Recent Advanced on the MXene–Organic Hybrids: Design, Synthesis, and Their Applications
Abstract
:1. Introduction
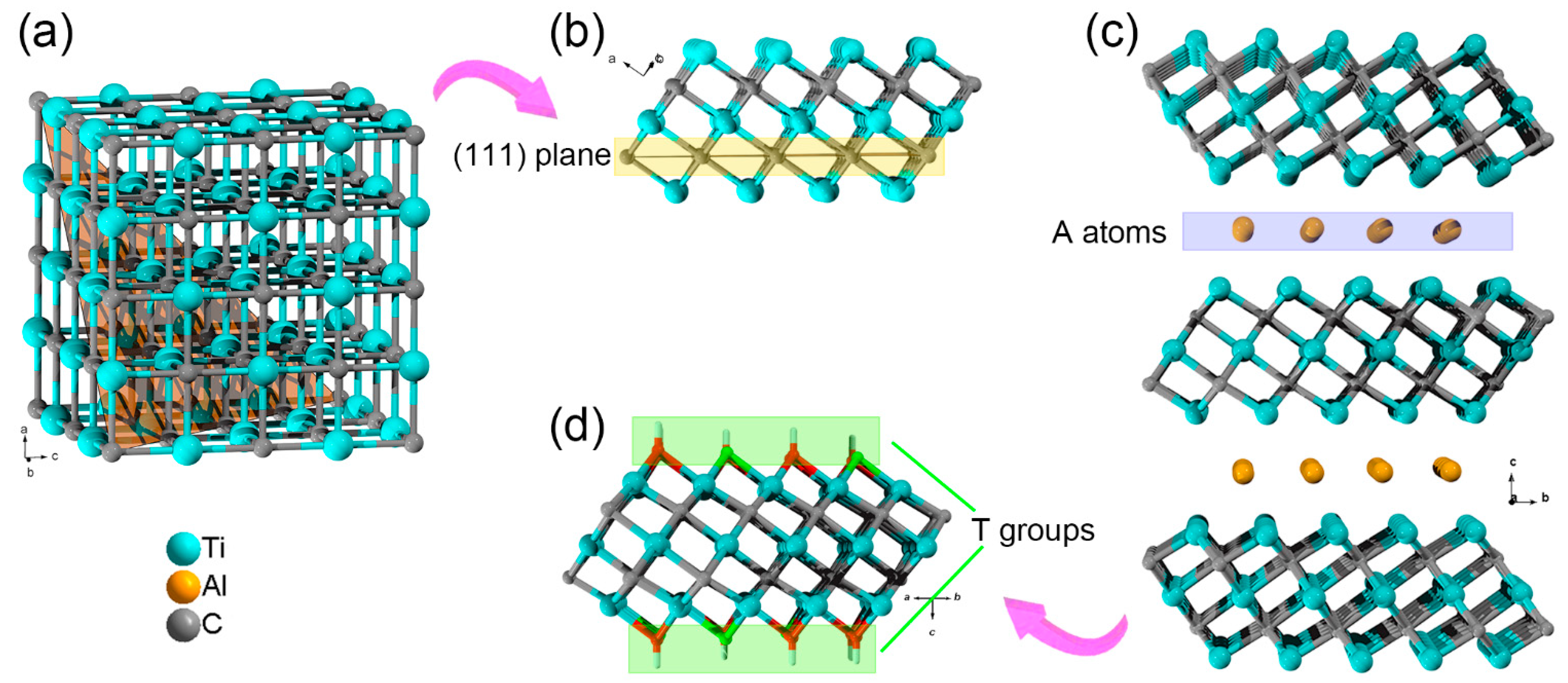
2. Structure and Surface Characters of MXenes
3. Strategies towards MXene-Organic Hybrids
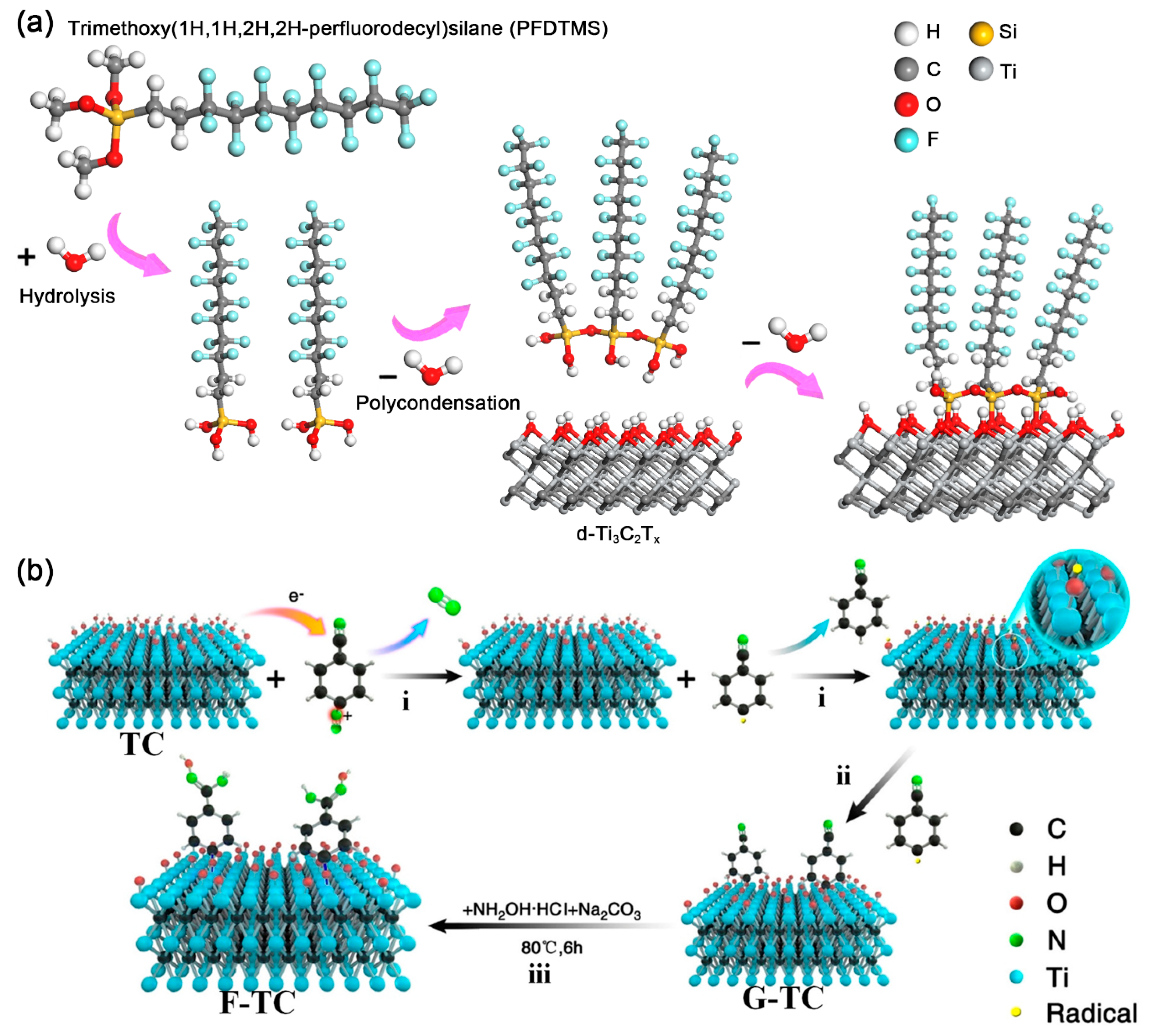
3.1. MXene-Organic Hybrids through Covalent Interaction
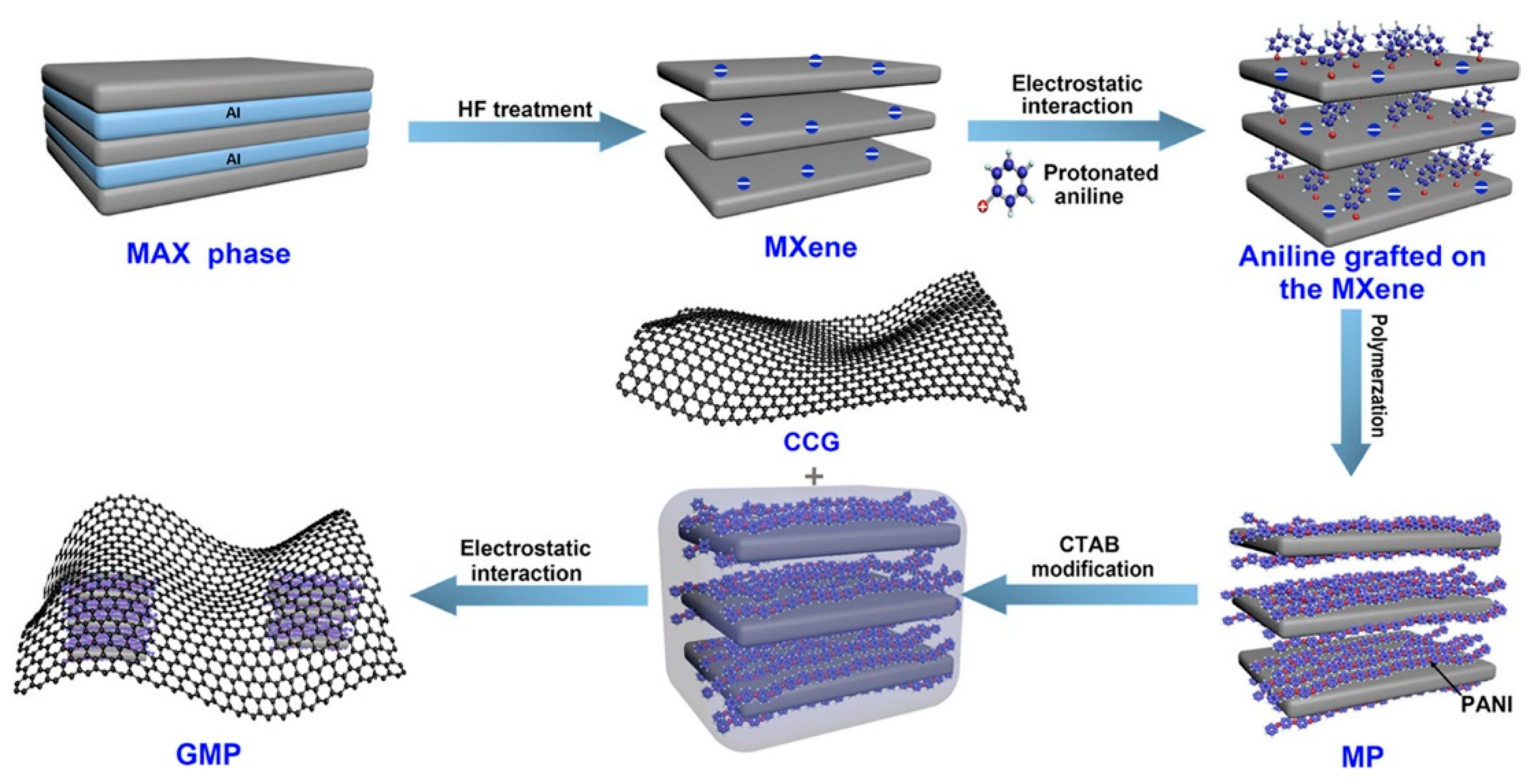
3.2. MXene-Organic Hybrids through Electrostatic Interaction
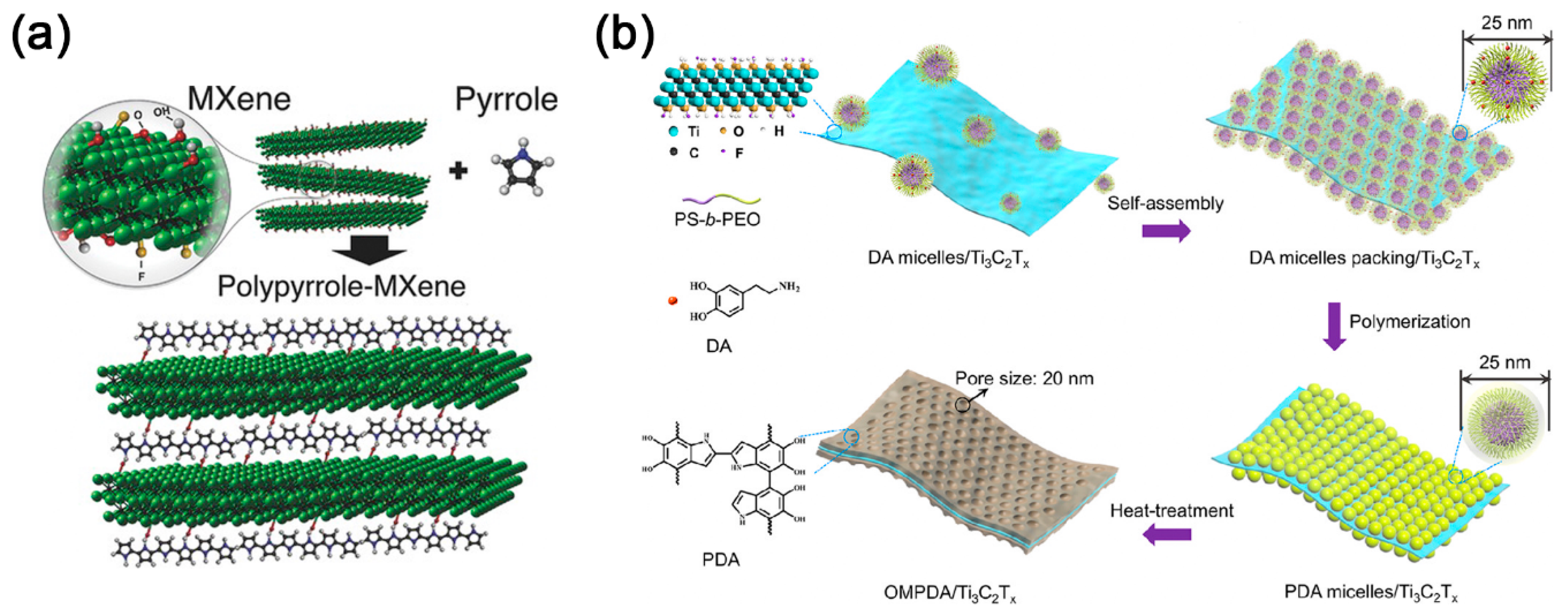
3.3. MXene-Organic Hybrids through Hydrogen Bonds and Other Supermolecular Interactions
4. MXene-Organic Hybrids for Flexible Devices
4.1. MXene-Organic Hybrids for Flexible Supercapacitors
4.2. MXene-Organic Hybrids for Flexible Metal-Ion Batteries
4.3. MXene-Organic Hybrids for Flexible Sensor
4.4. MXene-Organic Hybrids for Other Applications
5. Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, Y.; Chortos, A.; Xu, W.; Liu, Y.; Oh, J.Y.; Son, D.; Kang, J.; Foudeh, A.M.; Zhu, C.; Lee, Y.; et al. A Bioinspired Flexible Organic Artificial Afferent Nerve. Science 2018, 360, 998–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, S.; Cho, K.; Lee, T. Recent Progress in Inkjet-Printed Thin-Film Transistors. Adv. Sci. 2019, 6, 1801445. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Li, L.; Wang, L.; Shen, G. Recent Progress of Self-Powered Sensing Systems for Wearable Electronics. Small 2017, 13, 1701791. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-G.; Lee, S.; Park, J.-U. Recent Progress in Wireless Sensors for Wearable Electronics. Sensors 2019, 19, 4353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.; Zheng, L.; Liu, Z.; Wang, X. Two-dimensional materials: From mechanical properties to flexible mechanical sensors. InfoMat 2019, 2, 1077–1094. [Google Scholar] [CrossRef] [Green Version]
- Nathan, A.; Ahnood, A.; Cole, M.T.; Sungsik, L.; Suzuki, Y.; Hiralal, P.; Bonaccorso, F.; Hasan, T.; Garcia-Gancedo, L.; Dyadyusha, A.; et al. Flexible Electronics: The Next Ubiquitous Platform. Proc. IEEE 2012, 100, 1486–1517. [Google Scholar] [CrossRef]
- Ling, H.; Liu, S.; Zheng, Z.; Yan, F. Organic Flexible Electronics. Small Methods 2018, 2, 1800070. [Google Scholar] [CrossRef]
- Kim, H.; Wang, Z.; Alshareef, H.N. MXetronics: Electronic and photonic applications of MXenes. Nano. Energy 2019, 60, 179–197. [Google Scholar] [CrossRef]
- Zhang, F.P.; Shi, J.L.; Zhang, J.W.; Yang, X.Y.; Zhang, J.X. Grain alignment modulation and observed electrical transport properties of Ca3Co4O9 ceramics. Results Phys. 2019, 12, 321–326. [Google Scholar] [CrossRef]
- Tan, C.; Zhang, H. Two-dimensional transition metal dichalcogenide nanosheet-based composites. Chem. Soc. Rev. 2015, 44, 2713–2731. [Google Scholar] [CrossRef]
- Chhowalla, M.; Shin, H.S.; Eda, G.; Li, L.-J.; Loh, K.P.; Zhang, H. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 2013, 5, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Yang, J.; Li, W.; Wang, X.; Li, C.M. Quantifying the rigidity of 2D carbides (MXenes). Phys. Chem. Chem. Phys. 2020, 22, 2115–2121. [Google Scholar] [CrossRef] [PubMed]
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2017, 2, 16098. [Google Scholar] [CrossRef]
- Mendoza-Sánchez, B.; Gogotsi, Y. Synthesis of two-dimensional materials for capacitive energy storage. Adv. Mater. 2016, 28, 6104–6135. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wu, Y. 2D early transition metal carbides (MXenes) for catalysis. Small 2019, 15, e1804736. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Zhao, Y.; Li, L.; Wang, Y.; Wang, J.; Xiong, J.; Du, S.; Zhang, P.; Shi, X.; Yu, J. MXene/Polymer Nanocomposites: Preparation, Properties, and Applications. Polym. Rev. 2020, 1–36. [Google Scholar] [CrossRef]
- Hemanth, N.R.; Kandasubramanian, B. Recent advances in 2D MXenes for enhanced cation intercalation in energy harvesting Applications: A review. Chem. Eng. J. 2020, 392, 123678. [Google Scholar] [CrossRef]
