Determination of Cobalt Spin-Diffusion Length in Co/Cu Multilayered Heterojunction Nanocylinders Based on Valet–Fert Model
Abstract
1. Introduction
2. Materials and Methods
3. Demagnetization Factor and Valet–Fert Model in Multilayered Heterojunction Nanocylinders
4. Results and Discussion
4.1. Template Synthesis and Electrodeposition Process of Co/Cu Heterojunction Nanocylinders
4.2. Structure of Co/Cu Heterojunction Nanocylinders
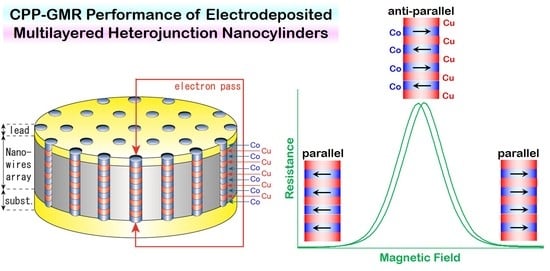
4.3. Magnetoresistance Properties of Co/Cu Multilayered Heterojunction Nanocylinders
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Baibich, M.N.; Broto, J.M.; Fert, A.; Nguyen, V.D.F.; Petroff, F. Giant Magnetoresistance of (001)Fe/(001)Cr Magnetic Super-Lattices. Phys. Rev. Lett. 1988, 61, 2472–2475. [Google Scholar] [CrossRef] [PubMed]
- Binasch, G.; Grünberg, P.; Saurenbach, F.; Zinn, W. Enhanced Magnetoresistance in Fe-Cr Layered Structures with Antiferro-Magnetic Interlayer Exchange. Phys. Rev. B 1989, 39, 4828. [Google Scholar] [CrossRef] [PubMed]
- Alper, M.; Attenborough, K.; Hart, R.; Lane, S.; Lashmore, D.S.; Younes, C.; Schwarzacher, W. Giant Magnetoresistance in Electrodeposited Superlattices. Appl. Phys. Lett. 1993, 63, 2144–2146. [Google Scholar] [CrossRef]
- Lenczowski, S.; Schönenberger, C.; Gijs, M.; De Jonge, W. Giant Magnetoresistance of Electrodeposited Co/Cu Multilayers. J. Magn. Magn. Mater. 1995, 148, 455–465. [Google Scholar] [CrossRef]
- Tóth, B.G.; Peter, L.; Dégi, J.; Revesz, A.; Oszetzky, D.; Molnár, G.; Bakonyi, I. Influence of Cu Deposition Potential on the Giant Magnetoresistance and Surface Roughness of Electrodeposited Ni–Co/Cu Multilayers. Electrochim. Acta 2013, 91, 122–129. [Google Scholar] [CrossRef]
- Sahin, T.; Kockar, H.; Alper, M. Properties of Electrodeposited CoFe/Cu Multilayers: The Effect of Cu Layer Thickness. J. Magn. Magn. Mater. 2015, 373, 128–131. [Google Scholar] [CrossRef]
- Zsurzsa, S.; Peter, L.; Kiss, L.; Bakonyi, I. Magnetic and Magnetoresistance Studies of Nanometric Electrodeposited Co Films and Co/Cu Layered Structures: Influence of Magnetic Layer Thickness. J. Magn. Magn. Mater. 2017, 421, 194–206. [Google Scholar] [CrossRef]
- Piraux, L.; George, J.-M.; Despres, J.F.; Leroy, C.; Ferain, E.; Legras, R.; Ounadjela, K.; Fert, A. Giant Magnetoresistance in Magnetic Multilayered Nanowires. Appl. Phys. Lett. 1994, 65, 2484–2486. [Google Scholar] [CrossRef]
- Blondel, A.; Meier, J.P.; Doudin, B.; Ansermet, J. Giant Magnetoresistance of Nanowires of Multilayers. Appl. Phys. Lett. 1994, 65, 3019–3021. [Google Scholar] [CrossRef]
- Evans, P.R.; Yi, G.; Schwarzacher, W. Current Perpendicular to Plane Giant Magnetoresistance of Multilayered Nanowires Electrodeposited in Anodic Aluminum Oxide Membranes. Appl. Phys. Lett. 2000, 76, 481–483. [Google Scholar] [CrossRef]
- Ohgai, T.; Hoffer, X.; Gravier, L.; Ansermet, J.P. Electrochemical Surface Modification of Aluminium Sheets for Application to Nano-Electronic Devices: Anodization Alminium and Cobalt-Copper Electrodeposition. J. Appl. Electrochem. 2004, 34, 1007–1012. [Google Scholar] [CrossRef]
