Bacterial Antifouling Characteristics of Helicene—Graphene Films
Abstract
:1. Introduction
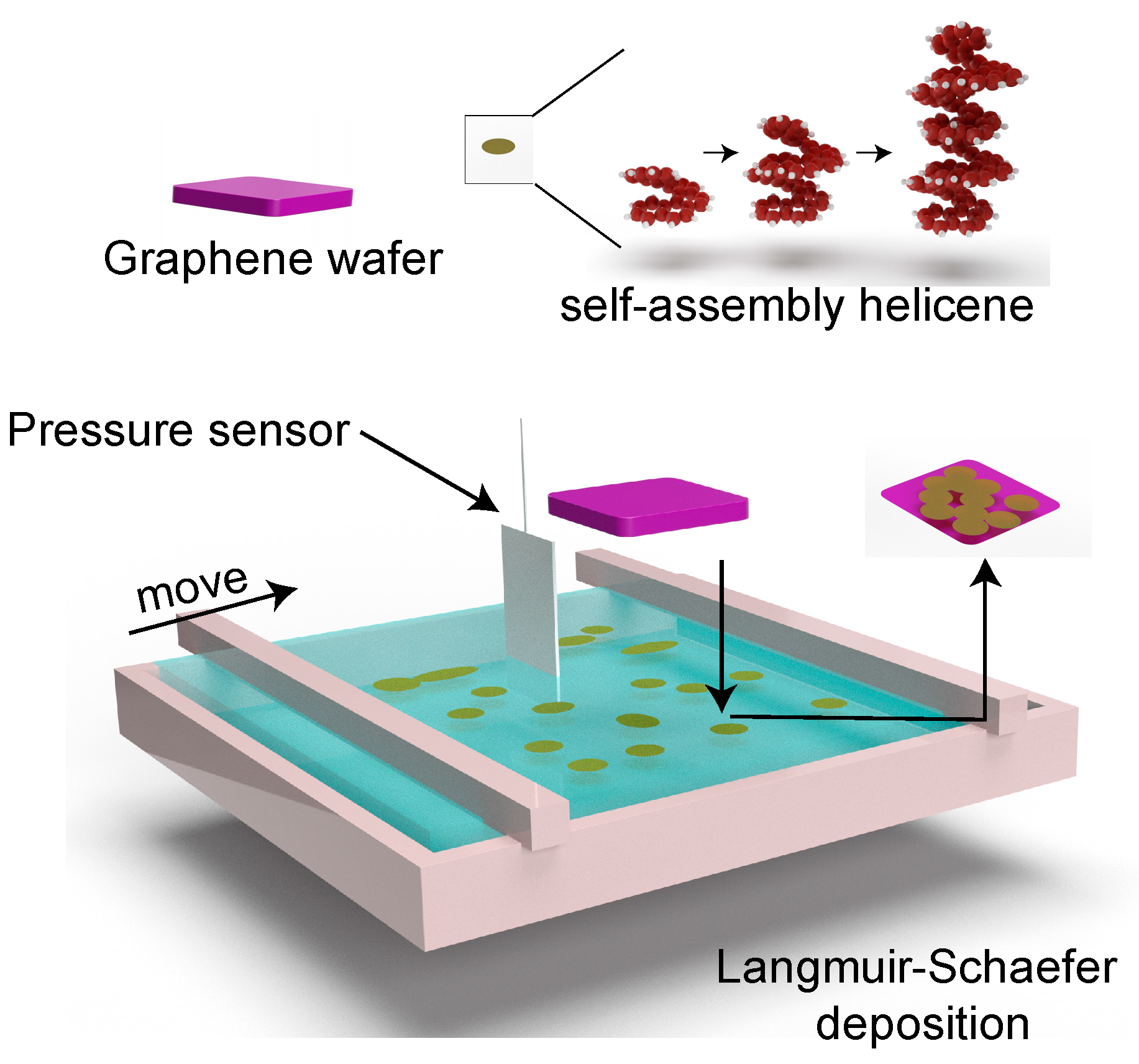
2. Methods and Experiments
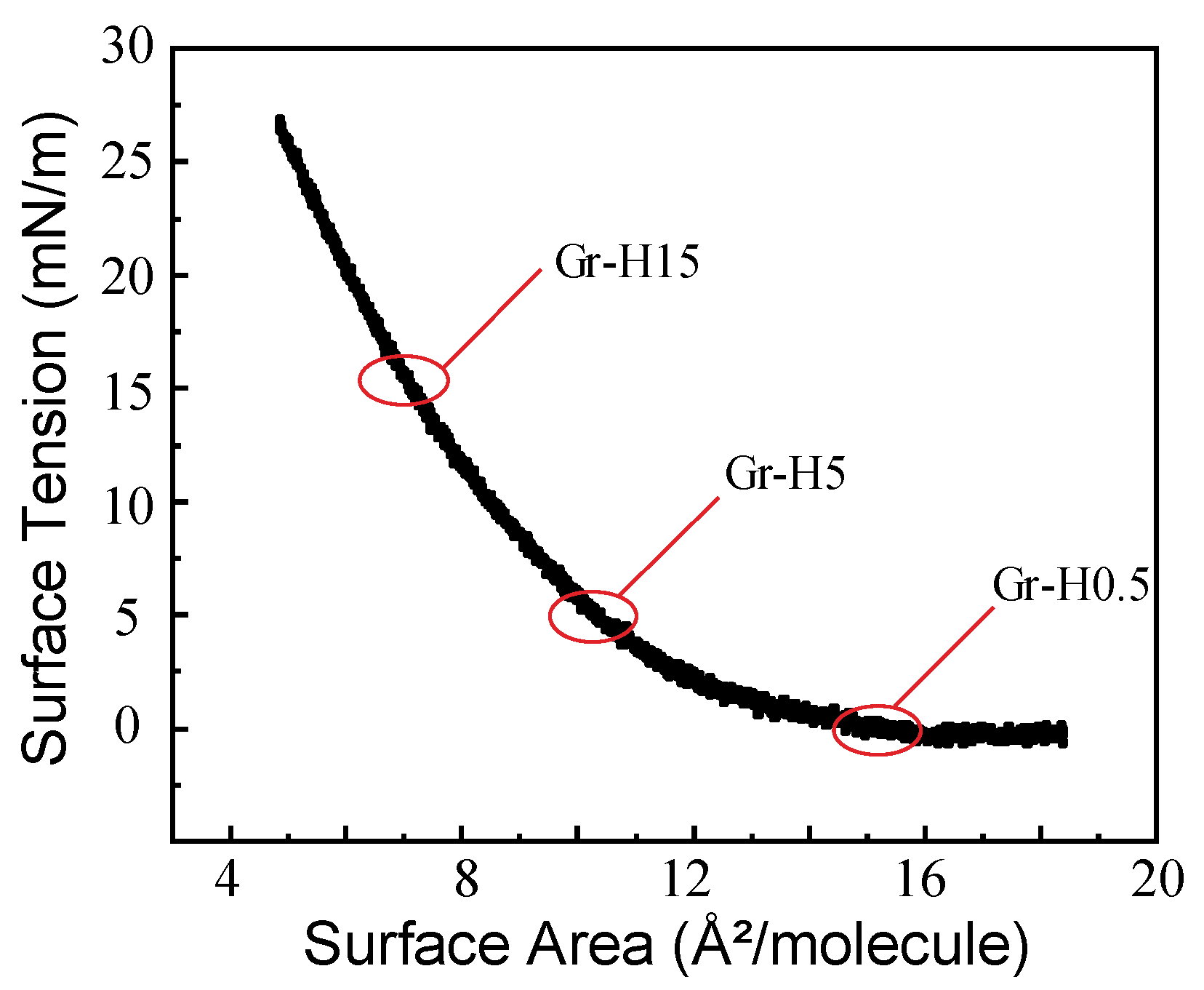
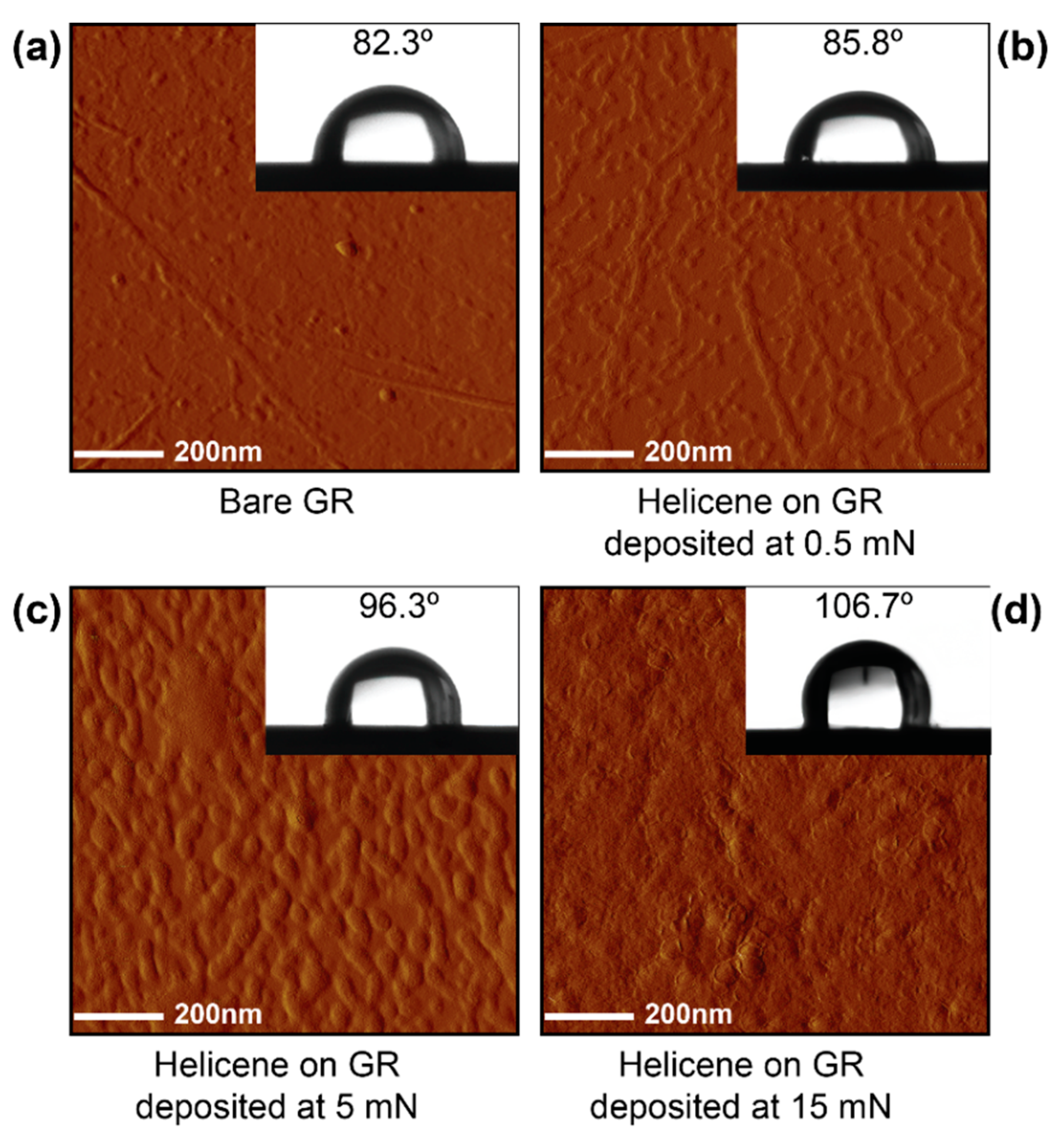
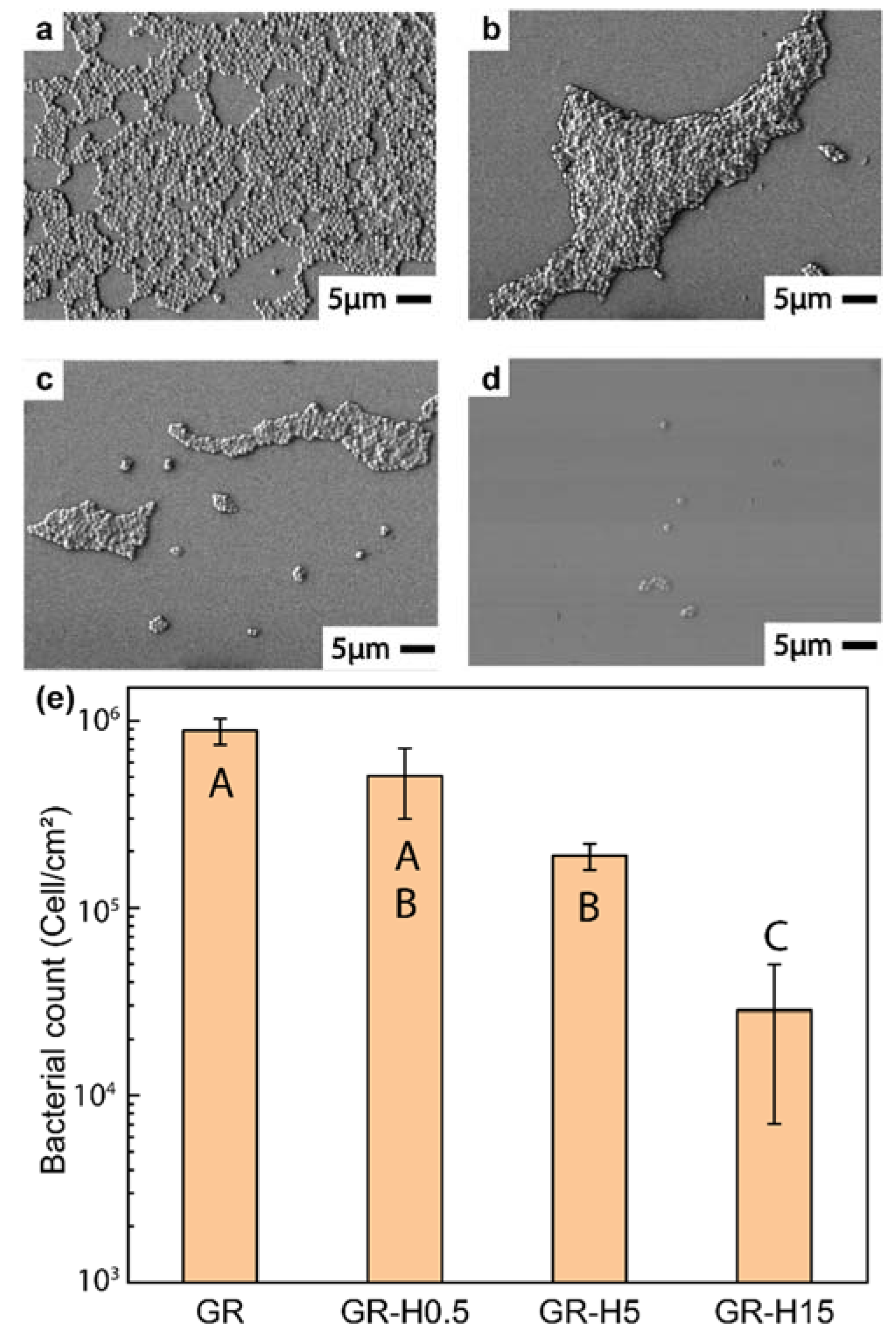
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Shen, Y.; Chen, C.-F. Helicenes: Synthesis and applications. Chem. Rev. 2012, 112, 1463–1535. [Google Scholar] [CrossRef]
- Gingras, M. One hundred years of helicene chemistry. Part 1: Non-stereoselective syntheses of carbohelicenes. Chem. Soc. Rev. 2013, 42, 968–1006. [Google Scholar] [CrossRef]
- Laarhoven, W.H.; Prinsen, W.J.C. Carbohelicenes and heterohelicenes. In Stereochemistry; Springer: Berlin/Heidelberg, Germany, 1984; pp. 63–130. [Google Scholar]
- Koetzner, L.; Webber, M.J.; Martinez, A.; De Fusco, C.; List, B. Asymmetric catalysis on the nanoscale: The organocatalytic approach to helicenes. Angew. Chemie Int. Ed. 2014, 53, 5202–5205. [Google Scholar] [CrossRef]
- Kiran, V.; Mathew, S.P.; Cohen, S.R.; Hernández Delgado, I.; Lacour, J.; Naaman, R. Helicenes—A new class of organic spin filter. Adv. Mater. 2016, 28, 1957–1962. [Google Scholar] [CrossRef] [PubMed]
- Coe, B.J.; Rusanova, D.; Joshi, V.D.; Sánchez, S.; Vávra, J.; Khobragade, D.; Severa, L.; Císařová, I.; Šaman, D.; Pohl, R. Helquat dyes: Helicene-like push–pull systems with large second-order nonlinear optical responses. J. Org. Chem. 2016, 81, 1912–1920. [Google Scholar] [CrossRef] [PubMed]
- Babič, A.; Pascal, S.; Duwald, R.; Moreau, D.; Lacour, J.; Allémann, E. [4]Helicene–Squalene Fluorescent Nanoassemblies for Specific Targeting of Mitochondria in Live-Cell Imaging. Adv. Funct. Mater. 2017, 27, 1701839. [Google Scholar] [CrossRef]
