Nanomaterial-Enabled Sensors and Therapeutic Platforms for Reactive Organophosphates
Abstract
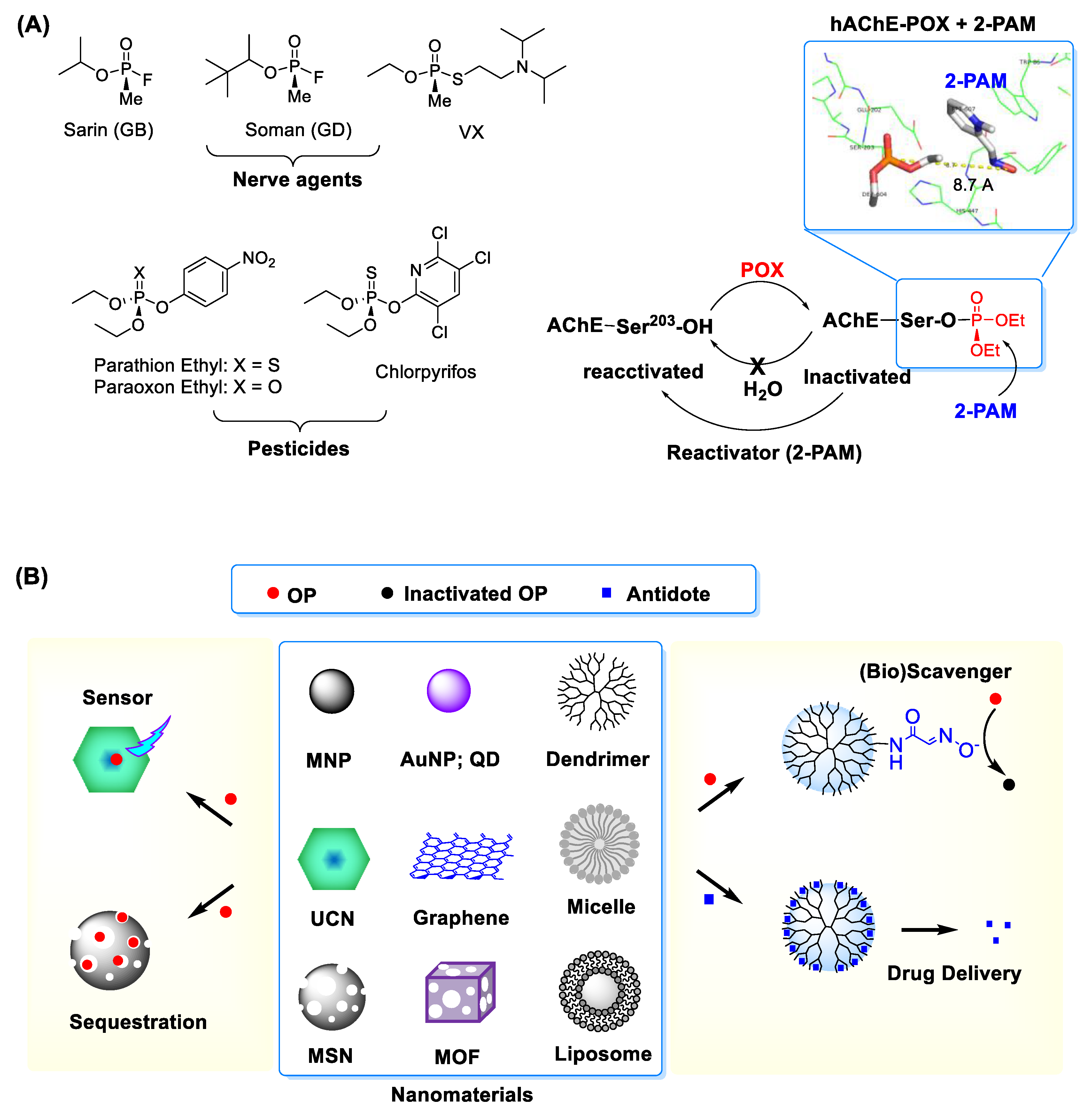
:1. Introduction
2. Nanosensors for Reactive Organophosphate Detection
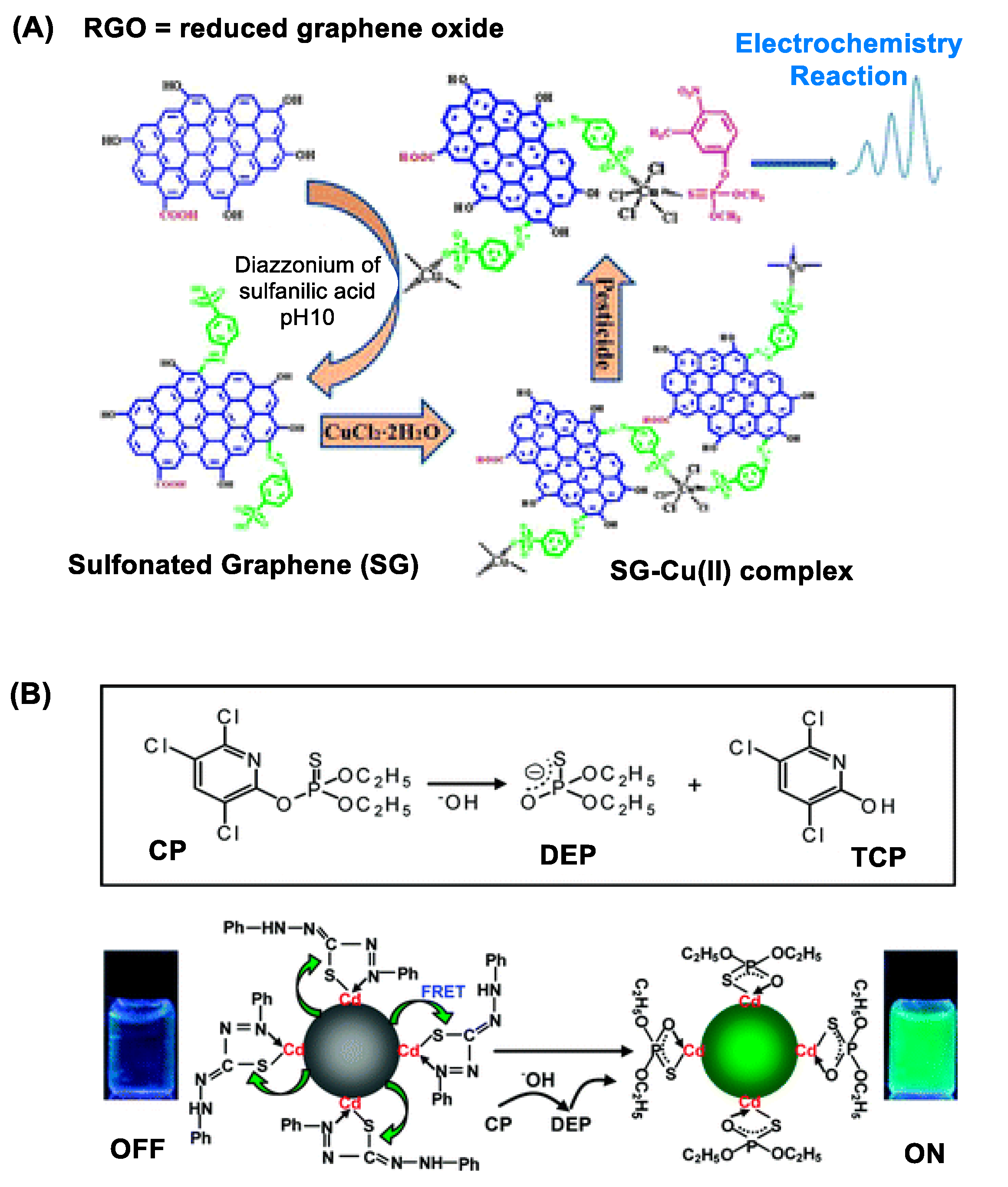
2.1. Electrochemistry
2.1.1. AChE-Immobilized Electrode
2.1.2. AChE-Immobilized Nanosensor
2.1.3. Antibody-Immobilized Nanosensor
2.1.4. OP-Responsive Nanosensor
2.2. Absorbance, Fluorescence and Luminescence Spectroscopy
2.2.1. Quantum Dot (QD) Nanosensors
2.2.2. Upconversion Nanocrystal (UCN) Nanosensors
2.2.3. Metal-Organic Framework Nanosensors
2.2.4. Plasmonic Nanomaterials
3. Therapeutic Platforms for Reactive Organophosphate Treatment
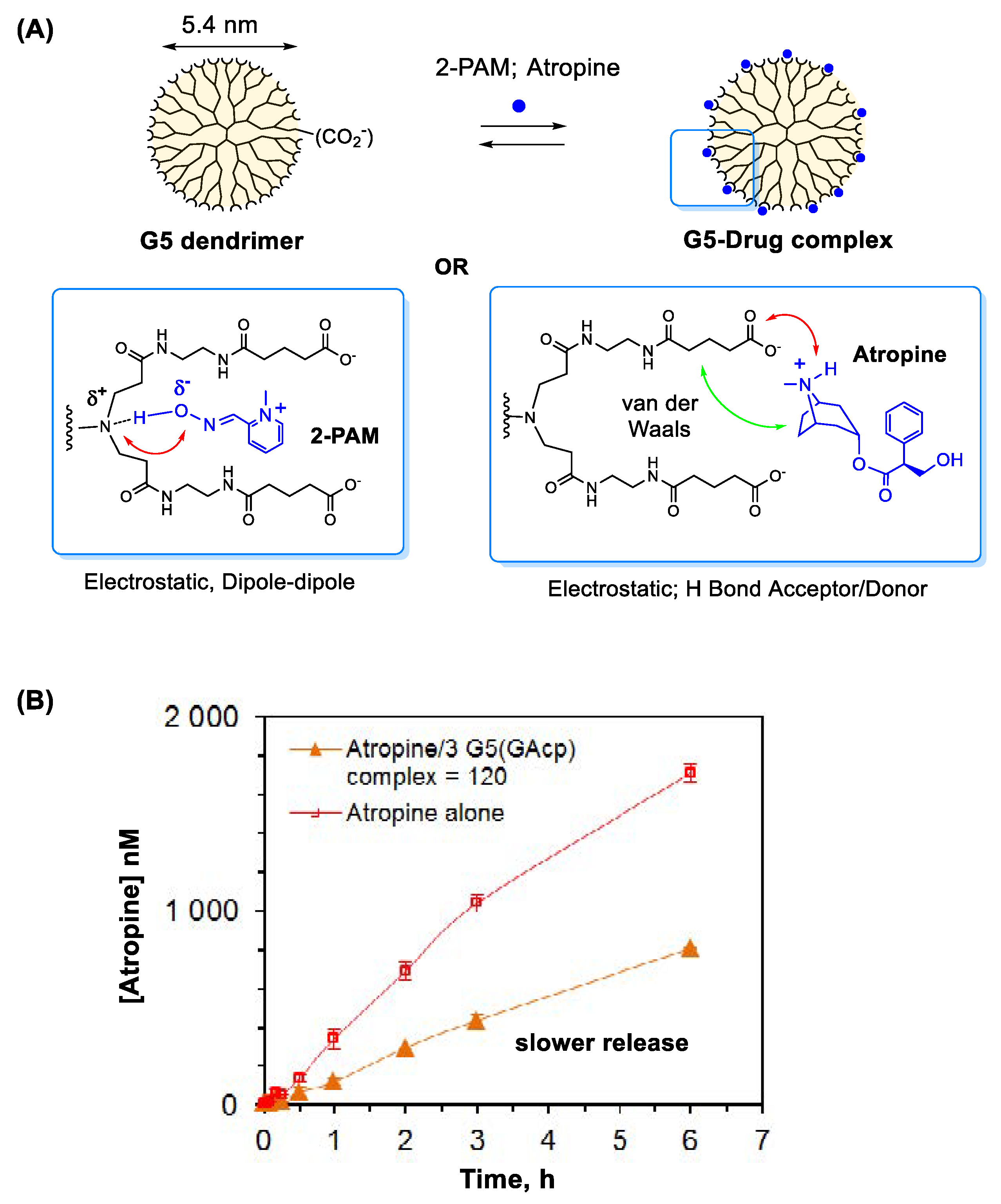
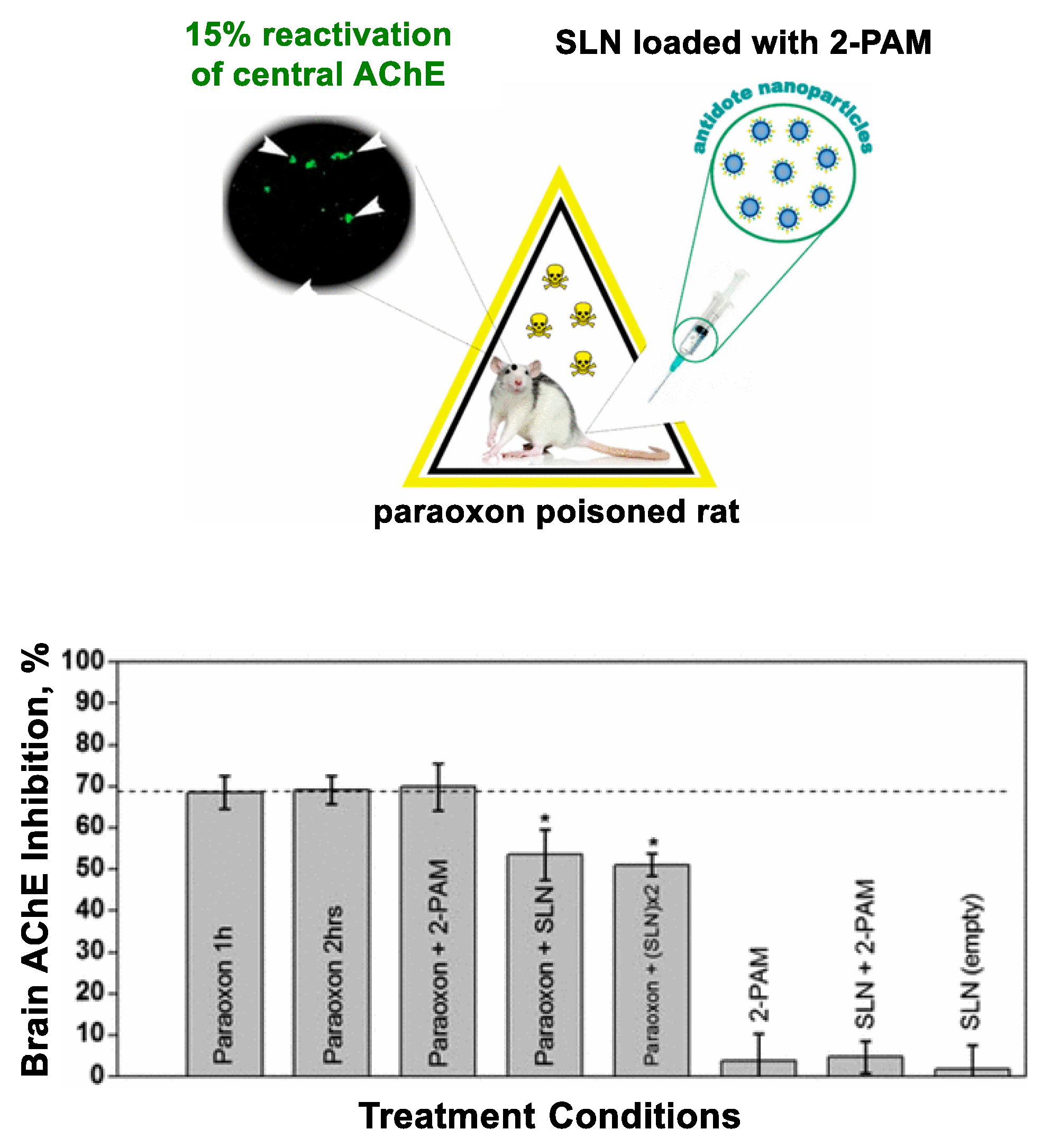
3.1. Delivery Systems for Antidotes
3.1.1. Oximes
3.1.2. Atropine
3.2. Bioscavengers
3.3. Chemical Scavengers
3.4. Nanoscavengers
3.4.1. Lipid Nanoparticles
3.4.2. Metal Ion Chelated Polymer
3.4.3. Mesoporous Silica Nanoparticle
3.4.4. Metal-Organic Framework
3.4.5. Metal Oxide Nanoparticle
3.4.6. PAMAM Dendrimers
4. Conclusions and Perspective
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Date Availability Statement
Conflicts of Interest
References
- Mercey, G.; Verdelet, T.; Renou, J.; Kliachyna, M.; Baati, R.; Nachon, F.; Jean, L.; Renard, P.-Y. Reactivators of Acetylcholinesterase Inhibited by Organophosphorus Nerve Agents. Acc. Chem. Res. 2012, 45, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Heldman, E.; Ashani, Y.; Raveh, L.; Rachaman, E.S. Sugar conjugates of pyridinium aldoximes as antidotes against organophosphate poisoning. Carbohydr. Res. 1986, 151, 337–347. [Google Scholar] [CrossRef]
- Xiong, S.; Deng, Y.; Zhou, Y.; Gong, D.; Xu, Y.; Yang, L.; Chen, H.; Chen, L.; Song, T.; Luo, A.; et al. Current progress in biosensors for organophosphorus pesticides based on enzyme functionalized nanostructures: A review. Anal. Methods 2018, 10, 5468–5479. [Google Scholar] [CrossRef]
- Sparling, D.W. Chapter 5—Current Use Pesticides. In Ecotoxicology Essentials; Sparling, D.W., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 109–152. [Google Scholar]
- Casida, J.E.; Quistad, G.B. Organophosphate Toxicology: Safety Aspects of Nonacetylcholinesterase Secondary Targets. Chem. Res. Toxicol. 2004, 17, 983–998. [Google Scholar] [CrossRef]
- D’Agostino, J.; Zhang, H.; Kenaan, C.; Hollenberg, P.F. Mechanism-Based Inactivation of Human Cytochrome P450 2B6 by Chlorpyrifos. Chem. Res. Toxicol. 2015, 28, 1484–1495. [Google Scholar] [CrossRef] [PubMed]
- Lin, V.S.; Volk, R.F.; DeLeon, A.J.; Anderson, L.N.; Purvine, S.O.; Shukla, A.K.; Bernstein, H.C.; Smith, J.N.; Wright, A.T. Structure Dependent Determination of Organophosphate Targets in Mammalian Tissues Using Activity-Based Protein Profiling. Chem. Res. Toxicol. 2020, 33, 414–425. [Google Scholar] [CrossRef]
- Schopfer, L.M.; Voelker, T.; Bartels, C.F.; Thompson, C.M.; Lockridge, O. Reaction Kinetics of Biotinylated Organophosphorus Toxicant, FP-biotin, with Human Acetylcholinesterase and Human Butyrylcholinesterase. Chem. Res. Toxicol. 2005, 18, 747–754. [Google Scholar] [CrossRef]
- Guo, J.-X.; Wu, J.J.Q.; Wright, J.B.; Lushington, G.H. Mechanistic Insight into Acetylcholinesterase Inhibition and Acute Toxicity of Organophosphorus Compounds: A Molecular Modeling Study. Chem. Res. Toxicol. 2006, 19, 209–216. [Google Scholar] [CrossRef]
- Franklin, M.C.; Rudolph, M.J.; Ginter, C.; Cassidy, M.S.; Cheung, J. Structures of Paraoxon-inhibited Human Acetylcholinesterase Reveal Perturbations of the Acyl Loop and the Dimer Interface. Proteins Struct. Funct. Bioinform. 2016, 84, 1246–1256. [Google Scholar] [CrossRef]
- Chambers, H.W.; Chambers, J.E. An investigation of acetylcholinesterase inhibition and aging and choline acetyltransferase activity following a high level acute exposure to paraoxon. Pestic. Biochem. Physiol. 1989, 33, 125–131. [Google Scholar] [CrossRef]
- Bajgar, J. Organophosphates/Nerve Agent Poisoning: Mechanism of Action, Diagnosis, Prophylaxis, and Treatment. In Advances in Clinical Chemistry; Gregory, S.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2004; Volume 38, pp. 151–216. [Google Scholar]
- Kim, K.-H.; Kabir, E.; Jahan, S.A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Sanson, B.; Nachon, F.; Colletier, J.-P.; Froment, M.-T.; Toker, L.; Greenblatt, H.M.; Sussman, J.L.; Ashani, Y.; Masson, P.; Silman, I.; et al. Crystallographic Snapshots of Nonaged and Aged Conjugates of Soman with Acetylcholinesterase, and of a Ternary Complex of the Aged Conjugate with Pralidoxime. J. Med. Chem. 2009, 52, 7593–7603. [Google Scholar] [CrossRef]
- Peter, J.V.; Sudarsan, T.I.; Moran, J.L. Clinical features of organophosphate poisoning: A review of different classification systems and approaches. Indian J. Crit. Care Med. 2014, 18, 735–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, P.; Bhattacharjee, S.; Cannon, J.; Tang, S.; Yang, K.; Bowden, S.; Varnau, V.; O′Konek, J.J.; Choi, S.K. Reactivity and Mechanism of α-Nucleophile Scaffolds as Catalytic Organophosphate Scavengers. Org. Biomol. Chem. 2019, 17, 3951–3963. [Google Scholar] [CrossRef] [PubMed]
- Clarke, J.F.; Cordery, S.F.; Morgan, N.A.; Knowles, P.K.; Guy, R.H. Dermal Absorption of Pesticide Residues. Chem. Res. Toxicol. 2018, 31, 1356–1363. [Google Scholar] [CrossRef] [PubMed]
- Fryer, A.D.; Lein, P.J.; Howard, A.S.; Yost, B.L.; Beckles, R.A.; Jett, D.A. Mechanisms of organophosphate insecticide-induced airway hyperreactivity. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 286, L963–L969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ragnarsdottir, K.V. Environmental fate and toxicology of organophosphate pesticides. J. Geol. Soc. 2000, 157, 859–876. [Google Scholar] [CrossRef]
