The Design, Characterization and Antibacterial Activity of Heat and Silver Crosslinked Poly(Vinyl Alcohol) Hydrogel Forming Dressings Containing Silver Nanoparticles
Abstract
:1. Introduction
2. Materials
3. Methods
3.1. Film Preparation
3.2. Film Swelling Studies
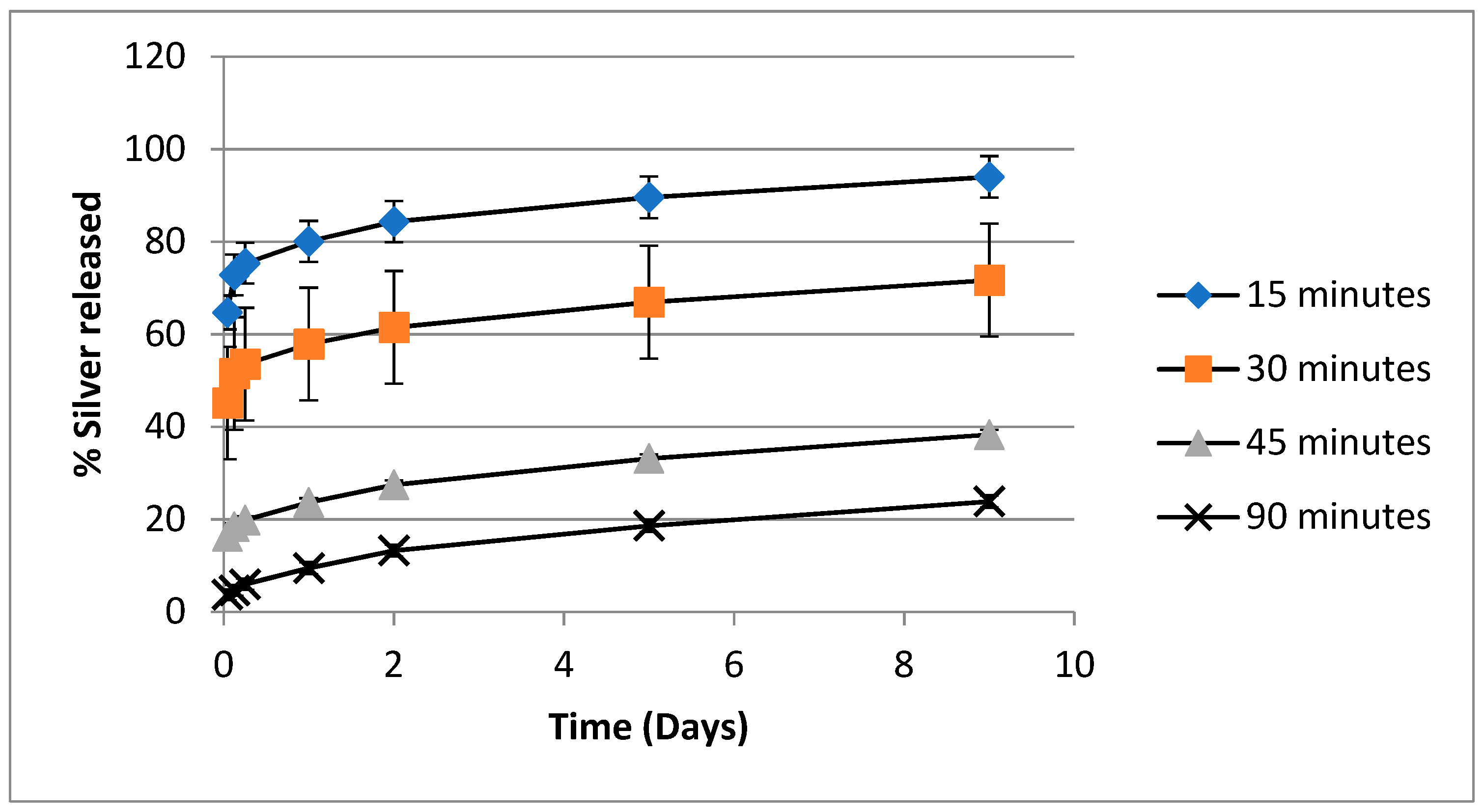
3.3. Silver Release Studies and Characterization
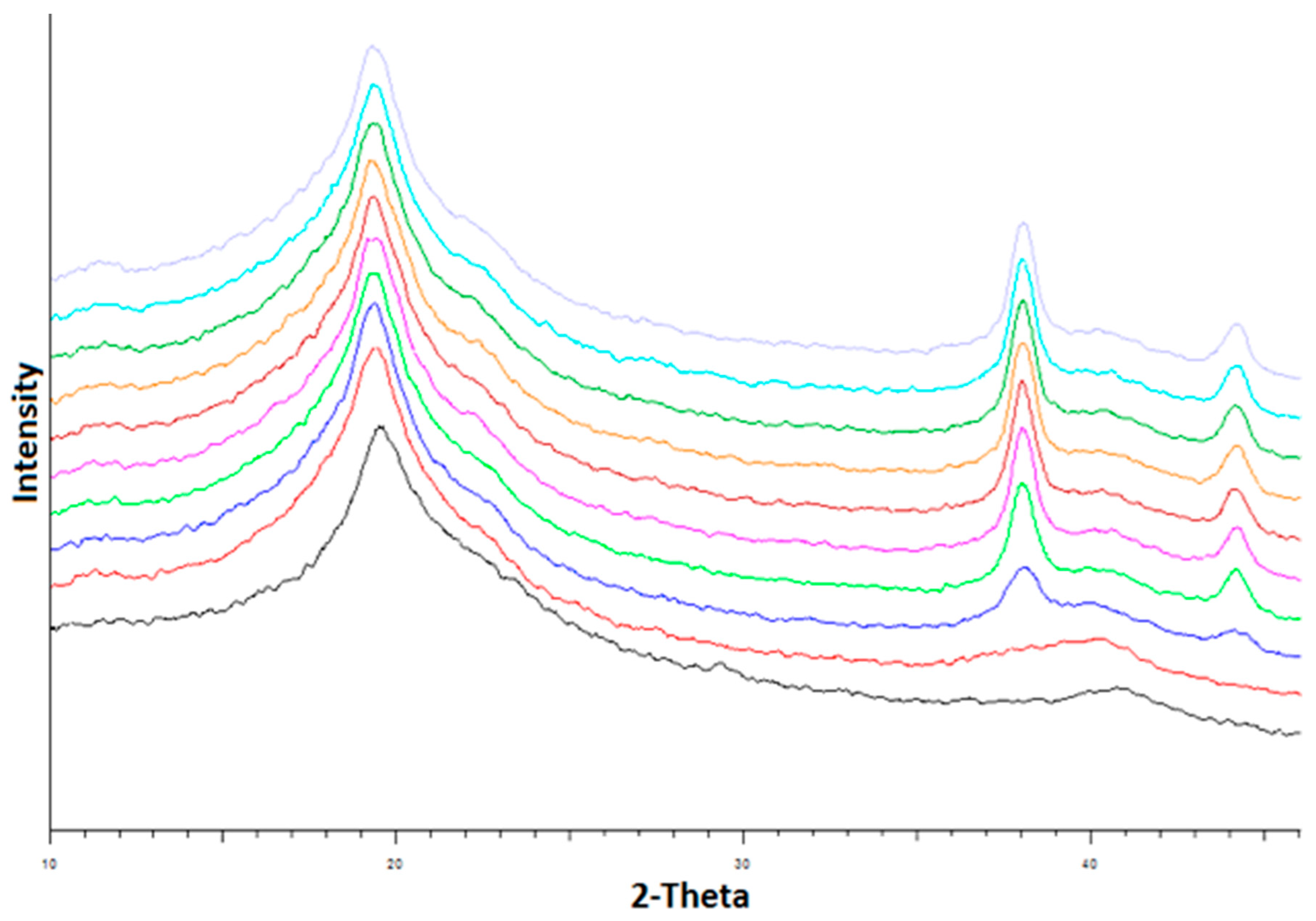
3.4. X-ray Diffraction of PVA-Silver Films
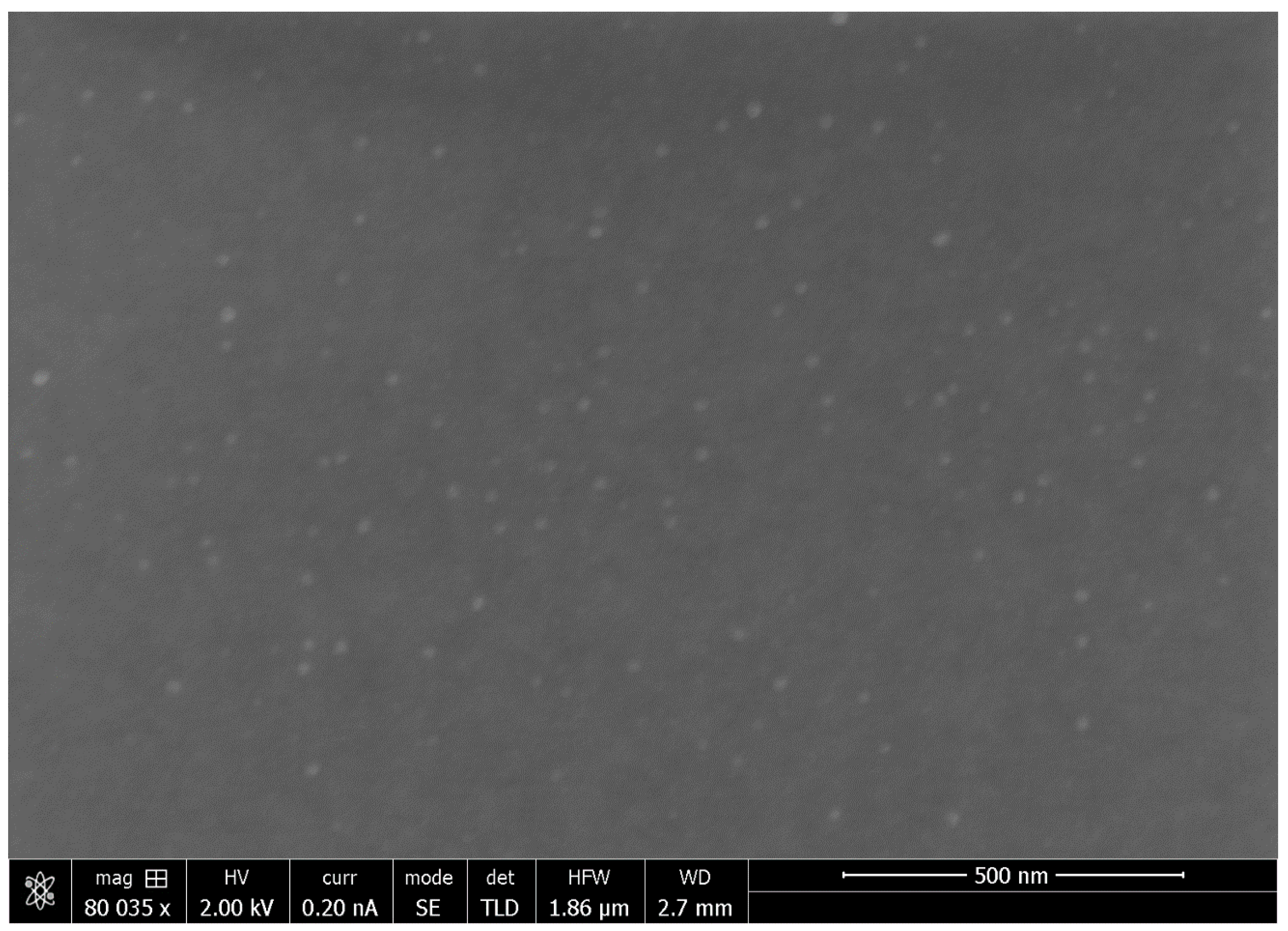
3.5. Scanning Electron Microscopy (SEM)
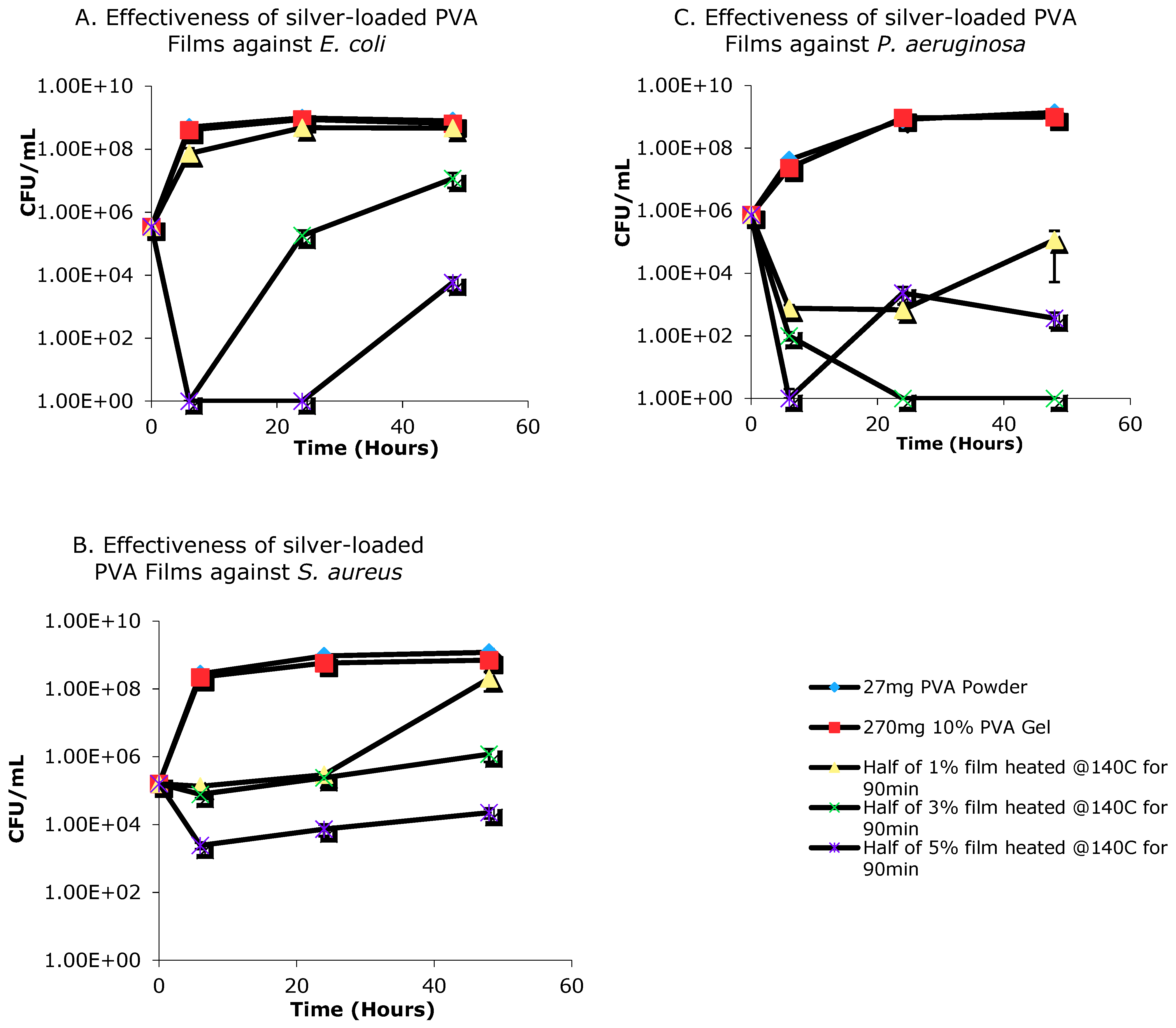
3.6. Antimicrobial Activity of Silver-Loaded PVA Films
3.7. Antimicrobial Efficacy of Aqueous Release Media from PVA-Silver Films
4. Results and Discussion
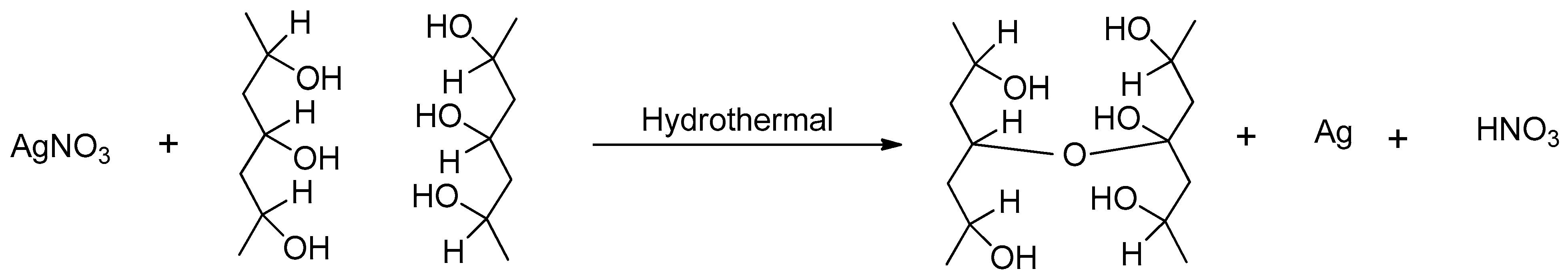
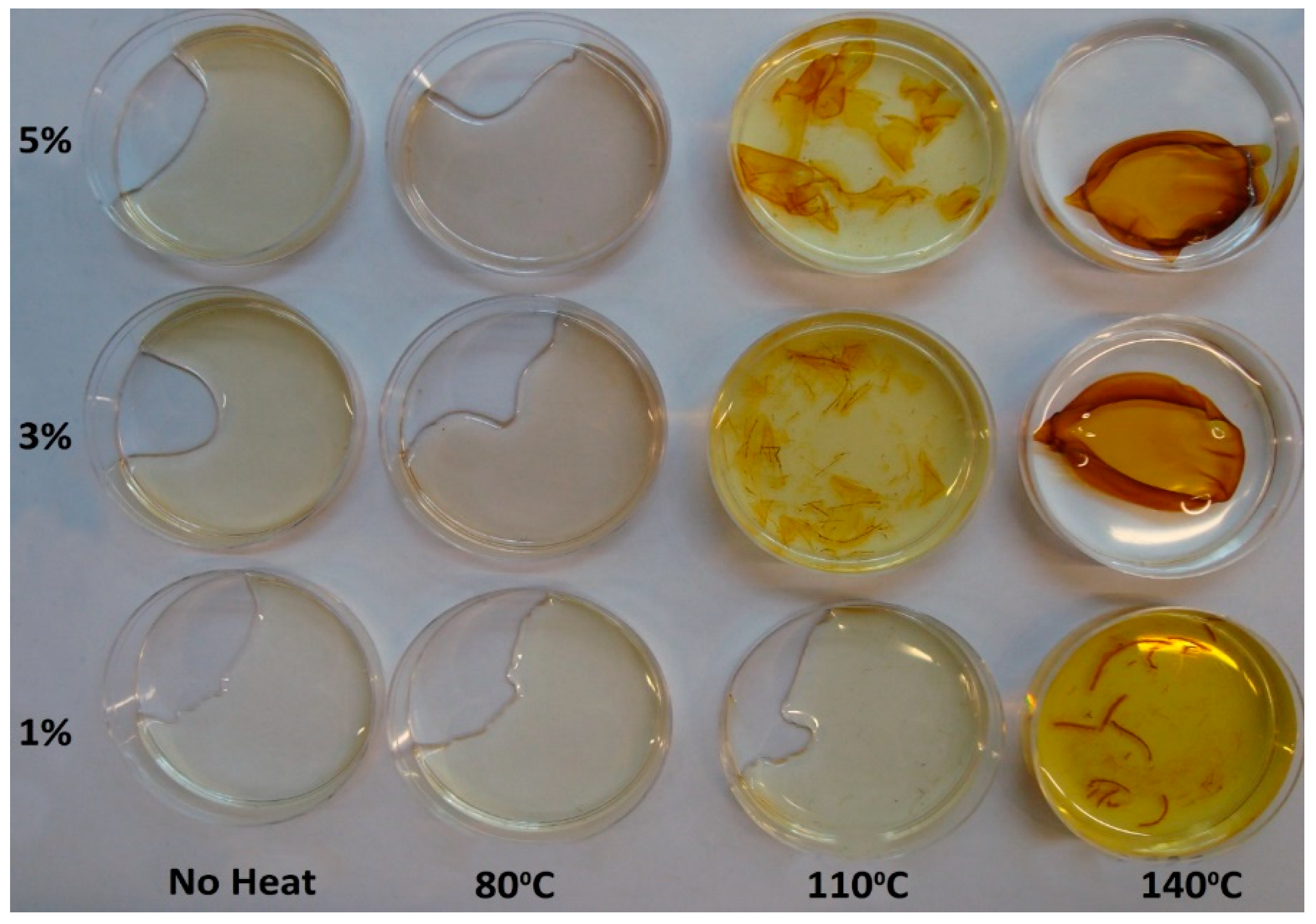
4.1. Appearance of PVA-Silver Films
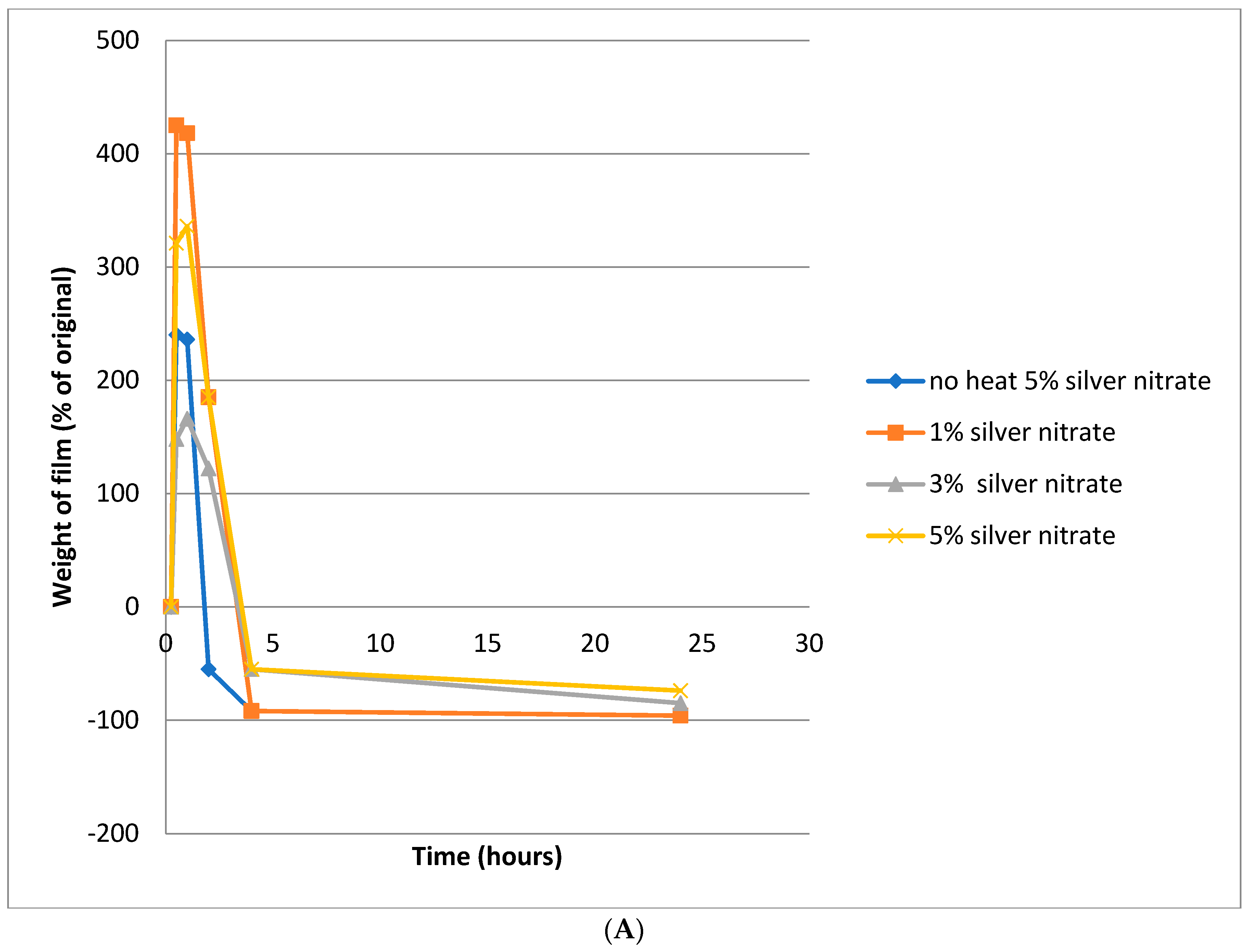
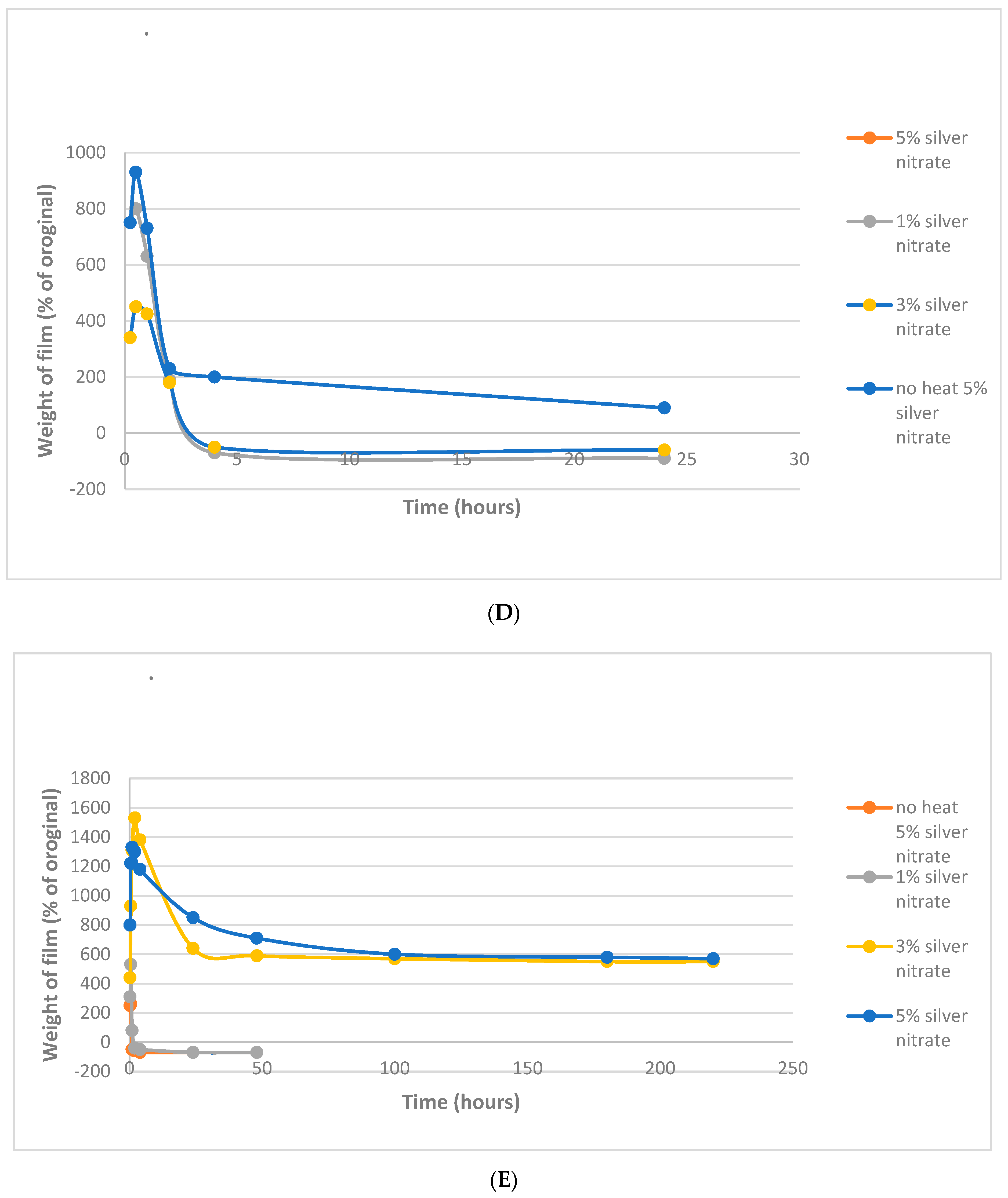
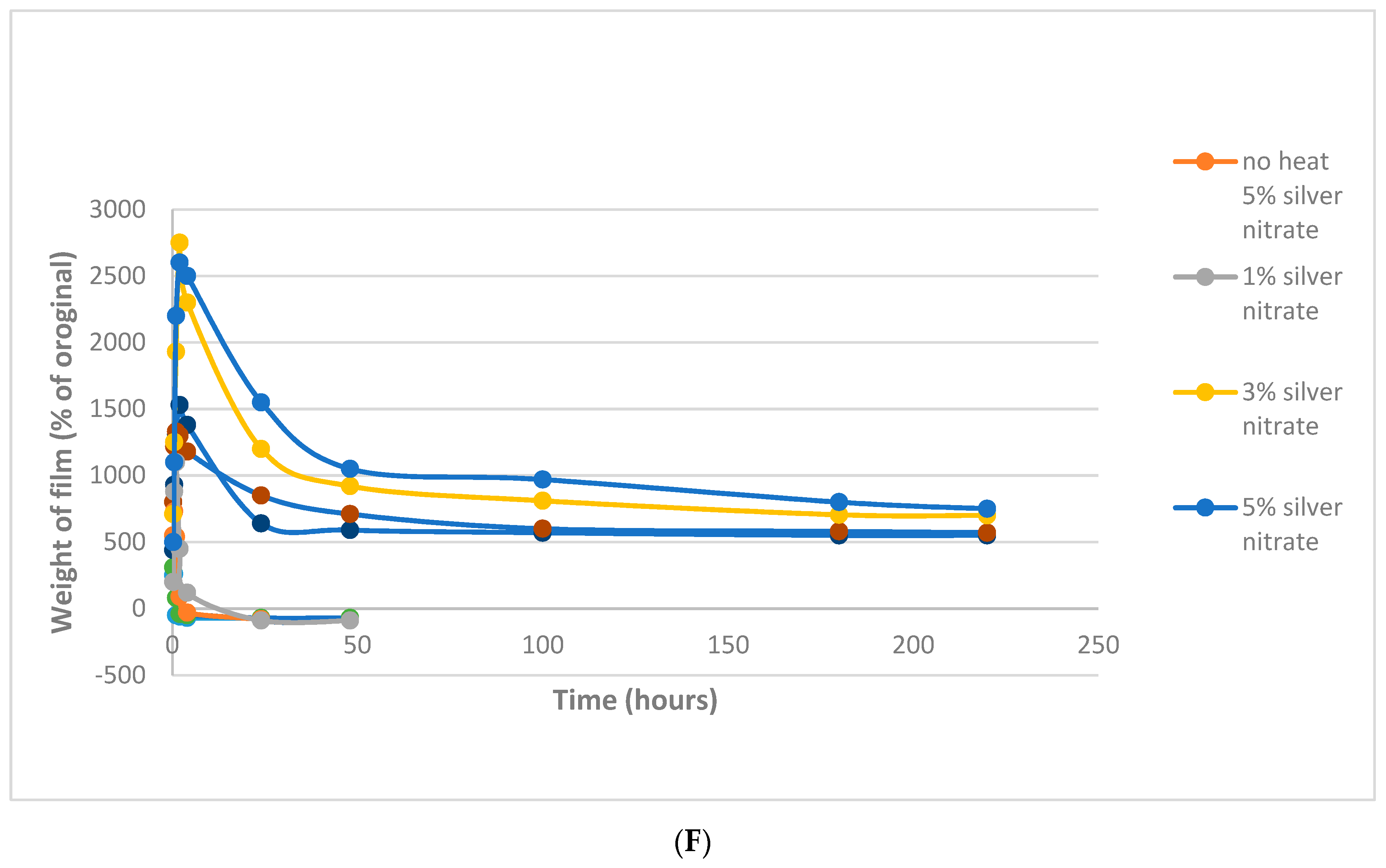
4.2. Film Swelling
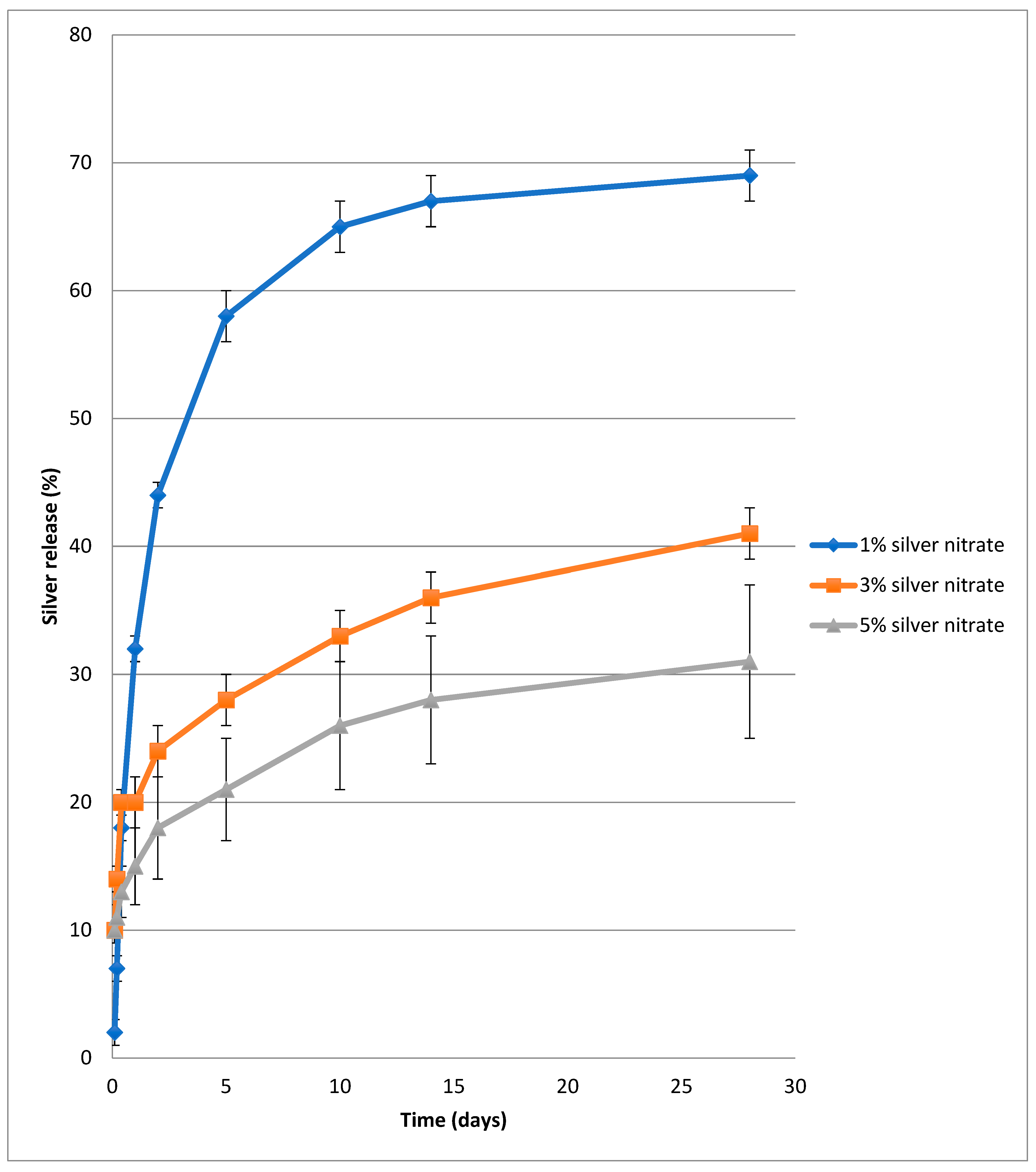
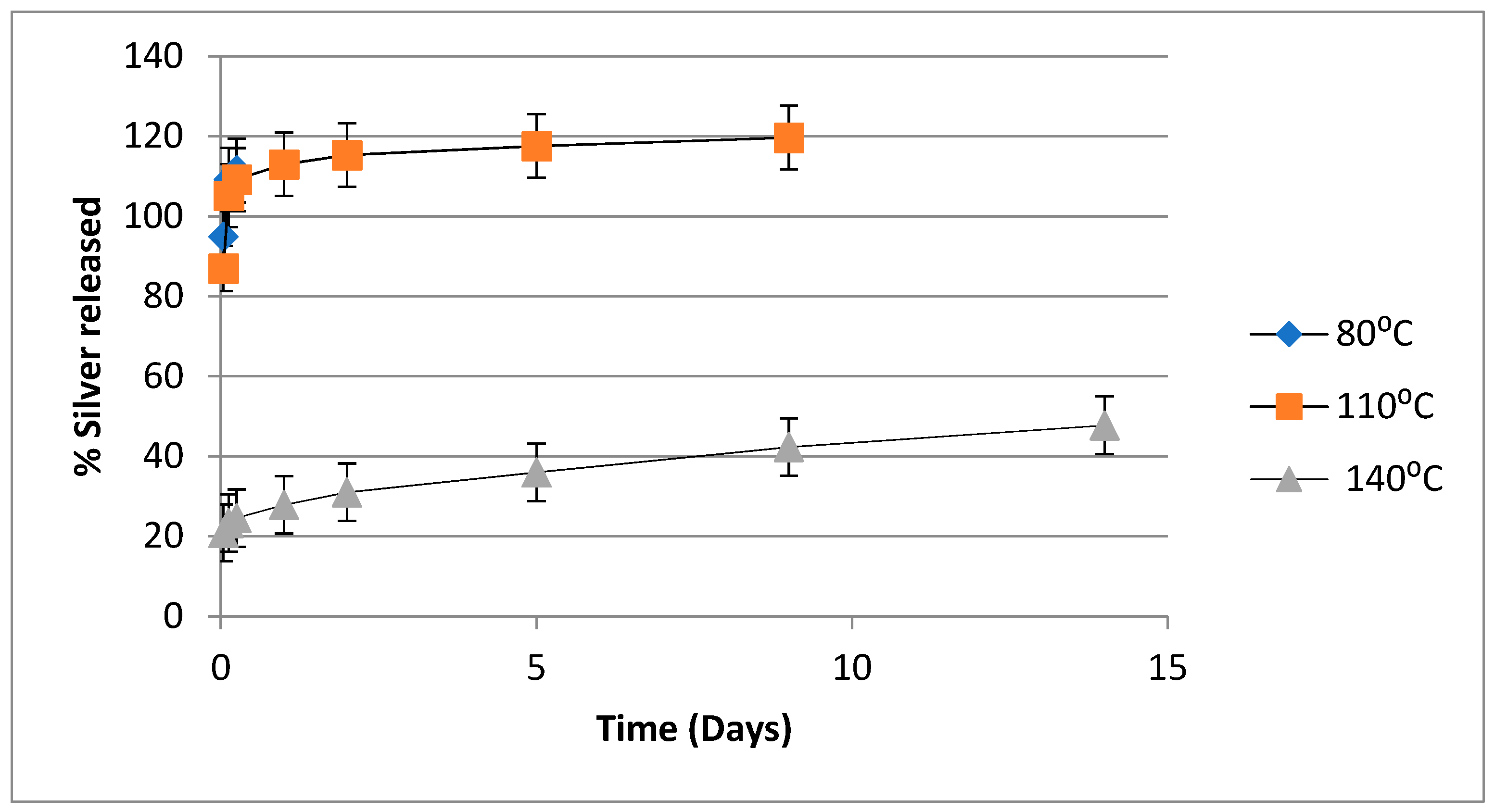
4.3. Release of Silver from PVA-Silver Films
