Magnetic Hyperthermia on ?-Fe2O3@SiO2 Core-Shell Nanoparticles for mi-RNA 122 Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of γ-Fe2O3@SiO2 Core-Shell Nanoparticles
2.2.1. Synthesis of γ-Fe2O3 Nanoparticles
2.2.2. Silica Coating Process
2.3. DNA Duplexes Conjugation on γ-Fe2O3@SiO2 Core-Shell Nanoparticles
2.4. Characterization of γ-Fe2O3@SiO2 Core-Shell Nanoparticles
2.5. Fluorescence Measurements
3. Results and Discussion
3.1. Characterization of γ-Fe2O3@SiO2 Core-Shell Nanoparticles
3.1.1. From a Spherical to a Rod-Like Morphology
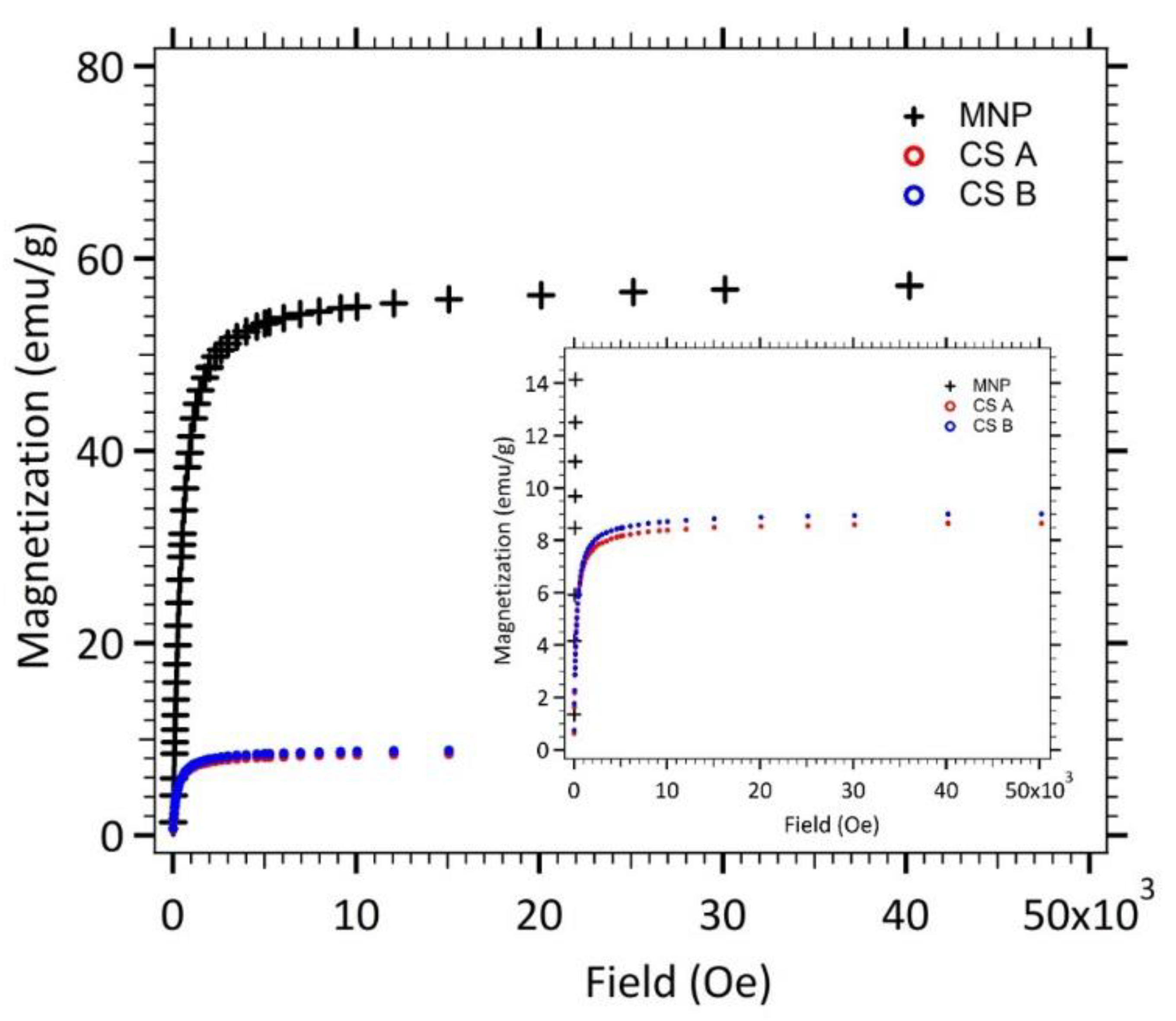
3.1.2. Magnetostatic Properties
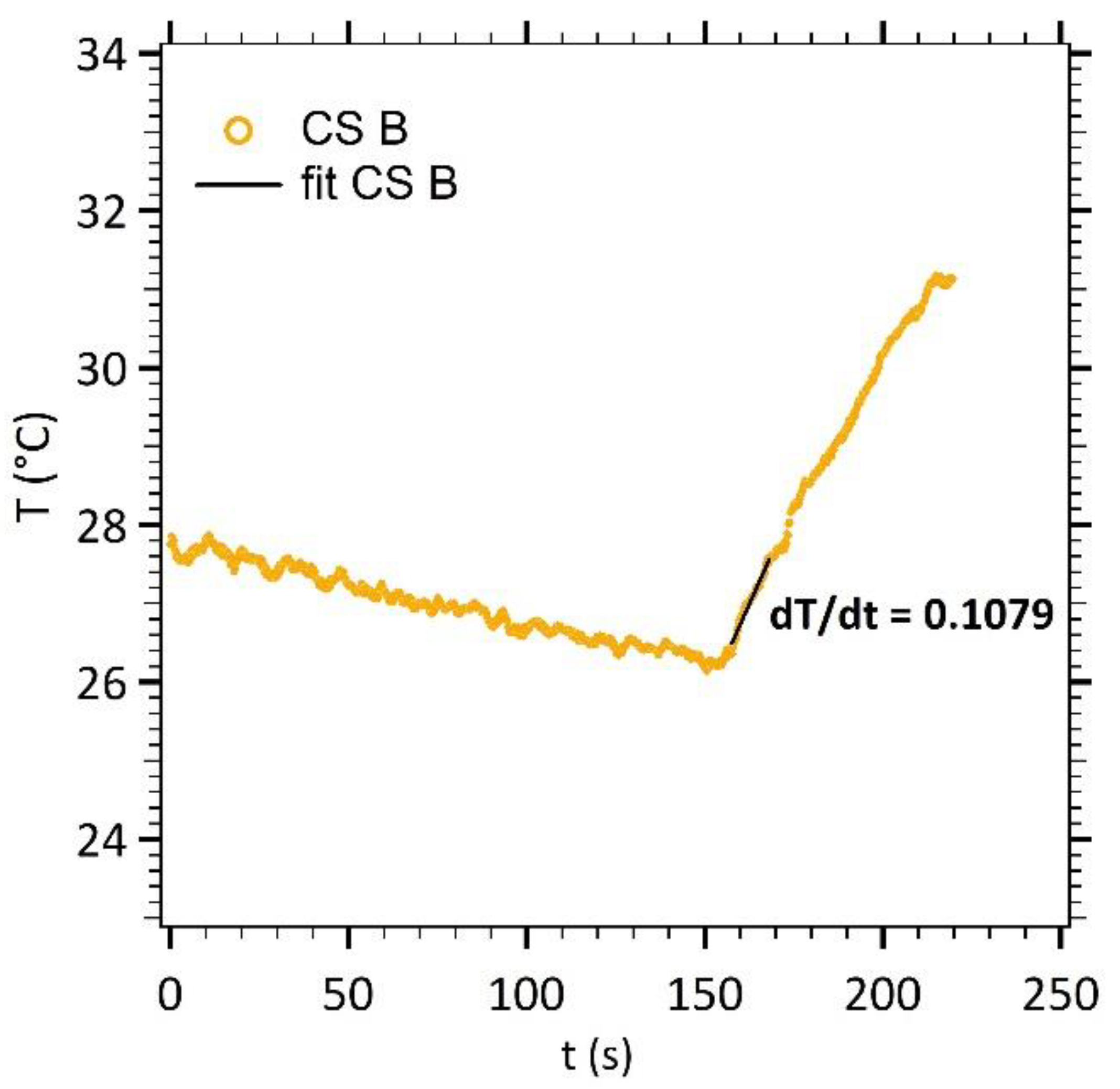
3.1.3. Heating Efficiency
3.2. DNA Release in Magnetic Hyperthermia
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

Appendix B

References
- Rosensweig, R.E.E. Heating magnetic fluid with alternating magnetic field. J. Magn. Magn. Mater. 2002, 252, 370–374. [Google Scholar] [CrossRef]
- Coral, D.F.; Mendoza Zélis, P.; Marciello, M.; Morales, M.D.P.; Craievich, A.; Sánchez, F.H.; van Raap, M.B. Effect of Nanoclustering and Dipolar Interactions in Heat Generation for Magnetic Hyperthermia. Langmuir 2016, 32, 1201–1213. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.T.; Moros, M.A.; Del Pino, P.; Rivera, S.; Graz, V.; De La Fuente, J.M. DNA as a molecular local thermal probe for the analysis of magnetic hyperthermia. Angew. Chem. Int. Ed. 2013, 52, 11526–11529. [Google Scholar] [CrossRef] [PubMed]
- Glöckl, G.; Hergt, R.; Zeisberger, M.; Dutz, S.; Nagel, S.; Weitschies, W. The effect of field parameters, nanoparticle properties and immobilization on the specific heating power in magnetic particle hyperthermia. J. Phys. Condens. Matter 2006, 18, S2935–S2949. [Google Scholar] [CrossRef]