- Farokhzad, O.C.; Langer, R. Impact of Nanotechnology on Drug Delivery. ACS Nano 2009, 3, 16–20. [Google Scholar] [CrossRef]
- Wong, P.T.; Choi, S.K. Mechanisms of Drug Release in Nanotherapeutic Delivery Systems. Chem. Rev. 2015, 115, 3388–3432. [Google Scholar] [CrossRef]
- Kukowska-Latallo, J.F.; Candido, K.A.; Cao, Z.; Nigavekar, S.S.; Majoros, I.J.; Thomas, T.P.; Balogh, L.P.; Khan, M.K.; Baker, J.R., Jr. Nanoparticle Targeting of Anticancer Drug Improves Therapeutic Response in Animal Model of Human Epithelial Cancer. Cancer Res. 2005, 65, 5317–5324. [Google Scholar] [CrossRef] [Green Version]
- Wong, P.T.; Chen, D.; Tang, S.; Yanik, S.; Payne, M.; Mukherjee, J.; Coulter, A.; Tang, K.; Tao, K.; Sun, K.; et al. Modular Integration of Upconversion Nanocrystal-Dendrimer Composites for Folate Receptor-Specific Near Infrared Imaging and Light Triggered Drug Release. Small 2015, 11, 6078–6090. [Google Scholar] [CrossRef] [PubMed]
- Agasti, S.S.; Rana, S.; Park, M.-H.; Kim, C.K.; You, C.-C.; Rotello, V.M. Nanoparticles for detection and diagnosis. Adv. Drug Delivery Rev. 2010, 62, 316–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biju, V. Chemical modifications and bioconjugate reactions of nanomaterials for sensing, imaging, drug delivery and therapy. Chem. Soc. Rev. 2014, 43, 744–764. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.K. Photoactivation Strategies for Therapeutic Release in Nanodelivery Systems. Adv. Ther. 2020, 3, 2000117. [Google Scholar] [CrossRef]
- Choi, S.K. Activation Strategies in Image-Guided Nanotherapeutic Delivery. J. Nanotheranostics 2020, 1, 79–105. [Google Scholar] [CrossRef]
- Stewart, M.E.; Anderton, C.R.; Thompson, L.B.; Maria, J.; Gray, S.K.; Rogers, J.A.; Nuzzo, R.G. Nanostructured Plasmonic Sensors. Chem. Rev. 2008, 108, 494–521. [Google Scholar] [CrossRef]
- Mu, Q.; Jiang, G.; Chen, L.; Zhou, H.; Fourches, D.; Tropsha, A.; Yan, B. Chemical Basis of Interactions Between Engineered Nanoparticles and Biological Systems. Chem. Rev. 2014, 114, 7740–7781. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Bai, Y.; Jia, J.; Gao, N.; Li, Y.; Zhang, R.; Jiang, G.; Yan, B. Perturbation of physiological systems by nanoparticles. Chem. Soc. Rev. 2014, 43, 3762–3809. [Google Scholar] [CrossRef]
- Dahl, M.; Liu, Y.; Yin, Y. Composite Titanium Dioxide Nanomaterials. Chem. Rev. 2014, 114, 9853–9889. [Google Scholar] [CrossRef]
- Daniel, M.-C.; Astruc, D. Gold Nanoparticles; Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications toward Biology, Catalysis, and Nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef]
- Eder, D. Carbon Nanotube−Inorganic Hybrids. Chem. Rev. 2010, 110, 1348–1385. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Qiu, H.; Prasad, P.N.; Chen, X. Upconversion Nanoparticles: Design, Nanochemistry, and Applications in Theranostics. Chem. Rev. 2014, 114, 5161–5214. [Google Scholar] [CrossRef] [PubMed]
- Haase, M.; Schäfer, H. Upconverting Nanoparticles. Angew. Chem. Int. Ed. 2011, 50, 5808–5829. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Li, W.; Cobley, C.M.; Chen, J.; Xia, X.; Zhang, Q.; Yang, M.; Cho, E.C.; Brown, P.K. Gold Nanocages: From Synthesis to Theranostic Applications. Acc. Chem. Res. 2011, 44, 914–924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, P.T.; Tang, S.; Cannon, J.; Yang, K.; Harrison, R.; Ruge, M.; O’Konek, J.J.; Choi, S.K. Shielded α-Nucleophile Nanoreactor for Topical Decontamination of Reactive Organophosphate. ACS Appl. Mater. Interfaces 2020, 12, 33500–33515. [Google Scholar] [CrossRef]
- Pang, Z.; Hu, C.-M.J.; Fang, R.H.; Luk, B.T.; Gao, W.; Wang, F.; Chuluun, E.; Angsantikul, P.; Thamphiwatana, S.; Lu, W.; et al. Detoxification of Organophosphate Poisoning Using Nanoparticle Bioscavengers. ACS Nano 2015, 9, 6450–6458. [Google Scholar] [CrossRef] [Green Version]
- Ju, H.; Kandimalla, V.B. CHAPTER 2—Biosensors for pesticides. In Electrochemical Sensors, Biosensors and Their Biomedical Applications; Zhang, X., Ju, H., Wang, J., Eds.; Academic Press: San Diego, CA, USA, 2008; pp. 31–56. [Google Scholar]
- Alonso, G.A.; Muñoz, R.; Marty, J.-L. Automatic Electronic Tongue for On-Line Detection and Quantification of Organophosphorus and Carbamate Pesticides Using Enzymatic Screen Printed Biosensors. Anal. Lett. 2013, 46, 1743–1757. [Google Scholar] [CrossRef]
- Cortina, M.; del Valle, M.; Marty, J.-L. Electronic Tongue Using an Enzyme Inhibition Biosensor Array for the Resolution of Pesticide Mixtures. Electroanalysis 2008, 20, 54–60. [Google Scholar] [CrossRef]
- Liu, X.; Song, M.; Hou, T.; Li, F. Label-Free Homogeneous Electroanalytical Platform for Pesticide Detection Based on Acetylcholinesterase-Mediated DNA Conformational Switch Integrated with Rolling Circle Amplification. ACS Sens. 2017, 2, 562–568. [Google Scholar] [CrossRef]
- Rodrigues, N.F.M.; Neto, S.Y.; Luz, R.D.C.S.; Damos, F.S.; Yamanaka, H. Ultrasensitive Determination of Malathion Using Acetylcholinesterase Immobilized on Chitosan-Functionalized Magnetic Iron Nanoparticles. Biosensors 2018, 8, 16. [Google Scholar] [CrossRef] [Green Version]