- Shchyrba, A.; Nguyen, M.-T.; Wäckerlin, C.; Martens, S.; Nowakowska, S.; Ivas, T.; Roose, J.; Nijs, T.; Boz, S.; Schär, M. Chirality transfer in 1D self-assemblies: Influence of H-bonding vs metal coordination between dicyano[7]helicene enantiomers. J. Am. Chem. Soc. 2013, 135, 15270–15273. [Google Scholar] [CrossRef] [Green Version]
- Nakakuki, Y.; Hirose, T.; Sotome, H.; Miyasaka, H.; Matsuda, K. Hexa-peri-hexabenzo[7]helicene: Homogeneously π-extended helicene as a primary substructure of helically twisted chiral graphenes. J. Am. Chem. Soc. 2018, 140, 4317–4326. [Google Scholar] [CrossRef]
- Fontana, F.; Melone, F.; Iannazzo, D.; Leonardi, S.G.; Neri, G. Synthesis, characterization and electrochemical properties of 5-aza[5]helicene-CH2O-CO-MWCNTs nanocomposite. Nanotechnology 2017, 28, 135501. [Google Scholar] [CrossRef]
- Feng, F.; Miyashita, T.; Okubo, H.; Yamaguchi, M. Spreading Behavior of Optically Active Macrocycloamides Consisting of Helical Chiral Units at the Air–Water Interface and the Formation of Langmuir-Blodgett Films. J. Am. Chem. Soc. 1998, 120, 10166–10170. [Google Scholar] [CrossRef]
- Karras, M.; Holec, J.; Bednárová, L.; Pohl, R.; Schmidt, B.; Stará, I.G.; Starý, I. Asymmetric synthesis of nonracemic 2-amino[6]helicenes and their self-assembly into langmuir films. J. Org. Chem. 2018, 83, 5523–5538. [Google Scholar] [CrossRef] [PubMed]
- Siltanen, M.; Vuorimaa, E.; Lemmetyinen, H.; Ihalainen, P.; Peltonen, J.; Kauranen, M. Nonlinear Optical and Structural Properties of Langmuir-Blodgett Films of Thiohelicenebisquinones. J. Phys. Chem. B 2008, 112, 1940–1945. [Google Scholar] [CrossRef] [PubMed]
