Sub-100-nm Nearly Monodisperse n-Paraffin/PMMA Phase Change Nanobeads
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Paraffin/PMMA Nanobeads
2.3. Characterization
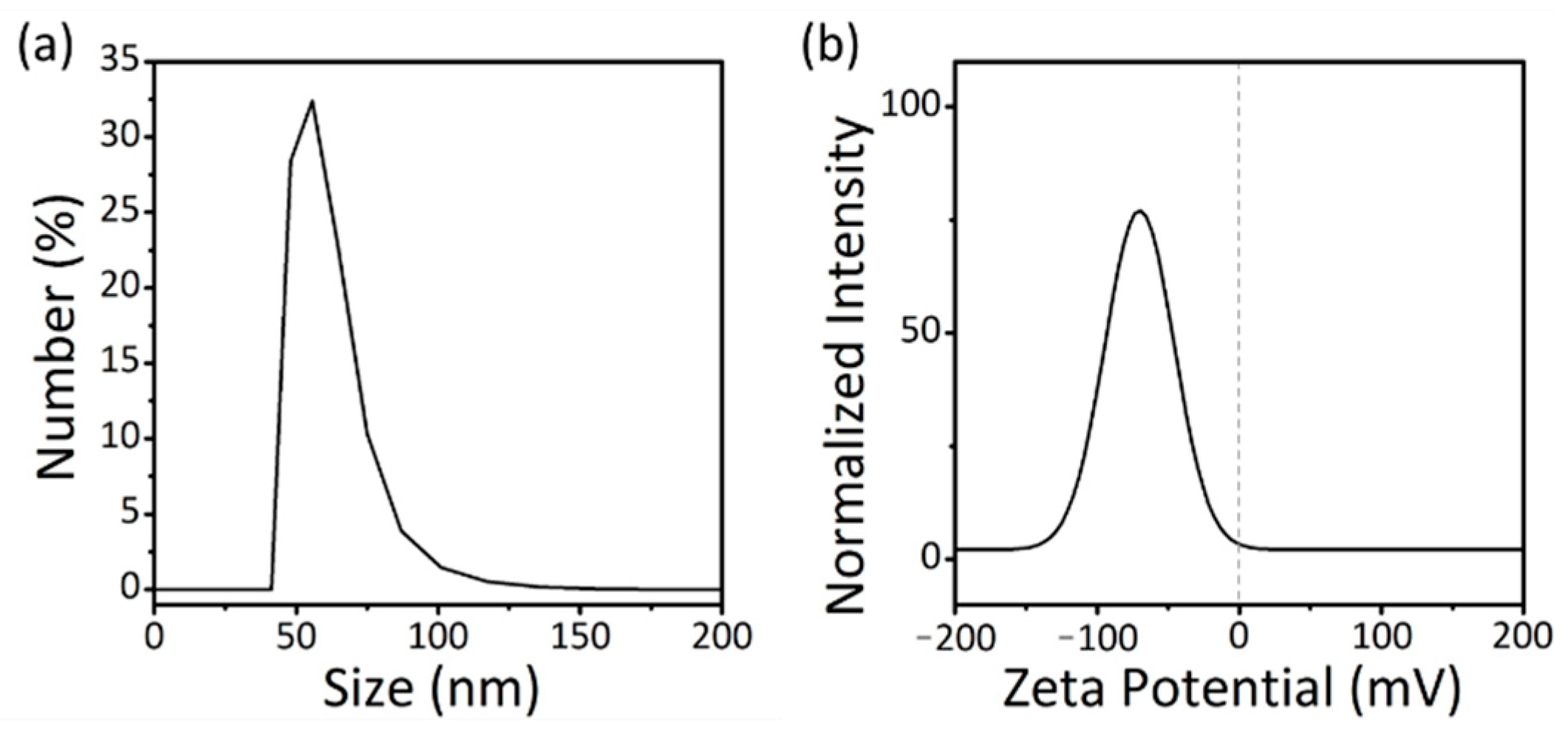
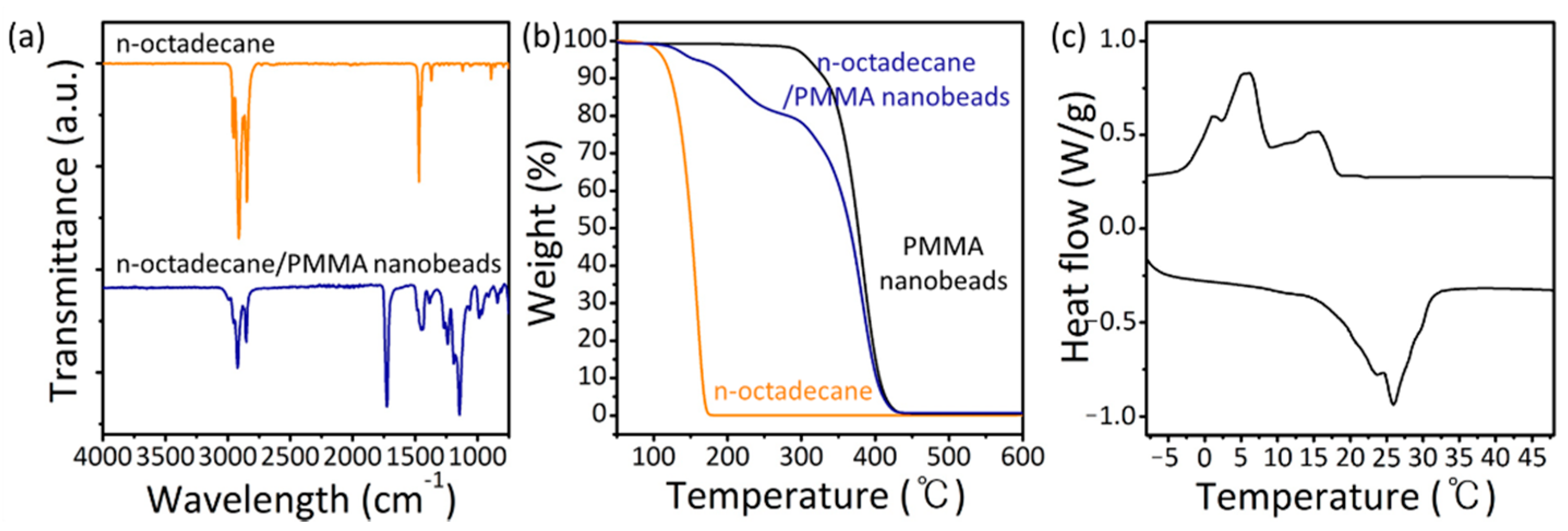
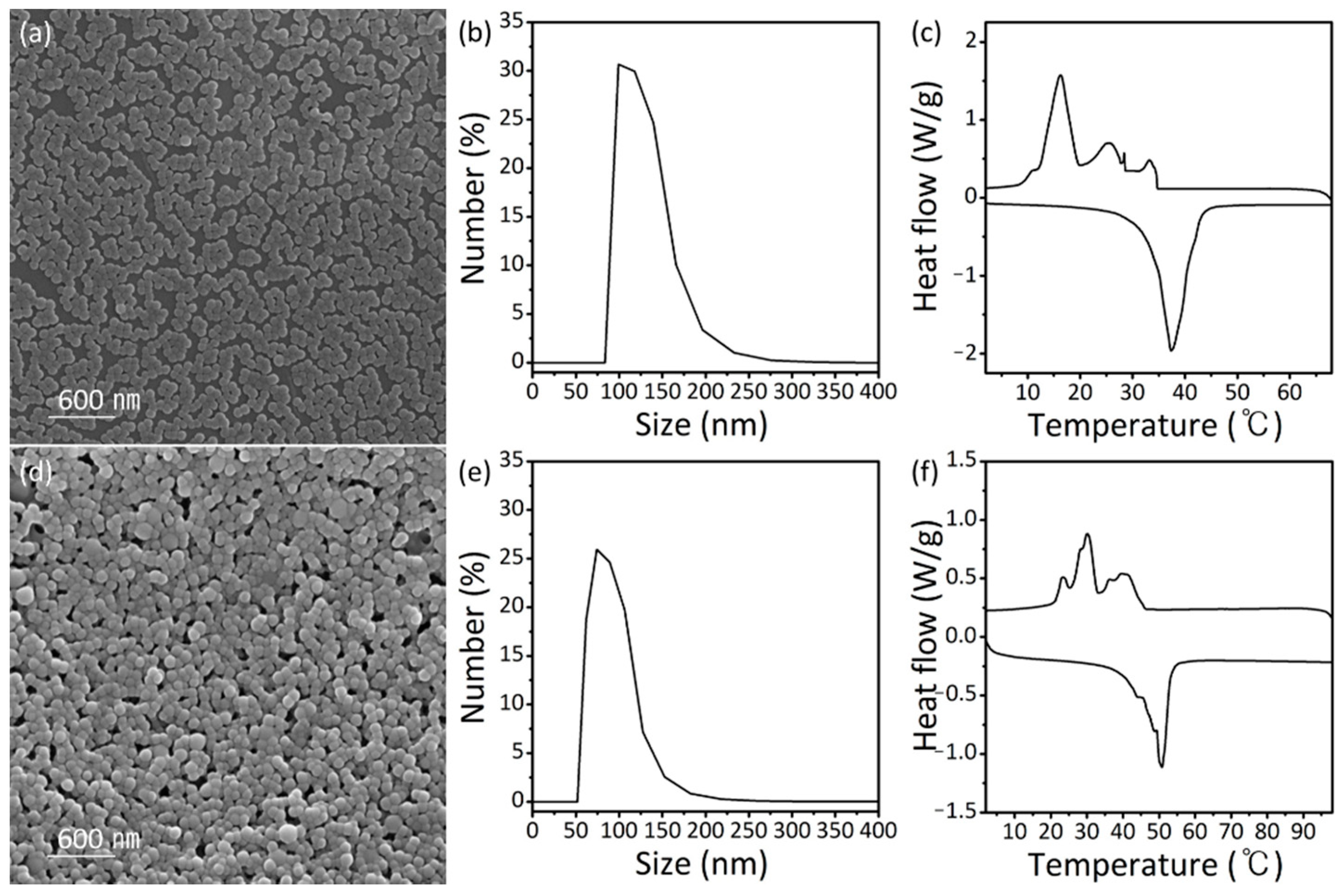
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sarı, A.; Alkan, C.; Karaipekli, A.; Uzun, O. Microencapsulated n-Octacosane as Phase Change Material. Sol. Energy 2009, 83, 1757–1763. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Zhang, G.H. Review on Microencapsulated Phase Change Materials (MEPCMs): Fabrication, Characterization and Applications. Renew. Sustain. Energy Rev. 2011, 15, 3813–3823. [Google Scholar] [CrossRef]
- Pielichowski, K.P.K. Phase Change Materials for Thermal Energy Storage. Prog. Mater. Sci. 2014, 65, 67–123. [Google Scholar] [CrossRef]
- Abhat, A. Low Temperature Latent Heat Thermal Energy Storage: Heat Storage Materials. Sol. Energy 1983, 30, 313–322. [Google Scholar] [CrossRef]
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on Thermal Energy Storage with Phase Change Materials and Applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Shukla, A.; Buddhi, D.; Sawhney, R.L. Solar Water Heaters with Phase Change Material Thermal Energy Storage Medium: A Review. Renew. Sustain. Energy Rev. 2009, 13, 2119–2125. [Google Scholar] [CrossRef]
- Kahwaji, S.; Johnson, M.B.; Kheirabadi, A.C.; Groulx, D.; White, M.A. A Comprehensive Study of Properties of Paraffin Phase Change Materials for Solar Thermal Energy Storage and Thermal Management Applications. Energy 2018, 162, 1169. [Google Scholar] [CrossRef]
- Konuklu, Y.; Ostry, M.; Paksoy, H.O.; Charvat, P. Review on Using Microencapsulated Phase Change Materials (PCM) in Building Applications. Energy Build. 2015, 106, 134–155. [Google Scholar] [CrossRef]
- Akeiber, H.; Nejat, P.; Majid, M.Z.A.; Wahid, M.A.; Jomehzadeh, F.; Famileh, I.Z.; Calautit, J.K.; Hughes, B.R.; Zaki, S.A. A Review on Phase Change Material (PCM) for Sustainable Passive Cooling in Building Envelopes. Renew. Sustain. Energy Rev. 2016, 60, 1470–1497. [Google Scholar] [CrossRef]