- El-Moghazy, A.Y.; Soliman, E.A.; Ibrahim, H.Z.; Noguer, T.; Marty, J.L.; Istamboulie, G. Ultra-sensitive biosensor based on genetically engineered acetylcholinesterase immobilized in poly (vinyl alcohol)/Fe–Ni alloy nanocomposite for phosmet detection in olive oil. Food Chem. 2016, 203, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Istamboulie, G.; Andreescu, S.; Marty, J.-L.; Noguer, T. Highly sensitive detection of organophosphorus insecticides using magnetic microbeads and genetically engineered acetylcholinesterase. Biosens. Bioelectron. 2007, 23, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, R.B.; Alonso, G.A.; Muñoz, R.; Hayat, A.; Marty, J.-L. Design of a novel magnetic particles based electrochemical biosensor for organophosphate insecticide detection in flow injection analysis. Sens. Actuators B Chem. 2015, 208, 491–496. [Google Scholar] [CrossRef]
- Chauhan, N.; Narang, J.; Pundir, C.S. Immobilization of rat brain acetylcholinesterase on porous gold-nanoparticle–CaCO3 hybrid material modified Au electrode for detection of organophosphorous insecticides. Int. J. Biol. Macromol. 2011, 49, 923–929. [Google Scholar] [CrossRef]
- Zheng, Q.; Yu, Y.; Fan, K.; Ji, F.; Wu, J.; Ying, Y. A nano-silver enzyme electrode for organophosphorus pesticide detection. Anal. Bioanal. Chem. 2016, 408, 5819–5827. [Google Scholar] [CrossRef]
- Palanivelu, J.; Chidambaram, R. Acetylcholinesterase with Mesoporous Silica: Covalent Immobilization, Physiochemical Characterization, and Its Application in Food for Pesticide Detection. J. Cell. Biochem. 2019, 120, 10777–10786. [Google Scholar] [CrossRef]
- Mehta, J.; Vinayak, P.; Tuteja, S.K.; Chhabra, V.A.; Bhardwaj, N.; Paul, A.K.; Kim, K.-H.; Deep, A. Graphene modified screen printed immunosensor for highly sensitive detection of parathion. Biosens. Bioelectron. 2016, 83, 339–346. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, H.; Ge, X.; Liang, Y.; An, X.; Yang, C.; Fang, B.; Xie, H.; Wei, J. A nanocomposite of copper(ii) functionalized graphene and application for sensing sulfurated organophosphorus pesticides. New J. Chem. 2013, 37, 3956–3963. [Google Scholar] [CrossRef]
- Facure, M.H.M.; Mercante, L.A.; Mattoso, L.H.C.; Correa, D.S. Detection of trace levels of organophosphate pesticides using an electronic tongue based on graphene hybrid nanocomposites. Talanta 2017, 167, 59–66. [Google Scholar] [CrossRef]
- Gong, J.; Miao, X.; Zhou, T.; Zhang, L. An enzymeless organophosphate pesticide sensor using Au nanoparticle-decorated graphene hybrid nanosheet as solid-phase extraction. Talanta 2011, 85, 1344–1349. [Google Scholar] [CrossRef]
- Huixiang, W.; Danqun, H.; Yanan, Z.; Na, M.; Jingzhou, H.; Miao, L.; Caihong, S.; Changjun, H. A non-enzymatic electro-chemical sensor for organophosphorus nerve agents mimics and pesticides detection. Sens. Actuators B Chem. 2017, 252, 1118–1124. [Google Scholar] [CrossRef]
- Yu, T.; Ying, T.-Y.; Song, Y.-Y.; Li, Y.-J.; Wu, F.-H.; Dong, X.-Q.; Shen, J.-S. A highly sensitive sensing system based on photoluminescent quantum dots for highly toxic organophosphorus compounds. RSC Adv. 2014, 4, 8321–8327. [Google Scholar] [CrossRef]
- Zhang, K.; Mei, Q.; Guan, G.; Liu, B.; Wang, S.; Zhang, Z. Ligand Replacement-Induced Fluorescence Switch of Quantum Dots for Ultrasensitive Detection of Organophosphorothioate Pesticides. Anal. Chem. 2010, 82, 9579–9586. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Yu, T.; Liu, F.; Sun, M.; Yu, H.; Liu, B.; Zhang, Z.; Jiang, H.; Wang, S. Selective Fluorescence Turn-On and Ratiometric Detection of Organophosphate Using Dual-Emitting Mn-Doped ZnS Nanocrystal Probe. Anal. Chem. 2014, 86, 11727–11733. [Google Scholar] [CrossRef]
- Lian, X.; Yan, B. Trace Detection of Organophosphorus Chemical Warfare Agents in Wastewater and Plants by Luminescent UIO-67(Hf) and Evaluating the Bioaccumulation of Organophosphorus Chemical Warfare Agents. ACS Appl. Mater. Interfaces 2018, 10, 14869–14876. [Google Scholar] [CrossRef]
- Li, X.; Cui, H.; Zeng, Z. A Simple Colorimetric and Fluorescent Sensor to Detect Organophosphate Pesticides Based on Adenosine Triphosphate-Modified Gold Nanoparticles. Sensors 2018, 18, 4302. [Google Scholar] [CrossRef] [Green Version]
- Dey, P.C.; Das, R. Ligand free surface of CdS nanoparticles enhances the energy transfer efficiency on interacting with Eosin Y dye—Helping in the sensing of very low level of chlorpyrifos in water. Spectrochim. Acta Part A 2019, 207, 156–163. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X.; Chen, X.; Cao, X.; Cao, J.; Xiong, X.; Zeng, W. A novel upconversion luminescence turn-on nanosensor for ratiometric detection of organophosphorus pesticides. RSC Adv. 2016, 6, 46317–46324. [Google Scholar] [CrossRef]
- Liu, Y.; Lv, B.; Liu, A.; Liang, G.; Yin, L.; Pu, Y.; Wei, W.; Gou, S.; Liu, S. Multicolor sensor for organophosphorus pesticides determination based on the bi-enzyme catalytic etching of gold nanorods. Sens. Actuators B Chem. 2018, 265, 675–681. [Google Scholar] [CrossRef]
- Dissanayake, N.M.; Arachchilage, J.S.; Samuels, T.A.; Obare, S.O. Highly sensitive plasmonic metal nanoparticle-based sensors for the detection of organophosphorus pesticides. Talanta 2019, 200, 218–227. [Google Scholar] [CrossRef]
- Sarkar, S.; Das, R. Presence of chlorpyrifos shows blue shift of the absorption peak of silver nanohexagons solution—An indication of etching of nanocrystals and sensing of chlorpyrifos. Sens. Actuators B Chem. 2018, 266, 149–159. [Google Scholar] [CrossRef]
- Chen, Y.; Tan, C.; Zhang, H.; Wang, L. Two-dimensional graphene analogues for biomedical applications. Chem. Soc. Rev. 2015, 44, 2681–2701. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, S.; Wales, M.; Good, T.; Grimsley, J.; Wild, J.; Simonian, A. Fluorescence-based sensing of p-nitrophenol and p-nitrophenyl substituent organophosphates. Anal. Chim. Acta 2007, 596, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Obare, S.O.; De, C.; Guo, W.; Haywood, T.L.; Samuels, T.A.; Adams, C.P.; Masika, N.O.; Murray, D.H.; Anderson, G.A.; Campbell, K.; et al. Fluorescent Chemosensors for Toxic Organophosphorus Pesticides: A Review. Sensors 2010, 10, 7018–7043. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yang, Y.; Zhang, C.-y. Toward Biocompatible Semiconductor Quantum Dots: From Biosynthesis and Bioconjugation to Biomedical Application. Chem. Rev. 2015, 115, 11669–11717. [Google Scholar] [CrossRef] [PubMed]