- Tang, X.-T.; Wang, G.-C.; Shima, M. Perpendicular Giant Magnetoresistance of Electrodeposited Co∕Cu-Multilayered Nanowires in Porous Alumina Templates. J. Appl. Phys. 2006, 99, 033906. [Google Scholar] [CrossRef]
- Tang, X.T.; Wang, G.C.; Shima, M. Layer Thickness Dependence of CPP Giant Magnetoresistance in Individual CoNi/Cu mul-tilayer Nanowire Grown by Electrodeposition. Phys. Rev. B 2007, 75, 134404. [Google Scholar] [CrossRef]
- Shakya, P.; Cox, B.; Davis, D. Giant Magnetoresistance and Coercivity of Electrodeposited Multilayered FeCoNi/Cu and CrFeCoNi/Cu. J. Magn. Magn. Mater. 2012, 324, 453–459. [Google Scholar] [CrossRef]
- Zhang, W.; Deng, H.; Li, H.; Su-Wei, Y.; Wang, H. Synthesis and Magnetic Properties of Ni–Fe/Cu/Co/Cu Multilayer Nanowire Arrays. J. Mater. Sci. Mater. Electron. 2015, 26, 2520–2524. [Google Scholar] [CrossRef]
- Han, J.; Qin, X.; Quan, Z.; Wang, L.; Xu, X. Perpendicular Giant Magnetoresistance and Magnetic Properties of Co/Cu Nan-Owire Arrays Affected by Period Number and Copper Layer Thickness. Adv. Condens. Matter Phys. 2016, 2016, 1–9. [Google Scholar] [CrossRef]
- Xi, H.; Gao, Y.; Liu, Z.; Han, G.; Lu, J.; Li, Y. Inter- and Intra-nanowire Magnetic Interaction in Co/Cu Multilayer Nanowires Deposited by Electrochemical Deposition. Phys. B Condens. Matter 2017, 518, 77–80. [Google Scholar] [CrossRef]
- Kamimura, H.; Hayashida, M.; Ohgai, T. CPP-GMR Performance of Electrochemically Synthesized Co/Cu Multilayered Nan-owire Arrays with Extremely Large Aspect Ratio. Nanomaterials 2020, 10, 5. [Google Scholar] [CrossRef]
- Lee, W.; Ji, R.; Gösele, U.; Nielsch, K. Fast Fabrication of Long-Range Ordered Porous Alumina Membranes by Hard Anodization. Nat. Mater. 2006, 5, 741–747. [Google Scholar] [CrossRef]
- Ohgai, T.; Mizumoto, M.; Nomura, S.; Kagawa, A. Electrochemical Fabrication of Metallic Nanowires and Metal Oxide na-Nopores. Mater. Manuf. Process. 2007, 22, 440–443. [Google Scholar] [CrossRef]
- Valet, T.; Fert, A. Theory of the Perpendicular Magnetoresistance in Magnetic Multilayers. Phys. Rev. B 1993, 48, 7099–7113. [Google Scholar] [CrossRef] [PubMed]
- Piraux, L.; Dubois, S.; Fert, A.; Belliard, L. The Temperature Dependence of the Perpendicular Giant Magnetoresistance in Co/Cu Multilayered Nanowires. Eur. Phys. J. B 1998, 4, 413–420. [Google Scholar] [CrossRef]
- Fert, A.; Piraux, L. Magnetic nanowires. J. Magn. Magn. Mater. 1999, 200, 338–358. [Google Scholar] [CrossRef]
- Nakano, H.; Ohgai, T.; Fukushima, H.; Akiyama, T.; Kammel, R. Factors Determining the Critical Current Density for Zinc Deposition in Sulfate Solutions. Metall 2001, 55, 676–681. [Google Scholar]
- Bockris, J.O.; Kita, H. Analysis of Galvanostatic Transients and Application to the Iron Electrode Reaction. J. Electrochem. Soc. 1961, 108, 676–685. [Google Scholar] [CrossRef]