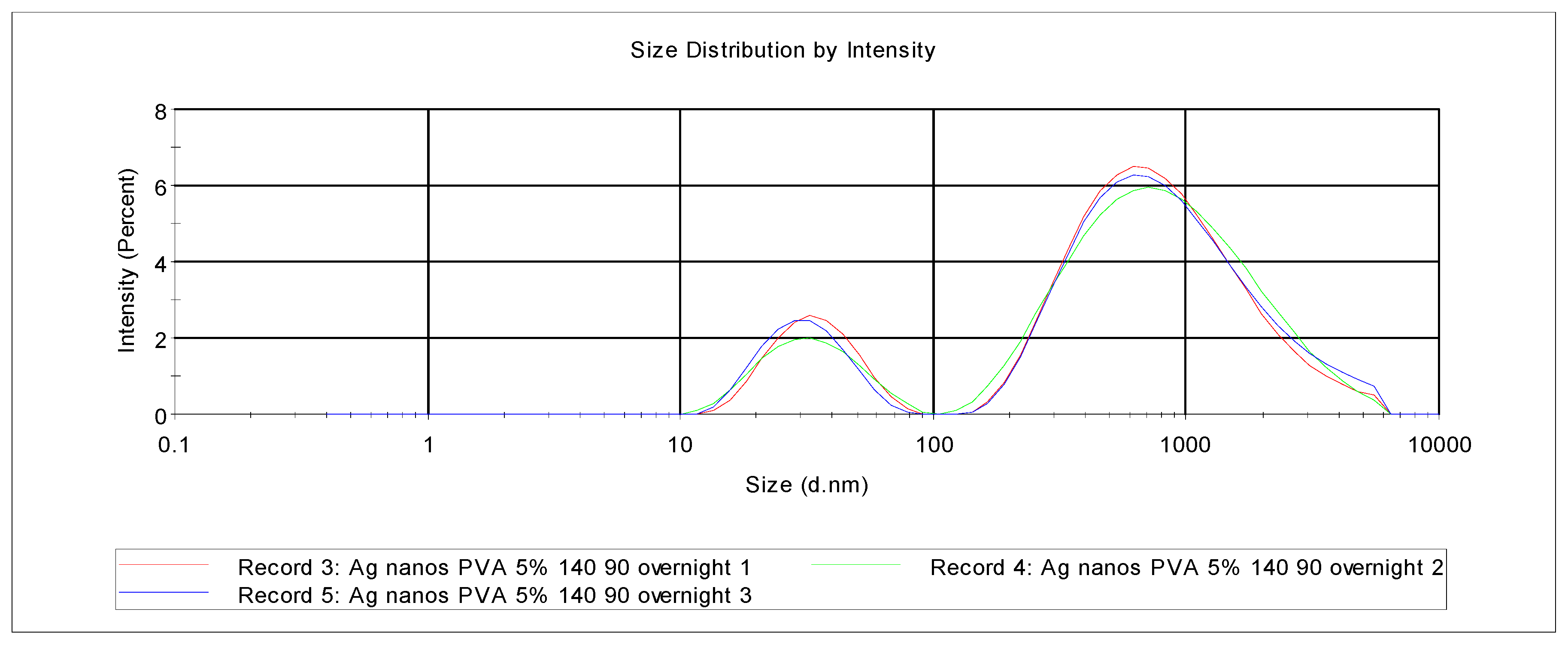
4.4. XRD and SEM Studies of PVA-Silver Films
4.5. Antimicrobial Activity of Silver-Loaded PVA Films
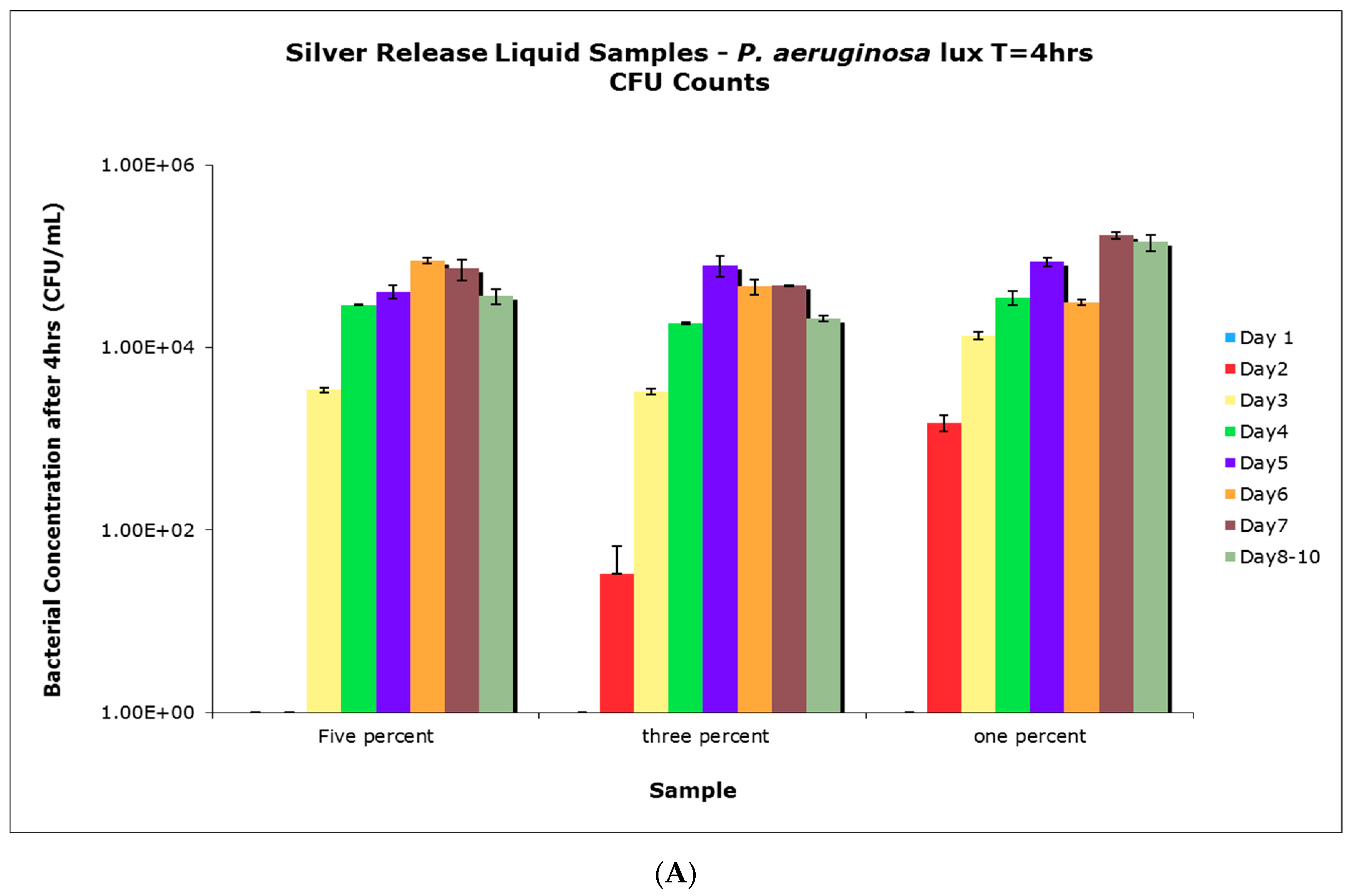
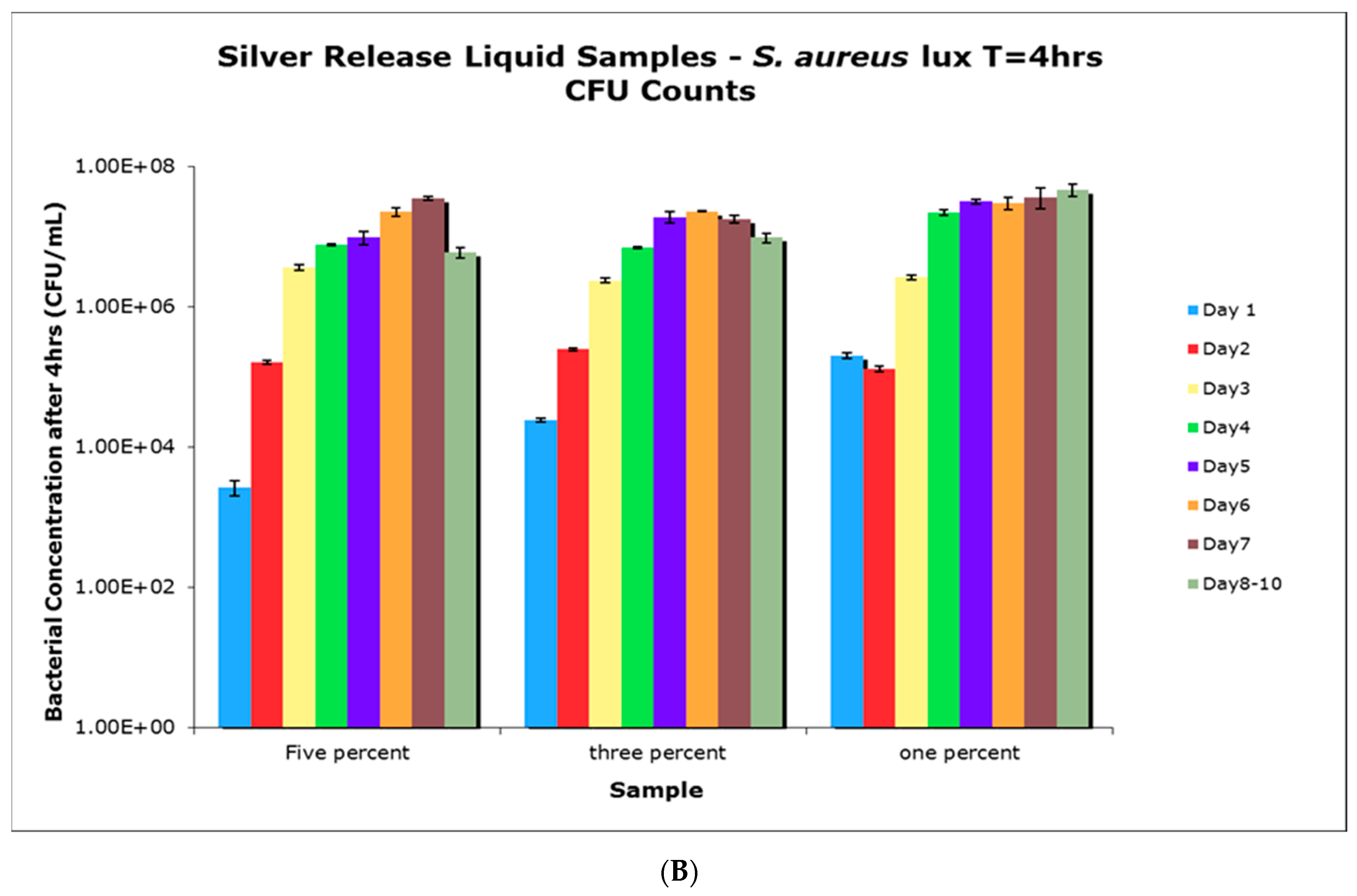
4.6. Antimicrobial Activity of Aqueous Release Media from Silver—Loaded PVA Films
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Albertyn, R.; Bickler, S.; Rode, H. Paediatric burn injuries in Sub Saharan Africa—An overview. Burns 2006, 32, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Rayner, R.; Prentice, J. Paediatric burns: A brief global review. Wound Pract. Res. 2011, 19, 39–46. [Google Scholar]
- Klasen, H.J. A historical review of the use of silver in the treatment of burns. Part 1 early uses. Burns 2000, 30, 1–9. [Google Scholar]
- Heggers, J.P.; Richard, J.W.; Spencer, B.A.; McCoy, L.F.; Carina, E.; Washington, J.; Edgar, P.; Rosenblatt, J.; Goodheart, R. Acticoat versus silver ion: The truth. J. Burn Care Rehabil. 2002, 23, S115. [Google Scholar] [CrossRef]
