Growth Mechanism of Micro/Nano Metal Dendrites and Cumulative Strategies for Countering Its Impacts in Metal Ion Batteries: A Review †
Abstract
:1. Introduction


2. Battery Insights—Material Perspectives
2.1. Materials for Li-Based Batteries
2.2. Materials for Na-Based Batteries
2.3. Materials for K-Based Batteries
3. Mechanism of Dendrite Formation
3.1. Dendrite Growth Theories
3.2. SEI Formation and Detrimental Effects of Dendrite Growth
3.3. Influential Factors for Dendrite Growth
3.4. Influence of Interfacial Viscosity and Crystal Solidification
4. Analytical Tools for SEI and Dendrite Assessment
5. Design Strategies Based on Growth Mechanism and Theoretical Models
5.1. Control of Pressure and Temperature
5.2. Current Density
5.3. Electrolyte Design
5.4. Electrode and Interphase Modification
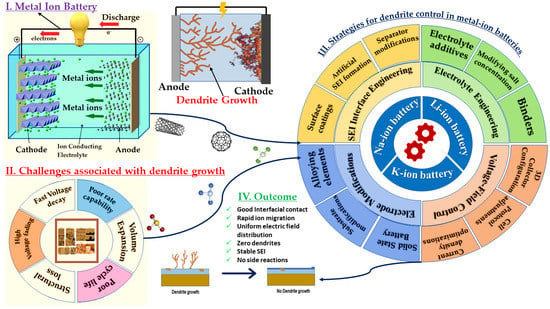
6. Dendrite Control Strategies
6.1. Strategies for LIB Design and Development with Dendrite-Free Structures
6.1.1. Electrolyte Solvent Modifications
6.1.2. Electrolyte Additives
6.1.3. Design of Stable Electrode–Electrolyte Structures
6.1.4. Interfacial Formulations with Artificial Films
6.1.5. Other Novel Methodologies
6.2. Dendrite Control in Na-Based Batteries
6.2.1. Electrolyte Design and Optimization
6.2.2. Binders and Additives for Electrolyte
6.2.3. Modifying Other Components
6.2.4. Interfacial Layer Alteration
6.2.5. Recent Approaches to Na Dendrite Suppression
6.3. K-Based Batteries
6.3.1. Altering the Salt and Design Chemistry
6.3.2. Optimizing Solvent Formulations
6.3.3. Use of Nanomaterials
6.3.4. Interfacial Modifications
6.3.5. New Methods for K Dendrite Blockage
6.4. Other Batteries (Mg-, Zn-, Ca-, and Al-Based)
7. Emerging Concepts
8. Computational Designs and Simulation
9. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alireza Hashemi, S.; Mojtaba Mousavi, S.; Reza Naderi, H.; Bahrani, S.; Arjmand, M.; Hagfeldt, A.; Chiang, W.H.; Ramakrishna, S. Reinforced polypyrrole with 2D graphene flakes decorated with interconnected nickel-tungsten metal oxide complex toward superiorly stable supercapacitor. Chem. Eng. J. 2021, 418, 129396. [Google Scholar] [CrossRef]
- Dincer, I.; Acar, C. Smart energy systems for a sustainable future. Appl. Energy 2017, 194, 225–235. [Google Scholar] [CrossRef]
- Hosseini-Fashami, F.; Motevali, A.; Nabavi-Pelesaraei, A.; Hashemi, S.J.; Chau, K. wing Energy-Life cycle assessment on applying solar technologies for greenhouse strawberry production. Renew. Sustain. Energy Rev. 2019, 116, 109411. [Google Scholar] [CrossRef]
- Mauger, A.; Julien, C.M.; Paolella, A.; Armand, M.; Zaghib, K. Building Better Batteries in the Solid State: A Review. Materials 2019, 12, 3892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Julien, C.; Mauger, A.; Vijh, A.; Zaghib, K. Lithium Batteries: Science and Technology. MRS Bull. 2016, 419, 41. [Google Scholar] [CrossRef] [Green Version]
- Sundaramurthy, J.; Aravindan, V.; Suresh Kumar, P.; Madhavi, S.; Ramakrishna, S. Electrospun TiO2-δ nanofibers as insertion anode for Li-ion battery applications. J. Phys. Chem. C 2014, 118, 16776–16781. [Google Scholar] [CrossRef]
- Sun, X.; Li, Z.; Wang, X.; Li, C. Technology development of electric vehicles: A review. Energies 2019, 13, 90. [Google Scholar] [CrossRef] [Green Version]
- Moseley, P.T.; Rand, D.A.J. Changes in the demands on automotive batteries require changes in battery design. J. Power Sources 2004, 133, 104–109. [Google Scholar] [CrossRef]
- Fan, X.; Liu, B.; Liu, J.; Ding, J.; Han, X.; Deng, Y.; Lv, X.; Xie, Y.; Chen, B.; Hu, W.; et al. Battery Technologies for Grid-Level Large-Scale Electrical Energy Storage. Trans. Tianjin Univ. 2020, 26, 92–103. [Google Scholar] [CrossRef] [Green Version]
- Lombardo, G.; Ebin, B.; St Foreman, M.R.J.; Steenari, B.M.; Petranikova, M. Chemical Transformations in Li-Ion Battery Electrode Materials by Carbothermic Reduction. ACS Sustain. Chem. Eng. 2019, 7, 13668–13679. [Google Scholar] [CrossRef]
- Li, P.; Hwang, J.Y.; Sun, Y.K. Nano/microstructured silicon-graphite composite anode for high-energy-density li-ion battery. ACS Nano 2019, 13, 2624–2633. [Google Scholar] [CrossRef]
- Tang, W.; Peng, C.X.; Nai, C.T.; Su, J.; Liu, Y.P.; Reddy, M.V.V.; Lin, M.; Loh, K.P. Ultrahigh capacity due to multi-electron conversion reaction in reduced graphene oxide-wrapped MoO2 porous nanobelts. Small 2015, 11, 2446–2453. [Google Scholar] [CrossRef] [PubMed]
- Darbar, D.; Reddy, M.V.; Sundarrajan, S.; Pattabiraman, R.; Ramakrishna, S.; Chowdari, B.V.R. Anodic electrochemical performances of MgCo2O4 synthesized by oxalate decomposition method and electrospinning technique for Li-ion battery application. Mater. Res. Bull. 2016, 73, 369–376. [Google Scholar] [CrossRef]
- Reddy, M.V.; Linh, T.T.; Hien, D.T.; Chowdari, B.V.R. SnO2 Based Materials and Their Energy Storage Studies. ACS Sustain. Chem. Eng. 2016, 4, 6268–6276. [Google Scholar] [CrossRef]
- Kumar, P.S.; Sakunthala, A.; Rao, R.P.; Adams, S.; Chowdari, B.V.R.; Reddy, M.V. Layered Li1+x(Ni0.33Co0.33Mn0.33)O2 cathode material prepared by microwave assisted solvothermal method for lithium ion batteries. Mater. Res. Bull. 2017, 93, 381–390. [Google Scholar] [CrossRef]
- Tan, T.Q.; Idris, M.S.; Osman, R.A.M.; Hambali, N.A.M.A.; Reddy, M.V. Comparison of structural and electrical behaviour of phospho-olivine LiNiPO4 and LiNi0.8Mn0.1Co0.1PO4 for high voltage rechargeable li-ion batteries. In Proceedings of the Solid State Phenomena; Trans Tech Publications Ltd.: Freienbach, Switzerland, 2018; Volume 280 SSP, pp. 50–57. [Google Scholar]
- Reddy, M.V.; Yao Quan, C.; Adams, S. Mg, Cu, Zn doped Fe2O3 as an electrode material for Li-ion batteries. Mater. Lett. 2018, 212, 186–192. [Google Scholar] [CrossRef]
- Kulkarni, P.; Ghosh, D.; Balakrishna, G.; Rawat, R.S.; Adams, S.; Reddy, M.V. Investigation of MnCo2O4/MWCNT composite as anode material for lithium ion battery. Ceram. Int. 2019, 45, 10619–10625. [Google Scholar] [CrossRef]
- Kulkarni, P.; Balkrishna, R.G.; Ghosh, D.; Rawat, R.S.; Medwal, R.; Chowdari, B.V.R.; Karim, Z.; Reddy, M.V. Molten salt synthesis of CoFe2O4 and its energy storage properties. Mater. Chem. Phys. 2021, 257, 123747. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, B.; Wang, Y.X.; Konstantinov, K.; Liu, H.K.; Dou, S.X. Alkali-Metal Sulfide as Cathodes toward Safe and High-Capacity Metal (M = Li, Na, K) Sulfur Batteries. Adv. Energy Mater. 2020, 10, 1–24. [Google Scholar] [CrossRef]
- Rakov, D.A.; Chen, F.; Ferdousi, S.A.; Li, H.; Pathirana, T.; Simonov, A.N.; Howlett, P.C.; Atkin, R.; Forsyth, M. Engineering high-energy-density sodium battery anodes for improved cycling with superconcentrated ionic-liquid electrolytes. Nat. Mater. 2020, 19, 1096–1101. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, W.; Ming, J.; Li, M.; Xie, L.; He, X.; Wang, J.; Liang, S.; Wu, Y. An Exploration of New Energy Storage System: High Energy Density, High Safety, and Fast Charging Lithium Ion Battery. Adv. Funct. Mater. 2019, 29, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Fan, L.; Wei, S.; Li, S.; Li, Q.; Lu, Y. Recent Progress of the Solid-State Electrolytes for High-Energy Metal-Based Batteries. Adv. Energy Mater. 2018, 8, 1702657. [Google Scholar] [CrossRef]
- Liu, C.; Chi, X.; Han, Q.; Liu, Y. A High Energy Density Aqueous Battery Achieved by Dual Dissolution/Deposition Reactions Separated in Acid-Alkaline Electrolyte. Adv. Energy Mater. 2020, 10, 1903589. [Google Scholar] [CrossRef]
- Lee, J.H.; Yoon, C.S.; Hwang, J.Y.; Kim, S.J.; Maglia, F.; Lamp, P.; Myung, S.T.; Sun, Y.K. High-energy-density lithium-ion battery using a carbon-nanotube-Si composite anode and a compositionally graded Li[Ni0.85Co0.05Mn0.10]O2 cathode. Energy Environ. Sci. 2016, 9, 2152–2158. [Google Scholar] [CrossRef]
- Yan, M.Y.; Yan, M.Y.; Li, G.; Li, G.; Zhang, J.; Tian, Y.F.; Tian, Y.F.; Yin, Y.X.; Yin, Y.X.; Zhang, C.J.; et al. Enabling SiOx/C Anode with High Initial Coulombic Efficiency through a Chemical Pre-Lithiation Strategy for High-Energy-Density Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 27202–27209. [Google Scholar] [CrossRef]
- Nanda, S.; Gupta, A.; Manthiram, A. Anode-Free Full Cells: A Pathway to High-Energy Density Lithium-Metal Batteries. Adv. Energy Mater. 2021, 11, 1–18. [Google Scholar] [CrossRef]