- Garg, R.; Agarwal, A.; Agarwal, M. A review on MXene for energy storage application: Effect of interlayer distance. Mater. Res. Express 2020, 7, 022001. [Google Scholar] [CrossRef]
- Jun, B.-M.; Kim, S.; Heo, J.; Park, C.M.; Her, N.; Jang, M.; Huang, Y.; Han, J.; Yoon, Y. Review of MXenes as new nanomaterials for energy storage/delivery and selected environmental applications. Nano. Res. 2019, 12, 471–487. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Li, C.; Huang, W.; Mei, S.; Lin, H.; Ou, Q.; Zhang, Y.; Guo, J.; Zhang, F.; Xu, S.; et al. MXene/Polymer Membranes: Synthesis, Properties, and Emerging Applications. Chem. Mater. 2020, 32, 1703–1747. [Google Scholar] [CrossRef]
- Pang, J.; Mendes, R.G.; Bachmatiuk, A.; Zhao, L.; Ta, H.Q.; Gemming, T.; Liu, H.; Liu, Z.; Rummeli, M.H. Applications of 2D MXenes in energy conversion and storage systems. Chem. Soc. Rev. 2019, 48, 72–133. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Wang, Y.; Jing, Y.; Ma, J.; Du, C.F.; Yan, Q. Surface modified MXene-based nanocomposites for electrochemical energy conversion and storage. Small 2019, 15, 1901503. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.J.; Pinilla, S.; McEvoy, N.; Cullen, C.P.; Anasori, B.; Long, E.; Park, S.-H.; Seral-Ascaso, A.; Shmeliov, A.; Krishnan, D.; et al. Oxidation Stability of Colloidal Two-Dimensional Titanium Carbides (MXenes). Chem. Mater. 2017, 29, 4848–4856. [Google Scholar] [CrossRef]
- Rozmyslowska, A.; Wojciechowski, T.; Ziemkowska, W.; Chlubny, L.; Olszyna, A.; Pozniak, S.; Tomkiewicz, K.; Jastrzebska, A.M. Colloidal Properties and Stability of 2D Ti3C2 and Ti2C MXenes in Water. Int. J. Electrochem. Sci. 2018, 13, 10837–10847. [Google Scholar] [CrossRef]
- Maleski, K.; Mochalin, V.N.; Gogotsi, Y. Dispersions of Two-Dimensional Titanium Carbide MXene in Organic Solvents. Chem. Mater. 2017, 29, 1632–1640. [Google Scholar] [CrossRef]
- Carey, M.; Hinton, Z.; Natu, V.; Pai, R.; Sokol, M.; Alvarez, N.J.; Kalra, V.; Barsoum, M.W. Dispersion and Stabilization of Alkylated 2D MXene in Nonpolar Solvents and Their Pseudocapacitive Behavior. Cell Rep. Phys. Sci. 2020, 1, 100042. [Google Scholar] [CrossRef]
- Du, Y.; Yu, B.; Wei, L.; Wang, Y.; Zhang, X.; Ye, S. Efficient removal of Pb(II) by Ti3C2Tx powder modified with a silane coupling agent. J. Mater. Sci. 2019, 54, 13283–13297. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, Y.; Yang, C.; Tian, Y.; Han, Y.; Liu, J.; Yin, X.; Que, W. A hydrophobic surface enabled salt-blocking 2D Ti3C2 MXene membrane for efficient and stable solar desalination. J. Mater. Chem. A 2018, 6, 16196–16204. [Google Scholar] [CrossRef]
- Lim, S.; Park, H.; Yang, J.; Kwak, C.; Lee, J. Stable colloidal dispersion of octylated Ti3C2-MXenes in a nonpolar solvent. Colloid Surf. A-Phys. Eng. Asp. 2019, 579, 123648. [Google Scholar] [CrossRef]
- Zhang, Q.; Yi, G.; Fu, Z.; Yu, H.; Chen, S.; Quan, X. Vertically Aligned Janus MXene-Based Aerogels for Solar Desalination with High Efficiency and Salt Resistance. ACS Nano 2019, 13, 13196–13207. [Google Scholar] [CrossRef] [PubMed]
- Tran, M.H.; Brilmayer, R.; Liu, L.; Zhuang, H.; Hess, C.; Andrieu-Brunsen, A.; Birkel, C.S. Synthesis of a Smart Hybrid MXene with Switchable Conductivity for Temperature Sensing. ACS Appl. Nano Mater. 2020, 3, 4069–4076. [Google Scholar] [CrossRef]
- Taloub, N.; Henniche, A.; Liu, L.; Li, J.; Rahoui, N.; Hegazy, M.; Huang, Y.D. Improving the mechanical properties, UV and hydrothermal aging resistance of PIPD fiber using MXene (Ti3C2(OH)2) nanosheets. Compos. Part B-Eng. 2019, 163, 260–271. [Google Scholar] [CrossRef]
- Riazi, H.; Anayee, M.; Hantanasirisakul, K.; Shamsabadi, A.A.; Anasori, B.; Gogotsi, Y.; Soroush, M. Surface Modification of a MXene by an Aminosilane Coupling Agent. Adv. Mater. Interfaces 2020, 7, 1902008. [Google Scholar] [CrossRef]
- Kim, D.; Ko, T.Y.; Kim, H.; Lee, G.H.; Cho, S.; Koo, C.M. Nonpolar Organic Dispersion of 2D Ti3C2Tx MXene Flakes via Simultaneous Interfacial Chemical Grafting and Phase Transfer Method. ACS Nano 2019, 13, 13818–13828. [Google Scholar] [CrossRef]