- Castellano, J.J.; Shafii, S.M.; Ko, F.; Donate, G.; Wright, T.E.; Mannari, R.J.; Payne, W.G.; Smith, D.J.; Robson, M.C. Comparative evaluation of silver-containing antimicrobial dressings and drugs. Int. Wound J. 2007, 4, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Khundkar, R.; Malic, C.; Burge, T. Use of Acticoat™ dressings in burns: What is the evidence? Burns 2010, 36, 751–758. [Google Scholar] [CrossRef]
- Barnea, Y.; Weiss, J.; Gur, E. A review of the clinical applications of the hydrofiber dressing with silver (Aquacel Ag®) in wound care. Ther. Clin. Risk Manag. 2010, 6, 21–27. [Google Scholar]
- Kobayashi, M.; Hyu, H.S. Development and evaluation of polyvinyl alcohol-hydrogels as an artificial articular cartilage for orthopedic implants. Materials 2010, 3, 2753–2771. [Google Scholar] [CrossRef] [Green Version]
- Baker, M.I.; Walsh, S.P.; Schwartz, Z.; Boyan, B.D. A review of polyvinyl alcohol and its use in cartilage and orthopedic applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 1451–1457. [Google Scholar] [CrossRef]
- Manna, U.; Patil, S. Borax mediated layer-by-layer self-assembly of neutral poly(vinyl alcohol) and chitosan. J. Phys. Chem. B 2009, 113, 9137–9142. [Google Scholar] [CrossRef]