- Demiroglu, I.; Peeters, F.M.; Gülseren, O.; Çaklr, D.; Sevik, C. Alkali metal intercalation in MXene/Graphene heterostructures: A new platform for ion battery applications. J. Phys. Chem. Lett. 2019, 10, 727–734. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.; Gallant, B.M. Advances in the chemistry and applications of alkali-metal–gas batteries. Nat. Rev. Chem. 2020, 4, 566–583. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Lv, T.; Chu, P.K.; Huo, K. Two-Dimensional Transition Metal Chalcogenides for Alkali Metal Ions Storage. ChemSusChem 2020, 13, 1114–1154. [Google Scholar] [CrossRef]
- Wu, Q.; Shao, Q.; Li, Q.; Duan, Q.; Li, Y.; Wang, H.G. Dual Carbon-Confined SnO2 Hollow Nanospheres Enabling High Performance for the Reversible Storage of Alkali Metal Ions. ACS Appl. Mater. Interfaces 2018, 10, 15642–15651. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, X.-B.; Huang, J.-Q.; Kaskel, S.; Chou, S.; Park, H.S.; Zhang, Q. Alloy Anodes for Rechargeable Alkali-Metal Batteries: Progress and Challenge. ACS Mater. Lett. 2019, 1, 217–229. [Google Scholar] [CrossRef]
- Li, Y.; Lu, Y.; Adelhelm, P.; Titirici, M.M.; Hu, Y.S. Intercalation chemistry of graphite: Alkali metal ions and beyond. Chem. Soc. Rev. 2019, 48, 4655–4687. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Chao, D.; Liu, E.; Jaroniec, M.; Zhao, N.; Qiao, S.Z. Transition metal dichalcogenides for alkali metal ion batteries: Engineering strategies at the atomic level. Energy Environ. Sci. 2020, 13, 1096–1131. [Google Scholar] [CrossRef]
- Yin, J.; Zhang, W.; Huang, G.; Alhebshi, N.A.; Salah, N.; Hedhili, M.N.; Alshareef, H.N. Fly Ash Carbon Anodes for Alkali Metal-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 26421–26430. [Google Scholar] [CrossRef]
- Lu, Y.; Goodenough, J.B.; Kim, Y. Aqueous cathode for next-generation alkali-ion batteries. J. Am. Chem. Soc. 2011, 133, 5756–5759. [Google Scholar] [CrossRef] [PubMed]
- Shao, T.; Liu, C.; Deng, W.; Li, C.; Wang, X.; Xue, M.; Li, R. Recent Research on Strategies to Improve Ion Conduction in Alkali Metal-Ion Batteries. Batter. Supercaps 2019, 2, 403–427. [Google Scholar] [CrossRef]
- Xiao, N.; Gourdin, G.; Wu, Y. Simultaneous Stabilization of Potassium Metal and Superoxide in K–O2 Batteries on the Basis of Electrolyte Reactivity. Angew. Chemie—Int. Ed. 2018, 57, 10864–10867. [Google Scholar] [CrossRef] [PubMed]
- Oswald, S. Binding energy referencing for XPS in alkali metal-based battery materials research (I): Basic model investigations. Appl. Surf. Sci. 2015, 351, 492–503. [Google Scholar] [CrossRef]
- Narayana, C.; Luo, H.; Orloff, J.; Ruoff, A.L. Solid hydrogen at 342 GPa: No evidence for an alkali metal. Nature 1998, 393, 46–49. [Google Scholar] [CrossRef]
- Chen, F.; Forsyth, M. Elucidation of transport mechanism and enhanced alkali ion transference numbers in mixed alkali metal-organic ionic molten salts. Phys. Chem. Chem. Phys. 2016, 18, 19336–19344. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, Y.; Liu, Y.; Wang, L.; Chou, S.; Liu, H. Strategies Toward Stable Nonaqueous Alkali Metal–O2 Batteries. Adv. Energy Mater. 2019, 9, 1–22. [Google Scholar] [CrossRef]
- McCloskey, B.D. Attainable Gravimetric and Volumetric Energy Density of Li-S and Li Ion Battery Cells with Solid Separator-Protected Li Metal Anodes. J. Phys. Chem. Lett. 2015, 6, 4581–4588. [Google Scholar] [CrossRef]
- Mesbahi, T.; Khenfri, F.; Rizoug, N.; Chaaban, K.; Bartholomeüs, P.; Le Moigne, P. Dynamical modeling of Li-ion batteries for electric vehicle applications based on hybrid Particle Swarm-Nelder-Mead (PSO-NM) optimization algorithm. Electr. Power Syst. Res. 2016, 131, 195–204. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Diouf, B.; Pode, R. Potential of lithium-ion batteries in renewable energy. Renew. Energy 2015, 76, 375–380. [Google Scholar] [CrossRef]
- Van Noorden, R. The rechargeable revolution: A better battery. Nature 2014, 507, 26–28. [Google Scholar] [CrossRef] [Green Version]
- Jia, J.; Tan, C.; Liu, M.; Li, D.; Chen, Y. Relaxation-Induced Memory Effect of LiFePO4 Electrodes in Li-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 24561–24567. [Google Scholar] [CrossRef] [PubMed]
- Pouraghajan, F.; Thompson, A.I.; Hunter, E.E.; Mazzeo, B.; Christensen, J.; Subbaraman, R.; Wray, M.; Wheeler, D. The effects of cycling on ionic and electronic conductivities of Li –ion battery electrodes. J. Power Sources 2021, 492, 229636. [Google Scholar] [CrossRef]
- Kwade, A.; Haselrieder, W.; Leithoff, R.; Modlinger, A.; Dietrich, F.; Droeder, K. Current status and challenges for automotive battery production technologies. Nat. Energy 2018, 3, 290–300. [Google Scholar] [CrossRef]
- Babu, B.; Simon, P.; Balducci, A. Fast Charging Materials for High Power Applications. Adv. Energy Mater. 2020, 10. [Google Scholar] [CrossRef]
- Pang, C.; Xu, G.; An, W.; Ding, G.; Liu, X.; Chai, J.; Ma, J.; Liu, H.; Cui, G. Three-Component Functional Additive in a LiPF6-Based Carbonate Electrolyte for a High-Voltage LiCoO2/Graphite Battery System. Energy Technol. 2017, 5, 1979–1989. [Google Scholar] [CrossRef]
- Rao, M.; Zhang, L.; Li, L.; Rong, L.; Ye, C.; Zhou, G.; Xu, H.; Qiu, Y. Investigation of lithium content changes to understand the capacity fading mechanism in LiFePO4/graphite battery. J. Electroanal. Chem. 2019, 853, 113544. [Google Scholar] [CrossRef]
- Hu, P.; Duan, Y.; Hu, D.; Qin, B.; Zhang, J.; Wang, Q.; Liu, Z.; Cui, G.; Chen, L. Rigid-flexible coupling high ionic conductivity polymer electrolyte for an enhanced performance of LiMn2O4/graphite battery at elevated temperature. ACS Appl. Mater. Interfaces 2015, 7, 4720–4727. [Google Scholar] [CrossRef] [PubMed]
- Lewerenz, M.; Sauer, D.U. Evaluation of cyclic aging tests of prismatic automotive LiNiMnCoO2-Graphite cells considering influence of homogeneity and anode overhang. J. Energy Storage 2018, 18, 421–434. [Google Scholar] [CrossRef]
- Reddy, M.V.; Raju, M.J.S.; Sharma, N.; Quan, P.Y.; Nowshad, S.H.; Emmanuel, H.E.-C.; Peterson, V.K.; Chowdari, B.V.R. Preparation of Li1.03Mn1.97O4 and Li1.06Mn1.94O4 by the Polymer Precursor Method and X-ray, Neutron Diffraction and Electrochemical Studies. J. Electrochem. Soc. 2011, 158, A1231. [Google Scholar] [CrossRef]
- Sakunthala, A.; Reddy, M.V.; Selvasekarapandian, S.; Chowdari, B.V.R.; Selvin, P.C. Preparation, Characterization, and Electrochemical Performance of Lithium Trivanadate Rods by a Surfactant-Assisted Polymer Precursor Method for Lithium Batteries. J. Phys. Chem. C 2010, 114, 8099–8107. [Google Scholar] [CrossRef]
- Sakunthala, A.; Reddy, M.V.; Selvasekarapandian, S.; Chowdari, B.V.R.; Selvin, P.C. Synthesis of compounds, Li(MMn11/6)O4 (M = Mn1/6, Co1/6, (Co1/12Cr1/12), (Co1/12Al1/12), (Cr1/12Al1/12)) by polymer precursor method and its electrochemical performance for lithium-ion batteries. Electrochim. Acta 2010, 55, 4441–4450. [Google Scholar] [CrossRef]
- Reddy, M.V.; Sakunthala, A.; SelvashekaraPandian, S.; Chowdari, B.V.R. Preparation, Comparative Energy Storage Properties, and Impedance Spectroscopy Studies of Environmentally Friendly Cathode, Li(MMn11/6)O4 (M = Mn1/6, Co1/6, (Co1/12Cr1/12)). J. Phys. Chem. C 2013, 117, 9056–9064. [Google Scholar] [CrossRef]
- Prabu, M.; Reddy, M.V.; Selvasekarapandian, S.; Subba Rao, G.V.; Chowdari, B.V.R. (Li, Al)-co-doped spinel, Li(Li0.1Al0.1Mn1.8)O4 as high performance cathode for lithium ion batteries. Electrochim. Acta 2013, 88, 745–755. [Google Scholar] [CrossRef]
- Reddy, M.V.; Cheng, H.Y.; Tham, J.H.; Yuan, C.Y.; Goh, H.L.; Chowdari, B.V.R. Preparation of Li(Ni0.5Mn1.5)O4 by polymer precursor method and its electrochemical properties. Electrochim. Acta 2012, 62, 269–275. [Google Scholar] [CrossRef]
- Zhao, X.; Reddy, M.V.; Liu, H.; Ramakrishna, S.; Rao, G.S.; Chowdari, B.V.R. Nano LiMn2O4 with spherical morphology synthesized by a molten salt method as cathodes for lithium ion batteries. RSC Adv. 2012, 2, 7462–7469. [Google Scholar] [CrossRef]
- Sodhro, A.H.; Pirbhulal, S.; Sangaiah, A.K. Convergence of IoT and product lifecycle management in medical health care. Futur. Gener. Comput. Syst. 2018, 86, 380–391. [Google Scholar] [CrossRef]
