Lab-on-Microsphere—FRET-Based Multiplex Sensor Platform
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of AIS/ZnS QDs
2.3. Preparation of AIS/ZnS-Doped Microspheres
2.4. Equipment
3. Results and Discussion
3.1. Spectroscopy of AIS QDs and Cyanine Dyes
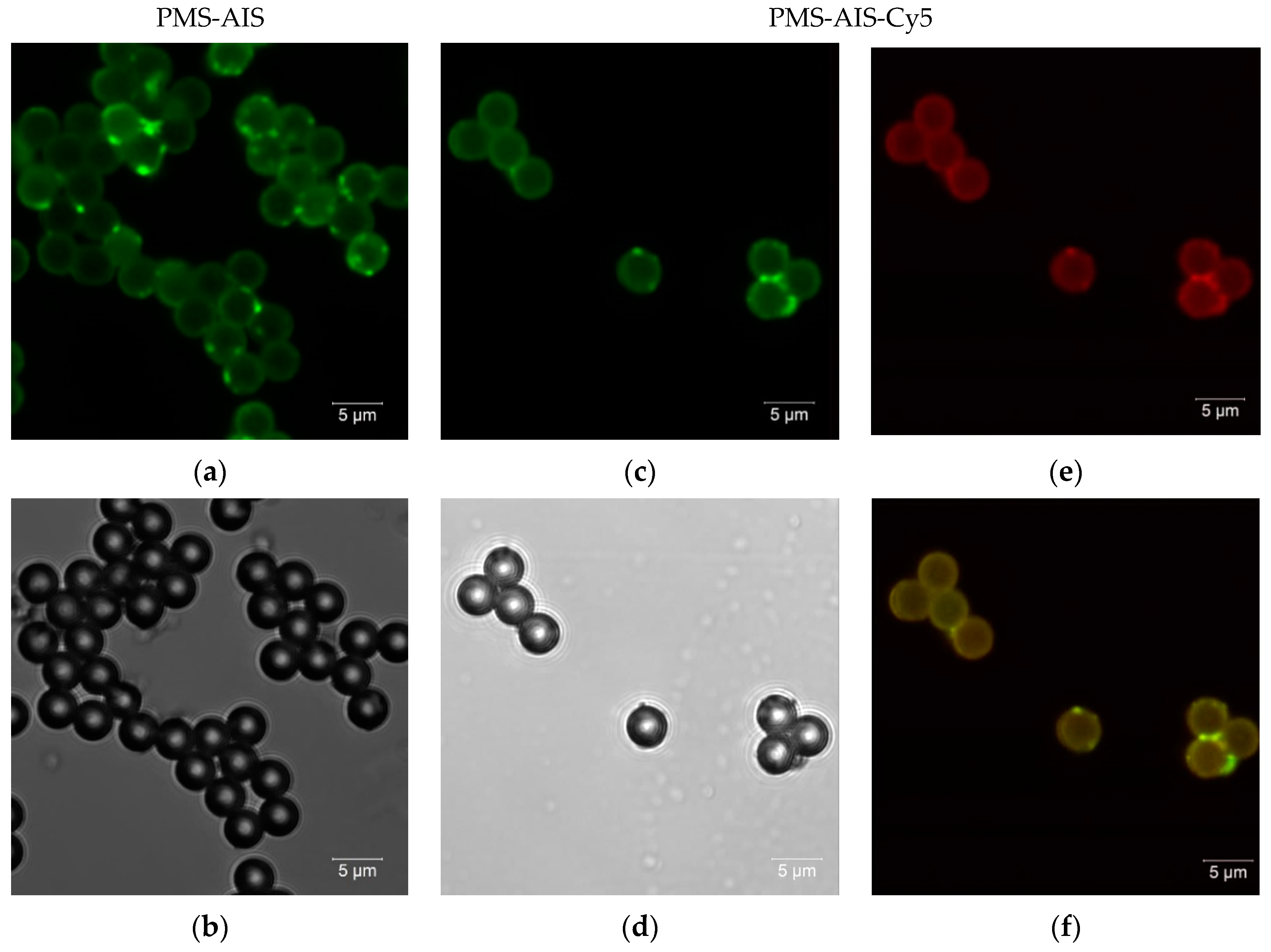
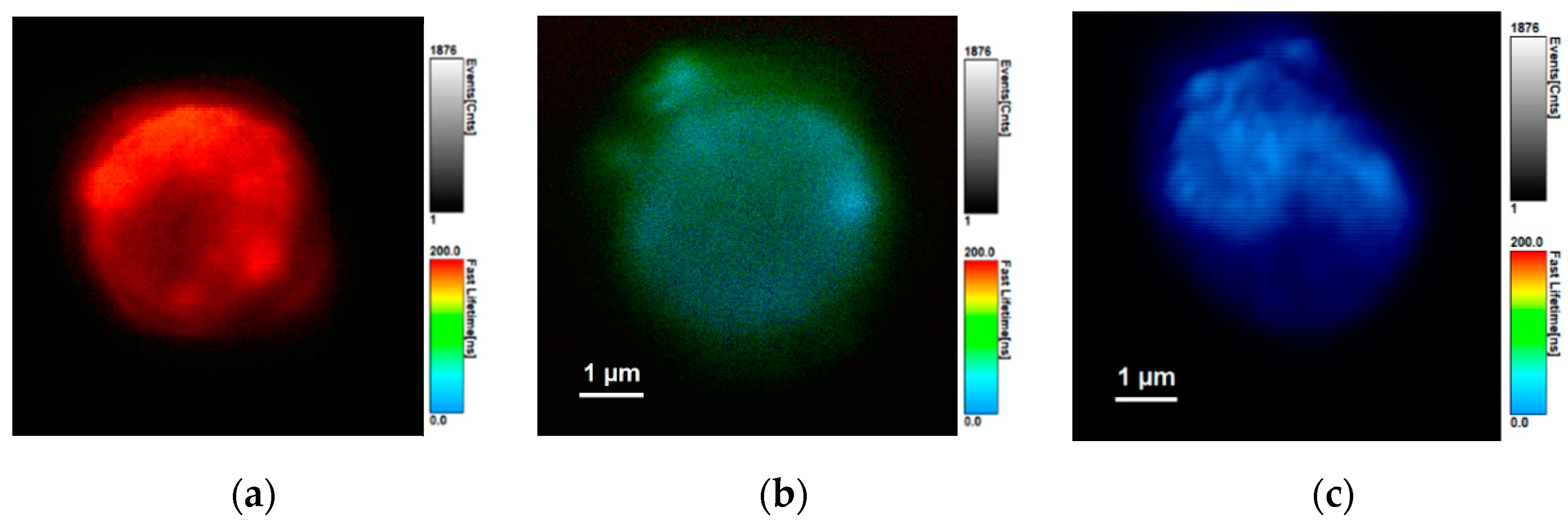
3.2. Confocal and Fluorescence Lifetime Imaging of PMS-AIS and PMS-AIS-Dyes
3.3. UV-Vis Spectroscopy of PMS-AIS and PMS-AIS-Dyes
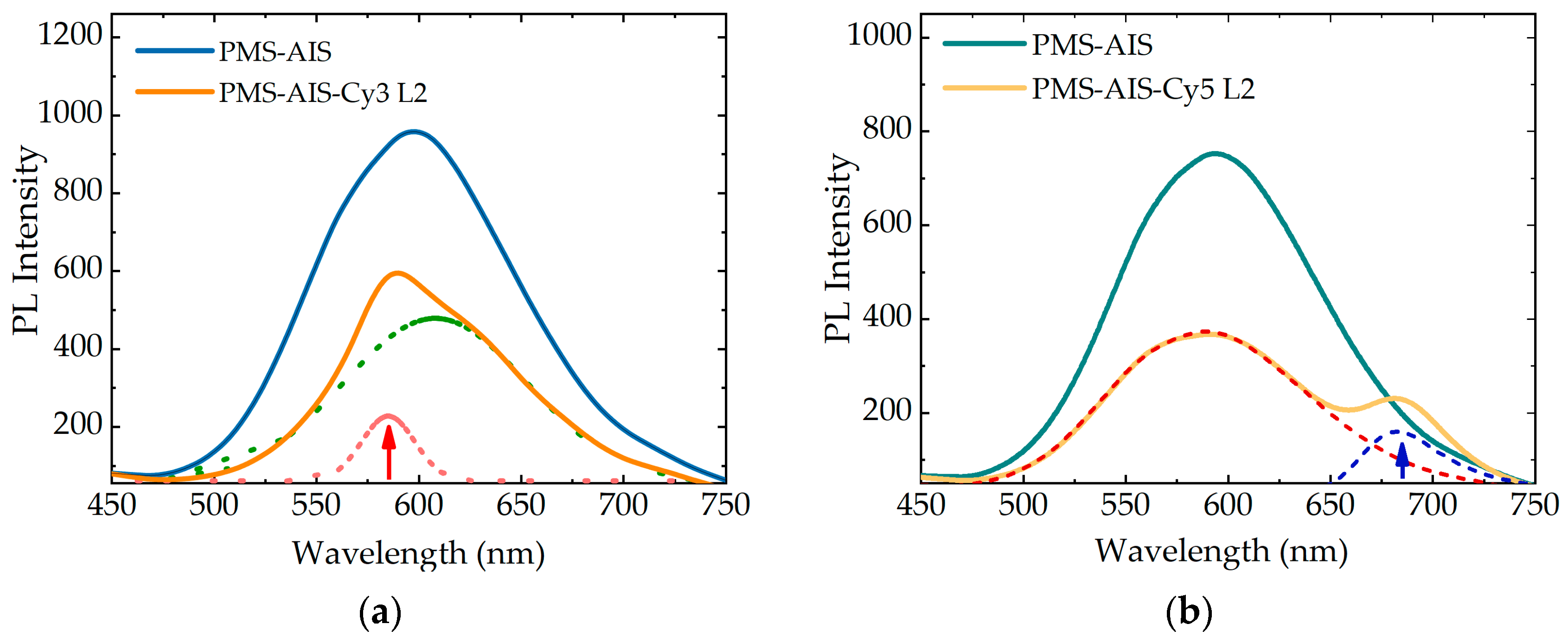
3.4. PL Spectra of PMS-AIS and PMS-AIS-Dyes Excited at 350 nm
3.5. PL Spectra of PMS-AIS and PMS-AIS-Dyes Excited at 520 and 600 nm
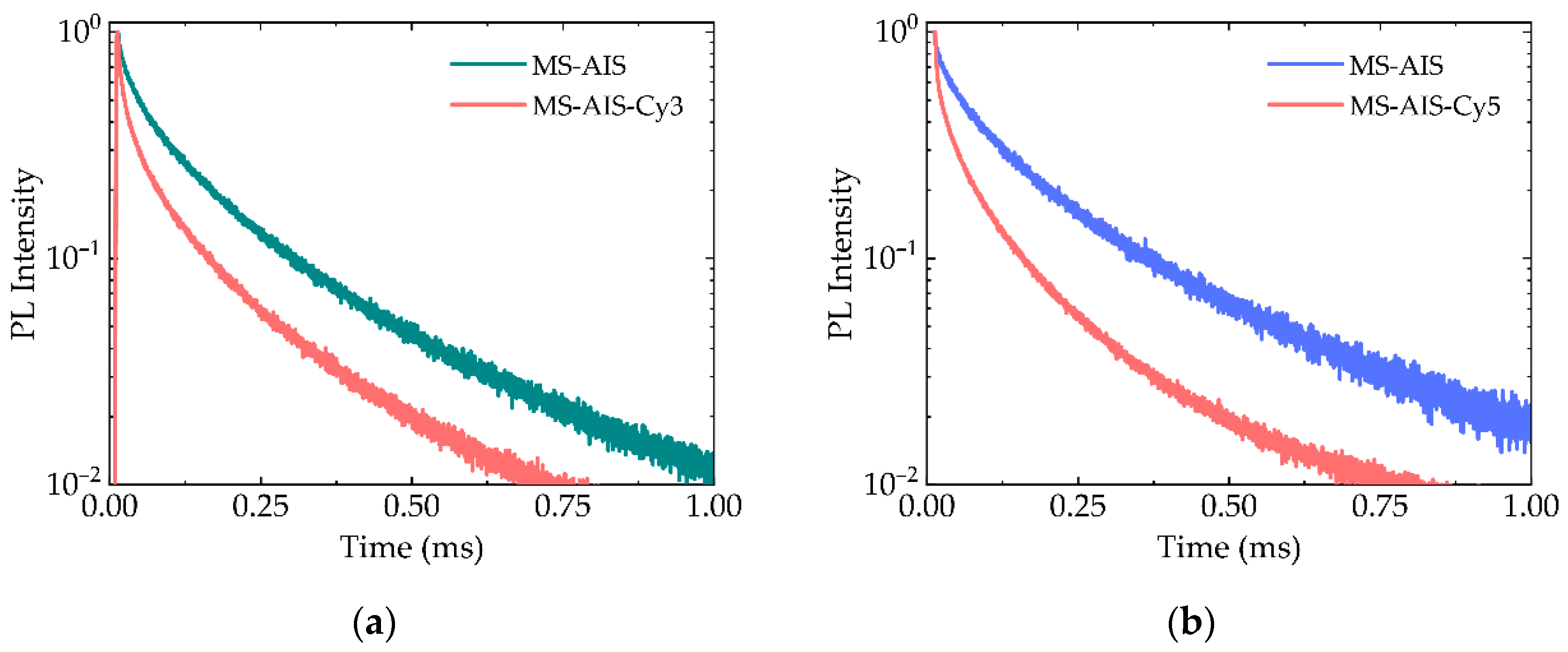
3.6. Time-Resolved PL Measurements of PMS-AIS and PMS-AIS-Dyes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Howes, P.D.; Chandrawati, R.; Stevens, M.M. Colloidal nanoparticles as advanced biological sensors. Science 2014, 346, 1247390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volkov, Y. Quantum dots in nanomedicine: Recent trends, advances and unresolved issues. Biochem. Biophys. Res. Commun. 2015, 468, 419–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramasamy, P.; Kim, N.; Kang, Y.-S.; Ramirez, O.; Lee, J.-S. Tunable, Bright, and Narrow-Band Luminescence from Colloidal Indium Phosphide Quantum Dots. Chem. Mater. 2017, 29, 6893–6899. [Google Scholar] [CrossRef]
- Willard, D.M.; Carillo, L.L.; Jung, J.; Van Orden, A. CdSe−ZnS Quantum Dots as Resonance Energy Transfer Donors in a Model Protein−Protein Binding Assay. Nano Lett. 2001, 1, 469–474. [Google Scholar] [CrossRef]
- Hyldahl, M.G.; Bailey, S.T.; Wittmershaus, B.P. Photo-stability and performance of CdSe/ZnS quantum dots in luminescent solar concentrators. Sol. Energy 2009, 83, 566–573. [Google Scholar] [CrossRef]