- Idris, N.M.; Jayakumar, M.K.G.; Bansal, A.; Zhang, Y. Upconversion nanoparticles as versatile light nanotransducers for photoactivation applications. Chem. Soc. Rev. 2015, 44, 1449–1478. [Google Scholar] [CrossRef]
- Heer, S.; Kömpe, K.; Güdel, H.U.; Haase, M. Highly Efficient Multicolour Upconversion Emission in Transparent Colloids of Lanthanide-Doped NaYF4 Nanocrystals. Adv. Mater. 2004, 16, 2102–2105. [Google Scholar] [CrossRef]
- Tao, K.; Sun, K.; Choi, S.K. Chapter 12—Upconversion nanocrystals for near-infrared-controlled drug delivery. In Photonanotechnology for Therapeutics and Imaging; Choi, S.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 345–371. [Google Scholar]
- Vikrant, K.; Tsang, D.C.W.; Raza, N.; Giri, B.S.; Kukkar, D.; Kim, K.-H. Potential Utility of Metal–Organic Framework-Based Platform for Sensing Pesticides. ACS Appl. Mater. Interfaces 2018, 10, 8797–8817. [Google Scholar] [CrossRef]
- Dhaka, S.; Kumar, R.; Deep, A.; Kurade, M.B.; Ji, S.-W.; Jeon, B.-H. Metal–organic frameworks (MOFs) for the removal of emerging contaminants from aquatic environments. Coord. Chem. Rev. 2019, 380, 330–352. [Google Scholar] [CrossRef]
- Yan, B. Lanthanide-Functionalized Metal–Organic Framework Hybrid Systems To Create Multiple Luminescent Centers for Chemical Sensing. Acc. Chem. Res. 2017, 50, 2789–2798. [Google Scholar] [CrossRef]
- Singha, D.K.; Majee, P.; Mondal, S.K.; Mahata, P. Highly Selective Aqueous Phase Detection of Azinphos-Methyl Pesticide in ppb Level Using a Cage-Connected 3D MOF. ChemistrySelect 2017, 2, 5760–5768. [Google Scholar] [CrossRef]
- Tang, J.; Ma, X.; Yang, J.; Feng, D.-D.; Wang, X.-Q. Recent advances in metal–organic frameworks for pesticide detection and adsorption. Dalton Trans. 2020, 49, 14361–14372. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Shao, L.; Li, Q.; Wang, J. Gold nanorods and their plasmonic properties. Chem. Soc. Rev. 2013, 42, 2679–2724. [Google Scholar] [CrossRef] [PubMed]
- Marrs, T.C.; Rice, P.; Vale, J.A. The Role of Oximes in the Treatment of Nerve Agent Poisoning in Civilian Casualties. Toxicol. Rev. 2006, 25, 297–323. [Google Scholar] [CrossRef] [PubMed]
- Snider, T.H.; Wilhelm, C.M.; Babin, M.C.; Platoff, G.E., Jr.; Yeung, D.T. Assessing the therapeutic efficacy of oxime therapies against percutaneous organophosphorus pesticide and nerve agent challenges in the Hartley guinea pig. J. Toxicol. Sci. 2015, 40, 759–775. [Google Scholar] [CrossRef] [Green Version]
- Reddy, S.D.; Reddy, D.S. Midazolam as an anticonvulsant antidote for organophosphate intoxication—A pharmacotherapeutic appraisal. Epilepsia 2015, 56, 813–821. [Google Scholar] [CrossRef] [Green Version]
- Sit, R.K.; Radić, Z.; Gerardi, V.; Zhang, L.; Garcia, E.; Katalinić, M.; Amitai, G.; Kovarik, Z.; Fokin, V.V.; Sharpless, K.B.; et al. New Structural Scaffolds for Centrally Acting Oxime Reactivators of Phosphylated Cholinesterases. J. Biol. Chem. 2011, 286, 19422–19430. [Google Scholar] [CrossRef] [Green Version]
- DeMar, J.C.; Clarkson, E.D.; Ratcliffe, R.H.; Campbell, A.J.; Thangavelu, S.G.; Herdman, C.A.; Leader, H.; Schulz, S.M.; Marek, E.; Medynets, M.A.; et al. Pro-2-PAM Therapy for Central and Peripheral Cholinesterases. Chem.-Biol. Interact. 2010, 187, 191–198. [Google Scholar] [CrossRef] [Green Version]
- Jovanović, D. Pharmacokinetics of Pralidoxime Chloride. Arch. Toxicol. 1989, 63, 416–418. [Google Scholar] [CrossRef]
- Pashirova, T.N.; Braïki, A.; Zueva, I.V.; Petrov, K.A.; Babaev, V.M.; Burilova, E.A.; Samarkina, D.A.; Rizvanov, I.K.; Souto, E.B.; Jean, L.; et al. Combination delivery of two oxime-loaded lipid nanoparticles: Time-dependent additive action for prolonged rat brain protection. J. Control. Release 2018, 290, 102–111. [Google Scholar] [CrossRef]
- Vilela, S.M.F.; Salcedo-Abraira, P.; Colinet, I.; Salles, F.; De Koning, M.C.; Joosen, M.J.A.; Serre, C.; Horcajada, P. Nanometric MIL-125-NH2 Metal–Organic Framework as a Potential Nerve Agent Antidote Carrier. Nanomaterials 2017, 7, 321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.K.; Thomas, T.P.; Leroueil, P.R.; Kotlyar, A.; Van Der Spek, A.F.L.; Baker, J.R. Specific and Cooperative Interactions between Oximes and PAMAM Dendrimers as Demonstrated by 1H NMR Study. J. Phys. Chem. B 2012, 116, 10387–10397. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.K.; Leroueil, P.; Li, M.-H.; Desai, A.; Zong, H.; Van Der Spek, A.F.L.; Baker, J.R., Jr. Specificity and Negative Cooperativity in Dendrimer–Oxime Drug Complexation. Macromolecules 2011, 44, 4026–4029. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Naylor, A.M.; William, A.; Goddard, I. Starburst Dendrimers: Molecular-Level Control of Size, Shape, Surface Chemistry, Topology, and Flexibility from Atoms to Macroscopic Matter. Angew. Chem. Int. Ed. 1990, 29, 138–175. [Google Scholar] [CrossRef]
- Mukherjee, J.; Wong, P.T.; Tang, S.; Gam, K.; Coulter, A.; Baker, J.R.; Choi, S.K. Mechanism of Cooperativity and Nonlinear Release Kinetics in Multivalent Dendrimer-Atropine Complexes. Mol. Pharm. 2015, 12, 4498–4508. [Google Scholar] [CrossRef]