- Gao, Y.; Zhu, C.; Zhang, X.; Guo, B. Implementation and evaluation of a practical electrochemical-thermal model of lithium-ion batteries for EV battery management system. Energy 2021, 221, 119688. [Google Scholar] [CrossRef]
- Lu, L.; Han, X.; Li, J.; Hua, J.; Ouyang, M. A review on the key issues for lithium-ion battery management in electric vehicles. J. Power Sources 2013, 226, 272–288. [Google Scholar] [CrossRef]
- Miao, Y.; Hynan, P.; Von Jouanne, A.; Yokochi, A. Current li-ion battery technologies in electric vehicles and opportunities for advancements. Energies 2019, 12, 1074. [Google Scholar] [CrossRef] [Green Version]
- Affanni, A.; Bellini, A.; Franceschini, G.; Guglielmi, P.; Tassoni, C. Battery choice and management for new-generation electric vehicles. IEEE Trans. Ind. Electron. 2005, 52, 1343–1349. [Google Scholar] [CrossRef] [Green Version]
- Selis, L.A.; Seminario, J.M. Dendrite formation in silicon anodes of lithium-ion batteries. RSC Adv. 2018, 8, 5255–5267. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhao, N.; Zhang, M.; Li, Y.; Chu, P.K.; Guo, X.; Di, Z.; Wang, X.; Li, H. Flexible and ion-conducting membrane electrolytes for solid-state lithium batteries: Dispersion of garnet nanoparticles in insulating polyethylene oxide. Nano Energy 2016, 28, 447–454. [Google Scholar] [CrossRef]
- Liu, S.; Imanishi, N.; Zhang, T.; Hirano, A.; Takeda, Y.; Yamamoto, O.; Yang, J. Lithium Dendrite Formation in Li/Poly(ethylene oxide)–Lithium Bis(trifluoromethanesulfonyl)imide and N-Methyl-N-propylpiperidinium Bis(trifluoromethanesulfonyl)imide/Li Cells. J. Electrochem. Soc. 2010, 157, A1092. [Google Scholar] [CrossRef]
- Bieker, G.; Winter, M.; Bieker, P. Electrochemical in situ investigations of SEI and dendrite formation on the lithium metal anode. Phys. Chem. Chem. Phys. 2015, 17, 8670–8679. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Sitapure, N.; Hwang, S.; Kwon, J.S.-I. Multiscale Modeling of Dendrite Formation in Lithium-ion Batteries. Comput. Chem. Eng. 2021, 107415. [Google Scholar] [CrossRef]
- Park, J.; Jeong, J.; Lee, Y.; Oh, M.; Ryou, M.H.; Lee, Y.M. Micro-Patterned Lithium Metal Anodes with Suppressed Dendrite Formation for Post Lithium-Ion Batteries. Adv. Mater. Interfaces 2016, 3, 1–9. [Google Scholar] [CrossRef]
- Hameed, A.S.; Reddy, M.V.; Chowdari, B.V.R.; Vittal, J.J. Carbon coated Li3V2(PO4)3 from the single-source precursor, Li2(VO)2(HPO4)2(C2O4)·6H2O as cathode and anode materials for Lithium ion batteries. Electrochim. Acta 2014, 128, 184–191. [Google Scholar] [CrossRef]
- Yaghtin, A.; Masoudpanah, S.M.; Hasheminiasari, M.; Salehi, A.; Safanama, D.; Ong, C.K.; Adams, S.; Reddy, M.V. Effect of Reducing Agent on Solution Synthesis of Li3V2(PO4)3 Cathode Material for Lithium Ion Batteries. Molecules 2020, 25, 3746. [Google Scholar] [CrossRef]
- Xu, K. Nonaqueous Liquid Electrolytes for Lithium-Based Rechargeable Batteries. Chem. Rev. 2004, 104, 4303–4417. [Google Scholar] [CrossRef] [PubMed]
- Manthiram, A. Materials challenges and opportunities of lithium ion batteries. J. Phys. Chem. Lett. 2011, 2, 176–184. [Google Scholar] [CrossRef]
- Xu, K. A Long Journey of Lithium: From the Big Bang to Our Smartphones. Energy Environ. Mater. 2019, 2, 229–233. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Chen, R.; Chen, T.; Lv, H.; Zhu, G.; Ma, L.; Wang, C.; Jin, Z.; Liu, J. Emerging non-lithium ion batteries. Energy Storage Mater. 2016, 4, 103–129. [Google Scholar] [CrossRef]
- Kubota, K.; Dahbi, M.; Hosaka, T.; Kumakura, S.; Komaba, S. Towards K-Ion and Na-Ion Batteries as “Beyond Li-Ion”. Chem. Rec. 2018, 18, 459–479. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Kim, G.; Casford, M.T.L.; Grey, C.P. Mechanistic Insights into the Challenges of Cycling a Nonaqueous Na-O2 Battery. J. Phys. Chem. Lett. 2016, 7, 4841–4846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, Z.; Hu, Y.; Wu, X.; Han, J.; Gu, Z. Main challenges for high performance NAS battery: Materials and interfaces. Adv. Funct. Mater. 2013, 23, 1005–1018. [Google Scholar] [CrossRef]
- Wu, X.; Leonard, D.P.; Ji, X. Emerging Non-Aqueous Potassium-Ion Batteries: Challenges and Opportunities. Chem. Mater. 2017, 29, 5031–5042. [Google Scholar] [CrossRef]
- Xiao, B.; Rojo, T.; Li, X. Hard Carbon as Sodium-Ion Battery Anodes: Progress and Challenges. ChemSusChem 2019, 12, 133–144. [Google Scholar] [CrossRef]
- Cho, M.H.; Trottier, J.; Gagnon, C.; Hovington, P.; Clément, D.; Vijh, A.; Kim, C.S.; Guerfi, A.; Black, R.; Nazar, L.; et al. The effects of moisture contamination in the Li-O2 battery. J. Power Sources 2014, 268, 565–574. [Google Scholar] [CrossRef]
- Mogensen, R.; Brandell, D.; Younesi, R. Solubility of the Solid Electrolyte Interphase (SEI) in Sodium Ion Batteries. ACS Energy Lett. 2016, 1, 1173–1178. [Google Scholar] [CrossRef]
- Xiang, Y.; Zheng, G.; Liang, Z.; Jin, Y.; Liu, X.; Chen, S.; Zhou, K.; Zhu, J.; Lin, M.; He, H.; et al. Visualizing the growth process of sodium microstructures in sodium batteries by in-situ 23Na MRI and NMR spectroscopy. Nat. Nanotechnol. 2020, 15, 883–890. [Google Scholar] [CrossRef]
- Rosso, M.; Brissot, C.; Teyssot, A.; Dollé, M.; Sannier, L.; Tarascon, J.M.; Bouchet, R.; Lascaud, S. Dendrite short-circuit and fuse effect on Li/polymer/Li cells. Electrochim. Acta 2006, 51, 5334–5340. [Google Scholar] [CrossRef]
- Dornbusch, D.A.; Hilton, R.; Lohman, S.D.; Suppes, G.J. Experimental Validation of the Elimination of Dendrite Short-Circuit Failure in Secondary Lithium-Metal Convection Cell Batteries. J. Electrochem. Soc. 2015, 162, A262–A268. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Pei, A.; Yan, K.; Sun, Y.; Wu, C.L.; Joubert, L.M.; Chin, R.; Koh, A.L.; Yu, Y.; et al. Atomic structure of sensitive battery materials and interfaces revealed by cryo–electron microscopy. Science 2017, 358, 506–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yufit, V.; Tariq, F.; Eastwood, D.S.; Biton, M.; Wu, B.; Lee, P.D.; Brandon, N.P. Operando Visualization and Multi-scale Tomography Studies of Dendrite Formation and Dissolution in Zinc Batteries. Joule 2019, 3, 485–502. [Google Scholar] [CrossRef] [Green Version]
- Hagopian, A.; Doublet, M.L.; Filhol, J.S. Thermodynamic origin of dendrite growth in metal anode batteries. Energy Environ. Sci. 2020, 13, 5186–5197. [Google Scholar] [CrossRef]
- Mandai, T.; Dokko, K.; Watanabe, M. Solvate Ionic Liquids for Li, Na, K, and Mg Batteries. Chem. Rec. 2019, 19, 708–722. [Google Scholar] [CrossRef]
- Lu, J.; Wu, T.; Amine, K. State-of-the-art characterization techniques for advanced lithium-ion batteries. Nat. Energy 2017, 2, 1–13. [Google Scholar] [CrossRef]
- Reddy, M.V.; Mauger, A.; Julien, C.M.; Paolella, A.; Zaghib, K. Brief History of Early Lithium-Battery Development. Materials 2020, 13, 1884. [Google Scholar] [CrossRef] [Green Version]
- Oh, P.; Yun, J.; Park, S.; Nam, G.; Liu, M.; Cho, J. Recent Advances and Prospects of Atomic Substitution on Layered Positive Materials for Lithium-Ion Battery. Adv. Energy Mater. 2021, 11, 2003197. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, X.; Chong, Y.; Yuan, H.; Huang, J.Q.; Zhang, Q. Advanced electrode processing of lithium ion batteries: A review of powder technology in battery fabrication. Particuology 2021, 57, 56–71. [Google Scholar] [CrossRef]
- Li, N.; Sun, M.Z.; Hwang, S.; Li, S.; Zhao, H.Y.; Du, Y.P.; Huang, B.L.; Su, D. Non-equilibrium insertion of lithium ions into graphite. J. Mater. Chem. A 2021, 9, 12080–12086. [Google Scholar] [CrossRef]
- Fujimoto, H.; Kiuchi, H.; Takagi, S.; Shimoda, K.; Okazaki, K.; Ogumi, Z.; Abe, T. Assessing Reaction Mechanisms of Graphite Negative Electrodes Based on Operando Synchrotron Radiation Diffraction Data. J. Electrochem. Soc. 2021, 168, 040509. [Google Scholar] [CrossRef]
- Xie, Y.; Han, Z.; Li, H.; Hu, J.; Zhang, L.; Wang, A.; Chang, S.; Xu, J.; Liu, C.; Lai, Y.; et al. Uniform nucleation of sodium/lithium in holey carbon nanosheet for stable Na/Li metal anodes. Chem. Eng. J. 2022, 427, 130959. [Google Scholar] [CrossRef]
- Wang, J.; Yang, J.; Xiao, Q.; Zhang, J.; Li, T.; Jia, L.; Wang, Z.; Cheng, S.; Li, L.; Liu, M.; et al. In Situ Self-Assembly of Ordered Organic/Inorganic Dual-Layered Interphase for Achieving Long-Life Dendrite-Free Li Metal Anodes in LiFSI-Based Electrolyte. Adv. Funct. Mater. 2021, 31, 2007434. [Google Scholar] [CrossRef]