- Jordan, A. Thermotherapy and Nanomedicine. In Hyperthermia in Cancer Treatment: A Primer; Springer: Boston, MA, USA, 2006; pp. 60–63. [Google Scholar] [CrossRef]
- Ruiz-Hernandez, E.; Baeza, A.; Vallet, R. Smart drug delivery through DNA/magnetic nanoparticle gates. ACS Nano 2011, 5, 1259–1266. [Google Scholar] [CrossRef]
- Chang, C.-M.; Chang, W.-H.; Wang, C.-H.; Wang, J.-H.; Mai, J.D.; Lee, G.-B. Nucleic acid amplification using microfluidic systems. Lab. Chip 2013, 13, 1225–1242. [Google Scholar] [CrossRef]
- Dong, J.; Zink, J.I. Taking the temperature of the interiors of magnetically heated nanoparticles. ACS Nano 2014, 8, 5199–5207. [Google Scholar] [CrossRef]
- Lassalle, V.; Agotegaray, M. Silica-Coated Magnetic Nanoparticles; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Yoon, T.-J.; Yu, K.N.; Kim, E.; Kim, J.S.; Kim, B.G.; Yun, S.-H.; Sohn, B.-H.; Cho, M.-H.; Lee, J.-K.; Park, S.B. Specific Targeting, Cell Sorting, and Bioimaging with Smart Magnetic Silica Core–Shell Nanomaterials. Small 2006, 2, 209–215. [Google Scholar] [CrossRef]
- Deng, Y.-H.; Wang, C.-C.; Hu, J.-H.; Yang, W.-L.; Fu, S.-K. Investigation of formation of silica-coated magnetite nanoparticles via sol–gel approach. Colloids Surf. Physicochem. Eng. Asp. 2005, 262, 87–93. [Google Scholar] [CrossRef]
- Li, B.; Gong, T.; Xu, N.; Cui, F.; Yuan, B.; Yuan, Q.; Sun, H.; Wang, L.; Liu, J. Improved Stability and Photothermal Performance of Polydopamine-Modified Fe3O4 Nanocomposites for Highly Efficient Magnetic Resonance Imaging-Guided Photothermal Therapy. Small 2020, 16, 2003969. [Google Scholar] [CrossRef]
- Massart, R.; Dubois, E.; Cabuil, V.; Hasmonay, E. Preparation and properties of monodisperse magnetic fluids. J. Magn. Magn. Mater. 1995, 149, 1–5. [Google Scholar] [CrossRef]