- Holec, J.; Rybáček, J.; Vacek, J.; Karras, M.; Bednárová, L.; Buděšínský, M.; Slušná, M.; Holý, P.; Schmidt, B.; Stará, I.G. Chirality-Controlled Self-Assembly of Amphiphilic Dibenzo[6]helicenes into Langmuir–Blodgett Thin Films. Chem. Eur. J. 2019, 25, 11494–11502. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.K.; Yegin, Y.; Yang, F.; Zhang, M.; Li, J.; Huang, S.; Verkhoturov, S.V.; Schweikert, E.A.; Perez-Lewis, K.; Scholar, E.A.; et al. The influence of surface chemistry on the kinetics and thermodynamics of bacterial adhesion. Sci. Rep. 2018, 8, 17247. [Google Scholar] [CrossRef]
- Sun, D.; Babar Shahzad, M.; Li, M.; Wang, G.; Xu, D. Antimicrobial materials with medical applications. Mater. Technol. 2015, 30, B90–B95. [Google Scholar] [CrossRef]
- Delaviz, Y.; Santerre, J.P.; Cvitkovitch, D.G. Infection Resistant Biomaterials. In Biomaterials and Medical Device—Associated Infections; Woodhead Publishing: Oxford, UK, 2015; pp. 223–254. ISBN 9780857097224. [Google Scholar]
- Amin, M.; Rowley-Neale, S.; Shalamanova, L.; Lynch, S.; Wilson-Nieuwenhuis, J.T.; El Mohtadi, M.; Banks, C.E.; Whitehead, K.A. Molybdenum Disulfide Surfaces to Reduce Staphylococcus aureus and Pseudomonas aeruginosa Biofilm Formation. ACS Appl. Mater. Interfaces 2020, 12, 21057–21069. [Google Scholar] [CrossRef]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Bayer, A.; Cosgrove, S.E.; Daum, R.S.; Fridkin, S.K.; Gorwitz, R.J.; Kaplan, S.L.; Karchmer, A.W.; Levine, D.P.; Murray, B.E.; et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 2011, 52, e18–e55. [Google Scholar] [CrossRef]
- Kobayashi, S.D.; Malachowa, N.; Deleo, F.R. Pathogenesis of Staphylococcus aureus abscesses. Am. J. Pathol. 2015, 185, 1518–1527. [Google Scholar] [CrossRef] [Green Version]