- Hao, J.; Zhou, J.; Zhang, C. A tri-n-octylphosphine-assisted successive ionic layer adsorption and reaction method to synthesize multilayered core–shell CdSe–ZnS quantum dots with extremely high quantum yield. Chem. Commun. 2013, 49, 6346–6348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hu, N.; Zeng, Z.; Lin, Q.; Zhang, F.; Tang, A.; Jia, Y.; Li, L.S.; Shen, H.; Teng, F.; et al. High-Efficiency Green InP Quantum Dot-Based Electroluminescent Device Comprising Thick-Shell Quantum Dots. Adv. Opt. Mater. 2019, 7, 1801602. [Google Scholar] [CrossRef]
- Frecker, T.; Bailey, D.; Arzeta-Ferrer, X.; McBride, J.; Rosenthal, S.J. Review—Quantum Dots and Their Application in Lighting, Displays, and Biology. ECS J. Solid State Sci. Technol. 2015, 5, R3019–R3031. [Google Scholar] [CrossRef]
- Resch-Genger, U.; Grabolle, M.; Cavaliere-Jaricot, S.; Nitschke, R.; Nann, T. Quantum dots versus organic dyes as fluorescent labels. Nat. Methods 2008, 5, 763. [Google Scholar] [CrossRef]
- Schliwa, A.; Winkelnkemper, M.; Bimberg, D. Impact of size, shape, and composition on piezoelectric effects and electronic properties of In (Ga) As/Ga As quantum dots. Phys. Rev. B 2007, 76, 205324. [Google Scholar] [CrossRef] [Green Version]
- Ngo, C.Y.; Yoon, S.F.; Fan, W.J.; Chua, S.J. Effects of size and shape on electronic states of quantum dots. Phys. Rev. B 2006, 74, 245331. [Google Scholar] [CrossRef]
- Kershaw, S.V.; Susha, A.S.; Rogach, A.L. Narrow bandgap colloidal metal chalcogenide quantum dots: Synthetic methods, heterostructures, assemblies, electronic and infrared optical properties. Chem. Soc. Rev. 2013, 42, 3033–3087. [Google Scholar] [CrossRef] [PubMed]