- Sun, W.J.; Zhao, Y.Y.; Cheng, X.F.; He, J.H.; Lu, J.M. Surface Functionalization of Single-Layered Ti3C2Tx MXene and Its Application in Multilevel Resistive Memory. ACS Appl. Mater. Interfaces 2020, 12, 9865–9871. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, L.; Du, K.; Wang, S.; Huang, Z.; Yuan, L.; Li, Z.; Wang, H.; Zheng, L.; Chai, Z.; et al. Effective removal of U(VI) and Eu(III) by carboxyl functionalized MXene nanosheets. J. Hazard. Mater. 2020, 396, 122731. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, L.; Huang, Z.; Yu, J.; Li, Z.; Deng, H.; Yin, T.; Yuan, L.; Gibson, J.K.; Mei, L.; et al. Aryl Diazonium-Assisted Amidoximation of MXene for Boosting Water Stability and Uranyl Sequestration via Electrochemical Sorption. ACS Appl. Mater. Interfaces 2020, 12, 15579–15587. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, J.; Wu, Y.; Huang, H.; Li, G.; Zhang, X.; Wang, Z. Surface modified MXene Ti3C2 multilayers by aryl diazonium salts leading to large-scale delamination. Appl. Surf. Sci. 2016, 384, 287–293. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, J.; Wu, Y.; Huang, H.; Jiang, Q. Chemically functionalized two-dimensional titanium carbide MXene by in situ grafting-intercalating with diazonium ions to enhance supercapacitive performance. J. Phys. Chem. Solids 2018, 115, 172–179. [Google Scholar] [CrossRef]
- Chen, K.; Yan, X.; Li, J.; Jiao, T.; Cai, C.; Zou, G.; Wang, R.; Wang, M.; Zhang, L.; Peng, Q. Preparation of Self-Assembled Composite Films Constructed by Chemically-Modified MXene and Dyes with Surface-Enhanced Raman Scattering Characterization. Nanomaterials 2019, 9, 284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boota, M.; Urbankowski, P.; Porzio, W.; Barba, L.; Osti, N.C.; Bleuel, M.; Keum, J.K.; Mamontov, E. Understanding Functionalization of Titanium Carbide (MXene) with Quinones and Their Pseudocapacitance. ACS Appl. Energy Mater. 2020, 3, 4127–4133. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.B.; Chen, Q.; Li, P.; Zhou, A.G.; Cao, X.X.; Hu, Q.K. Preparation, mechanical and anti-friction performance of MXene/polymer composites. Mater. Des. 2016, 92, 682–689. [Google Scholar] [CrossRef]
- Sliozberg, Y.; Andzelm, J.; Hatter, C.B.; Anasori, B.; Gogotsi, Y.; Hall, A. Interface binding and mechanical properties of MXene-epoxy nanocomposites. Compos. Sci. Technol. 2020, 192, 108124. [Google Scholar] [CrossRef]
- Zhi, W.; Xiang, S.; Bian, R.; Lin, R.; Wu, K.; Wang, T.; Cai, D. Study of MXene-filled polyurethane nanocomposites prepared via an emulsion method. Compos. Sci. Technol. 2018, 168, 404–411. [Google Scholar] [CrossRef]
- Malaki, M.; Varma, R.S. Mechanotribological Aspects of MXene-Reinforced Nanocomposites. Adv. Mater. 2020, 32, 2003154. [Google Scholar] [CrossRef]
- Hai, Y.; Jiang, S.; Zhou, C.; Sun, P.; Huang, Y.; Niu, S. Fire-safe unsaturated polyester resin nanocomposites based on MAX and MXene: A comparative investigation of their properties and mechanism of fire retardancy. Dalton Trans. 2020, 49, 5803–5814. [Google Scholar] [CrossRef]
- He, L.; Wang, J.; Wang, B.; Wang, X.; Zhou, X.; Cai, W.; Mu, X.; Hou, Y.; Hu, Y.; Song, L. Large-scale production of simultaneously exfoliated and Functionalized Mxenes as promising flame retardant for polyurethane. Compos. Part B-Eng. 2019, 179, 107486. [Google Scholar] [CrossRef]
- Chen, J.; Chen, K.; Tong, D.; Huang, Y.; Zhang, J.; Xue, J.; Huang, Q.; Chen, T. CO2 and temperature dual responsive “Smart” MXene phases. Chem. Commun. 2015, 51, 314–317. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, X.-J.; Li, P.; Zhao, Y.; Niu, Q.J. Fabrication of novel MXene (Ti3C2)/polyacrylamide nanocomposite hydrogels with enhanced mechanical and drug release properties. Soft Matter 2020, 16, 162–169. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, J.; Wang, X.; Qin, J.; Cao, M. A Hybrid Assembly of MXene with NH2−Si Nanoparticles Boosting Lithium Storage Performance. Chem. Asian J. 2020, 15, 1376–1383. [Google Scholar] [CrossRef]