- Wagner, S.; Kufleitner, J.; Zensi, A.; Dadparvar, M.; Wien, S.; Bungert, J.; Vogel, T.; Worek, F.; Kreuter, J.; von Briesen, H. Nanoparticulate Transport of Oximes over an In Vitro Blood-Brain Barrier Model. PLoS ONE 2010, 5, e14213. [Google Scholar] [CrossRef] [Green Version]
- Pashirova, T.N.; Zueva, I.V.; Petrov, K.A.; Babaev, V.M.; Lukashenko, S.S.; Rizvanov, I.K.; Souto, E.B.; Nikolsky, E.E.; Zakharova, L.Y.; Masson, P.; et al. Nanoparticle-Delivered 2-PAM for Rat Brain Protection against Paraoxon Central Toxicity. ACS Appl. Mater. Interfaces 2017, 9, 16922–16932. [Google Scholar] [CrossRef]
- Hinderling, P.H.; Gundert-Remy, U.; Schmidlin, O. Integrated pharmacokinetics and pharmacodynamics of atropine in healthy humans I: Pharmacokinetics. J. Pharm. Sci. 1985, 74, 703–710. [Google Scholar] [CrossRef]
- Han, J.; Lim, S.-J.; Lee, M.-K.; Kim, C.-K. Altered Pharmacokinetics and Liver Targetability of Methotrexate by Conjugation with Lactosylated Albumins. Drug Deliv. 2001, 8, 125–134. [Google Scholar]
- Morgan, M.T.; Nakanishi, Y.; Kroll, D.J.; Griset, A.P.; Carnahan, M.A.; Wathier, M.; Oberlies, N.H.; Manikumar, G.; Wani, M.C.; Grinstaff, M.W. Dendrimer-Encapsulated Camptothecins: Increased Solubility, Cellular Uptake, and Cellular Retention Affords Enhanced Anticancer Activity In vitro. Cancer Res. 2006, 66, 11913–11921. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Zeng, S.; Abd El-Aty, A.M.; Hacımüftüoğlu, A.; Kalekristos Yohannes, W.; Khan, M.; She, Y. Development of Water-Compatible Molecularly Imprinted Polymers Based on Functionalized β-Cyclodextrin for Controlled Release of Atropine. Polymers 2020, 12, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacquet, P.; Daudé, D.; Bzdrenga, J.; Masson, P.; Elias, M.; Chabrière, E. Current and emerging strategies for organophosphate decontamination: Special focus on hyperstable enzymes. Environ. Sci. Pollut. Res. 2016, 23, 8200–8218. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, M.; Ashani, Y. Catalytic bioscavengers as countermeasures against organophosphate nerve agents. Chem.-Biol. Interact. 2018, 292, 50–64. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, A.R.S.; Pande, A.H. Organophosphate-Hydrolyzing Enzymes as First-Line of Defence Against Nerve Agent-Poisoning: Perspectives and the Road Ahead. Protein J. 2016, 35, 424–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schenk, G.; Mateen, I.; Ng, T.-K.; Pedroso, M.M.; Mitić, N.; Jafelicci, M.; Marques, R.F.C.; Gahan, L.R.; Ollis, D.L. Organophosphate-degrading metallohydrolases: Structure and function of potent catalysts for applications in bioremediation. Coord. Chem. Rev. 2016, 317, 122–131. [Google Scholar] [CrossRef] [Green Version]
- Radić, Z.; Dale, T.; Kovarik, Z.; Berend, S.; Garcia, E.; Zhang, L.; Amitai, G.; Green, C.; Radić, B.; Duggan, B.M.; et al. Catalytic detoxification of nerve agent and pesticide organophosphates by butyrylcholinesterase assisted with non-pyridinium oximes. Biochem. J. 2013, 450, 231–242. [Google Scholar]
- Sit, R.K.; Fokin, V.V.; Amitai, G.; Sharpless, K.B.; Taylor, P.; Radić, Z. Imidazole Aldoximes Effective in Assisting Butyrylcholinesterase Catalysis of Organophosphate Detoxification. J. Med. Chem. 2014, 57, 1378–1389. [Google Scholar] [CrossRef]
- Huang, Y.-J.; Huang, Y.; Baldassarre, H.; Wang, B.; Lazaris, A.; Leduc, M.; Bilodeau, A.S.; Bellemare, A.; Côté, M.; Herskovits, P.; et al. Recombinant human butyrylcholinesterase from milk of transgenic animals to protect against organophosphate poisoning. Proc. Natl. Acad. Sci. USA 2007, 104, 13603–13608. [Google Scholar] [CrossRef] [Green Version]
- Hemmert, A.C.; Otto, T.C.; Wierdl, M.; Edwards, C.C.; Fleming, C.D.; MacDonald, M.; Cashman, J.R.; Potter, P.M.; Cerasoli, D.M.; Redinbo, M.R. Human Carboxylesterase 1 Stereoselectively Binds the Nerve Agent Cyclosarin and Spontaneously Hydrolyzes the Nerve Agent Sarin. Mol. Pharmacol. 2010, 77, 508–516. [Google Scholar] [CrossRef] [Green Version]
- Lenz, D.E.; Yeung, D.; Smith, J.R.; Sweeney, R.E.; Lumley, L.A.; Cerasoli, D.M. Stoichiometric and catalytic scavengers as protection against nerve agent toxicity: A mini review. Toxicology 2007, 233, 31–39. [Google Scholar] [CrossRef]
- Valiyaveettil, M.; Alamneh, Y.; Rezk, P.; Biggemann, L.; Perkins, M.W.; Sciuto, A.M.; Doctor, B.P.; Nambiar, M.P. Protective efficacy of catalytic bioscavenger, paraoxonase 1 against sarin and soman exposure in guinea pigs. Biochem. Pharmacol. 2011, 81, 800–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Liu, E.J.; Tsao, C.; Kasten, S.A.; Boeri, M.V.; Dao, T.L.; DeBus, S.J.; Cadieux, C.L.; Baker, C.A.; Otto, T.C.; et al. Nanoscavenger provides long-term prophylactic protection against nerve agents in rodents. Sci. Transl. Med. 2019, 11, eaau7091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sogorb, M.A.; García-Argüelles, S.; Carrera, V.; Vilanova, E. Serum Albumin is as Efficient as Paraxonase in the Detoxication of Paraoxon at Toxicologically Relevant Concentrations. Chem. Res. Toxicol. 2008, 21, 1524–1529. [Google Scholar] [CrossRef] [PubMed]
- Cohen, O.; Kronman, C.; Raveh, L.; Mazor, O.; Ordentlich, A.; Shafferman, A. Comparison of Polyethylene Glycol-Conjugated Recombinant Human Acetylcholinesterase and Serum Human Butyrylcholinesterase as Bioscavengers of Organophosphate Compounds. Mol. Pharmacol. 2006, 70, 1121–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jun, D.; Musilová, L.; Link, M.; Loiodice, M.; Nachon, F.; Rochu, D.; Renault, F.; Masson, P. Preparation and Characterization of Methoxy Polyethylene Glycol-conjugated Phosphotriesterase As a Potential Catalytic Bioscavenger against Organophosphate Poisoning. Chem.-Biol. Interact. 2010, 187, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Worek, F.; Thiermann, H.; Wille, T. Oximes in Organophosphate Poisoning: 60 Years of Hope and Despair. Chem.-Biol. Interact. 2016, 259, 93–98. [Google Scholar] [CrossRef]