- Boyle, D.T.; Huang, W.; Wang, H.; Li, Y.; Chen, H.; Yu, Z.; Zhang, W.; Bao, Z.; Cui, Y. Corrosion of lithium metal anodes during calendar ageing and its microscopic origins. Nat. Energy 2021, 6, 487–494. [Google Scholar] [CrossRef]
- May, R.; Fritzsching, K.J.; Livitz, D.; Denny, S.R.; Marbella, L.E. Rapid Interfacial Exchange of Li Ions Dictates High Coulombic Efficiency in Li Metal Anodes. ACS Energy Lett. 2021, 6, 1162–1169. [Google Scholar] [CrossRef]
- Tavassol, H.; Buthker, J.W.; Ferguson, G.A.; Curtiss, L.A.; Gewirth, A.A. Solvent Oligomerization during SEI Formation on Model Systems for Li-Ion Battery Anodes. J. Electrochem. Soc. 2012, 159, A730–A738. [Google Scholar] [CrossRef]
- Zhang, Y.; Viswanathan, V. Design Rules for Selecting Fluorinated Linear Organic Solvents for Li Metal Batteries. J. Phys. Chem. Lett. 2021, 12, 5821–5828. [Google Scholar] [CrossRef]
- Reddy, M.V.; Adams, S.; Liang, G.T.J.; Mingze, I.F.; Van Tu An, H.; Chowdari, B.V.R. Low temperature molten salt synthesis of anatase TiO2 and its electrochemical properties. Solid State Ionics 2014, 262, 120–123. [Google Scholar] [CrossRef]
- Peljo, P.; Girault, H.H. Electrochemical potential window of battery electrolytes: The HOMO–LUMO misconception. Energy Environ. Sci. 2018, 11, 2306–2309. [Google Scholar] [CrossRef]
- Shen, Y.; Qian, J.; Yang, H.; Zhong, F.; Ai, X. Chemically Prelithiated Hard-Carbon Anode for High Power and High Capacity Li-Ion Batteries. Small 2020, 16, 1907602. [Google Scholar] [CrossRef] [PubMed]
- Dai, R.; Sun, W.; Lv, L.P.; Wu, M.; Liu, H.; Wang, G.; Wang, Y. Bimetal-Organic-Framework Derivation of Ball-Cactus-Like Ni-Sn-P@C-CNT as Long-Cycle Anode for Lithium Ion Battery. Small 2017, 13, 1700521. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Li, X.; Wei, T.; Yin, Z.; Li, X. Improved compatibility of graphite anode for lithium ion battery using sulfuric esters. Electrochim. Acta 2016, 196, 622–628. [Google Scholar] [CrossRef]
- Zhao, J.; Shi, Q.; Xiang, Y.; Xia, Y. Preparation of LiTi2O4 as a Lithium-ion Battery Anode by a Carbon-thermal Reduction Method. Int. J. Electrochem. Sci 2018, 13. [Google Scholar] [CrossRef]
- Subramanyan, K.; Natarajan, S.; Lee, Y.S.; Aravindan, V. Exploring the usage of LiCrTiO4 as cathode towards constructing 1.4 V class Li-ion cells with graphite anode recovered from spent Li-Ion battery. Chem. Eng. J. 2020, 397, 125472. [Google Scholar] [CrossRef]
- Uceda, M.R. Highly Homogeneously Structured Lithium-ion Storage Electrodes via Electrophoretic Deposition; McGill University: Montréal, QC, Canada, 2020. [Google Scholar]
- Li, Y.; Liu, H.-Y.; Shi, L.-N.; Zhu, Y.-R.; Yi, T.-F. Improved lithium storage performance of CeO2-decorated SrLi2Ti6O14 material as an anode for Li-ion battery. J. Ind. Eng. Chem. 2021, 101, 144–152. [Google Scholar] [CrossRef]
- He, Z.; Jiang, Y.; Zhu, J.; Li, Y.; Jiang, Z.; Zhou, H.; Meng, W.; Wang, L.; Dai, L. Boosting the performance of LiTi2(PO4)3/C anode for aqueous lithium ion battery by Sn doping on Ti sites. J. Alloys Compd. 2018, 731, 32–38. [Google Scholar] [CrossRef]
- Du, D.J.; Du, Y.E.; Yue, W.B.; Yang, X.J. Lithium storage performance of {010}-faceted and [111]-faceted anatase TiO2 nanocrystals. J. Cent. South Univ. 2019, 26, 1530–1539. [Google Scholar] [CrossRef]
- Wang, S.; Yang, Y.; Dong, Y.; Zhang, Z.; Tang, Z. Recent progress in Ti-based nanocomposite anodes for lithium ion batteries. J. Adv. Ceram. 2019, 8, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Wei, Y.; Wang, C.; Qiao, R.; Yang, W.; Messersmith, P.B.; Liu, G. Mussel-Inspired Conductive Polymer Binder for Si-Alloy Anode in Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 5440–5446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Li, T.; Cui, Y.; Meyerson, M.L.; Weeks, J.A.; Mullins, C.B.; Jin, Y.; Liu, Y.; Zhu, L. In Situ and Operando Morphology Study of Germanium-Selenium Alloy Anode for Lithium-Ion Batteries. ACS Appl. Energy Mater. 2020, 3, 6115–6120. [Google Scholar] [CrossRef]
- Wan, M.; Kang, S.; Wang, L.; Lee, H.W.; Zheng, G.W.; Cui, Y.; Sun, Y. Mechanical rolling formation of interpenetrated lithium metal/lithium tin alloy foil for ultrahigh-rate battery anode. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Wei, Y.; Zhai, T.; Li, H. Antimony-based materials as promising anodes for rechargeable lithium-ion and sodium-ion batteries. Mater. Chem. Front. 2018, 2, 437–455. [Google Scholar] [CrossRef]
- Kebede, M.A. Tin oxide–based anodes for both lithium-ion and sodium-ion batteries. Curr. Opin. Electrochem. 2020, 21, 182–187. [Google Scholar] [CrossRef]
- Wang, M.S.; Wang, Z.Q.; Jia, R.; Yang, Y.; Zhu, F.Y.; Yang, Z.L.; Huang, Y.; Li, X.; Xu, W. Facile electrostatic self-assembly of silicon/reduced graphene oxide porous composite by silica assist as high performance anode for Li-ion battery. Appl. Surf. Sci. 2018, 456, 379–389. [Google Scholar] [CrossRef]
- Shao, J.; Feng, J.; Zhou, H.; Yuan, A. Graphene aerogel encapsulated Fe-Co oxide nanocubes derived from Prussian blue analogue as integrated anode with enhanced Li-ion storage properties. Appl. Surf. Sci. 2019, 471, 745–752. [Google Scholar] [CrossRef]
- Wu, S.; Fu, G.; Lv, W.; Wei, J.; Chen, W.; Yi, H.; Gu, M.; Bai, X.; Zhu, L.; Tan, C.; et al. A Single-Step Hydrothermal Route to 3D Hierarchical Cu2O/CuO/rGO Nanosheets as High-Performance Anode of Lithium-Ion Batteries. Small 2018, 14, 1702667. [Google Scholar] [CrossRef]
- Li, T.; Bai, Y.; Wang, Y.; Xu, H.; Jin, H. Advances in transition-metal (Zn, Mn, Cu)-based MOFs and their derivatives for anode of lithium-ion batteries. Coord. Chem. Rev. 2020, 410, 213221. [Google Scholar] [CrossRef]
- McNulty, D.; Geaney, H.; O’Dwyer, C. Carbon-Coated Honeycomb Ni-Mn-Co-O Inverse Opal: A High Capacity Ternary Transition Metal Oxide Anode for Li-ion Batteries. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Zhao, Y.; Dong, W.; Riaz, M.S.; Ge, H.; Wang, X.; Liu, Z.; Huang, F. “electron-Sharing” Mechanism Promotes Co@Co3O4/CNTs Composite as the High-Capacity Anode Material of Lithium-Ion Battery. ACS Appl. Mater. Interfaces 2018, 10, 43641–43649. [Google Scholar] [CrossRef] [PubMed]
- Petibon, R.; Chevrier, V.L.; Aiken, C.P.; Hall, D.S.; Hyatt, S.R.; Shunmugasundaram, R.; Dahn, J.R. Studies of the Capacity Fade Mechanisms of LiCoO2/Si-Alloy: Graphite Cells. J. Electrochem. Soc. 2016, 163, A1146–A1156. [Google Scholar] [CrossRef]
- Yu, X.; Yu, W.A.; Manthiram, A. Advances and Prospects of High-Voltage Spinel Cathodes for Lithium-Based Batteries. Small Methods 2021, 5, 2001196. [Google Scholar] [CrossRef]
- Kwon, N.H.; Conder, J.; Srout, M.; Fromm, K.M. Surface modifications of positive-electrode materials for lithium ion batteries. Chimia 2019, 73, 880–893. [Google Scholar] [CrossRef] [Green Version]
- Das, B.; Reddy, M.V.; Chowdari, B.V.R. SnO and SnO·CoO nanocomposite as high capacity anode materials for lithium ion batteries. Mater. Res. Bull. 2016, 74, 291–298. [Google Scholar] [CrossRef] [Green Version]
- Reddy, M.V.; Rao, G.V.S.; Chowdari, B.V.R. Metal Oxides and Oxysalts as Anode Materials for Li Ion Batteries. Chem. Rev. 2013, 113, 5364–5457. [Google Scholar] [CrossRef]
- Chang, W.; Bommier, C.; Mohr, R.; Steingart, D. Impact of Non-Arrhenius Temperature Behavior on the Fast-Charging Capabilities of LiCoO2-Graphite Lithium-Ion Batteries. J. Phys. Chem. C 2021, 125, 1731–1741. [Google Scholar] [CrossRef]
- Darbar, D.; Anilkumar, M.R.; Rajagopalan, V.; Bhattacharya, I.; Elim, H.I.; Ramakrishnappa, T.; Ezema, F.I.; Jose, R.; Reddy, M.V. Studies on spinel cobaltites, MCo2O4 (M = Mn, Zn, Fe, Ni and Co) and their functional properties. Ceram. Int. 2018, 44, 4630–4639. [Google Scholar] [CrossRef]
- Nithyadharseni, P.; Abhilash, K.P.; Petnikota, S.; Anilkumar, M.R.; Jose, R.; Ozoemena, K.I.; Vijayaraghavan, R.; Kulkarni, P.; Balakrishna, G.; Chowdari, B.V.R.; et al. Synthesis and Lithium Storage Properties of Zn, Co and Mg doped SnO2 Nano Materials. Electrochim. Acta 2017, 247, 358–370. [Google Scholar] [CrossRef] [Green Version]