- Xu, B.; Zhu, M.; Zhang, W.; Zhen, X.; Pei, Z.; Xue, Q.; Zhi, C.; Shi, P. Ultrathin MXene-Micropattern-Based Field-Effect Transistor for Probing Neural Activity. Adv. Mater. 2016, 28, 3333–3339. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zheng, J.; Nai, J.; Jin, C.; Yuan, H.; Sheng, O.; Liu, Y.; Fang, R.; Zhang, W.; Huang, H.; et al. Atomic Sulfur Covalently Engineered Interlayers of Ti3C2 MXene for Ultra-Fast Sodium-Ion Storage by Enhanced Pseudocapacitance. Adv. Funct. Mater. 2019, 29, 1808107. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, W.; Yuan, H.; Jin, C.; Zhang, L.; Huang, H.; Liang, C.; Xia, Y.; Zhang, J.; Gan, Y.; et al. Pillared Structure Design of MXene with Ultralarge Interlayer Spacing for High-Performance Lithium-Ion Capacitors. ACS Nano 2017, 11, 2459–2469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Si, J.Y.; Tawiah, B.; Sun, W.L.; Lin, B.; Wang, C.; Yuen, A.C.Y.; Yu, B.; Li, A.; Yang, W.; Lu, H.D.; et al. Functionalization of MXene Nanosheets for Polystyrene towards High Thermal Stability and Flame Retardant Properties. Polymers 2019, 11, 976. [Google Scholar] [CrossRef] [Green Version]
- Bian, R.; Lin, R.; Wang, G.; Lu, G.; Zhi, W.; Xiang, S.; Wang, T.; Clegg, P.S.; Cai, D.; Huang, W. 3D assembly of Ti3C2-MXene directed by water/oil interfaces. Nanoscale 2018, 10, 3621–3625. [Google Scholar] [CrossRef]
- Boota, M.; Pasini, M.; Galeotti, F.; Porzio, W.; Zhao, M.-Q.; Halim, J.; Gogotsi, Y. Interaction of Polar and Nonpolar Polyfluorenes with Layers of Two-Dimensional Titanium Carbide (MXene): Intercalation and Pseudocapacitance. Chem. Mater. 2017, 29, 2731–2738. [Google Scholar] [CrossRef]
- Elumalai, S.; Yoshimura, M.; Ogawa, M. Simultaneous Delamination and Rutile Formation on the Surface of Ti3C2Tx MXene for Copper Adsorption. Chem. Asian J. 2020, 15, 1044–1051. [Google Scholar] [CrossRef]
- Chen, C.; Boota, M.; Urbankowski, P.; Anasori, B.; Miao, L.; Jiang, J.J.; Gogotsi, Y. Effect of glycine functionalization of 2D titanium carbide (MXene) on charge storage. J. Mater. Chem. A 2018, 6, 4617–4622. [Google Scholar] [CrossRef]
- Carey, M.; Hinton, Z.; Sokol, M.; Alvarez, N.J.; Barsoum, M.W. Nylon-6/Ti3C2Tz MXene Nanocomposites Synthesized by in Situ Ring Opening Polymerization of epsilon-Caprolactam and Their Water Transport Properties. ACS Appl. Mater. Interfaces 2019, 11, 20425–20436. [Google Scholar] [CrossRef]
- Carey, M.S.; Sokol, M.; Palmese, G.R.; Barsoum, M.W. Water Transport and Thermomechanical Properties of Ti3C2Tz MXene Epoxy Nanocomposites. ACS Appl. Mater. Interfaces 2019, 11, 39143–39149. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, X.; Zhang, L.J.; Wu, X.M.; Wang, Q.G.; Luo, C.Y.; Wu, G.L. Synthesis of Ti3C2/Fe3O4/PANI hierarchical architecture composite as an efficient wide-band electromagnetic absorber. Appl. Surf. Sci. 2019, 480, 830–838. [Google Scholar] [CrossRef]
- Fu, J.; Yun, J.; Wu, S.; Li, L.; Yu, L.; Kim, K.H. Architecturally Robust Graphene-Encapsulated MXene Ti2CTx@Polyaniline Composite for High-Performance Pouch-Type Asymmetric Supercapacitor. ACS Appl. Mater. Interfaces 2018, 10, 34212–34221. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zheng, D.; Liu, F.; Li, W.; Lin, J. Synthesis of an MXene/polyaniline composite with excellent electrochemical properties. J. Mater. Chem. A 2020, 8, 5853–5858. [Google Scholar] [CrossRef]
- Chen, C.; Boota, M.; Xie, X.; Zhao, M.; Anasori, B.; Ren, C.E.; Miao, L.; Jiang, J.; Gogotsi, Y. Charge transfer induced polymerization of EDOT confined between 2D titanium carbide layers. J. Mater. Chem. A 2017, 5, 5260–5265. [Google Scholar] [CrossRef]
- Qin, L.; Tao, Q.; Liu, X.; Fahlman, M.; Halim, J.; Perssona, P.O.A.; Rosen, J.; Zhang, F. Polymer-MXene composite films formed by MXene-facilitated electrochemical polymerization for flexible solid-state microsupercapacitors. Nano Energy 2019, 60, 734–742. [Google Scholar] [CrossRef]