- Kovarik, Z.; Maček Hrvat, N.; Katalinić, M.; Sit, R.K.; Paradyse, A.; Žunec, S.; Musilek, K.; Fokin, V.V.; Taylor, P.; Radić, Z. Catalytic Soman Scavenging by the Y337A/F338A Acetylcholinesterase Mutant Assisted with Novel Site-Directed Aldoximes. Chem. Res. Toxicol. 2015, 28, 1036–1044. [Google Scholar] [CrossRef] [Green Version]
- Trovaslet-Leroy, M.; Musilova, L.; Renault, F.; Brazzolotto, X.; Misik, J.; Novotny, L.; Froment, M.-T.; Gillon, E.; Loiodice, M.; Verdier, L.; et al. Organophosphate hydrolases as catalytic bioscavengers of organophosphorus nerve agents. Toxicol. Lett. 2011, 206, 14–23. [Google Scholar] [CrossRef]
- Kronman, C.; Cohen, O.; Raveh, L.; Mazor, O.; Ordentlich, A.; Shafferman, A. Polyethylene-glycol conjugated recombinant human acetylcholinesterase serves as an efficacious bioscavenger against soman intoxication. Toxicology 2007, 233, 40–46. [Google Scholar] [CrossRef]
- Noy-Porat, T.; Cohen, O.; Ehrlich, S.; Epstein, E.; Alcalay, R.; Mazor, O. Acetylcholinesterase-Fc Fusion Protein (AChE-Fc): A Novel Potential Organophosphate Bioscavenger with Extended Plasma Half-Life. Bioconjug. Chem. 2015, 26, 1753–1758. [Google Scholar] [CrossRef]
- Misik, J.; Pavlikova, R.; Josse, D.; Cabal, J.; Kuca, K. In vitro skin permeation and decontamination of the organophosphorus pesticide paraoxon under various physical conditions—Evidence for a wash-in effect. Toxicol. Mech. Methods 2012, 22, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Bjarnason, S.; Mikler, J.; Hill, I.; Tenn, C.; Garrett, M.; Caddy, N.; Sawyer, T. Comparison of Selected Skin Decontaminant Products and Regimens Against VX In Domestic Swine. Hum. Exp. Toxicol. 2008, 27, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Moon, S.-Y.; Guelta, M.A.; Lin, L.; Gómez-Gualdrón, D.A.; Snurr, R.Q.; Harvey, S.P.; Hupp, J.T.; Farha, O.K. Nanosizing a Metal–Organic Framework Enzyme Carrier for Accelerating Nerve Agent Hydrolysis. ACS Nano 2016, 10, 9174–9182. [Google Scholar] [CrossRef] [PubMed]
- Amitai, G.; Murata, H.; Andersen, J.D.; Koepsel, R.R.; Russell, A.J. Decontamination of Chemical and Biological Warfare Agents with a Single Multi-functional Material. Biomaterials 2010, 31, 4417–4425. [Google Scholar] [CrossRef]
- Morales, J.I.; Figueroa, R.; Rojas, M.; Millán, D.; Tapia, R.A.; Pavez, P. Dual function of amino acid ionic liquids (Bmim[AA]) on the degradation of the organophosphorus pesticide, Paraoxon®. Org. Biomol. Chem. 2018, 16, 7446–7453. [Google Scholar] [CrossRef]
- Singh, N.; Karpichev, Y.; Sharma, R.; Gupta, B.; Sahu, A.K.; Satnami, M.L.; Ghosh, K.K. From α-nucleophiles to functionalized aggregates: Exploring the reactivity of hydroxamate ion towards esterolytic reactions in micelles. Org. Biomol. Chem. 2015, 13, 2827–2848. [Google Scholar] [CrossRef]
- Tsang, J.S.W.; Neverov, A.A.; Brown, R.S. La3+-catalyzed methanolysis of O,O-diethyl S-(p-nitrophenyl) phosphorothioate and O,O-diethyl S-phenyl phosphorothioate. Millions-fold acceleration of the destruction of V-agent simulants. Org. Biomol. Chem. 2004, 2, 3457–3463. [Google Scholar] [CrossRef]
- Braue, E.H.; Smith, K.H.; Doxzon, B.F.; Lumpkin, H.L.; Clarkson, E.D. Efficacy Studies of Reactive Skin Decontamination Lotion, M291 Skin Decontamination Kit, 0.5% Bleach, 1% Soapy Water, and Skin Exposure Reduction Paste Against Chemical Warfare Agents, Part 1: Guinea Pigs Challenged with VX. Cutan. Ocul. Toxicol. 2011, 30, 15–28. [Google Scholar] [CrossRef]
- Fentabil, M.; Gebremedhin, M.; Purdon, J.G.; Cochrane, L.; Goldman, V.S. Degradation of Pesticides with RSDL® (Reactive Skin Decontamination Lotion Kit) Lotion: LC–MS Investigation. Toxicol. Lett. 2018, 293, 241–248. [Google Scholar] [CrossRef]
- Tang, S.; Wong, P.T.; Cannon, J.; Yang, K.; Bowden, S.; Bhattacharjee, S.; O’Konek, J.J.; Choi, S.K. Hydrophilic Scaffolds of Oxime as the Potent Catalytic Inactivator of Reactive Organophosphate. Chem.-Biol. Interact. 2019, 297, 67–79. [Google Scholar] [CrossRef]
- Fryer, M.W.; Gage, P.W.; Neering, I.R.; Dulhunty, A.F.; Lamh, G.D. Paralysis of skeletal muscle by butanedione monoxime, a chemical phosphatase. Pflugers Arch-Eur. J. Physiol. 1988, 411, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Um, I.-H.; Jeon, S.-E.; Baek, M.-H.; Park, H.-R. Significant and differential acceleration of dephosphorylation of the insecticides, paraoxon and parathion, caused by alkali metal ethoxides. Chem. Commun. 2003, 24, 3016–3017. [Google Scholar] [CrossRef] [PubMed]
- Orth, E.S.; Almeida, T.G.; Silva, V.B.; Oliveira, A.R.M.; Ocampos, F.M.M.; Barison, A. Mechanistic insight on the catalytic detoxification of Paraoxon mediated by imidazole: Furnishing optimum scaffolds for scavenging organophosphorus agents. J. Mol. Catal. A Chem. 2015, 403, 93–98. [Google Scholar] [CrossRef]
- Wilson, C.; Cooper, N.J.; Briggs, M.E.; Cooper, A.I.; Adams, D.J. Investigating the breakdown of the nerve agent simulant methyl paraoxon and chemical warfare agents GB and VX using nitrogen containing bases. Org. Biomol. Chem. 2018, 16, 9285–9291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terrier, F.; Rodriguez-Dafonte, P.; Le Guevel, E.; Moutiers, G. Revisiting the Reactivity of Oximate a-Nucleophiles with Electrophilic Phosphorus Centers. Relevance to Detoxification of Sarin, Soman and DFP Under Mild Conditions. Org. Biomol. Chem. 2006, 4, 4352–4363. [Google Scholar] [CrossRef]