- Cherian, C.T.; Zheng, M.; Reddy, M.V.; Chowdari, B.V.R.; Sow, C.H. Zn2SnO4 Nanowires versus Nanoplates: Electrochemical Performance and Morphological Evolution during Li-Cycling. ACS Appl. Mater. Interfaces 2013, 5, 6054–6060. [Google Scholar] [CrossRef] [PubMed]
- Pralong, V.; Reddy, M.A.; Caignaert, V.; Malo, S.; Lebedev, O.I.; Varadaraju, U.V.; Raveau, B. A New Form of LiNbO3 with a Lamellar Structure Showing Reversible Lithium Intercalation. Chem. Mater. 2011, 23, 1915–1922. [Google Scholar] [CrossRef]
- Tan, K.S.; Reddy, M.V.; Rao, G.V.S.; Chowdari, B.V.R. Effect of AlPO4-coating on cathodic behaviour of Li(Ni0.8Co0.2)O2. J. Power Sources 2005, 141, 129–142. [Google Scholar] [CrossRef]
- Zhu, H.; Lyu, F.; Du, M.; Zhang, M.; Wang, Q.; Yao, J.; Guo, B. Design of Two-Dimensional, Ultrathin MoS2 Nanoplates Fabricated within One-Dimensional Carbon Nanofibers with Thermosensitive Morphology: High-Performance Electrocatalysts for the Hydrogen Evolution Reaction. ACS Appl. Mater. Interfaces 2014, 6, 22126–22137. [Google Scholar] [CrossRef]
- Khan, I.A.; Medwal, R.; Fareed, S.; Farid, A.; Vas, J.V.; Reddy, M.V.; Rawat, R.S. Nanostructured polycrystalline Ni3S2 as electrode material for lithium ion batteries. Mater. Res. Express 2020, 7, 015517. [Google Scholar] [CrossRef]
- Reddy, M.V.; Madhavi, S.; Subba Rao, G.V.; Chowdari, B.V.R. Metal oxyfluorides TiOF2 and NbO2F as anodes for Li-ion batteries. J. Power Sources 2006, 162, 1312–1321. [Google Scholar] [CrossRef]
- Jose, R.; Archana, P.S.; Le Viet, A.; Reddy, M.V.; Yusoff, M.M.; Ramakrishna, S. Electrospun metal oxides nanostructures for energy related devices. In Proceedings of the IEEE Conference on Clean Energy and Technology (CET), Kuala Lumpur, Malaysia, 27–29 June 2011; pp. 161–165. [Google Scholar] [CrossRef]
- Ramakrishna, S.; Le Viet, A.; Reddy, M.V.; Jose, R.; Chowdari, B.V.R. Nanostructured Nb2O5 polymorphs by electrospinning for rechargeable lithium batteries. J. Phys. Chem. C 2010, 114, 664–671. [Google Scholar] [CrossRef]
- Zhang, C.; He, Y.; Wang, Y.; Liang, Y.; Majeed, A.; Yang, Z.; Yao, S.; Shen, X.; Li, T.; Qin, S. CoFe2O4 nanoparticles loaded N-doped carbon nanofibers networks as electrocatalyst for enhancing redox kinetics in Li-S batteries. Appl. Surf. Sci. 2021, 560, 149908. [Google Scholar] [CrossRef]
- Zhou, L.; Danilov, D.L.; Eichel, R.A.; Notten, P.H.L. Host Materials Anchoring Polysulfides in Li–S Batteries Reviewed. Adv. Energy Mater. 2021, 11, 2001304. [Google Scholar] [CrossRef]
- Liu, Y.; He, P.; Zhou, H. Rechargeable Solid-State Li–Air and Li–S Batteries: Materials, Construction, and Challenges. Adv. Energy Mater. 2018, 8, 1701602. [Google Scholar] [CrossRef]
- Wang, L.; Ye, Y.; Chen, N.; Huang, Y.; Li, L.; Wu, F.; Chen, R. Development and Challenges of Functional Electrolytes for High-Performance Lithium–Sulfur Batteries. Adv. Funct. Mater. 2018, 28, 1800919. [Google Scholar] [CrossRef]
- Zhang, H.; Armand, M. History of Solid Polymer Electrolyte-Based Solid-State Lithium Metal Batteries: A Personal Account. Isr. J. Chem. 2021, 61, 94–100. [Google Scholar] [CrossRef]
- Xu, X.; Ye, S.; Liu, S.; Yan, T. Silver Iodide as a Host Material of Sulfur for Li–S Battery. J. Electrochem. Soc. 2021, 168, 060536. [Google Scholar] [CrossRef]
- Huang, S.; Huixiang, E.; Yang, Y.; Zhang, Y.; Ye, M.; Li, C.C. Transition metal phosphides: New generation cathode host/separator modifier for Li–S batteries. J. Mater. Chem. A 2021, 9, 7458–7480. [Google Scholar] [CrossRef]
- Gu, X.; Deng, L.; Ren, X. Metal Atom-Decorated Carbon Nanomaterials for Enhancing Li-S/Se Batteries Performances: A Mini Review. Front. Energy Res. 2021, 9, 37. [Google Scholar] [CrossRef]
- Wei, H.; Liu, Y.; Zhai, X.; Wang, F.; Ren, X.; Tao, F.; Li, T.; Wang, G.; Ren, F. Application of Carbon Nanotube-Based Materials as Interlayers in High-Performance Lithium-Sulfur Batteries: A Review. Front. Energy Res. 2020, 8, 221. [Google Scholar] [CrossRef]
- Li, S.; Fan, Z. Encapsulation methods of sulfur particles for lithium-sulfur batteries: A review. Energy Storage Mater. 2021, 34, 107–127. [Google Scholar] [CrossRef]
- Wei, C.; Tian, M.; Fan, Z.; Yu, L.; Song, Y.; Yang, X.; Shi, Z.; Wang, M.; Yang, R.; Sun, J. Concurrent realization of dendrite-free anode and high-loading cathode via 3D printed N-Ti3C2 MXene framework toward advanced Li–S full batteries. Energy Storage Mater. 2021, 41, 141–151. [Google Scholar] [CrossRef]
- Chen, Y.; Zou, K.; Dai, X.; Bai, H.; Zhang, S.; Zhou, T.; Li, C.; Liu, Y.; Pang, W.K.; Guo, Z. Polysulfide Filter and Dendrite Inhibitor: Highly Graphitized Wood Framework Inhibits Polysulfide Shuttle and Lithium Dendrites in Li–S Batteries. Adv. Funct. Mater. 2021, 31, 2102458. [Google Scholar] [CrossRef]
- Gao, Y.; Guo, Q.; Zhang, Q.; Cui, Y.; Zheng, Z. Fibrous Materials for Flexible Li–S Battery. Adv. Energy Mater. 2021, 11, 2002580. [Google Scholar] [CrossRef]
- Landi, B.J.; Ganter, M.J.; Cress, C.D.; DiLeo, R.A.; Raffaelle, R.P. Carbon nanotubes for lithium ion batteries. Energy Environ. Sci. 2009, 2, 638–654. [Google Scholar] [CrossRef]
- Deng, Z.; Mo, Y.; Ong, S.P. Computational studies of solid-state alkali conduction in rechargeable alkali-ion batteries. NPG Asia Mater. 2016, 8, e254. [Google Scholar] [CrossRef] [Green Version]
- Luo, W.; Shen, F.; Bommier, C.; Zhu, H.; Ji, X.; Hu, L. Na-Ion Battery Anodes: Materials and Electrochemistry. Acc. Chem. Res. 2016, 49, 231–240. [Google Scholar] [CrossRef]
- Mortazavi, M.; Ye, Q.; Birbilis, N.; Medhekar, N.V. High capacity group-15 alloy anodes for Na-ion batteries: Electrochemical and mechanical insights. J. Power Sources 2015, 285, 29–36. [Google Scholar] [CrossRef]
- Hameed, A.S.; Reddy, M.V.; Chen, J.L.T.; Chowdari, B.V.R.; Vittal, J.J. RGO/Stibnite Nanocomposite as a Dual Anode for Lithium and Sodium Ion Batteries. ACS Sustain. Chem. Eng. 2016, 4, 2479–2486. [Google Scholar] [CrossRef]
- Bommier, C.; Ji, X. Recent development on anodes for Na-ion batteries. Isr. J. Chem. 2015, 55, 486–507. [Google Scholar] [CrossRef]
- Kang, H.; Liu, Y.; Cao, K.; Zhao, Y.; Jiao, L.; Wang, Y.; Yuan, H. Update on anode materials for Na-ion batteries. J. Mater. Chem. A 2015, 3, 17899–17913. [Google Scholar] [CrossRef]
- Phadke, S.; Mysyk, R.; Anouti, M. Effect of cation (Li+, Na+, K+, Rb+, Cs+) in aqueous electrolyte on the electrochemical redox of Prussian blue analogue (PBA) cathodes. J. Energy Chem. 2020, 40, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Xiao, P.; Song, J.; Wang, L.; Goodenough, J.B.; Henkelman, G. Theoretical study of the structural evolution of a Na2FeMn(CN)6 cathode upon Na intercalation. Chem. Mater. 2015, 27, 3763–3768. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Zhao, J.; Wang, E.; Liu, X.; Shen, X.; Rong, X.; Zheng, Q.; Ren, G.; Zhang, N.; Liu, X.; et al. A Novel NASICON-Typed Na4VMn0.5Fe0.5(PO4)3 Cathode for High-Performance Na-Ion Batteries. Adv. Energy Mater. 2021, 11, 2100729. [Google Scholar] [CrossRef]
- Ghosh, S.; Barman, N.; Mazumder, M.; Pati, S.K.; Rousse, G.; Senguttuvan, P. High Capacity and High-Rate NASICON-Na3.75V1.25Mn0.75(PO4)3 Cathode for Na-Ion Batteries via Modulating Electronic and Crystal Structures. Adv. Energy Mater. 2020, 10, 1902918. [Google Scholar] [CrossRef]
- Aragón, M.J.; Lavela, P.; Ortiz, G.F.; Alcántara, R.; Tirado, J.L. Insight into the Electrochemical Sodium Insertion of Vanadium Superstoichiometric NASICON Phosphate. Inorg. Chem. 2017, 56, 11845–11853. [Google Scholar] [CrossRef] [PubMed]
- Dhir, S.; Wheeler, S.; Capone, I.; Pasta, M. Outlook on K-Ion Batteries. Chem 2020, 6, 2442–2460. [Google Scholar] [CrossRef]
- Hurlbutt, K.; Wheeler, S.; Capone, I.; Pasta, M. Prussian Blue Analogs as Battery Materials. Joule 2018, 2, 1950–1960. [Google Scholar] [CrossRef] [Green Version]
- Reeves, K.G.; Ma, J.; Fukunishi, M.; Salanne, M.; Komaba, S.; Dambournet, D. Insights into Li+, Na+, and K+ Intercalation in Lepidocrocite-Type Layered TiO2 Structures. ACS Appl. Energy Mater. 2018, 1, 2078–2086. [Google Scholar] [CrossRef]
- Jiang, Y.; Guo, Y.; Lu, W.; Feng, Z.; Xi, B.; Kai, S.; Zhang, J.; Feng, J.; Xiong, S. Rationally Incorporated MoS2/SnS2 Nanoparticles on Graphene Sheets for Lithium-Ion and Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 27697–27706. [Google Scholar] [CrossRef]