- Hou, C.; Yu, H. Modifying the nanostructures of PEDOT:PSS/Ti3C2TX composite hole transport layers for highly efficient polymer solar cells. J. Mater. Chem. C 2020, 8, 4169–4180. [Google Scholar] [CrossRef]
- Wang, X.; Sun, K.; Li, K.; Li, X.; Gogotsi, Y. Ti3C2Tx/PEDOT:PSS hybrid materials for room-temperature methanol sensor. Chin. Chem. Lett. 2020, 31, 1018–1021. [Google Scholar] [CrossRef]
- Liu, R.; Miao, M.; Li, Y.; Zhang, J.; Cao, S.; Feng, X. Ultrathin Biomimetic Polymeric Ti3C2Tx MXene Composite Films for Electromagnetic Interference Shielding. ACS Appl. Mater. Interfaces 2018, 10, 44787–44795. [Google Scholar] [CrossRef]
- Li, L.; Zhang, N.; Zhang, M.; Zhang, X.; Zhang, Z. Flexible Ti3C2Tx/PEDOT:PSS films with outstanding volumetric capacitance for asymmetric supercapacitors. Dalton Trans. 2019, 48, 1747–1756. [Google Scholar] [CrossRef]
- Qin, L.; Tao, Q.; El Ghazaly, A.; Fernandez-Rodriguez, J.; Persson, P.O.Å.; Rosen, J.; Zhang, F. High-Performance Ultrathin Flexible Solid-State Supercapacitors Based on Solution Processable Mo1.33C MXene and PEDOT:PSS. Adv. Funct. Mater. 2018, 28. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Seyedin, S.; Qin, S.; Wang, Z.; Moradi, S.; Yang, F.; Lynch, P.A.; Yang, W.; Liu, J.; Wang, X.; et al. Highly Conductive Ti3C2Tx MXene Hybrid Fibers for Flexible and Elastic Fiber-Shaped Supercapacitors. Small 2019, 15, 1804732. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Ren, C.E.; Zhao, M.Q.; Yang, J.; Giammarco, J.M.; Qiu, J.S.; Barsoum, M.W.; Gogotsi, Y. Flexible and conductive MXene films and nanocomposites with high capacitance. Proc. Natl. Acad. Sci. USA 2014, 111, 16676–16681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirkhani, S.A.; Zeraati, A.S.; Aliabadian, E.; Naguib, M.; Sundararaj, U. High Dielectric Constant and Low Dielectric Loss via Poly(vinyl alcohol)/Ti3C2Tx MXene Nanocomposites. ACS Appl. Mater. Interfaces 2019, 11, 18599–18608. [Google Scholar] [CrossRef]
- Ajnsztajn, A.; Ferguson, S.; Thostenson, J.O.; Ngaboyamahina, E.; Parker, C.B.; Glass, J.T.; Stiff-Roberts, A.D. Transparent MXene-Polymer Supercapacitive Film Deposited Using RIR-MAPLE. Crystals 2020, 10, 152. [Google Scholar] [CrossRef] [Green Version]
- Boota, M.; Anasori, B.; Voigt, C.; Zhao, M.Q.; Barsoum, M.W.; Gogotsi, Y. Pseudocapacitive Electrodes Produced by Oxidant-Free Polymerization of Pyrrole between the Layers of 2D Titanium Carbide (MXene). Adv. Mater. 2016, 28, 1517–1522. [Google Scholar] [CrossRef]
- Zhu, M.; Huang, Y.; Deng, Q.; Zhou, J.; Pei, Z.; Xue, Q.; Huang, Y.; Wang, Z.; Li, H.; Huang, Q.; et al. Highly Flexible, Freestanding Supercapacitor Electrode with Enhanced Performance Obtained by Hybridizing Polypyrrole Chains with MXene. Adv. Energy Mater. 2016, 6, 1600969. [Google Scholar] [CrossRef]
- Zhang, X.; Li, J.; Li, J.; Han, L.; Lu, T.; Zhang, X.; Zhu, G.; Pan, L. 3D TiO2@nitrogen-doped carbon/Fe7S8 composite derived from polypyrrole-encapsulated alkalized MXene as anode material for high-performance lithium-ion batteries. Chem. Eng. J. 2020, 385, 123394. [Google Scholar] [CrossRef]
- Dong, X.; Ding, B.; Guo, H.; Dou, H.; Zhang, X. Superlithiated Polydopamine Derivative for High-Capacity and High-Rate Anode for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 38101–38108. [Google Scholar] [CrossRef]
- Sun, T.; Li, Z.J.; Wang, H.G.; Bao, D.; Meng, F.L.; Zhang, X.B. A Biodegradable Polydopamine-Derived Electrode Material for High-Capacity and Long-Life Lithium-Ion and Sodium-Ion Batteries. Angew. Chem. Int. Ed. Engl. 2016, 55, 10662–10666. [Google Scholar] [CrossRef]