- Lefebure, S.; Dubois, E.; Cabuil, V.; Neveu, S.; Massart, R. Monodisperse magnetic nanoparticles: Preparation and dispersion in water and oils. J. Mater. Res. 1998, 13, 2975–2981. [Google Scholar] [CrossRef]
- Maurice, V.; Georgelin, T.; Siaugue, J.-M.; Cabuil, V. Synthesis and characterization of functionalized core–shell γFe2O3–SiO2 nanoparticles. J. Magn. Magn. Mater. 2009, 321, 1408–1413. [Google Scholar] [CrossRef]
- Georgelin, T.; Bombard, S.; Siaugue, J.-M.; Cabuil, V. Nanoparticle-Mediated Delivery of Bleomycin. Angew. Chem. Int. Ed. 2010, 49, 8897–8901. [Google Scholar] [CrossRef] [PubMed]
- Etoc, F.; Vicario, C.; Lisse, D.; Siaugue, J.-M.; Piehler, J.; Coppey, M.; Dahan, M. Magnetogenetic Control of Protein Gradients Inside Living Cells with High Spatial and Temporal Resolution. Nano Lett. 2015, 15, 3487–3494. [Google Scholar] [CrossRef] [PubMed]
- Invitrogen Detection Technologies. Quant-iT™ Assays for high-throughput quantitation of DNA, RNA, and oligos. In Nucleic Acid Quantitation; Invitrogen Molecular Probes: Waltham, MA, USA, 2008; pp. 1–7. [Google Scholar]
- Kaiser, R.; Miskolczy, G. Magnetic properties of stable dispersions of subdomain magnetite particles. J. Appl. Phys. 1970, 41, 1064–1072. [Google Scholar] [CrossRef]
- Fortin, J.P.; Wilhelm, C.; Servais, J.; Mnager, C.; Bacri, J.C.; Gazeau, F. Size-Sorted Anionic Iron Oxide Nanomagnets as Colloidal Mediators for Magnetic Hyperthermia. J. Am. Chem. Soc. 2007, 129, 2628–2635. [Google Scholar] [CrossRef]
- Bacri, J.C.; Perzynski, R.; Salin, D.; Cabuil, V.; Massart, R. Magnetic colloidal properties of ionic ferrofluids. J. Magn. Magn. Mater. 1986, 1986 62, 36–46. [Google Scholar] [CrossRef]
- Mornet, S. Synthèse et Modification Chimique de la Surface de Nanoparticules de Maghémite à des Fins D'applications Biomédicales; Université Sciences et Technologies-Bordeaux I: Bordeaux, France, 2002. [Google Scholar]