- Masese, T.; Miyazaki, Y.; Mbiti Kanyolo, G.; Takahashi, T.; Ito, M.; Senoh, H.; Saito, T. Evidence of Unique Stacking and Related Topological Defects in the Honeycomb Layered Oxide: K2Ni2TeO6. Chem Rxiv 2020, 2, 1–30. [Google Scholar] [CrossRef]
- Li, J.; Yang, F.; Ye, J.; Cheng, Y.T. Whisker formation on a thin film tin lithium-ion battery anode. J. Power Sources 2011, 196, 1474–1477. [Google Scholar] [CrossRef]
- Biton, M.; Tariq, F.; Yufit, V.; Chen, Z.; Brandon, N. Integrating multi-length scale high resolution 3D imaging and modelling in the characterisation and identification of mechanical failure sites in electrochemical dendrites. Acta Mater. 2017, 141, 39–46. [Google Scholar] [CrossRef]
- Maslyn, J.A.; Loo, W.S.; McEntush, K.D.; Oh, H.J.; Harry, K.J.; Parkinson, D.Y.; Balsara, N.P. Growth of Lithium Dendrites and Globules through a Solid Block Copolymer Electrolyte as a Function of Current Density. J. Phys. Chem. C 2018, 122, 26797–26804. [Google Scholar] [CrossRef] [Green Version]
- Brissot, C.; Rosso, M.; Chazalviel, J.N.; Lascaud, S. Concentration measurements in lithium/polymer-electrolyte/lithium cells during cycling. J. Power Sources 2001, 94, 212–218. [Google Scholar] [CrossRef]
- Cheng, E.J.; Sharafi, A.; Sakamoto, J. Intergranular Li metal propagation through polycrystalline Li6.25Al0.25La3Zr2O12 ceramic electrolyte. Electrochim. Acta 2017, 223, 85–91. [Google Scholar] [CrossRef] [Green Version]
- Qian, J.; Henderson, W.A.; Xu, W.; Bhattacharya, P.; Engelhard, M.; Borodin, O.; Zhang, J.G. High rate and stable cycling of lithium metal anode. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Strickland, J.; Nenchev, B.; Dong, H. On directional dendritic growth and primary spacing—A review. Crystals 2020, 10, 627. [Google Scholar] [CrossRef]
- Glicksman, M.E.; Lupulescu, A.O. Dendritic crystal growth in pure materials. J. Cryst. Growth 2004, 264, 541–549. [Google Scholar] [CrossRef]
- Kavousi, S.; Novak, B.R.; Zaeem, M.A.; Moldovan, D. Combined molecular dynamics and phase field simulation investigations of crystal-melt interfacial properties and dendritic solidification of highly undercooled titanium. Comput. Mater. Sci. 2019, 163, 218–229. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Liu, S.; Wang, X.; Xie, D.; Xia, X.; Gu, C.; Tu, J. Original growth mechanism for ultra-stable dendrite-free potassium metal electrode. Nano Energy 2019, 62, 367–375. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, X.-B.; Jin, Z.; Zhang, R.; Wang, G.; Chen, L.-Q.; Liu, Q.-B.; Huang, J.-Q.; Zhang, Q. Recent advances in understanding dendrite growth on alkali metal anodes. EnergyChem 2019, 1, 100003. [Google Scholar] [CrossRef]
- Li, G.; Monroe, C.W. Dendrite nucleation in lithium-conductive ceramics. Phys. Chem. Chem. Phys. 2019, 21, 20354–20359. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Wang, M.; Wang, J.; Ye, F.; Wang, S.; Xu, Y.; Liu, J.; Xu, G.; Zhang, Y.; Zhang, Y.; et al. Asymmetric gel polymer electrolyte with high lithium ion conductivity for dendrite-free lithium metal batteries. J. Mater. Chem. A 2020, 8, 8033–8040. [Google Scholar] [CrossRef]
- Barai, P.; Higa, K.; Srinivasan, V. Effect of Initial State of Lithium on the Propensity for Dendrite Formation: A Theoretical Study. J. Electrochem. Soc. 2017, 164, A180–A189. [Google Scholar] [CrossRef] [Green Version]
- Hagopian, A.; Kopač, D.; Filhol, J.S.; Kopač Lautar, A. Morphology evolution and dendrite growth in Li- and Mg-metal batteries: A potential dependent thermodynamic and kinetic multiscale ab initio study. Electrochim. Acta 2020, 353. [Google Scholar] [CrossRef]
- Jäckle, M.; Groß, A. Microscopic properties of lithium, sodium, and magnesium battery anode materials related to possible dendrite growth. J. Chem. Phys. 2014, 141. [Google Scholar] [CrossRef]
- Hamidah, N.L.; Wang, F.M.; Nugroho, G. The understanding of solid electrolyte interface (SEI) formation and mechanism as the effect of flouro-o-phenylenedimaleimaide (F-MI) additive on lithium-ion battery. Surf. Interface Anal. 2019, 51, 345–352. [Google Scholar] [CrossRef]
- Aslam, M.K.; Niu, Y.; Hussain, T.; Tabassum, H.; Tang, W.; Xu, M.; Ahuja, R. How to avoid dendrite formation in metal batteries: Innovative strategies for dendrite suppression. Nano Energy 2021, 86, 106142. [Google Scholar] [CrossRef]
- Peled, E. Film forming reaction at the lithium/electrolyte interface. J. Power Sources 1983, 9, 253–266. [Google Scholar] [CrossRef]
- Cheng, X.B.; Zhang, R.; Zhao, C.Z.; Wei, F.; Zhang, J.G.; Zhang, Q. A review of solid electrolyte interphases on lithium metal anode. Adv. Sci. 2015, 3, 1–20. [Google Scholar] [CrossRef]
- An, S.J.; Li, J.; Daniel, C.; Mohanty, D.; Nagpure, S.; Wood, D.L. The state of understanding of the lithium-ion-battery graphite solid electrolyte interphase (SEI) and its relationship to formation cycling. Carbon 2016, 105, 52–76. [Google Scholar] [CrossRef] [Green Version]
- Parimalam, B.S.; MacIntosh, A.D.; Kadam, R.; Lucht, B.L. Decomposition Reactions of Anode Solid Electrolyte Interphase (SEI) Components with LiPF6. J. Phys. Chem. C 2017, 121, 22733–22738. [Google Scholar] [CrossRef]
- Zhou, J.; Guo, Y.; Liang, C.; Cao, L.; Pan, H.; Yang, J.; Wang, J. A new ether-based electrolyte for lithium sulfur batteries using a S@pPAN cathode. Chem. Commun. 2018, 54, 5478–5481. [Google Scholar] [CrossRef]
- Yamagata, M.; Matsui, Y.; Sugimoto, T.; Kikuta, M.; Higashizaki, T.; Kono, M.; Ishikawa, M. High-performance graphite negative electrode in a bis(fluorosulfonyl)imide- based ionic liquid. J. Power Sources 2013, 227, 60–64. [Google Scholar] [CrossRef]
- Dollé, M.; Grugeon, S.; Beaudoin, B.; Dupont, L.; Tarascon, J.M. In situ TEM study of the interface carbon/electrolyte. J. Power Sources 2001, 97–98, 104–106. [Google Scholar] [CrossRef]
- Yan, K.; Zhao, S.; Zhang, J.; Safaei, J.; Yu, X.; Wang, T.; Wang, S.; Sun, B.; Wang, G. Dendrite-Free Sodium Metal Batteries Enabled by the Release of Contact Strain on Flexible and Sodiophilic Matrix. Nano Lett. 2020, 20, 6112–6119. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, H.; Matios, E.; Luo, J. Combining theories and experiments to understand the sodium nucleation behavior towards safe sodium metal batteries. Chem. Soc. Rev. 2020, 49, 3783–3805. [Google Scholar] [CrossRef]
- Michaels, T.C.T.; Buell, A.K.; Terentjev, E.M.; Knowles, T.P.J. Quantitative analysis of diffusive reactions at the solid-liquid interface in finite systems. J. Phys. Chem. Lett. 2014, 5, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Raman, S.; Hoyt, J.J.; Saidi, P.; Asta, M. Molecular dynamics study of the thermodynamic and kinetic properties of the solid-liquid interface in FeMn. Comput. Mater. Sci. 2020, 182, 109773. [Google Scholar] [CrossRef]
- Ely, D.R.; García, R.E. Heterogeneous Nucleation and Growth of Lithium Electrodeposits on Negative Electrodes. J. Electrochem. Soc. 2013, 160, A662–A668. [Google Scholar] [CrossRef]
- Wang, H.; Chew, H.B. Nanoscale Mechanics of the Solid Electrolyte Interphase on Lithiated-Silicon Electrodes. ACS Appl. Mater. Interfaces 2017, 9, 25662–25667. [Google Scholar] [CrossRef]
- Mu, W.; Liu, X.; Wen, Z.; Liu, L. Numerical simulation of the factors affecting the growth of lithium dendrites. J. Energy Storage 2019, 26, 100921. [Google Scholar] [CrossRef]
- Shin, H.; Park, J.; Han, S.; Sastry, A.M.; Lu, W. Component-/structure-dependent elasticity of solid electrolyte interphase layer in Li-ion batteries: Experimental and computational studies. J. Power Sources 2015, 277, 169–179. [Google Scholar] [CrossRef] [Green Version]
- Shi, S.; Qi, Y.; Li, H.; Hector, L.G. Defect thermodynamics and diffusion mechanisms in Li2CO3 and implications for the solid electrolyte interphase in Li-ion batteries. J. Phys. Chem. C 2013, 117, 8579–8593. [Google Scholar] [CrossRef]
- Ye, H.; Zhang, Y.; Yin, Y.X.; Cao, F.F.; Guo, Y.G. An Outlook on Low-Volume-Change Lithium Metal Anodes for Long-Life Batteries. ACS Cent. Sci. 2020, 6, 661–671. [Google Scholar] [CrossRef]
- Sun, F.; He, X.; Jiang, X.; Osenberg, M.; Li, J.; Zhou, D.; Dong, K.; Hilger, A.; Zhu, X.; Gao, R.; et al. Advancing knowledge of electrochemically generated lithium microstructure and performance decay of lithium ion battery by synchrotron X-ray tomography. Mater. Today 2019, 27, 21–32. [Google Scholar] [CrossRef]