- Li, T.; Ding, B.; Wang, J.; Qin, Z.; Fernando, J.F.S.; Bando, Y.; Nanjundan, A.K.; Kaneti, Y.V.; Golberg, D.; Yamauchi, Y. Sandwich-Structured Ordered Mesoporous Polydopamine/MXene Hybrids as High-Performance Anodes for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 14993–15001. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Qiu, J.; Deng, S.; Du, Z.; Cheng, X.; Wang, H. Ti3C2Tx@PDA-Integrated Polyurethane Phase Change Composites with Superior Solar-Thermal Conversion Efficiency and Improved Thermal Conductivity. ACS Sustain. Chem. Eng. 2020, 8, 5799–5806. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Lee, K.H.; Anjum, D.H.; Sougrat, R.; Jiang, Q.; Kim, H.; Alshareef, H.N. MXenes stretch hydrogel sensor performance to new limits. Sci. Adv. 2018, 4, eaat0098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Wan, L.; Gao, Y.; Fang, X.; Lu, T.; Pan, L.; Xuan, F. Highly Stretchable and Self-Healable MXene/Polyvinyl Alcohol Hydrogel Electrode for Wearable Capacitive Electronic Skin. Adv. Electron. Mater. 2019, 5, 1900285. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, K.; Li, Y.; Lan, J.; Yan, B.; Shi, L.; Ran, R. High-Strength, Self-Healable, Temperature-Sensitive, MXene-Containing Composite Hydrogel as a Smart Compression Sensor. ACS Appl. Mater. Interfaces 2019, 11, 47350–47357. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Z.; El-Demellawi, J.K.; Jiang, Q.; Ge, G.; Liang, H.; Lee, K.; Dong, X.; Alshareef, H.N. MXene hydrogels: Fundamentals and applications. Chem. Soc. Rev. 2020, 49, 7229–7251. [Google Scholar] [CrossRef]
- Liao, H.; Guo, X.; Wan, P.; Yu, G. Conductive MXene Nanocomposite Organohydrogel for Flexible, Healable, Low-Temperature Tolerant Strain Sensors. Adv. Funct. Mater. 2019, 29, 1904507. [Google Scholar] [CrossRef]
- Wu, X.; Liao, H.; Ma, D.; Chao, M.; Wang, Y.; Jia, X.; Wan, P.; Zhang, L. A wearable, self-adhesive, long-lastingly moist and healable epidermal sensor assembled from conductive MXene nanocomposites. J. Mater. Chem. C 2020, 8, 1788–1795. [Google Scholar] [CrossRef]
- Le, T.A.; Tran, N.Q.; Hong, Y.; Lee, H. Intertwined Titanium Carbide MXene within a 3 D Tangled Polypyrrole Nanowires Matrix for Enhanced Supercapacitor Performances. Chem. Eur. J. 2019, 25, 1037–1043. [Google Scholar] [CrossRef]
- Kumar, S.; Arti; Kumar, P.; Singh, N.; Verma, V. Steady microwave absorption behavior of two-dimensional metal carbide MXene and Polyaniline composite in X-band. J. Magn. Magn. Mater. 2019, 488, 165364. [Google Scholar] [CrossRef]
- Zhao, S.; Li, L.; Zhang, H.-B.; Qian, B.; Luo, J.-Q.; Deng, Z.; Shi, S.; Russell, T.P.; Yu, Z.-Z. Janus MXene nanosheets for macroscopic assemblies. Mater. Chem. Front. 2020, 4, 910–917. [Google Scholar] [CrossRef]
- Xiong, D.; Li, X.; Bai, Z.; Lu, S. Recent Advances in Layered Ti3C2Tx MXene for Electrochemical Energy Storage. Small 2018, 14, 1703419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukatskaya, M.R.; Mashtalir, O.; Ren, C.E.; Dall’Agnese, Y.; Rozier, P.; Taberna, P.L.; Naguib, M.; Simon, P.; Barsoum, M.W.; Gogotsi, Y. Cation Intercalation and High Volumetric Capacitance of Two-Dimensional Titanium Carbide. Science 2013, 341, 1502–1505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gund, G.S.; Park, J.H.; Harpalsinh, R.; Kota, M.; Shin, J.H.; Kim, T.-I.; Gogotsi, Y.; Park, H.S. MXene/Polymer Hybrid Materials for Flexible AC-Filtering Electrochemical Capacitors. Joule 2019, 3, 164–176. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.; Liu, J.; Yang, C.; Yang, X.; Wei, J.; Xia, Y.; Gong, X.; Suo, Z. Hydrogel Paint. Adv. Mater. 2019, 31, e1903062. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xie, X.; Xin, X.; Tang, Z.R.; Xu, Y.J. Ti3C2Tx-Based Three-Dimensional Hydrogel by a Graphene Oxide-Assisted Self-Convergence Process for Enhanced Photoredox Catalysis. ACS Nano 2019, 13, 295–304. [Google Scholar] [CrossRef] [PubMed]
- VahidMohammadi, A.; Moncada, J.; Chen, H.; Kayali, E.; Orangi, J.; Carrero, C.A.; Beidaghi, M. Thick and freestanding MXene/PANI pseudocapacitive electrodes with ultrahigh specific capacitance. J. Mater. Chem. A 2018, 6, 22123–22133. [Google Scholar] [CrossRef]