- Cabeza, F.; Castell, A.; Barrenechea, C.; De Gracia, A.; Fernández, A.I. Materials Used as PCM in Thermal Energy Storage in Buildings: A Review. Renew. Sustain. Energy Rev. 2011, 15, 1675–1695. [Google Scholar] [CrossRef]
- Browne, M.C.; Norton, B.; McCormack, S.J. Phase Change Materials for Photovoltaic Thermal Management. Renew. Sustain. Energy Rev. 2015, 47, 762–782. [Google Scholar] [CrossRef]
- Ling, Z.; Zhang, Z.; Shi, G.; Fang, X.; Wang, L.; Gao, X.; Fang, Y.; Xu, T.; Wang, S.; Liu, X. Review on Thermal Management Systems Using Phase Change Materials for Electronic Components, Li-ion Batteries and Photovoltaic Modules. Renew. Sustain. Energy Rev. 2015, 31, 427–438. [Google Scholar] [CrossRef] [Green Version]
- Bakan, G.; Gerislioglu, B.; Dirisaglik, F.; Jurado, Z.; Sullivan, L.; Dana, A.; Lam, C.; Gokirmak, A.; Silva, H. Extracting the temperature distribution on a phase-change memory cell during crystallization. J. Appl. Phys. 2016, 120, 164504. [Google Scholar] [CrossRef] [Green Version]
- Gerislioglu, B.; Bakan, G.; Ahuja, R.; Adam, J.; Mishra, Y.K.; Ahmadivand, A. The role of Ge2Sb2Te5 in enhancing the performance of functional plasmonic devices. Mater. Today Phys. 2020, 12, 100178. [Google Scholar] [CrossRef]
- Gerislioglu, B.; Ahmadivand, A.; Karabiyik, M.; Sinha, R.; Pala, N. VO2-Based Reconfigurable Antenna Platform with Addressable Microheater Matrix. Adv. Electron. Mater. 2017, 3, 1700170. [Google Scholar] [CrossRef]
- Mondal, S. Phase Change Materials for Smart Textiles—An Overview. Appl. Therm. Eng. 2007, 28, 1536–1550. [Google Scholar] [CrossRef]
- Sarier, N.; Onder, E. Organic Phase Change Materials and their Textile Applications: An Overview. Thermochim. Acta 2012, 540, 7–60. [Google Scholar] [CrossRef]
- Sharma, R.K.; Ganesan, P.; Tyagi, V.V.; Metselaar, H.S.C.; Sandaran, S.C. Developments in Organic Solid–Liquid Phase Change Materials and Their Applications in Thermal Energy Storage. Energy Conv. Manag. 2015, 95, 193–228. [Google Scholar] [CrossRef] [Green Version]
- Su, W.; Darkwa, J.; Kokogiannakis, G. Review of Solid–Liquid Phase Change Materials and Their Encapsulation Technologies. Renew. Sustain. Energy Rev. 2015, 48, 373–391. [Google Scholar] [CrossRef]