- Girma, W.M.; Fahmi, M.Z.; Permadi, A.; Abate, M.A.; Chang, J.-Y. Synthetic strategies and biomedical applications of I–III–VI ternary quantum dots. J. Mater. Chem. B 2017, 5, 6193–6216. [Google Scholar] [CrossRef] [PubMed]
- Long, Z.; Zhang, W.; Tian, J.; Chen, G.; Liu, Y.; Liu, R. Recent research on the luminous mechanism, synthetic strategies, and applications of CuInS2 quantum dots. Inorg. Chem. Front. 2020. [Google Scholar] [CrossRef]
- Ghasemi, Y.; Peymani, P.; Afifi, S. Quantum dot: Magic nanoparticle for imaging, detection and targeting. Acta Biomed. 2009, 80, 156–165. [Google Scholar] [PubMed]
- Hines, M.A.; Scholes, G.D. Colloidal PbS nanocrystals with size-tunable near-infrared emission: Observation of post-synthesis self-narrowing of the particle size distribution. Adv. Mater. 2003, 15, 1844–1849. [Google Scholar] [CrossRef]
- Li, J.J.; Wang, Y.A.; Guo, W.; Keay, J.C.; Mishima, T.D.; Johnson, M.B.; Peng, X. Large-Scale Synthesis of Nearly Monodisperse CdSe/CdS Core/Shell Nanocrystals Using Air-Stable Reagents via Successive Ion Layer Adsorption and Reaction. J. Am. Chem. Soc. 2003, 125, 12567–12575. [Google Scholar] [CrossRef] [PubMed]
- Tsoi, K.M.; Dai, Q.; Alman, B.A.; Chan, W.C.W. Are Quantum Dots Toxic? Exploring the Discrepancy Between Cell Culture and Animal Studies. Acc. Chem. Res. 2013, 46, 662–671. [Google Scholar] [CrossRef]
- Oh, E.; Liu, R.; Nel, A.; Gemill, K.B.; Bilal, M.; Cohen, Y.; Medintz, I.L. Meta-analysis of cellular toxicity for cadmium-containing quantum dots. Nat. Nanotechnol. 2016, 11, 479–486. [Google Scholar] [CrossRef]
- Kurshanov, D.; Gromova, Y.; Cherevkov, S.; Ushakova, E.; Kormilina, T.; Dubavik, A.; Fedorov, A.; Baranov, A. Non-Toxic Ternary Quantum Dots AgInS2 and AgInS2/ZnS: Synthesis and Optical Properties. Opt. Spectrosc. 2018, 125, 1041–1046. [Google Scholar] [CrossRef]