- Thomer, L.; Schneewind, O.; Missiakas, D. Pathogenesis of Staphylococcus aureus Bloodstream Infections. Annu. Rev. Pathol. Mech. Dis. 2016, 11, 343–364. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Shi, H.; Yu, H.; Yan, S.; Luan, S. The recent advances in surface antibacterial strategies for biomedical catheters. Biomater. Sci. 2020, 8, 4095–4108. [Google Scholar] [CrossRef] [PubMed]
- Breyre, A.; Frazee, B.W. Skin and Soft Tissue Infections in the Emergency Department. Emerg. Med. Clin. N. Am. 2018, 36, 723–750. [Google Scholar] [CrossRef] [PubMed]
- van Hal, S.J.; Jensen, S.O.; Vaska, V.L.; Espedido, B.A.; Paterson, D.L.; Gosbell, I.B. Predictors of mortality in staphylococcus aureus bacteremia. Clin. Microbiol. Rev. 2012, 25, 362–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tleyjeh, I.M.; Abdel-Latif, A.; Rahbi, H.; Scott, C.G.; Bailey, K.R.; Steckelberg, J.M.; Wilson, W.R.; Baddour, L.M. A systematic review of population-based studies of infective endocarditis. Chest 2007, 132, 1025–1035. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Zheng, J.; Hao, L.; Yegin, Y.; Bae, M.; Ulugun, B.; Taylor, T.M.; Scholar, E.A.; Cisneros-Zevallos, L.; Oh, J.K. Dual-Functional, Superhydrophobic Coatings with Bacterial Anticontact and Antimicrobial Characteristics. ACS Appl. Mater. Interfaces 2020, 12, 21311–21321. [Google Scholar] [CrossRef]
- Encinas, N.; Yang, C.Y.; Geyer, F.; Kaltbeitzel, A.; Baumli, P.; Reinholz, J.; Mailänder, V.; Butt, H.J.; Vollmer, D. Submicrometer-Sized Roughness Suppresses Bacteria Adhesion. ACS Appl. Mater. Interfaces 2020, 12, 21192–21200. [Google Scholar] [CrossRef]