- Shchukina, E.M.; Graham, M.; Zheng, Z.; Shchukin, D.G. Nanoencapsulation of Phase Change Materials for Advanced Thermal Energy Storage Systems. Chem. Soc. Rev. 2018, 47, 4156–4175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamekhorshid, A.; Sadrameli, S.M.; Farid, M. A Review of Microencapsulation Methods of Phase Change Materials (PCMs) as a Thermal Energy Storage (TES) Medium. Renew. Sustain. Energy Rev. 2014, 31, 531–542. [Google Scholar] [CrossRef]
- Aftab, W.; Huang, X.; Wu, W.; Liang, Z.; Mahmood, A.; Zou, R. Nanoconfined Phase Change Materials for Thermal Energy Applications. Energy Environ. Sci. 2018, 11, 1392–1424. [Google Scholar] [CrossRef]
- Castro, P.F.D.; Ahmed, A.; Shchukin, D.G. Confined-Volume Effect on the Thermal Properties of Encapsulated Phase Change Materials for Thermal Energy Storage. Chem. A Eur. J. 2016, 22, 4389–4394. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Zhang, D.; Ling, X.; Li, Y.; Wang, Y.; Yu, Q.; She, X.; Li, Y.; Ding, Y. n-Alkanes Phase Change Materials and Their Microencapsulation for Thermal Energy Storage: A Critical Review. Energy Fuels 2018, 32, 7262–7293. [Google Scholar] [CrossRef]
- Yu, S.; Wang, X.; Wu, D. Self-Assembly Synthesis of Microencapsulated n-Eicosane Phase-Change Materials with Crystalline-Phase-Controllable Calcium Carbonate Shell. Energy Fuels 2014, 28, 3519–3529. [Google Scholar] [CrossRef]
- Huang, X.; Chen, X.; Li, A.; Atinafu, D.; Gao, H.; Dong, W.; Wang, G. Shape-Stabilized Phase Change Materials Based on Porous Supports for Thermal Energy Storage Applications. Chem. Eng. J. 2019, 356, 641–661. [Google Scholar] [CrossRef]
- Gao, H.; Wang, J.; Chen, X.; Wang, G.; Huang, X.; Li, A.; Dong, W. Nanoconfinement Effects on Thermal Properties of Nanoporous Shape-Stabilized Composite PCMs: A Review. Nano Energy 2018, 53, 769–797. [Google Scholar] [CrossRef]