- Yun, J.; Echols, I.; Flouda, P.; Wang, S.; Easley, A.; Zhao, X.; Tan, Z.; Prehn, E.; Zi, G.; Radovic, M.; et al. Layer-by-Layer Assembly of Polyaniline Nanofibers and MXene Thin-Film Electrodes for Electrochemical Energy Storage. ACS Appl. Mater. Interfaces 2019, 11, 47929–47938. [Google Scholar] [CrossRef]
- Li, K.; Wang, X.; Li, S.; Urbankowski, P.; Li, J.; Xu, Y.; Gogotsi, Y. An Ultrafast Conducting Polymer@MXene Positive Electrode with High Volumetric Capacitance for Advanced Asymmetric Supercapacitors. Small 2020, 16, 1906851. [Google Scholar] [CrossRef]
- Tian, W.; VahidMohammadi, A.; Wang, Z.; Ouyang, L.; Beidaghi, M.; Hamedi, M.M. Layer-by-layer self-assembly of pillared two-dimensional multilayers. Nat. Commun. 2019, 10, 2558. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Huang, B.; Lv, R.; Wang, Q.; Wang, Y. Highly flexible and low capacitance loss supercapacitor electrode based on hybridizing decentralized conjugated polymer chains with MXene. Chem. Eng. J. 2019, 378, 122246. [Google Scholar] [CrossRef]
- Lipomi, D.J.; Lee, J.A.; Vosgueritchian, M.; Tee, B.C.K.; Bolander, J.A.; Bao, Z. Electronic Properties of Transparent Conductive Films of PEDOT:PSS on Stretchable Substrates. Chem. Mater. 2012, 24, 373–382. [Google Scholar] [CrossRef]
- Wang, H.; Li, L.; Zhu, C.; Lin, S.; Wen, J.; Jin, Q.; Zhang, X. In situ polymerized Ti3C2Tx/PDA electrode with superior areal capacitance for supercapacitors. J. Alloy. Compd. 2019, 778, 858–865. [Google Scholar] [CrossRef]
- Zhao, M.Q.; Xie, X.; Ren, C.E.; Makaryan, T.; Anasori, B.; Wang, G.; Gogotsi, Y. Hollow MXene Spheres and 3D Macroporous MXene Frameworks for Na-Ion Storage. Adv. Mater. 2017, 29, 1702410. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kamdem, P.; Jin, X.-J. Hierarchical architecture of MXene/PANI hybrid electrode for advanced asymmetric supercapacitors. J. Alloy. Compd. 2021, 850, 156608. [Google Scholar] [CrossRef]
- Siriwardane, E.M.D.; Demiroglu, I.; Sevik, C.; Cakir, D. Achieving Fast Kinetics and Enhanced Li Storage Capacity for Ti3C2O2 by Intercalation of Quinone Molecules. ACS Appl. Energy Mater. 2019, 2, 1251–1258. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Qin, J.; Xie, X.; Yang, R.; Cao, M. Surface Charge Engineering for Covalently Assembling Three-Dimensional MXene Network for All-Climate Sodium Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 39181–39194. [Google Scholar] [CrossRef]
- Yang, K.; Yin, F.; Xia, D.; Peng, H.; Yang, J.; Yuan, W. A highly flexible and multifunctional strain sensor based on a network-structured MXene/polyurethane mat with ultra-high sensitivity and a broad sensing range. Nanoscale 2019, 11, 9949–9957. [Google Scholar] [CrossRef]
- Cai, Y.-W.; Zhang, X.-N.; Wang, G.-G.; Li, G.-Z.; Zhao, D.-Q.; Sun, N.; Li, F.; Zhang, H.-Y.; Han, J.-C.; Yang, Y. A flexible ultra-sensitive triboelectric tactile sensor of wrinkled PDMS/MXene composite films for E-skin. Nano Energy 2021, 81, 105663. [Google Scholar] [CrossRef]
- Zhou, S.J.; Gu, C.X.; Li, Z.Z.; Yang, L.Y.; He, L.H.; Wang, M.H.; Huang, X.Y.; Zhou, N.; Zhang, Z.H. Ti3C2Tx MXene and polyoxometalate nanohybrid embedded with polypyrrole: Ultra-sensitive platform for the detection of osteopontin. Appl. Surf. Sci. 2019, 498, 143889. [Google Scholar] [CrossRef]
- Galizia, M.; Chi, W.S.; Smith, Z.P.; Merkel, T.C.; Baker, R.W.; Freeman, B.D. 50th Anniversary Perspective: Polymers and Mixed Matrix Membranes for Gas and Vapor Separation: A Review and Prospective Opportunities. Macromolecules 2017, 50, 7809–7843. [Google Scholar] [CrossRef]