- Luo, L.-B.; Yu, S.-H.; Qian, H.; Zhou, T. Large-Scale fabrication of flexible silver/cross-linked poly(vinyl alcohol) coaxial nanocables by a facile solution approach. JACS Commun. 2005, 127, 2822–2823. [Google Scholar] [CrossRef] [PubMed]
- Marambio-Jones, C.; Hoek, E.M.V. A review of the antibacterial effect of silver nanomaterials and potential implications for human health and the environment. J. Nanopart. Res. 2010, 12, 1531. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Yngard, R.A.; Lin, Y. Silver nanoparticles: Green synthesis and their antimicrobial activities. Adv. Colloid Interface Sci. 2009, 145, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Xiu, Z.M.; Zhang, Q.B.; Puppala, H.L.; Colvin, V.L.; Alaverez, P.J.J. Negligible particle-specific antibacterial activity of silver nanoparticles. Nano Lett. 2012, 12, 4271–4275. [Google Scholar] [CrossRef]
- Cencetti, C.; Bellini, D.; Pavesio, A.; Senigaglia, D.; Passariello, C.; Virga, A.; Matricardi, P. Preparation and characterization of antimicrobial wound dressings based on silver, gellan, PVA and borax. Carbohydr. Polym. 2012, 90, 1362–1370. [Google Scholar] [CrossRef]