- Xia, Y.; Cui, W.; Zhang, H.; Xu, F.; Sun, L.; Zou, Y.; Chu, H.; Yan, E. Synthesis of Three-Dimensional Graphene Aerogel Encapsulated n-Octadecane for Enhancing Phase-Change Behavior and Thermal Conductivity. J. Mater. Chem. A 2017, 5, 15191–15199. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, J.; Yu, F.; Zhang, Z.; Gao, X. Preparation and Properties of Graphene Oxide-Modified Poly(melamine-formaldehyde) Microcapsules Containing Phase Change Material n-Dodecanol for Thermal Energy Storage. J. Mater. Chem. A 2015, 3, 11624–11630. [Google Scholar] [CrossRef]
- Zhang, G.H.; Bon, S.A.F.; Zhao, C.Y. Synthesis, Characterization and Thermal Properties of Novel Nanoencapsulated Phase Change Materials for Thermal Eergy Storage. Sol. Energy 2012, 86, 1149–1154. [Google Scholar] [CrossRef]
- Tumirah, K.; Hussein, M.Z.; Zulkarnain, Z.; Rafeadah, R. Nano-Encapsulated Organic Phase Change Material Based on Copolymer Nanocomposites for Thermal Energy Storage. Energy 2014, 66, 881–890. [Google Scholar] [CrossRef]
- Sánchez-Silva, L.; Rodríguez, J.F.; Romero, A.; Borreguero, A.M.; Carmona, M.; Sánchez, P. Microencapsulation of PCMs with a styrene-methyl methacrylate copolymer shell by suspension-like polymerisation. Chem. Eng. J. 2010, 157, 216–222. [Google Scholar] [CrossRef]
- Chaiyasat, P.; Noppalit, S.; Okubo, M.; Chaiyasat, A. Innovative synthesis of high performance poly (methyl methacrylate) microcapsules with encapsulated heat storage material by microsuspension iodine transfer polymerization (ms ITP). Sol. Energy Mater. Sol. Cells 2016, 157, 996–1003. [Google Scholar] [CrossRef]
- Sarı, A.; Alkan, C.; Bilgin, C. Micro/nano encapsulation of some paraffin eutectic mixtures with poly (methyl methacrylate) shell: Preparation, characterization and latent heat thermal energy storage properties. Appl. Energy 2014, 136, 217–227. [Google Scholar] [CrossRef]
- Jegadheeswaran, S.; Pohekar, S.D. Performance enhancement in latent heat thermal storage system: A review. Renew. Sustain. Energy Rev. 2009, 13, 2225–2244. [Google Scholar] [CrossRef]
- Navya, P.; Kaphle, A.; Srinivas, S.; Bhargava, S.K.; Rotello, V.M.; Daima, H.K. Current trends and challenges in cancer management and therapy using designer nanomaterials. Nano Converg. 2019, 6, 23. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, K.; Kimura, S.-I.; Itai, S.; Kondo, H.; Iwao, Y. In vivo temperature-sensitive drug release system trigged by cooling using low-melting-point microcrystalline wax. J. Control. Release 2019, 303, 281–288. [Google Scholar] [CrossRef]