- Neampet, S.; Ruecha, N.; Qin, J.; Wonsawat, W.; Chailapakul, O.; Rodthongkum, N. A nanocomposite prepared from platinum particles, polyaniline and a Ti3C2 MXene for amperometric sensing of hydrogen peroxide and lactate. Microchim. Acta 2019, 186, 752. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Y.; Lai, S.N.; Yen, C.C.; Jiang, X.; Peroulis, D.; Stanciu, L.A. Surface Functionalization of Ti3C2Tx MXene with Highly Reliable Superhydrophobic Protection for Volatile Organic Compounds Sensing. ACS Nano 2020, 14, 11490–11501. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, J.L.; Jiang, Y.D.; He, Z.Z.; Liu, B.H.; Xie, H.K.; Li, H.; Li, Z.M.; Wang, Y.; Tai, H.L. Toward agricultural ammonia volatilization monitoring: A flexible polyaniline/Ti3C2Tx hybrid sensitive films based gas sensor. Sens. Actuators B-Chem. 2020, 316, 128144. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.; Sun, Y.; Meng, X.; Dall’Agnese, Y.; Chen, G.; Dall’Agnese, C.; Ren, H.; Sasaki, S.-i.; Tamiaki, H.; et al. Chlorosome-Like Molecular Aggregation of Chlorophyll Derivative on Ti3C2Tx MXene Nanosheets for Efficient Noble Metal-Free Photocatalytic Hydrogen Evolution. Adv. Mater. Interfaces 2020, 7, 1902080. [Google Scholar] [CrossRef]
- An, H.; Habib, T.; Shah, S.; Gao, H.; Patel, A.; Echols, I.; Zhao, X.; Radovic, M.; Green, M.J.; Lutkenhaus, J.L. Water Sorption in MXene/Polyelectrolyte Multilayers for Ultrafast Humidity Sensing. ACS Appl. Nano Mater. 2019, 2, 948–955. [Google Scholar] [CrossRef]
- Liu, G.Z.; Shen, J.; Ji, Y.F.; Liu, Q.; Liu, G.P.; Yang, J.; Jin, W.Q. Two-dimensional Ti2CTx MXene membranes with integrated and ordered nanochannels for efficient solvent dehydration. J. Mater. Chem. A 2019, 7, 12095–12104. [Google Scholar] [CrossRef]

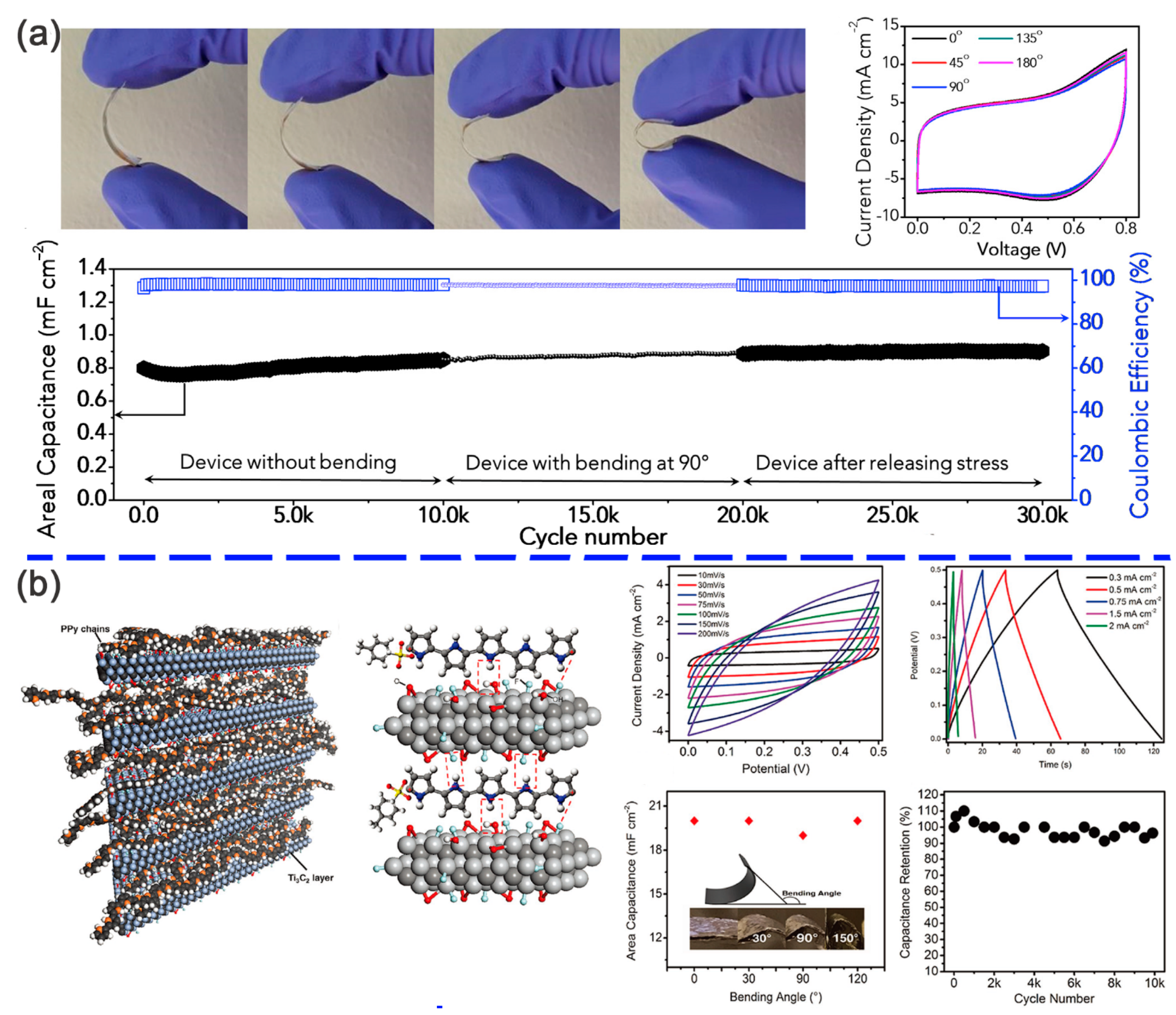
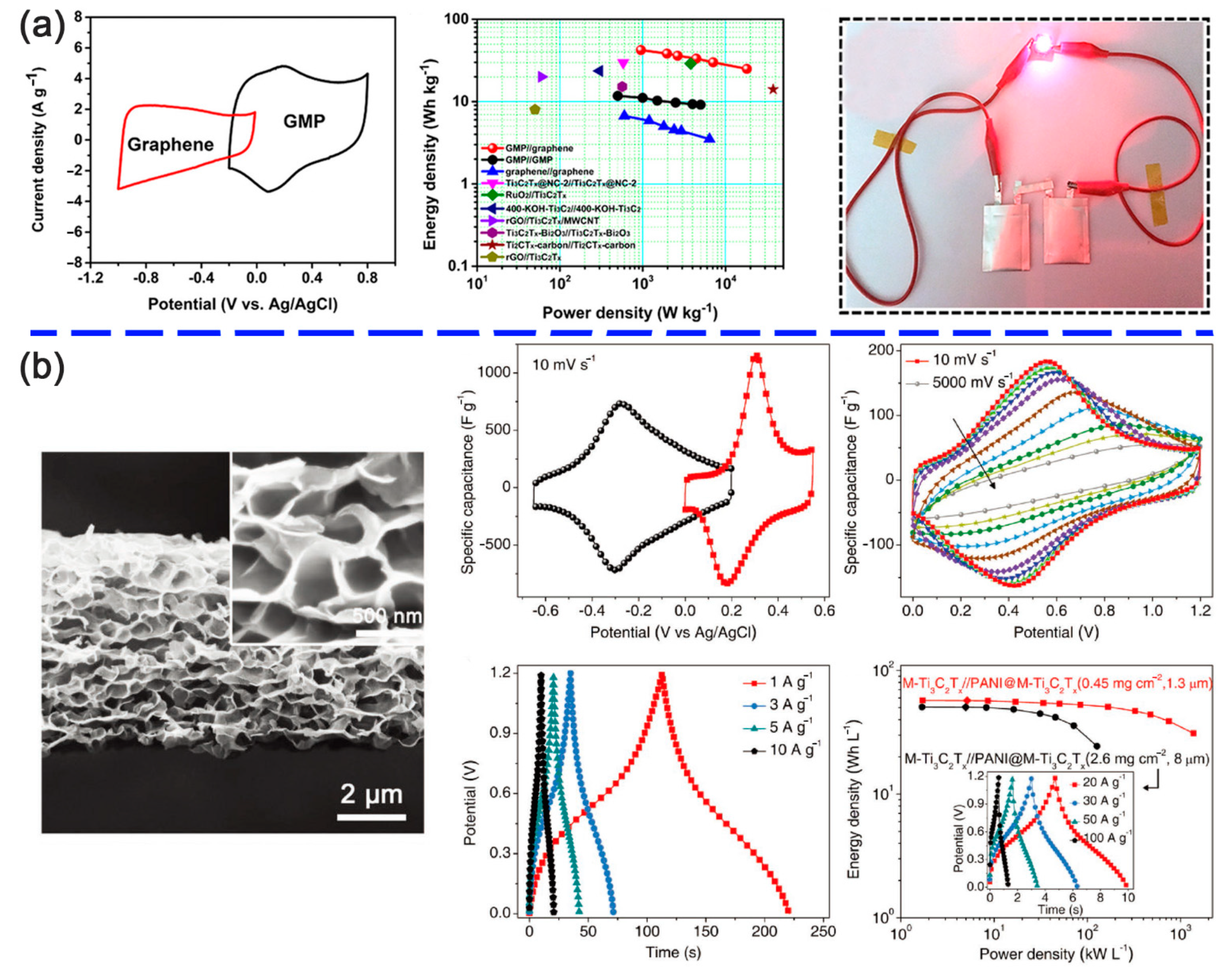
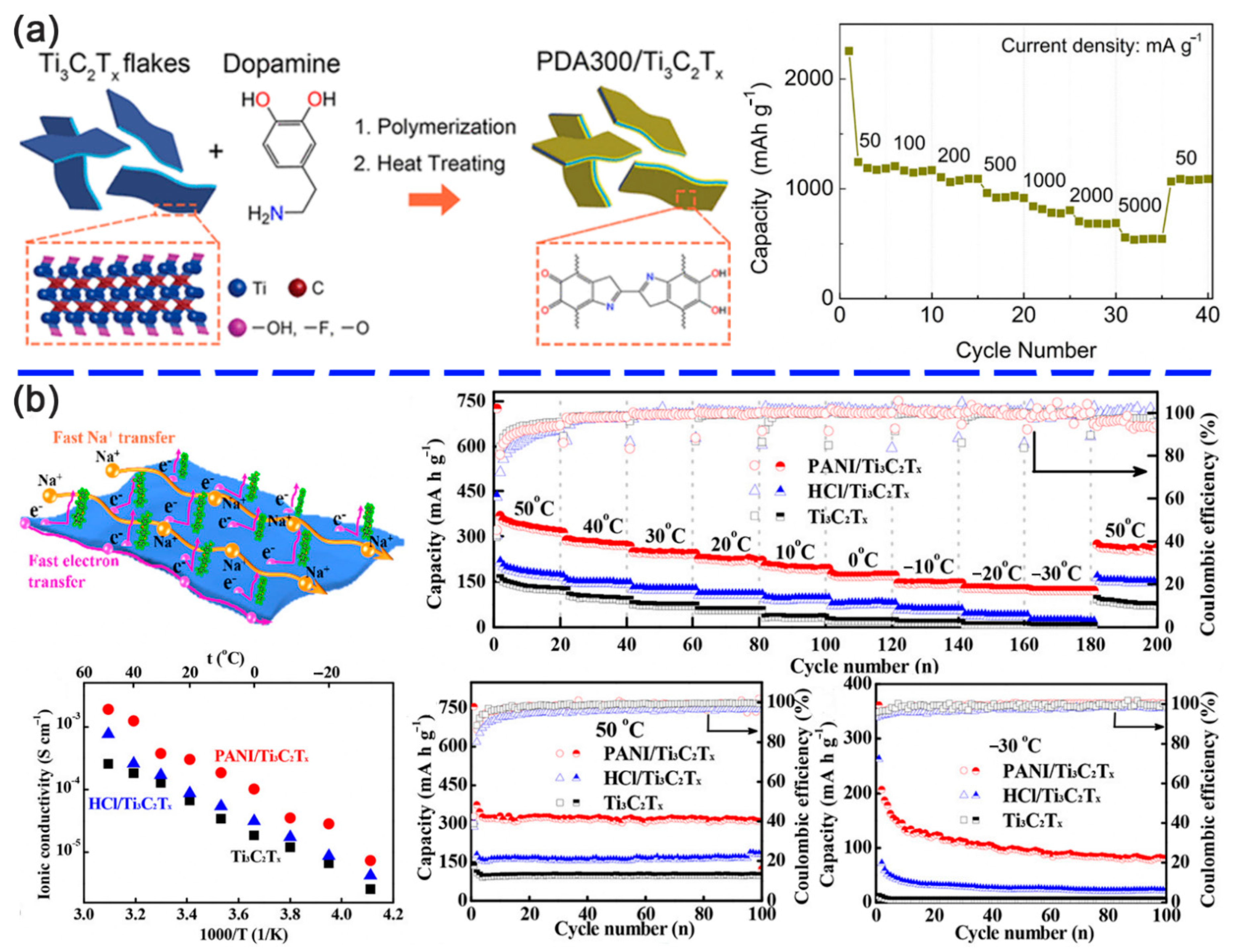
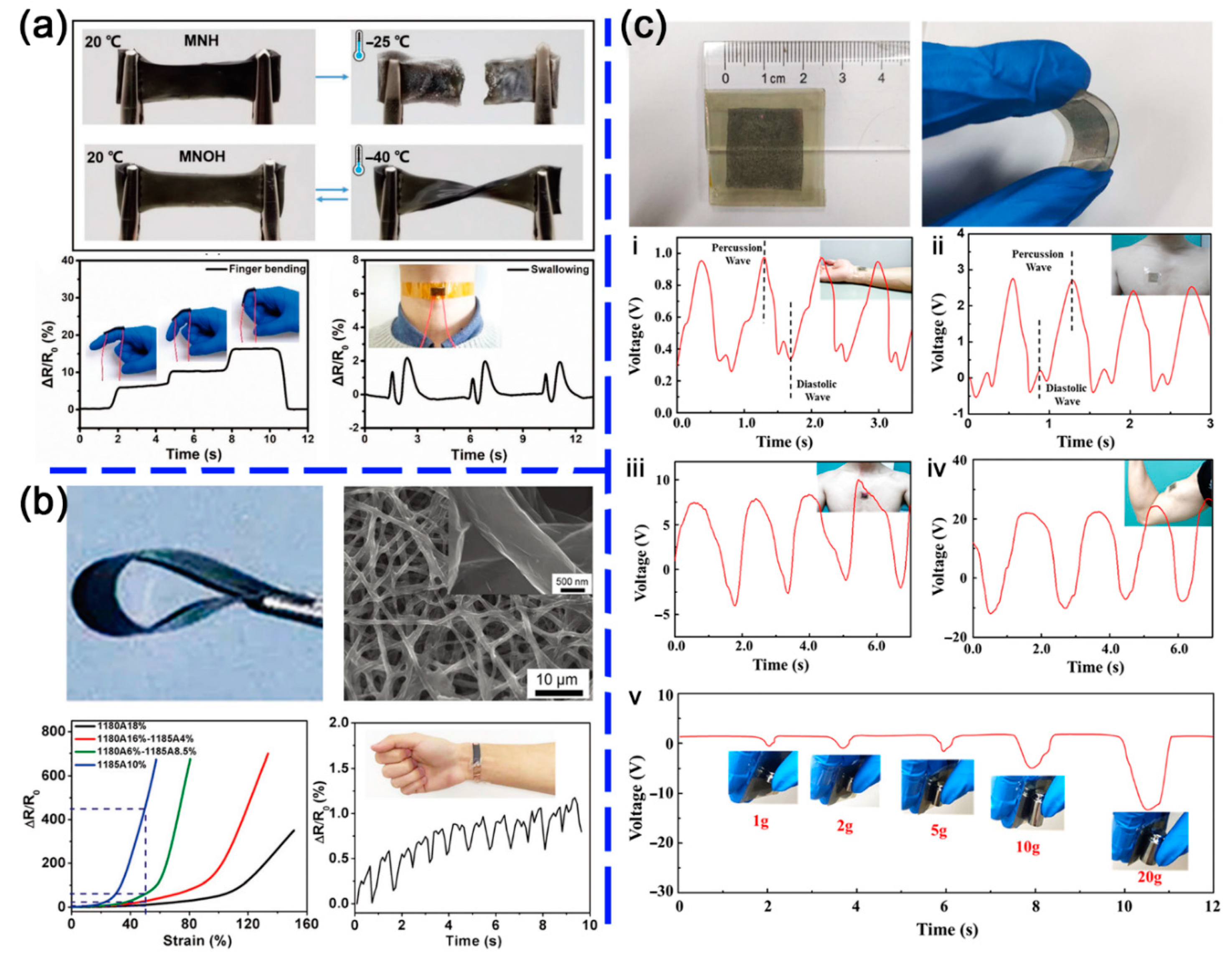

| Interaction Type | Typical Modifier | Binding Forms |
|---|---|---|
| Covalent interaction | Silane | a Si–O–M |
| Diazonium salts | C–O–M | |
| Alkyl phosphoric acid | P–O–M | |
| Electrostatic interaction | Alkyl ammonium salts | b R4N⊕ ⊝T–M |
| Amino acids | –H3N⊕ ⊝T–M | |
| PFO | R4N⊕ ⊝T–M | |
| PANI | –H3N⊕ ⊝T–M | |
| PEDOT:PSS | SO3H⊕ ⊝T–M | |
| Hydrogen bonds | Pyrrole/PPy | NH···O–M |
| Dopamine/PDA | OH···O–M | |
| PAM | CONH2···O–M | |
| PVA | OH···O–M | |
| PSS | SO3H···O–M |
| MXene Type | Capacitance@Rate | Electrolyte | Cycle Number | Cycling Stability (%) | Refs. |
|---|---|---|---|---|---|
| PDT/Ti3C2Tx | 284 mF cm−2@50 mA cm−2 | 0.5 M H2SO4 | 10,000 | 100 | [101] |
| PPy/l-Ti3C2Tx | 203 mF cm−2 | 0.5 M H2SO4 | 20,000 | 100 | [77] |
| PPy/Ti3C2Tx (1:2) | 1000 F cm−3@5 mV s−1 | 1 M H2SO4 | 25,000 | 92 | [76] |
| Mo1.33C/PEDOT:PSS | 1310 F cm−3@2 mV s−1 | 1 M H2SO4 | 10,000 | 90 | [71] |
| Ti3C2Tx/P-100-H | 1065 F cm−3@2 mV s−1 | 1 M H2SO4 | 10,000 | 96 | [70] |
| GMP | 635 F g−1@1 A g−1 | 1 M H2SO4 | 10,000 | 97.54 | [102] |
| Ti3C2Tx/PDA | 715 mF cm−2@2 mV s−1 | 1 M H2SO4 | 10,000 | 95.5 | [103] |
| P3@Ti3C2Tx | 380 F g−1@ 2 mV s−1 | 1 M H2SO4 | 10,000 | 98 | [57] |
| MXene/PANI | 556.2 F g−1@0.5 A g−1 | 1 M H2SO4 | 5000 | 91.6 | [64] |
| MXene/PANI | 503 F g−1@2 mV s−1 | 3 M H2SO4 | 10,000 | 98.3 | [104] |
| d-Ti3C2Tx/glycine | 324 F g−1@ 10 mV s−1 140 F g−1@ 1000 mV s−1 | 3 M H2SO4 | 20,000 | ~100 | [59] |
| PANI@M-Ti3C2Tx | 1632 F cm−3@10 mV s−1 | 3 M H2SO4 | 20,000 | 85.7 | [99] |
| Ti3C2Tx@PPy NW | 610 F g−1@0.5 A g−1 | 3 M KOH | 20,000 | 100 | [89] |
| MXene/PANI (1:3) | 592 F g−1@ 0.5 A g−1 | 7 M KOH | 10,000 | 95.3 | [105] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, C.-F.; Zhao, X.; Wang, Z.; Yu, H.; Ye, Q. Recent Advanced on the MXene–Organic Hybrids: Design, Synthesis, and Their Applications. Nanomaterials 2021, 11, 166. https://doi.org/10.3390/nano11010166
Du C-F, Zhao X, Wang Z, Yu H, Ye Q. Recent Advanced on the MXene–Organic Hybrids: Design, Synthesis, and Their Applications. Nanomaterials. 2021; 11(1):166. https://doi.org/10.3390/nano11010166
Chicago/Turabian StyleDu, Cheng-Feng, Xiangyuan Zhao, Zijiao Wang, Hong Yu, and Qian Ye. 2021. "Recent Advanced on the MXene–Organic Hybrids: Design, Synthesis, and Their Applications" Nanomaterials 11, no. 1: 166. https://doi.org/10.3390/nano11010166
APA StyleDu, C.-F., Zhao, X., Wang, Z., Yu, H., & Ye, Q. (2021). Recent Advanced on the MXene–Organic Hybrids: Design, Synthesis, and Their Applications. Nanomaterials, 11(1), 166. https://doi.org/10.3390/nano11010166