- Oh, J.K.; Perez, K.; Kohli, N.; Kara, V.; Li, J.; Min, Y.; Castillo, A.; Taylor, M.; Jayaraman, A.; Cisneros-Zevallos, L.; et al. Hydrophobically-modified silica aerogels: Novel food-contact surfaces with bacterial anti-adhesion properties. Food Control 2015, 52, 132–141. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Zhao, Y.Q.; Zhang, X.Y.; Ding, X.; Ding, X.; Yu, B.; Duan, S.; Xu, F.J. Self-adaptive antibacterial surfaces with bacterium-triggered antifouling-bactericidal switching properties. Biomater. Sci. 2020, 8. [Google Scholar] [CrossRef]
- Borjihan, Q.; Yang, J.; Song, Q.; Gao, L.; Xu, M.; Gao, T.; Liu, W.; Li, P.; Li, Q.; Dong, A. Povidone-iodine-functionalized fluorinated copolymers with dual-functional antibacterial and antifouling activities. Biomater. Sci. 2019, 7, 3334–3347. [Google Scholar] [CrossRef]
- Huang, D.; Wang, J.; Ren, K.; Ji, J. Functionalized biomaterials to combat biofilms. Biomater. Sci. 2020, 8, 4052–4066. [Google Scholar] [CrossRef]
- Devine, R.; Singha, P.; Handa, H. Versatile biomimetic medical device surface: Hydrophobin coated, nitric oxide-releasing polymer for antimicrobial and hemocompatible applications. Biomater. Sci. 2019, 7, 3438–3449. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wan, X.; Xiao, K.; Lin, W.; Li, J.; Li, Z.; Luo, F.; Tan, H.; Li, J.; Fu, Q. Anti-biofilm surfaces from mixed dopamine-modified polymer brushes: Synergistic role of cationic and zwitterionic chains to resist staphyloccocus aureus. Biomater. Sci. 2019, 7, 5369–5382. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhu, W.; Xin, B.; Lin, S.; Jin, L.; Wang, H. Development of an antibacterial surface with a self-defensive and pH-responsive function. Biomater. Sci. 2019, 7, 3795–3800. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Choi, W.T.; Johnson, C.T.; García, A.J.; Singh, P.M.; Breedveld, V.; Hess, D.W.; Champion, J.A. Inhibition of bacterial adhesion on nanotextured stainless steel 316L by electrochemical etching. ACS Biomater. Sci. Eng. 2018, 4, 90–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tripathy, A.; Sen, P.; Su, B.; Briscoe, W.H. Natural and bioinspired nanostructured bactericidal surfaces. Adv. Colloid Interface Sci. 2017, 248, 85–104. [Google Scholar] [CrossRef] [PubMed]
- Kouloumpis, A.; Thomou, E.; Chalmpes, N.; Dimos, K.; Spyrou, K.; Bourlinos, A.B.; Koutselas, I.; Gournis, D.; Rudolf, P. Graphene/carbon dot hybrid thin films prepared by a modified langmuir–schaefer method. ACS omega 2017, 2, 2090–2099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinicke, S.; Rees, H.C.; Espeel, P.; Vanparijs, N.; Bisterfeld, C.; Dick, M.; Rosencrantz, R.R.; Brezesinski, G.; de Geest, B.G.; Du Prez, F.E. Immobilization of 2-deoxy-d-ribose-5-phosphate aldolase in polymeric thin films via the Langmuir–Schaefer technique. ACS Appl. Mater. Interfaces 2017, 9, 8317–8326. [Google Scholar] [CrossRef] [Green Version]
- Rossos, A.K.; Katsiaflaka, M.; Cai, J.; Myers, S.M.; Koenig, E.; Bücker, R.; Keskin, S.; Kassier, G.; Gengler, R.Y.N.; Miller, R.J.D. Photochromism of Amphiphilic Dithienylethenes as Langmuir–Schaefer Films. Langmuir 2018, 34, 10905–10912. [Google Scholar] [CrossRef]
- Oh, J.K.; Lu, X.; Min, Y.; Cisneros-Zevallos, L.; Akbulut, M. Bacterially Antiadhesive, Optically Transparent Surfaces Inspired from Rice Leaves. ACS Appl. Mater. Interfaces 2015, 7. [Google Scholar] [CrossRef]