- Choi, S.W.; Zhang, Y.; Xia, Y. A temperature-sensitive drug release system based on phase-change materials. Angew. Chem. Int. Ed. 2010, 49, 7904–7908. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Li, Q.; Yang, N.; Shi, Y.; Ge, W.; Wang, W.; Huang, W.; Song, X.; Dong, X. Phase-Change Materials Based Nanoparticles for Controlled Hypoxia Modulation and Enhanced Phototherapy. Adv. Funct. Mater. 2019, 29, 1906805. [Google Scholar] [CrossRef]
- Salapa, J.; Bushman, A.; Lowe, K.; Irudayaraj, J. Nano drug delivery systems in upper gastrointestinal cancer therapy. Nano Converg. 2020, 7, 1–17. [Google Scholar] [CrossRef]
- Chung, T.; Han, J.; Kim, Y.S. Nanocomposite hydrogel actuators hybridized with various dimensional nanomaterials for stimuli responsiveness enhancement. Nano Converg. 2019, 6, 1–21. [Google Scholar]
- Hsu, L.; Weder, C.; Rowan, S.J. Stimuli-responsive, mechanically-adaptive polymer nanocomposites. J. Mater. Chem. 2011, 21, 2812–2822. [Google Scholar] [CrossRef] [Green Version]
- Li, X.-H.; Liu, C.; Feng, S.-P.; Fang, N.X. Broadband light management with thermochromic hydrogel microparticles for smart windows. Joule 2019, 3, 290–302. [Google Scholar] [CrossRef] [Green Version]
- Nasseri, R.; Deutschman, C.; Han, L.; Pope, M.; Tam, K. Cellulose nanocrystals in smart and stimuli-responsive materials: A review. Mater. Today Adv. 2020, 5, 100055. [Google Scholar] [CrossRef]
- Chern, C. Emulsion polymerization mechanisms and kinetics. Prog. Polym. Sci. 2006, 31, 443–486. [Google Scholar] [CrossRef]
- Vanderhoff, J.; Bradford, E.; Tarkowski, H.; Shaffer, J.; Wiley, R. Inverse emulsion polymerization. Adv. Chem. Ser. 1962, 34, 32–51. [Google Scholar]
- Van Rijswijk, K.; Bersee, H. Reactive processing of textile fiber-reinforced thermoplastic composites–An overview. Compos. Part A Appl. Sci. Manuf. 2007, 38, 666–681. [Google Scholar] [CrossRef]