- Liu, W.; Liu, P.; Mitlin, D. Tutorial review on structure-dendrite growth relations in metal battery anode supports. Chem. Soc. Rev. 2020, 49, 7284–7300. [Google Scholar] [CrossRef]
- Frenck, L.; Sethi, G.K.; Maslyn, J.A.; Balsara, N.P. Factors That Control the Formation of Dendrites and Other Morphologies on Lithium Metal Anodes. Front. Energy Res. 2019, 7. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Luo, Y.; Zhang, H.; Qu, C.; Zhang, H.; Li, X. The Challenge of Lithium Metal Anodes for Practical Applications. Small Methods 2019, 3, 1–23. [Google Scholar] [CrossRef]
- Jeuss Knrpnrnrcr, R. Crystal Growth from the Melt: A Review. Am. Mineral. 1975, 60, 798–814. [Google Scholar]
- Suresh, P.; Shukla, A.K.; Munichandraiah, N. Temperature dependence studies of a.c. impedance of lithium-ion cells. J. Appl. Electrochem. 2002, 32, 267–273. [Google Scholar] [CrossRef]
- Nakatani, N.; Kishida, K.; Nakagawa, K. Effect of SEI Component on Graphite Electrode Performance for Li-Ion Battery Using Ionic Liquid Electrolyte. J. Electrochem. Soc. 2018, 165, A1621–A1625. [Google Scholar] [CrossRef]
- Zurek, E.; Edwards, P.P.; Hoffmann, R. A molecular perspective on lithium-ammonia solutions. Angew. Chemie—Int. Ed. 2009, 48, 8198–8232. [Google Scholar] [CrossRef]
- Marcinek, M.; Syzdek, J.; Marczewski, M.; Piszcz, M.; Niedzicki, L.; Kalita, M.; Plewa-Marczewska, A.; Bitner, A.; Wieczorek, P.; Trzeciak, T.; et al. Electrolytes for Li-ion transport—Review. Solid State Ionics 2015, 276, 107–126. [Google Scholar] [CrossRef]
- Tripathi, A.M.; Su, W.-N.; Hwang, B.J. In situ analytical techniques for battery interface analysis. Chem. Soc. Rev. 2018, 47, 736–851. [Google Scholar] [CrossRef]
- Wang, S.; Xu, H.; Li, W.; Dolocan, A.; Manthiram, A. Interfacial Chemistry in Solid-State Batteries: Formation of Interphase and Its Consequences. J. Am. Chem. Soc. 2017, 140, 250–257. [Google Scholar] [CrossRef]
- Golozar, M.; Hovington, P.; Paolella, A.; Bessette, S.; Lagacé, S.; Bouchard, P.; Demers, H.; Gauvin, R.; Zaghib, K. In Situ Scanning Electron Microscopy Detection of Carbide Nature of Dendrites in Li−Polymer Batteries. Nano Lett. 2018, 18, 7583–7589. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Lipson, A.L.; Karmel, H.J.; Emery, J.D.; Fister, T.T.; Fenter, P.A.; Hersam, M.C.; Bedzyk, M.J. In Situ X-ray Study of the Solid Electrolyte Interphase (SEI) Formation on Graphene as a Model Li-ion Battery Anode. Chem. Mater. 2012, 24, 3038–3043. [Google Scholar] [CrossRef]
- Sacci, R.L.; Black, J.M.; Balke, N.; Dudney, N.J.; More, K.L.; Unocic, R.R. Nanoscale Imaging of Fundamental Li Battery Chemistry: Solid-Electrolyte Interphase Formation and Preferential Growth of Lithium Metal Nanoclusters. Nano Lett. 2015, 15, 2011–2018. [Google Scholar] [CrossRef]
- Tang, S.; Gu, Y.; Yi, J.; Zeng, Z.; Ding, S.-Y.; Yan, J.-W.; Wu, D.-Y.; Ren, B.; Tian, Z.-Q.; Mao, B.-W. An electrochemical surface-enhanced Raman spectroscopic study on nanorod-structured lithium prepared by electrodeposition. J. Raman Spectrosc. 2016, 47, 1017–1023. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhang, X.; Bustillo, K.; Niu, K.; Gammer, C.; Xu, J.; Zheng, H. In Situ Study of Lithiation and Delithiation of MoS2 Nanosheets Using Electrochemical Liquid Cell Transmission Electron Microscopy. Nano Lett. 2015, 15, 5214–5220. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, Y.; Han, H.-L.; Ross, P.N.; Somorjai, G.A. In Situ Potentiodynamic Analysis of the Electrolyte/Silicon Electrodes Interface Reactions—A Sum Frequency Generation Vibrational Spectroscopy Study. J. Am. Chem. Soc. 2016, 138, 726–729. [Google Scholar] [CrossRef] [Green Version]
- Nicolau, B.G.; García-Rey, N.; Dryzhakov, B.; Dlott, D.D. Interfacial Processes of a Model Lithium Ion Battery Anode Observed, in Situ, with Vibrational Sum-Frequency Generation Spectroscopy. J. Phys. Chem. C 2015, 119, 10227–10233. [Google Scholar] [CrossRef]
- Pyun, S.; Ryu, Y.G. In-situ spectroelectrochemical analysis of the passivating surface film formed on a graphite electrode during the electrochemical reduction of lithium salts and organic carbonate solvent. J. Electroanal. Chem. 1998, 455, 11–17. [Google Scholar] [CrossRef]
- Lanz, P.; Novák, P. Combined In Situ Raman and IR Microscopy at the Interface of a Single Graphite Particle with Ethylene Carbonate/Dimethyl Carbonate. J. Electrochem. Soc. 2014, 161, A1555. [Google Scholar] [CrossRef]
- Vanpeene, V.; Etiemble, A.; Bonnin, A.; Maire, E.; Roué, L. In-situ X-ray tomographic study of the morphological changes of a Si/C paper anode for Li-ion batteries. J. Power Sources 2017, 350, 18–27. [Google Scholar] [CrossRef]
- Haregewoin, A.M.; Shie, T.-D.; Lin, S.D.; Hwang, B.-J.; Wang, F.-M. An Effective In Situ Drifts Analysis of the Solid Electrolyte Interface in Lithium-Ion Battery. ECS Trans. 2013, 53, 23. [Google Scholar] [CrossRef]
- Son, I.H.; Park, J.H.; Kwon, S.; Mun, J.; Choi, J.W. Self-Terminated Artificial SEI Layer for Nickel-Rich Layered Cathode Material via Mixed Gas Chemical Vapor Deposition. Chem. Mater. 2015, 27, 7370–7379. [Google Scholar] [CrossRef]
- Li, J.-T.; Chen, S.-R.; Fan, X.-Y.; Huang, L.; Sun, S.-G. Studies of the Interfacial Properties of an Electroplated Sn Thin Film Electrode/Electrolyte Using in Situ MFTIRS and EQCM. Langmuir 2007, 23, 13174–13180. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; McLarnon, F. Spectroscopic ellipsometry of lithium/polymer electrolyte interfaces. J. Power Sources 2000, 89, 180–189. [Google Scholar] [CrossRef]
- Wenzel, S.; Leichtweiss, T.; Krüger, D.; Sann, J.; Janek, J. Interphase formation on lithium solid electrolytes—An in situ approach to study interfacial reactions by photoelectron spectroscopy. Solid State Ionics 2015, 278, 98–105. [Google Scholar] [CrossRef]
- Li, J.; Hang, S.U.; Huang, L.; Shigang, S. Investigation of interfacial processes in graphite thin film anodes of lithium-ion batteries by both in situ and ex situ infrared spectroscopy. Sci. China Chem. 2013, 56, 992–996. [Google Scholar] [CrossRef]
- Cabo-Fernandez, L.; Mueller, F.; Passerini, S.; Hardwick, L.J. In situ Raman spectroscopy of carbon-coated ZnFe 2 O 4 anode material in Li-ion batteries—Investigation of SEI growth. Chem. Commun. 2016, 52, 3970–3973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Y.-W.; Shi, P.; Li, B.-Q.; Chen, X.; Zhao, C.-X.; Chen, W.-J.; Zhang, X.-Q.; Chen, X.; Zhang, Q. Covalent Organic Frameworks Construct Precise Lithiophilic Sites for Uniform Lithium Deposition. Matter 2021, 4, 253–264. [Google Scholar] [CrossRef]
- Qin, L.; Wang, K.; Xu, H.; Zhou, M.; Yu, G.; Liu, C.; Sun, Z.; Chen, J. The role of mechanical pressure on dendritic surface toward stable lithium metal anode. Nano Energy 2020, 77, 105098. [Google Scholar] [CrossRef]
- Louli, A.J.; Genovese, M.; Weber, R.; Hames, S.G.; Logan, E.R.; Dahn, J.R. Exploring the Impact of Mechanical Pressure on the Performance of Anode-Free Lithium Metal Cells. J. Electrochem. Soc. 2019, 166, A1291. [Google Scholar] [CrossRef]
- Aryanfar, A.; Brooks, D.J.; Colussi, A.J.; Merinov, B.V.; III, W.A.G.; Hoffmann, M.R. Thermal relaxation of lithium dendrites. Phys. Chem. Chem. Phys. 2015, 17, 8000–8005. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.; Yang, Y.; Shao, H. Substrate effects on Li+ electrodeposition in Li secondary batteries with a competitive kinetics model. Phys. Chem. Chem. Phys. 2015, 17, 20398–20406. [Google Scholar] [CrossRef]
- Pei, A.; Zheng, G.; Shi, F.; Li, Y.; Cui, Y. Nanoscale Nucleation and Growth of Electrodeposited Lithium Metal. Nano Lett. 2017, 17, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, X.; Sadd, M.; Kapitanova, O.O.; Krivchenko, V.A.; Ban, J.; Wang, J.; Jiao, X.; Song, Z.; Song, J.; et al. Insight into the Critical Role of Exchange Current Density on Electrodeposition Behavior of Lithium Metal. Adv. Sci. 2021, 8, 2003301. [Google Scholar] [CrossRef] [PubMed]
- Ein-Eli, Y.; McDevitt, S.F.; Aurbach, D.; Markovsky, B.; Schechter, A. Methyl Propyl Carbonate: A Promising Single Solvent for Li-Ion Battery Electrolytes. J. Electrochem. Soc. 1997, 144, L180. [Google Scholar] [CrossRef]