- Herron, M.; Agarwal, A.; Kierski, P.R.; Calderon, D.F.; Teixeira, L.B.C.; Schurr, M.J.; Murphy, C.J.; Czuprynski, C.J.; McAnulty, J.F.; Abbott, N.L. Reduction in wound bioburden using a silver-loaded dissolvable film construct. Adv. Healthcare Mater. 2014, 3, 916–928. [Google Scholar] [CrossRef]
- Oliveira, R.N.; Rouzé, R.; Quilty, B.; Alves, G.G.; Soares, G.D.A.; Thiré, R.M.S.M.; McGuinness, G.B. Mechanical properties and in vitro characterization of polyvinyl alcohol-nano-silver hydrogel wound dressings. Interface Focus 2014, 4, 20130049. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, K.H. Synthesis, characterization, optical and antimicrobial studies of polyvinyl alcohol-silver nanocomposites. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 138, 434–440. [Google Scholar] [CrossRef]
- Bhowmick, S.; Koul, V. Assessment of PVA/silver nanocomposite hydrogel patch as antimicrobial dressing scaffold: Synthesis, characterization and biological evaluation. Mater. Sci. Eng. C 2016, 59, 109–119. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, Y.; Wang, Z.; Zhang, X.; Zhao, Y.; Sun, L. Electrospinning of Ag Nanowires/polyvinyl alcohol hybrid nanofibers for their antibacterial properties. Mater. Sci. Eng. C 2017, 78, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Longenberger, L.; Mills, G. Formation of metal particles in aqueous solutions by reactions of metal complexes with polymers. J. Phys. Chem. 1995, 99, 475–478. [Google Scholar] [CrossRef]
- Porel, S.; Singh, S.; Harsha, S.S.; Rao, D.N.; Radhakrishnan, T.P. Nanoparticle-Embedded polymer: In situ synthesis, free-standing films with highly monodisperse silver nanoparticles and optical limiting. Chem. Mater. 2005, 17, 9–12. [Google Scholar] [CrossRef]
- Khanna, P.; Singh, N.; Charan, S.; Subbarao, V.; Gokhale, R.; Mulik, U. Synthesis and characterization of Ag/PVA nanocomposite by chemical reduction method. Mater. Chem. Phys. 2005, 93, 117–121. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 10, 2346–2353. [Google Scholar] [CrossRef] [Green Version]
- Kanmani, P.; Rhim, J.W. Physicochemical properties of gelatin/silver nanoparticle antimicrobial composite films. Food Chem. 2014, 148, 162–169. [Google Scholar] [CrossRef]