- Mao, B.; Chuang, C.-H.; Lu, F.; Sang, L.; Zhu, J.; Burda, C. Study of the Partial Ag-to-Zn Cation Exchange in AgInS2/ZnS Nanocrystals. J. Phys. Chem. C 2013, 117, 648–656. [Google Scholar] [CrossRef]
- Dai, M.; Ogawa, S.; Kameyama, T.; Okazaki, K.; Kudo, A.; Kuwabata, S.; Tsuboi, Y.; Torimoto, T. Tunable photoluminescence from the visible to near-infrared wavelength region of non-stoichiometric AgInS2 nanoparticles. J. Mater. Chem. 2012, 22, 12851–12858. [Google Scholar] [CrossRef]
- Chevallier, T.; Le Blevennec, G.; Chandezon, F. Photoluminescence properties of AgInS2–ZnS nanocrystals: The critical role of the surface. Nanoscale 2016, 8, 7612–7620. [Google Scholar] [CrossRef]
- Zhong, H.; Zhou, Y.; Ye, M.; He, Y.; Ye, J.; He, C.; Yang, C.; Li, Y. Controlled Synthesis and Optical Properties of Colloidal Ternary Chalcogenide CuInS2 Nanocrystals. Chem. Mater. 2008, 20, 6434–6443. [Google Scholar] [CrossRef]
- Sun, J.; Ikezawa, M.; Wang, X.; Jing, P.; Li, H.; Zhao, J.; Masumoto, Y. Photocarrier recombination dynamics in ternary chalcogenide CuInS2 quantum dots. Phys. Chem. Chem. Phys. 2015, 17, 11981–11989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, C.; Zhai, L.; Ma, Y.; Zou, C.; Zhang, L.; Yang, Y.; Huang, S. Synthesis of AgInS2 quantum dots with tunable photoluminescence for sensitized solar cells. J. Power Sources 2017, 341, 11–18. [Google Scholar] [CrossRef]
- Xiang, W.; Xie, C.; Wang, J.; Zhong, J.; Liang, X.; Yang, H.; Luo, L.; Chen, Z. Studies on highly luminescent AgInS2 and Ag–Zn–In–S quantum dots. J. Alloys Compd. 2014, 588, 114–121. [Google Scholar] [CrossRef]
- Ogawa, T.; Kuzuya, T.; Hamanaka, Y.; Sumiyama, K. Synthesis of Ag–In binary sulfide nanoparticles—structural tuning and their photoluminescence properties. J. Mater. Chem. 2010, 20, 2226–2231. [Google Scholar] [CrossRef]