- Ohgai, T.; Tanaka, Y.; Fujimaru, T. Soft Magnetic Properties of Ni-Cr and Co-Cr Alloy Thin Films Electrodeposited from Aqueous Solutions Containing Trivalent Chromium Ions and Glycine. J. Appl. Electrochem. 2012, 42, 893–899. [Google Scholar] [CrossRef]
- Rafaja, D.; Schimpf, C.; Klemm, V.; Schreiber, G.; Bakonyi, I.; Péter, L. Formation of Microstructural Defects in Electrodeposited Co/Cu Multilayers. Acta Mater. 2009, 57, 3211–3222. [Google Scholar] [CrossRef]
- Ohgai, T.; Washio, R.; Tanaka, Y. Anisotropic Magnetization Behavior of Electrodeposited Nanocrystalline Ni-Mo Alloy Thin Films and Nanowires Array. J. Electrochem. Soc. 2012, 159, H800–H804. [Google Scholar] [CrossRef]
- Dulal, S.; Charles, E. Effect of Interface Number on Giant Magnetoresistance. J. Phys. Chem. Solids 2010, 71, 309–313. [Google Scholar] [CrossRef]










| Authors | FM/NM | GMR/% | D/nm | L/μm | L/D | tCo/nm | tCu/nm | Source Title | Year | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Piraux et al. | Co/Cu | 15 | 40 | 10 | 250 | 10 | 10 | Appl. Phys. Lett. | 1994 | [8] |
| Blondel et al. | Co/Cu | 14 | 80 | 6 | 75 | 5 | 5 | Appl. Phys. Lett. | 1994 | [9] |
| Evans et al. | CoNi/Cu | 55 | 300 | 60 | 200 | 5 | 2 | Appl. Phys. Lett. | 2000 | [10] |
| Ohgai et al. | Co/Cu | 15 | 60 | 2 | 33 | 10 | 10 | J. Appl. Electrochem. | 2004 | [11] |
| Tang et al. | Co/Cu | 14 | 300 | 60 | 200 | 8 | 10 | J. Appl. Phys. | 2006 | [12] |
| Tang et al. | CoNi/Cu | 23 | 300 | 60 | 200 | 10 | 4 | Phys. Rev. B | 2007 | [13] |
| Shakya et al. | FeCoNi/Cu | 15 | 300 | 60 | 200 | 14 | 10 | J. Magn. Magn. Mater | 2012 | [14] |
| Zhang et al. | FeNi/Cu/Co | 45 | 120 | 2 | 17 | 25 | 15 | J. Mater. Sci. M. E. | 2015 | [15] |
| Han et al. | Co/Cu | 13 | 50 | 11 | 220 | 50 | 5 | Adv. Cond. Mat. Phys. | 2016 | [16] |
| Xi et al. | Co/Cu | 0.16 | 80 | 3 | 38 | 200 | 5 | Physica B | 2017 | [17] |
| Kamimura et al. | Co/Cu | 24 | 75 | 70 | 933 | 19 | 1.4 | Nanomaterials | 2020 | [18] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizoguchi, S.; Hayashida, M.; Ohgai, T. Determination of Cobalt Spin-Diffusion Length in Co/Cu Multilayered Heterojunction Nanocylinders Based on Valet–Fert Model. Nanomaterials 2021, 11, 218. https://doi.org/10.3390/nano11010218
Mizoguchi S, Hayashida M, Ohgai T. Determination of Cobalt Spin-Diffusion Length in Co/Cu Multilayered Heterojunction Nanocylinders Based on Valet–Fert Model. Nanomaterials. 2021; 11(1):218. https://doi.org/10.3390/nano11010218
Chicago/Turabian StyleMizoguchi, Saeko, Masamitsu Hayashida, and Takeshi Ohgai. 2021. "Determination of Cobalt Spin-Diffusion Length in Co/Cu Multilayered Heterojunction Nanocylinders Based on Valet–Fert Model" Nanomaterials 11, no. 1: 218. https://doi.org/10.3390/nano11010218
APA StyleMizoguchi, S., Hayashida, M., & Ohgai, T. (2021). Determination of Cobalt Spin-Diffusion Length in Co/Cu Multilayered Heterojunction Nanocylinders Based on Valet–Fert Model. Nanomaterials, 11(1), 218. https://doi.org/10.3390/nano11010218