- Shliomis, M.I.; Pshenichnikov, A.F.; Morozov, K.I.; Shurubor, I.Y. Magnetic properties of ferrocolloids. J. Magn. Magn. Mater. 1990, 85, 40–46. [Google Scholar] [CrossRef]
- Andreu, I.; Natividad, E. Accuracy of available methods for quantifying the heat power generation of nanoparticles for magnetic hyperthermia. Int. J. Hyperth. 2013, 29, 739–751. [Google Scholar] [CrossRef] [Green Version]
- Kallumadil, M.; Tada, M.; Nakagawa, T.; Abe, M.; Southern, P.; Pankhurst, Q.A. Suitability of commercial colloids for magnetic hyperthermia. J. Magn. Magn. Mater. 2009, 321, 1509–1513. [Google Scholar] [CrossRef]
- Das, R.; Alonso, J.; Nemati Porshokouh, Z.; Kalappattil, V.; Torres, D.; Phan, M.-H.; Garaio, E.; García, J.Á.; Sanchez Llamazares, J.L.; Srikanth, H. Tunable High Aspect Ratio Iron Oxide Nanorods for Enhanced Hyperthermia. J. Phys. Chem. C 2016, 120, 10086–10093. [Google Scholar] [CrossRef]
- Martinez-Boubeta, C.; Simeonidis, K.; Makridis, A.; Angelakeris, M.; Iglesias, O.; Guardia, P.; Cabot, A.; Yedra, L.; Estradé, S.; Peiró, F.; et al. Learning from Nature to Improve the Heat Generation of Iron-Oxide Nanoparticles for Magnetic Hyperthermia Applications. Sci. Rep. 2013, 3, 1652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serantes, D.; Simeonidis, K.; Angelakeris, M.; Chubykalo-Fesenko, O.; Marciello, M.; Morales, M.D.P.; Baldomir, D.; Martinez-Boubeta, C. Multiplying Magnetic Hyperthermia Response by Nanoparticle Assembling. J. Phys. Chem. C 2014, 118, 5927–5934. [Google Scholar] [CrossRef]
- Meunier-Prest, R.; Raveau, S.; Finot, E.; Legay, G.; Cherkaoui-Malki, M.; Latruffe, N. Direct measurement of the melting temperature of supported DNA by electrochemical method. Nucleic Acids Res. 2003, 31, e150. [Google Scholar] [CrossRef] [Green Version]