- Wegner, K.D.; Lanh, P.T.; Jennings, T.; Oh, E.; Jain, V.; Fairclough, S.M.; Smith, J.M.; Giovanelli, E.; Lequeux, N.; Pons, T.; et al. Influence of Luminescence Quantum Yield, Surface Coating, and Functionalization of Quantum Dots on the Sensitivity of Time-Resolved FRET Bioassays. ACS Appl. Mater. Interfaces 2013, 5, 2881–2892. [Google Scholar] [CrossRef]
- Miropoltsev, M.; Kuznetsova, V.; Tkach, A.; Cherevkov, S.; Sokolova, A.; Osipova, V.; Gromova, Y.; Baranov, M.; Fedorov, A.; Gun’ko, Y.; et al. FRET-Based Analysis of AgInS2/ZnAgInS/ZnS Quantum Dot Recombination Dynamics. Nanomaterials 2020, 10, 2455. [Google Scholar] [CrossRef]
- Kuznetsova, V.; Tkach, A.; Cherevkov, S.; Sokolova, A.; Gromova, Y.; Osipova, V.; Baranov, M.; Ugolkov, V.; Fedorov, A.; Baranov, A. Spectral-Time Multiplexing in FRET Complexes of AgInS2/ZnS Quantum Dot and Organic Dyes. Nanomaterials 2020, 10, 1569. [Google Scholar] [CrossRef] [PubMed]
- Evstigneev, R.; Parfenov, P.; Dubavik, A.; Cherevkov, S.; Fedorov, A.; Martynenko, I.; Resch-Genger, U.; Ushakova, E.; Baranov, A. Time-resolved FRET in AgInS2/ZnS-CdSe/ZnS quantum dot systems. Nanotechnology 2019, 30. [Google Scholar] [CrossRef] [PubMed]
- Martynenko, I.; Kuznetsova, V.; Orlova, A.; Kanaev, P.; Gromova, Y.; Maslov, V.; Baranov, A.; Fedorov, A. ZnSe/ZnS quantum dots—Photosensitizer complexes: Optical properties and cancer cell photodynamic destruction effect. In Proceedings of the SPIE—The International Society for Optical Engineering, SPIE Photonics Europe, Brussels, Belgium, 14–17 April 2014; Volume 9126. [Google Scholar]
- Martynenko, I.V.; Baimuratov, A.S.; Weigert, F.; Soares, J.X.; Dhamo, L.; Nickl, P.; Doerfel, I.; Pauli, J.; Rukhlenko, I.D.; Baranov, A.V.; et al. Photoluminescence of Ag-In-S/ZnS quantum dots: Excitation energy dependence and low-energy electronic structure. Nano Res. 2019, 12, 1595–1603. [Google Scholar] [CrossRef]