- Oh, J.K.J.K.; Rapisand, W.; Zhang, M.; Yegin, Y.; Min, Y.; Castillo, A.; Cisneros-Zevallos, L.; Akbulut, M. Surface modification of food processing and handling gloves for enhanced food safety and hygiene. J. Food Eng. 2016, 187, 82–91. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, G.E.G.; Morais, T.R.; Lago, J.H.G.; Caseli, L. Incorporation of polygodial in Langmuir films of selected lipids. Thin Solid Films 2019, 669, 19–28. [Google Scholar] [CrossRef]
- Berova, N.; Di Bari, L.; Pescitelli, G. Application of electronic circular dichroism in configurational and conformational analysis of organic compounds. Chem. Soc. Rev. 2007, 36, 914–931. [Google Scholar] [CrossRef] [PubMed]
- Islam, N.; Pandith, A.H. Optoelectronic and nonlinear optical properties of triarylamine helicenes: A DFT study. J. Mol. Model. 2014, 20, 2535. [Google Scholar] [CrossRef] [PubMed]
- Belman, N.; Jin, K.; Golan, Y.; Israelachvili, J.N.; Pesika, N.S. Origin of the contact angle hysteresis of water on chemisorbed and physisorbed self-assembled monolayers. Langmuir 2012, 28, 14609–14617. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.K.; Kohli, N.; Zhang, Y.; Min, Y.; Jayaraman, A.; Cisneros-Zevallos, L.; Akbulut, M. Nanoporous aerogel as a bacteria repelling hygienic material for healthcare environment. Nanotechnology 2016, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Absolom, D.R.; Lamberti, F.V.; Policova, Z.; Zingg, W.; van Oss, C.J.; Neumann, A.W. Surface thermodynamics of bacterial adhesion. Appl. Environ. Microbiol. 1983, 46, 90–97. [Google Scholar] [CrossRef] [Green Version]
- Rijnaarts, H.H.M.; Norde, W.; Bouwer, E.J.; Lyklema, J.; Zehnder, A.J.B. Bacterial adhesion under static and dynamic conditions. Appl. Environ. Microbiol. 1993, 59, 3255–3265. [Google Scholar] [CrossRef] [Green Version]
