed] [Green Version]










| Emitters a | Von b (V) | EQEmax c (%) | Lmax d (cd m−2) | Lifetime e | Reference |
|---|---|---|---|---|---|
| CsTFA-derived CsPbBr3 | 2.8 | 10.5 | 16,436 | T50 = 250 h at 100 cd m−2 | [28] |
| CsPbBr3-PEO-CF | 2.6 | 4.76 | 51,890 | 82% of the initial efficiency after 80 h | [124] |
| OPA-CsPbBr3 | 2.8 | 6.5 | 7085 | >50% of the initial efficiency after 30 min | [137] |
| DDAB-CsPbBr3 NPLs | 3.6 | 1.42 | 41.8 | T50 ≈ 42 s at 1 mA cm−2 | [138] |
| FA0.8Cs0.2PbBr3 | 3.5 | 2.8 | 55,005 | T50 ≈ 85 s | [158] |
| CsPb0.9Mg0.1Br3 | ~2.7 | 3.6 | 25,450 | T50 = 138 min at ~100 cd m−2 | [167] |
| CsPbBr3 | 5.8 | 0.35 | 2983 | T50 ≈ 120 s at 7 V | [193] |
| CsPbBr3 | 4.8 | 1.43 | 452 | T50 = 460 s at 9 V | [202] |
| CsPbBr3 | 3.0 | 2.39 | 3809 | ~80% of the initial efficiency after 10 h | [211] |
| FAPbBr3 | - | 11.3 | 79,700 | T50 ≈ 6 min at 10 mA cm−2 | [229] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, P.; Yu, Y.; Cheng, J.; Chen, Y.; Yuan, S.; Chen, J.; Yuan, J.; Liu, B. Advances in Perovskite Light-Emitting Diodes Possessing Improved Lifetime. Nanomaterials 2021, 11, 103. https://doi.org/10.3390/nano11010103
Xiao P, Yu Y, Cheng J, Chen Y, Yuan S, Chen J, Yuan J, Liu B. Advances in Perovskite Light-Emitting Diodes Possessing Improved Lifetime. Nanomaterials. 2021; 11(1):103. https://doi.org/10.3390/nano11010103
Chicago/Turabian StyleXiao, Peng, Yicong Yu, Junyang Cheng, Yonglong Chen, Shengjin Yuan, Jianwen Chen, Jian Yuan, and Baiquan Liu. 2021. "Advances in Perovskite Light-Emitting Diodes Possessing Improved Lifetime" Nanomaterials 11, no. 1: 103. https://doi.org/10.3390/nano11010103
APA StyleXiao, P., Yu, Y., Cheng, J., Chen, Y., Yuan, S., Chen, J., Yuan, J., & Liu, B. (2021). Advances in Perovskite Light-Emitting Diodes Possessing Improved Lifetime. Nanomaterials, 11(1), 103. https://doi.org/10.3390/nano11010103