- Petryayeva, E.; Algar, W.R.; Medintz, I.L. Quantum Dots in Bioanalysis: A Review of Applications Across Various Platforms for Fluorescence Spectroscopy and Imaging. Appl. Spectrosc. 2013, 67, 215–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snee, P.T. Semiconductor quantum dot FRET: Untangling energy transfer mechanisms in bioanalytical assays. TrAC Trends Anal. Chem. 2020, 123, 115750. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, C.; Su, L.; Wang, H.; Gong, X.; Wang, H.; Liu, J.; Chang, J. Multifunctional Microspheres Encoded with Upconverting Nanocrystals and Magnetic Nanoparticles for Rapid Separation and Immunoassays. ACS Appl. Mater. Interfaces 2016, 8, 745–753. [Google Scholar] [CrossRef]
- Han, M.; Gao, X.; Su, J.Z.; Nie, S. Quantum-dot-tagged microbeads for multiplexed optical coding of biomolecules. Nat. Biotechnol. 2001, 19, 631–635. [Google Scholar] [CrossRef]
- Buranda, T.; Huang, J.; Ramarao, G.V.; Ista, L.K.; Larson, R.S.; Ward, T.L.; Sklar, L.A.; Lopez, G.P. Biomimetic Molecular Assemblies on Glass and Mesoporous Silica Microbeads for Biotechnology. Langmuir 2003, 19, 1654–1663. [Google Scholar] [CrossRef]
- Sonawane, S.L.; Asha, S.K. Fluorescent Polystyrene Microbeads as Invisible Security Ink and Optical Vapor Sensor for 4-Nitrotoluene. ACS Appl. Mater. Interfaces 2016, 8, 10590–10599. [Google Scholar] [CrossRef]
- Gao, X.; Nie, S. Quantum dot-encoded beads. In NanoBiotechnology Protocols; Springer: Berlin, Germany, 2005; pp. 61–71. [Google Scholar]