- Parreira, P.; Magalhães, A.; Gonçalves, I.C.; Gomes, J.; Vidal, R.; Reis, C.A.; Leckband, D.E.; Martins, M.C.L. Effect of surface chemistry on bacterial adhesion, viability, and morphology. J. Biomed. Mater. Res. Part A 2011, 99, 344–353. [Google Scholar] [CrossRef]
- Cunliffe, D.; Smart, C.A.; Alexander, C.; Vulfson, E.N. Bacterial adhesion at synthetic surfaces. Appl. Environ. Microbiol. 1999, 65, 4995–5002. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Wang, L.; Levänen, E. Superhydrophobic surfaces for the reduction of bacterial adhesion. RSC Adv. 2013, 3, 12003–12020. [Google Scholar] [CrossRef]
- Jenkins, J.; Mantell, J.; Neal, C.; Gholinia, A.; Verkade, P.; Nobbs, A.H.; Su, B. Antibacterial effects of nanopillar surfaces are mediated by cell impedance, penetration and induction of oxidative stress. Nat. Commun. 2020, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.; Akbulut, M.; Kristiansen, K.; Golan, Y.; Israelachvili, J. The role of interparticle and external forces in nanoparticle assembly. Nat. Mater. 2008, 7, 527–538. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Bae, M.; Hao, L.; Oh, J.K.; White, A.R.; Min, Y.; Cisneros-Zevallos, L.; Akbulut, M. Bacterial Antifouling Characteristics of Helicene—Graphene Films. Nanomaterials 2021, 11, 89. https://doi.org/10.3390/nano11010089
Liu S, Bae M, Hao L, Oh JK, White AR, Min Y, Cisneros-Zevallos L, Akbulut M. Bacterial Antifouling Characteristics of Helicene—Graphene Films. Nanomaterials. 2021; 11(1):89. https://doi.org/10.3390/nano11010089
Chicago/Turabian StyleLiu, Shuhao, Michael Bae, Li Hao, Jun Kyun Oh, Andrew R. White, Younjin Min, Luis Cisneros-Zevallos, and Mustafa Akbulut. 2021. "Bacterial Antifouling Characteristics of Helicene—Graphene Films" Nanomaterials 11, no. 1: 89. https://doi.org/10.3390/nano11010089