- Ge, D.; Wang, X.; Williams, K.; Levicky, R. Thermostable DNA Immobilization and Temperature Effects on Surface Hybridization. Langmuir 2012, 28, 8446–8455. [Google Scholar] [CrossRef]






| Oligonucleotide | Sequence (5′ to 3′) |
|---|---|
| Probe (P) | 5′-Carboxy C6-CAA ACA CCA TTG TCA CAC TGC-3′ |
| Target (T) | 5′-GC AGT GTG ACA ATG GTG TTT G-3′ |
| dmag (nm) | σ | Maghemite Mass Ratio (%) | |||
|---|---|---|---|---|---|
| MNP | 10.9 | 0.37 | 11.7 | 56.5 | N/A |
| CS A | 9.4 | 0.30 | 9.83 | 8.63 | 15.3 |
| CS B | 8.5 | 0.41 | 9.25 | 8.99 | 15.9 |
| Particles | (nm) † | [Fe2O3] (mg·mL−1) | dT/dt (°C·s−1) | SLP (W·g−1) | ILP (m2·nH·kg−1) |
|---|---|---|---|---|---|
| MNP | 11.7 | 6.16 | 0.077 | 52 | 0.88 |
| CS A | 9.83 | 5.60 | 0.085 | 64 | 1.06 |
| CS B | 9.25 | 5.36 | 0.105 | 82 | 1.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horny, M.-C.; Gamby, J.; Dupuis, V.; Siaugue, J.-M. Magnetic Hyperthermia on ?-Fe2O3@SiO2 Core-Shell Nanoparticles for mi-RNA 122 Detection. Nanomaterials 2021, 11, 149. https://doi.org/10.3390/nano11010149
Horny M-C, Gamby J, Dupuis V, Siaugue J-M. Magnetic Hyperthermia on ?-Fe2O3@SiO2 Core-Shell Nanoparticles for mi-RNA 122 Detection. Nanomaterials. 2021; 11(1):149. https://doi.org/10.3390/nano11010149
Chicago/Turabian StyleHorny, Marie-Charlotte, Jean Gamby, Vincent Dupuis, and Jean-Michel Siaugue. 2021. "Magnetic Hyperthermia on ?-Fe2O3@SiO2 Core-Shell Nanoparticles for mi-RNA 122 Detection" Nanomaterials 11, no. 1: 149. https://doi.org/10.3390/nano11010149
APA StyleHorny, M.-C., Gamby, J., Dupuis, V., & Siaugue, J.-M. (2021). Magnetic Hyperthermia on ?-Fe2O3@SiO2 Core-Shell Nanoparticles for mi-RNA 122 Detection. Nanomaterials, 11(1), 149. https://doi.org/10.3390/nano11010149