- Kage, D.; Hoffmann, K.; Nifontova, G.; Krivenkov, V.; Sukhanova, A.; Nabiev, I.; Resch-Genger, U. Tempo-spectral multiplexing in flow cytometry with lifetime detection using QD-encoded polymer beads. Sci. Rep. 2020, 10, 653. [Google Scholar] [CrossRef] [Green Version]
- Kage, D.; Hoffmann, K.; Wittkamp, M.; Ameskamp, J.; Göhde, W.; Resch-Genger, U. Luminescence lifetime encoding in time-domain flow cytometry. Sci. Rep. 2018, 8, 16715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilan, R.; Nabiev, I.; Sukhanova, A. Quantum dot-based nanotools for bioimaging, diagnostics, and drug delivery. ChemBioChem 2016, 17, 2103–2114. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, J.; Li, Z.; Lv, X.; Liang, L.; Yuan, Q. Recent Progress in Time-Resolved Biosensing and Bioimaging Based on Lanthanide-Doped Nanoparticles. Small 2019, 15, 1804969. [Google Scholar] [CrossRef] [PubMed]
- Wolska, E.; Kaszewski, J.; Kiełbik, P.; Grzyb, J.; Godlewski, M.M.; Godlewski, M. Rare earth activated ZnO nanoparticles as biomarkers. Opt. Mater. 2014, 36, 1655–1659. [Google Scholar] [CrossRef]
- Raevskaya, A.; Lesnyak, V.; Haubold, D.; Dzhagan, V.; Stroyuk, O.; Gaponik, N.; Zahn, D.R.T.; Eychmüller, A. A Fine Size Selection of Brightly Luminescent Water-Soluble Ag–In–S and Ag–In–S/ZnS Quantum Dots. J. Phys. Chem. C 2017, 121, 9032–9042. [Google Scholar] [CrossRef] [Green Version]
- Gromova, Y.; Sokolova, A.; Kurshanov, D.; Korsakov, I.; Osipova, V.; Cherevkov, S.; Dubavik, A.; Maslov, V.; Perova, T.; Gun’ko, Y.; et al. Investigation of AgInS2/ZnS Quantum Dots by Magnetic Circular Dichroism Spectroscopy. Materials 2019, 12, 3616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michel, M.; Toniazzo, V.; Ruch, D.; Vincent, B. Deposition Mechanisms in Layer-by-Layer or Step-by-Step Deposition Methods: From Elastic and Impermeable Films to Soft Membranes with Ion Exchange Properties. ISRN Mater. Sci. 2012, 2012. [Google Scholar] [CrossRef] [Green Version]