- Bharathi, S.; Wong, P.T.; Desai, A.; Lykhytska, O.; Choe, V.; Kim, H.; Thomas, T.P.; Baker, J.R.; Choi, S.K. Design and Mechanistic Investigation of Oxime-conjugated PAMAM Dendrimers As the Catalytic Scavenger of Reactive Organophosphate. J. Mater. Chem. B 2014, 2, 1068–1078. [Google Scholar] [CrossRef]
- Behrman, E.J.; Biallas, M.J.; Brass, H.J.; Edwards, J.O.; Isaks, M. Reactions of Phosphonic Acid Esters with Nucleophiles. II. Survey of Nucleophiles Reacting with p-Nitrophenyl Methylphosphonate Anion. J. Org. Chem. 1970, 35, 3069–3075. [Google Scholar] [CrossRef]
- Le Provost, R.; Wille, T.; Louise, L.; Masurier, N.; Muller, S.; Reiter, G.; Renard, P.-Y.; Lafont, O.; Worek, F.; Estour, F. Optimized strategies to synthesize b-cyclodextrin-oxime conjugates as a new generation of organophosphate scavengers. Org. Biomol. Chem. 2011, 9, 3026–3032. [Google Scholar] [CrossRef]
- Masurier, N.; Estour, F.; Froment, M.-T.; Lefèvre, B.; Debouzy, J.-C.; Brasme, B.; Masson, P.; Lafont, O. Synthesis of 2-substituted β-cyclodextrin derivatives with a hydrolytic activity against the organophosphorylester paraoxon. Eur. J. Med. Chem. 2005, 40, 615–623. [Google Scholar] [CrossRef]
- Han, X.; Balakrishnan, V.K.; van Loon, G.W.; Buncel, E. Degradation of the Pesticide Fenitrothion as Mediated by Cationic Surfactants and a-Nucleophilic Reagents. Langmuir 2006, 22, 9009–9017. [Google Scholar] [CrossRef]
- Kandpal, N.; Dewangan, H.K.; Nagwanshi, R.; Ghosh, K.K.; Satnami, M.L. Micellar-accelerated hydrolysis of organophosphate and thiophosphates by pyridine oximate. Int. J. Chem. Kinet. 2018, 50, 827–835. [Google Scholar] [CrossRef]
- Gonçalves, L.M.; Kobayakawa, T.G.; Zanette, D.; Chaimovich, H.; Cuccovia, I.M. Effects of Micelles and Vesicles on the Oximolysis of p-Nitrophenyl Diphenyl Phosphate: A Model System for Surfactant-Based Skin-Defensive Formulations against Organophosphates. J. Pharm. Sci. 2009, 98, 1040–1052. [Google Scholar] [CrossRef] [PubMed]
- Kapitanov, I.V.; Mirgorodskaya, A.B.; Valeeva, F.G.; Gathergood, N.; Kuca, K.; Zakharova, L.Y.; Karpichev, Y. Physicochemical properties and esterolytic reactivity of oxime functionalized surfactants in pH-responsive mixed micellar system. Colloids Surf. A Physicochem. Eng. Asp. 2017, 524, 143–159. [Google Scholar] [CrossRef]
- Kandpal, N.; Dewangan, H.K.; Nagwanshi, R.; Ghosh, K.K.; Satnami, M.L. An investigation of kinetic and physicochemical properties of vesicular surfactants with oximate and hydroxamate ions: Hydrolytic reactions of organophosphorus pesticides. J. Mol. Liq. 2017, 243, 178–186. [Google Scholar] [CrossRef]
- Alves, N.J.; Moore, M.; Johnson, B.J.; Dean, S.N.; Turner, K.B.; Medintz, I.L.; Walper, S.A. Environmental Decontamination of a Chemical Warfare Simulant Utilizing a Membrane Vesicle-Encapsulated Phosphotriesterase. ACS Appl. Mater. Interfaces 2018, 10, 15712–15719. [Google Scholar] [CrossRef] [Green Version]
- Totten, R.K.; Weston, M.H.; Park, J.K.; Farha, O.K.; Hupp, J.T.; Nguyen, S.T. Catalytic Solvolytic and Hydrolytic Degradation of Toxic Methyl Paraoxon with La(catecholate)-Functionalized Porous Organic Polymers. ACS Catal. 2013, 3, 1454–1459. [Google Scholar] [CrossRef]
- Hartshorn, C.M.; Singh, A.; Chang, E.L. Metal-chelator polymers as organophosphate hydrolysis catalysts. J. Mater. Chem. 2002, 12, 602–605. [Google Scholar] [CrossRef]
- Wu, S.-H.; Mou, C.-Y.; Lin, H.-P. Synthesis of mesoporous silica nanoparticles. Chem. Soc. Rev. 2013, 42, 3862–3875. [Google Scholar] [CrossRef]
- Tarn, D.; Ashley, C.E.; Xue, M.; Carnes, E.C.; Zink, J.I.; Brinker, C.J. Mesoporous Silica Nanoparticle Nanocarriers: Biofunctionality and Biocompatibility. Acc. Chem. Res. 2013, 46, 792–801. [Google Scholar] [CrossRef] [Green Version]
- Xu, P.; Guo, S.; Yu, H.; Li, X. Mesoporous Silica Nanoparticles (MSNs) for Detoxification of Hazardous Organophorous Chemicals. Small 2014, 10, 2404–2412. [Google Scholar] [CrossRef]
- Horcajada, P.; Gref, R.; Baati, T.; Allan, P.K.; Maurin, G.; Couvreur, P.; Férey, G.; Morris, R.E.; Serre, C. Metal–Organic Frameworks in Biomedicine. Chem. Rev. 2012, 112, 1232–1268. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.J.; Moon, S.-Y.; Mondloch, J.E.; Beyzavi, M.H.; Stephenson, C.J.; Hupp, J.T.; Farha, O.K. Exploiting parameter space in MOFs: A 20-fold enhancement of phosphate-ester hydrolysis with UiO-66-NH2. Chem. Sci. 2015, 6, 2286–2291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katz, M.J.; Mondloch, J.E.; Totten, R.K.; Park, J.K.; Nguyen, S.T.; Farha, O.K.; Hupp, J.T. Simple and Compelling Biomimetic Metal–Organic Framework Catalyst for the Degradation of Nerve Agent Simulants. Angew. Chem. Int. Ed. 2014, 53, 497–501. [Google Scholar] [CrossRef] [PubMed]
- de Koning, M.C.; van Grol, M.; Breijaert, T. Degradation of Paraoxon and the Chemical Warfare Agents VX, Tabun, and Soman by the Metal–Organic Frameworks UiO-66-NH2, MOF-808, NU-1000, and PCN-777. Inorg. Chem. 2017, 56, 11804–11809. [Google Scholar] [CrossRef]
- Salerno, A.; Devers, T.; Bolzinger, M.-A.; Pelletier, J.; Josse, D.; Briançon, S. In Vitro Skin Decontamination of the Organophosphorus Pesticide Paraoxon with Nanometric Cerium Oxide CeO2. Chem.-Biol. Interact. 2017, 267, 57–66. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Sakellarides, T.M.; Sakkas, V.A.; Albanis, T.A. Photocatalytic Degradation of Selected s-Triazine Herbicides and Organophosphorus Insecticides over Aqueous TiO2 Suspensions. Environ. Sci. Technol. 2001, 35, 398–405. [Google Scholar] [CrossRef]
- Choudhary, M.K.; Kataria, J.; Bhardwaj, V.K.; Sharma, S. Green biomimetic preparation of efficient Ag–ZnO heterojunctions with excellent photocatalytic performance under solar light irradiation: A novel biogenic-deposition-precipitation approach. Nanoscale Adv. 2019, 1, 1035–1044. [Google Scholar] [CrossRef] [Green Version]