- Sadanand, V.; Rajini, N.; Satyanarayana, B.; Varada Rajulu, A. Preparation and properties of cellulose/silver na noparticle composites with in situ-generated silver nanoparticles using Ocimum sanctum leaf extract. Int. J. Polym. Anal. Charact. 2016, 21, 408–416. [Google Scholar] [CrossRef]
- Tankhiwale, R.; Bajpai, S.K. Silver-Nanoparticle-Loaded chitosan lactate films with fair antibacterial properties. J. Appl. Polym. Sci. 2010, 115, 1894–1900. [Google Scholar] [CrossRef]
- Rhim, J.W.; Wang, L.F.; Hong, S.I. Preparation and characterization of agar/silver nanoparticles composite films with antimicrobial activity. Food Hydrocoll. 2013, 13, 327–335. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J.W.; Won, K. Preparation of poly (lactide) lignin/silver nanoparticles composite films with UV light barrier and antibacterial properties. Int. J. Biol. Macromol. 2018, 107, 1724–1731. [Google Scholar] [CrossRef]
- Gilchrist, E.; Lange, D.; Letchford, K.; Bach, H.; Fazli, L.; Burt, H.M. Fusidic acid and rifampicin co-loaded PLGA nanofibers for the prevention of orthopedic implant associated infections. J. Cont. Release 2013, 170, 64–73. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jackson, J.; Burt, H.; Lange, D.; Whang, I.; Evans, R.; Plackett, D. The Design, Characterization and Antibacterial Activity of Heat and Silver Crosslinked Poly(Vinyl Alcohol) Hydrogel Forming Dressings Containing Silver Nanoparticles. Nanomaterials 2021, 11, 96. https://doi.org/10.3390/nano11010096
Jackson J, Burt H, Lange D, Whang I, Evans R, Plackett D. The Design, Characterization and Antibacterial Activity of Heat and Silver Crosslinked Poly(Vinyl Alcohol) Hydrogel Forming Dressings Containing Silver Nanoparticles. Nanomaterials. 2021; 11(1):96. https://doi.org/10.3390/nano11010096
Chicago/Turabian StyleJackson, John, Helen Burt, Dirk Lange, In Whang, Robin Evans, and David Plackett. 2021. "The Design, Characterization and Antibacterial Activity of Heat and Silver Crosslinked Poly(Vinyl Alcohol) Hydrogel Forming Dressings Containing Silver Nanoparticles" Nanomaterials 11, no. 1: 96. https://doi.org/10.3390/nano11010096