- Hamanaka, Y.; Ozawa, K.; Kuzuya, T. Enhancement of Donor–Acceptor Pair Emissions in Colloidal AgInS2 Quantum Dots with High Concentrations of Defects. J. Phys. Chem. C 2014, 118, 14562–14568. [Google Scholar] [CrossRef]




| Sample Name | ||||
|---|---|---|---|---|
| PMS-AIS (580 nm) | 460 ± 5 | 130 ± 5 | — | 130 ± 5 |
| PMS-AIS-Cy3 (580 nm) | 440 ± 5 | 120 ± 5 | 20 ± 5 | 80 ± 5 |
| PMS-AIS (665 nm) | 745 ± 5 | 225 ± 5 | — | 245 ± 5 |
| PMS-AIS-Cy5 (665 nm) | 705 ± 5 | 210 ± 5 | 30 ± 5 | 160 ± 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuznetsova, V.; Osipova, V.; Tkach, A.; Miropoltsev, M.; Kurshanov, D.; Sokolova, A.; Cherevkov, S.; Zakharov, V.; Fedorov, A.; Baranov, A.; et al. Lab-on-Microsphere—FRET-Based Multiplex Sensor Platform. Nanomaterials 2021, 11, 109. https://doi.org/10.3390/nano11010109
Kuznetsova V, Osipova V, Tkach A, Miropoltsev M, Kurshanov D, Sokolova A, Cherevkov S, Zakharov V, Fedorov A, Baranov A, et al. Lab-on-Microsphere—FRET-Based Multiplex Sensor Platform. Nanomaterials. 2021; 11(1):109. https://doi.org/10.3390/nano11010109
Chicago/Turabian StyleKuznetsova, Vera, Viktoria Osipova, Anton Tkach, Maksim Miropoltsev, Danil Kurshanov, Anastasiia Sokolova, Sergei Cherevkov, Viktor Zakharov, Anatoly Fedorov, Alexander Baranov, and et al. 2021. "Lab-on-Microsphere—FRET-Based Multiplex Sensor Platform" Nanomaterials 11, no. 1: 109. https://doi.org/10.3390/nano11010109
APA StyleKuznetsova, V., Osipova, V., Tkach, A., Miropoltsev, M., Kurshanov, D., Sokolova, A., Cherevkov, S., Zakharov, V., Fedorov, A., Baranov, A., & Gun’ko, Y. (2021). Lab-on-Microsphere—FRET-Based Multiplex Sensor Platform. Nanomaterials, 11(1), 109. https://doi.org/10.3390/nano11010109