- Svenson, S.; Tomalia, D.A. Dendrimers in Biomedical Applications--Reflections on the Field. Adv. Drug Deliv. Rev. 2005, 57, 2106–2129. [Google Scholar] [CrossRef]
- Shcharbin, D.; Janaszewska, A.; Klajnert-Maculewicz, B.; Ziemba, B.; Dzmitruk, V.; Halets, I.; Loznikova, S.; Shcharbina, N.; Milowska, K.; Ionov, M.; et al. How to Study Dendrimers and Dendriplexes III. Biodistribution, Pharmacokinetics and Toxicity In Vivo. J. Control. Release 2014, 181, 40–52. [Google Scholar] [CrossRef]
- Roberts, J.C.; Bhalgat, M.K.; Zera, R.T. Preliminary Biological Evaluation of Polyamidoamine (PAMAM) StarburstTM Dendrimers. J. Biomed. Mater. Res. 1996, 30, 53–65. [Google Scholar] [CrossRef]
- Durán-Lara, E.F.; Ávila-Salas, F.; Galaz, S.; John, A.; Maricán, A.; Gutiérrez, M.; Nachtigall, F.M.; Gonzalez-Nilo, F.D.; Santos, L.S. Nano-Detoxification of Organophosphate Agents by PAMAM Derivatives. J. Braz. Chem. Soc. 2015, 26, 580–591. [Google Scholar] [CrossRef]
- Shi, X.; Wang, S.H.; Swanson, S.D.; Ge, S.; Cao, Z.; Van Antwerp, M.E.; Landmark, K.J.; Baker, J.R., Jr. Dendrimer-Functionalized Shell-crosslinked Iron Oxide Nanoparticles for In-Vivo Magnetic Resonance Imaging of Tumors. Adv. Mater. 2008, 20, 1671–1678. [Google Scholar] [CrossRef] [Green Version]
- Lim, E.-K.; Kim, T.; Paik, S.; Haam, S.; Huh, Y.-M.; Lee, K. Nanomaterials for Theranostics: Recent Advances and Future Challenges. Chem. Rev. 2015, 115, 327–394. [Google Scholar] [CrossRef] [PubMed]
- Rosi, N.L.; Mirkin, C.A. Nanostructures in Biodiagnostics. Chem. Rev. 2005, 105, 1547–1562. [Google Scholar] [CrossRef] [PubMed]
- Aragay, G.; Pino, F.; Merkoçi, A. Nanomaterials for Sensing and Destroying Pesticides. Chem. Rev. 2012, 112, 5317–5338. [Google Scholar] [CrossRef]
- Yang, Y.; Sunoqrot, S.; Stowell, C.; Ji, J.; Lee, C.-W.; Kim, J.W.; Khan, S.A.; Hong, S. Effect of Size, Surface Charge, and Hydrophobicity of Poly(amidoamine) Dendrimers on Their Skin Penetration. Biomacromolecules 2012, 13, 2154–2162. [Google Scholar] [CrossRef] [Green Version]

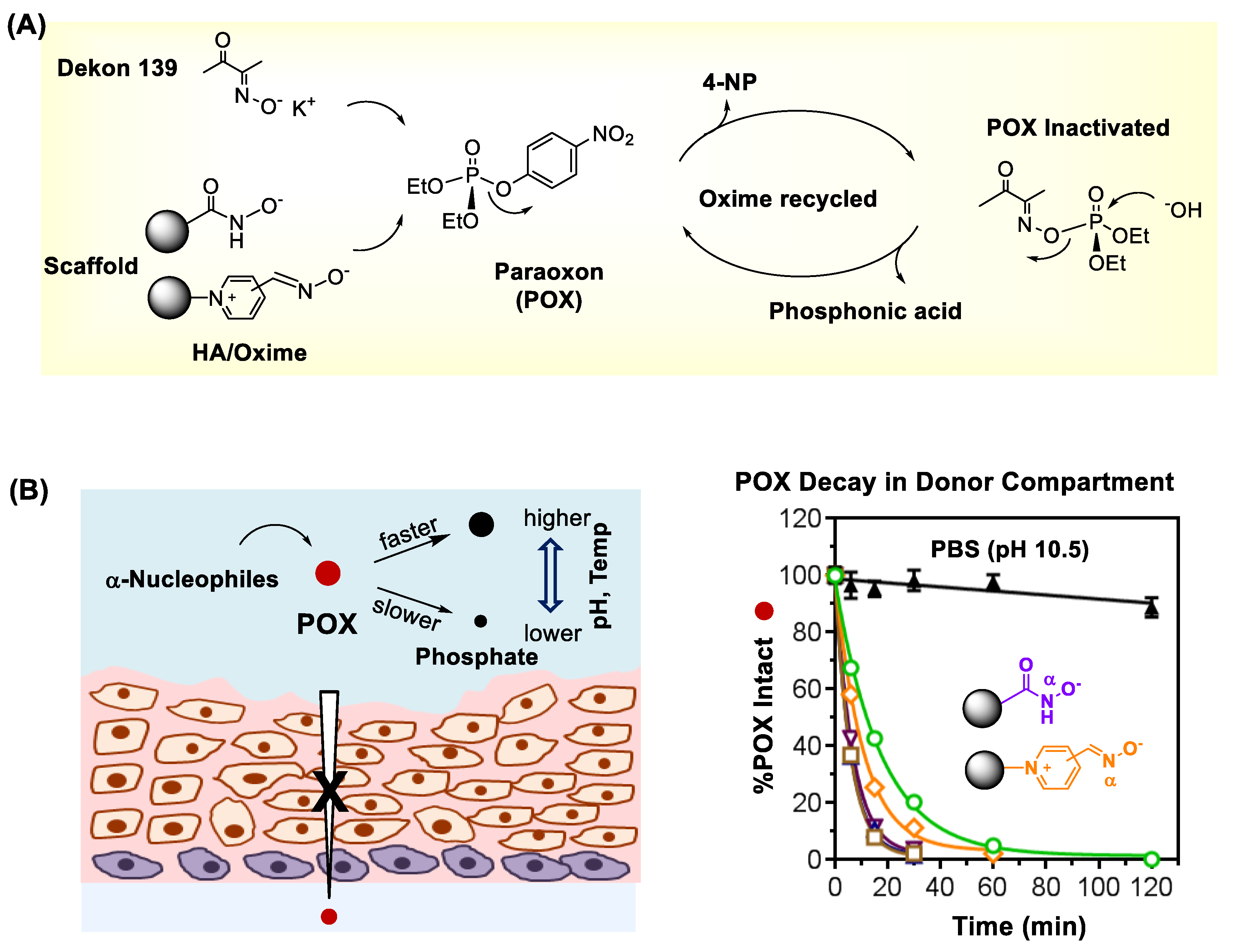
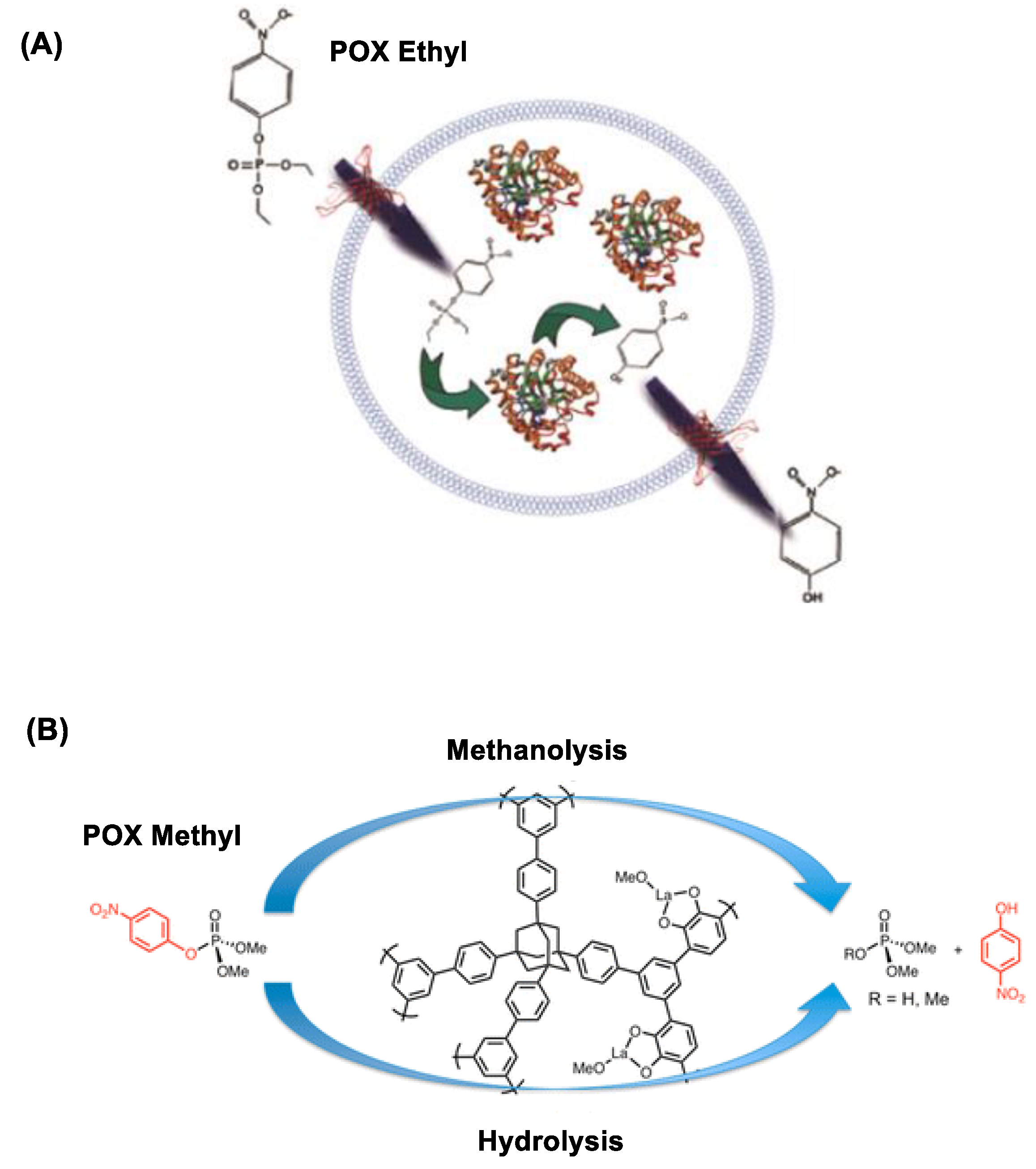


| Detection | Concept | Design | OP Analyte (LOD) | Ref |
|---|---|---|---|---|
| Electrochemistry | AChE Inhibition | IONP@AChE | Chlorpyrifos oxon, malathion (0.3 nM) | [43,45,46] |
| nano Fe-Ni@AChE | Phosmet (0.1 nM) | [44] | ||
| AuNP-CaCO3@AChE | Malathion, chlorpyrifos (0.1 nM) | [47] | ||
| nano Ag@Chitosan-AChE | POX (15 nM) | [48] | ||
| MSN@AChE | Dimethoate (6.5 nM) | [49] | ||
| Anti-OP Antibody | GNS@Anti-parathion Ab | Parathion (0.2 fM) | [50] | |
| OP Adsorption | rGO@Cu | Parathion, fenitrothion, malathion (3 nM) | [51] | |
| rGO@AuNP-polymer | Malathion (0.1 nM) | [52] | ||
| GNS@AuNP | Parathion methyl (2 nM) | [53] | ||
| OP Reaction | GO@AuNP-acetophenone oxime | Diethyl cyanophosphonate, dimethoate, fenitrothion | [54] | |
| Fluorescence (Luminescence) Spectroscopy | AChE Inhibition | Cd-Te QD | Paraoxon, GB, VX (0.1–8.0 nM) | [55] |
| OP Adsorption | CdTe QD | Chlorpyrifos (0.1 nM) | [56] | |
| ZnS-Mn QD | Diethyl phosphorothioate | [57] | ||
| Hf-doped MOF | Methylphosphonate | [58] | ||
| AuNP@Rhodamine | Ethoprophos (37 nM) | [59] | ||
| OP Reaction | CdS QD + Eosin Y | Chlorpyrifos (29 nM) | [60] | |
| UCN@Oxime probe | Dimethoate (0.14 μM) | [61] | ||
| Colorimetry & Spectrophotometry | AChE Inhibition | AuNR + AChE | Dichlorvos (45 fM) | [62] |
| OP Adsorption | AuNP, AgNP | Ethion, parathion | [63] | |
| AuNP@Rhodamine | Ethoprophos (37 nM) | [59] | ||
| Nano Ag@PVP | Chlorpyrifos (14 nM) | [64] |
| Nanomaterial | Design Feature | Tested OP | Function | Ref |
|---|---|---|---|---|
| Lipid based (micelle, liposome) | Oxime, HA presented | Fenitrothion | Catalytic inactivation | [120,134,135,136] |
| Phosphotriesterase encapsulated | POX | Catalytic inactivation | [139] | |
| 2-PAM encapsulated | POX | Rat brain delivery | [84,91] | |
| Polymer | Cu (II)-bipyridyl chelated | Parathion methyl | Catalytic inactivation | [141] |
| La (catecholate) chelated | POX, Nerve agents | Catalytic inactivation | [140] | |
| MSN | Unmodified | Dichlorvos | Adsorption; inactivation | [144] |
| MOF | UiO-66 | OP | Adsorption | [73] |
| UiO-66-NH2 | POX, VX, Soman | Catalytic inactivation | [146,147,148] | |
| OPAA immobilized | Soman | Catalytic inactivation | [117] | |
| Metal Oxide | CeO2 | POX | Catalytic inactivation | [149] |
| TiO2 | Parathion | Photocatalytic inactivation | [150] | |
| Ag-ZnO | Chlorpyrifos | Photocatalytic inactivation | [151] | |
| PAMAM dendrimer | Amino acid conjugated | Azhinophos methyl | Adsorption | [155] |
| Oxime/HA conjugated | POX, Malathion | Catalytic inactivation | [37,130] | |
| 2-PAM, atropine encapsulated | - | Extended drug release | [86,87,89] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.K. Nanomaterial-Enabled Sensors and Therapeutic Platforms for Reactive Organophosphates. Nanomaterials 2021, 11, 224. https://doi.org/10.3390/nano11010224
Choi SK. Nanomaterial-Enabled Sensors and Therapeutic Platforms for Reactive Organophosphates. Nanomaterials. 2021; 11(1):224. https://doi.org/10.3390/nano11010224
Chicago/Turabian StyleChoi, Seok Ki. 2021. "Nanomaterial-Enabled Sensors and Therapeutic Platforms for Reactive Organophosphates" Nanomaterials 11, no. 1: 224. https://doi.org/10.3390/nano11010224
APA StyleChoi, S. K. (2021). Nanomaterial-Enabled Sensors and Therapeutic Platforms for Reactive Organophosphates. Nanomaterials, 11(1), 224. https://doi.org/10.3390/nano11010224