- Alkan, C.; Sarı, A.; Karaipekli, A.; Uzun, O. Preparation, characterization, and thermal properties of microencapsulated phase change material for thermal energy storage. Sol. Energy Mater. Sol. Cells 2009, 93, 143–147. [Google Scholar] [CrossRef]
- Qiu, X.; Li, W.; Song, G.; Chu, X.; Tang, G. Microencapsulated n-octadecane with different methylmethacrylate-based copolymer shells as phase change materials for thermal energy storage. Energy 2012, 46, 188–199. [Google Scholar] [CrossRef]
- Cao, F.; Yang, B. Supercooling suppression of microencapsulated phase change materials by optimizing shell composition and structure. Appl. Energy 2014, 113, 1512–1518. [Google Scholar] [CrossRef]
- Zhang, X.-X.; Fan, Y.-F.; Tao, X.-M.; Yick, K.-L. Crystallization and prevention of supercooling of microencapsulated n-alkanes. J. Colloid Interface Sci. 2005, 281, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.L.; McKenna, G.B. The melting behavior of organic materials confined in porous solids. J. Chem. Phys. 1990, 93, 9002–9011. [Google Scholar] [CrossRef]
- Alcoutlabi, M.; McKenna, G.B. Effects of confinement on material behaviour at the nanometre size scale. J. Phys. Condens. Matter 2005, 17, R461. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Wu, D. Silica encapsulation of n-octadecane via sol–gel process: A novel microencapsulated phase-change material with enhanced thermal conductivity and performance. J. Colloid Interface Sci. 2010, 343, 246–255. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woo, H.Y.; Lee, D.W.; Yoon, T.Y.; Kim, J.B.; Chae, J.-Y.; Paik, T. Sub-100-nm Nearly Monodisperse n-Paraffin/PMMA Phase Change Nanobeads. Nanomaterials 2021, 11, 204. https://doi.org/10.3390/nano11010204
Woo HY, Lee DW, Yoon TY, Kim JB, Chae J-Y, Paik T. Sub-100-nm Nearly Monodisperse n-Paraffin/PMMA Phase Change Nanobeads. Nanomaterials. 2021; 11(1):204. https://doi.org/10.3390/nano11010204
Chicago/Turabian StyleWoo, Ho Young, Da Won Lee, Tae Yeol Yoon, Jong Bae Kim, Ji-Yeon Chae, and Taejong Paik. 2021. "Sub-100-nm Nearly Monodisperse n-Paraffin/PMMA Phase Change Nanobeads" Nanomaterials 11, no. 1: 204. https://doi.org/10.3390/nano11010204