- Shangguan, X.; Xu, G.; Cui, Z.; Wang, Q.; Du, X.; Chen, K.; Huang, S.; Jia, G.; Li, F.; Wang, X.; et al. Additive-Assisted Novel Dual-Salt Electrolyte Addresses Wide Temperature Operation of Lithium–Metal Batteries. Small 2019, 15, e1900269. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, D.; Qin, X.; Lin, K.; Kang, F.; Li, B.; Shanmukaraj, D.; Rojo, T.; Armand, M.; Wang, G. A room-temperature sodium–sulfur battery with high capacity and stable cycling performance. Nat. Commun. 2018, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ye, W.; Wang, H.; Ning, J.; Zhong, Y.; Hu, Y. New types of hybrid electrolytes for supercapacitors. J. Energy Chem. 2021, 57, 219–232. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, D.; Liang, Z.; Zhao, J.; Yan, K.; Cui, Y. Lithium-coated polymeric matrix as a minimum volume-change and dendrite-free lithium metal anode. Nat. Commun. 2016, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chi, S.-S.; Liu, Y.; Song, W.-L.; Fan, L.-Z.; Zhang, Q. Prestoring Lithium into Stable 3D Nickel Foam Host as Dendrite-Free Lithium Metal Anode. Adv. Funct. Mater. 2017, 27, 1700348. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, W.; Hong, L.; Xu, W.; Yang, H.; Wang, F.; Duan, H.; Tang, M.; Jiang, H. Stress-driven lithium dendrite growth mechanism and dendrite mitigation by electroplating on soft substrates. Nat. Energy 2018, 3, 227–235. [Google Scholar] [CrossRef]
- Mauger, A.; Julien, C.M.; Paolella, A.; Armand, M.; Zaghib, K. A comprehensive review of lithium salts and beyond for rechargeable batteries: Progress and perspectives. Mater. Sci. Eng. R Rep. 2018, 134, 1–21. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, K.; Zhao, Z.; Li, Q.; Ma, R.; Sasaki, T.; Geng, F. Giant two-dimensional titania sheets for constructing a flexible fiber sodium-ion battery with long-term cycling stability. Energy Storage Mater. 2020, 24, 504–511. [Google Scholar] [CrossRef]
- Deng, K.; Zeng, Q.; Wang, D.; Liu, Z.; Wang, G.; Qiu, Z.; Zhang, Y.; Xiao, M.; Meng, Y. Nonflammable organic electrolytes for high-safety lithium-ion batteries. Energy Storage Mater. 2020, 32, 425–447. [Google Scholar] [CrossRef]
- Bushkova, O.V.; Yaroslavtseva, T.V.; Dobrovolsky, Y.A. New lithium salts in electrolytes for lithium-ion batteries (Review). Russ. J. Electrochem. 2017, 53, 677–699. [Google Scholar] [CrossRef]
- Fang, Z.; Wang, J.; Wu, H.; Li, Q.; Fan, S.; Wang, J. Progress and challenges of flexible lithium ion batteries. J. Power Sources 2020, 454, 227932. [Google Scholar] [CrossRef]
- Parimalam, B.S.; Lucht, B.L. Reduction Reactions of Electrolyte Salts for Lithium Ion Batteries: LiPF6, LiBF4, LiDFOB, LiBOB, and LiTFSI. J. Electrochem. Soc. 2018, 165, A251–A255. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Gu, S.; Wang, S.; Zhang, X.; Chen, S. A LiPO2F2/LiPF6 dual-salt electrolyte enabled stable cycling performance of nickel-rich lithium ion batteries. RSC Adv. 2020, 10, 1704–1710. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Bo, S.H.; Xia, Y. Solid-electrolyte interphase formation process on Li2TiSiO5 anode in LiPF6-based carbonate electrolyte. J. Power Sources 2020, 467, 228292. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Xia, S.; Dong, P.; Jin, L.; Song, J. Challenges in the Development of Film-Forming Additives for Lithium Ion Battery: A Review. Am. J. Anal. Chem. 2013, 04, 7–12. [Google Scholar] [CrossRef] [Green Version]
- Arai, J.; Matsuo, A.; Fujisaki, T.; Ozawa, K. A novel high temperature stable lithium salt (Li2B12F12) for lithium ion batteries. J. Power Sources 2009, 193, 851–854. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, J.; Jansen, A.N.; Girishkumar, G.; Casteel, B.; Amine, K. Lithium borate cluster salts as redox shuttles for overcharge protection of lithium-ion cells. Electrochem. Solid-State Lett. 2010, 13, 4–7. [Google Scholar] [CrossRef]
- Read, J.A.; Cresce, A.V.; Ervin, M.H.; Xu, K. Dual-graphite chemistry enabled by a high voltage electrolyte. Energy Environ. Sci. 2014, 7, 617–620. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, J.; Li, Q.; Vatamanu, J.; Ji, X.; Pollard, T.P.; Cui, C.; Hou, S.; Chen, J.; Yang, C.; et al. A 63 m Superconcentrated Aqueous Electrolyte for High-Energy Li-Ion Batteries. ACS Energy Lett. 2020, 5, 968–974. [Google Scholar] [CrossRef]
- Petnikota, S.; Srikanth, V.V.S.S.; Toh, J.J.; Srinivasan, M.; Bobba, C.V.R.; Adams, S.; Reddy, M.V. Electrochemistry-related aspects of safety of graphene-based non-aqueous electrochemical supercapacitors: A case study with MgO-decorated few-layer graphene as an electrode material. New J. Chem. 2019, 43, 9793–9801. [Google Scholar] [CrossRef]
- Li, X.; Tian, Y.; Shen, L.; Qu, Z.; Ma, T.; Sun, F.; Liu, X.; Zhang, C.; Shen, J.; Li, X.; et al. Electrolyte Interphase Built from Anionic Covalent Organic Frameworks for Lithium Dendrite Suppression. Adv. Funct. Mater. 2021, 31, 2009718. [Google Scholar] [CrossRef]
- Hwang, J.; Okada, H.; Haraguchi, R.; Tawa, S.; Matsumoto, K.; Hagiwara, R. Ionic liquid electrolyte for room to intermediate temperature operating Li metal batteries: Dendrite suppression and improved performance. J. Power Sources 2020, 453, 227911. [Google Scholar] [CrossRef]
- Bakierska, M.; Świętosławski, M.; Chudzik, K.; Lis, M.; Molenda, M. Enhancing the lithium ion diffusivity in LiMn2O4−ySy cathode materials through potassium doping. Solid State Ionics 2018, 317, 190–193. [Google Scholar] [CrossRef]
- Kalluri, S.; Yoon, M.; Jo, M.; Park, S.; Myeong, S.; Kim, J.; Dou, S.X.; Guo, Z.; Cho, J. Surface Engineering Strategies of Layered LiCoO2 Cathode Material to Realize High-Energy and High-Voltage Li-Ion Cells. Adv. Energy Mater. 2017, 7. [Google Scholar] [CrossRef]
- Shetti, N.P.; Mehta, A.; Basu, S.; Mishra, A.; Malode, S.J.; Shukla, S.S.; Nadagouda, M.N.; Aminabhavi, T.M. Electrode materials for lithium-ion batteries. Mater. Sci. Technol. 2018, 1, 182–187. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Y.; Ji, S.; Tang, Y.; Chen, W.; Li, A.; Zhao, J.; Xiong, Y.; Wu, Y.; Gong, Y.; et al. Iridium single-atom catalyst on nitrogen-doped carbon for formic acid oxidation synthesized using a general host–guest strategy. Nat. Chem. 2020, 12, 764–772. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Gao, M.Y.; Liu, S.; Li, G.R.; Gao, X.P. Yttrium Surface Gradient Doping for Enhancing Structure and Thermal Stability of High-Ni Layered Oxide as Cathode for Li-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 7343–7354. [Google Scholar] [CrossRef] [PubMed]
- Thackeray, M.M. Exploiting the Spinel Structure for Li-ion Battery Applications: A Tribute to John B. Goodenough. Adv. Energy Mater. 2020, 11, 2001117. [Google Scholar] [CrossRef]
- Mosquera, N.; Bedoya-Lora, F.; Vásquez, V.; Vásquez, F.; Calderón, J. Capacity fading of high specific capacity spinel LixMn2-yTiyO4 as cathode material for Li-ion batteries. J. Appl. Electrochem. 2021, 1, 3. [Google Scholar] [CrossRef]
- Tang, D.; Zhang, W.; Qiao, Z.A.; Liu, Y.; Wang, D. Polyanthraquinone/CNT nanocomposites as cathodes for rechargeable lithium ion batteries. Mater. Lett. 2018, 214, 107–110. [Google Scholar] [CrossRef]
- Lahiru Sandaruwan, R.D.; Cong, L.; Ma, L.; Ma, S.; Wang, H. Tackling the Interfacial Issues of Spinel LiNi0.5Mn1.5O4 by Room-Temperature Spontaneous Dediazonation Reaction. ACS Appl. Mater. Interfaces 2021, 13, 13264–13272. [Google Scholar] [CrossRef] [PubMed]
- Hameed, A.S.; Reddy, M.V.; Nagarathinam, M.; Runčevski, T.; Dinnebier, R.E.; Adams, S.; Chowdari, B.V.R.; Vittal, J.J. Room temperature large-scale synthesis of layered frameworks as low-cost 4 V cathode materials for lithium ion batteries. Sci. Rep. 2015, 5, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Prabu, M.; Reddy, M.V.; Selvasekarapandian, S.; Rao, G.V.S.; Chowdari, B.V.R. Synthesis, impedance and electrochemical studies of lithium iron fluorophosphate, LiFePO4F cathode. Electrochim. Acta 2012, 85, 572–578. [Google Scholar] [CrossRef]
- Hameed, A.S.; Nagarathinam, M.; Reddy, M.V.; Chowdari, B.V.R.; Vittal, J.J. Synthesis and electrochemical studies of layer-structured metastable αI-LiVOPO4. J. Mater. Chem. 2012, 22, 7206–7213. [Google Scholar] [CrossRef]
- Chu, C.T.; Mondal, A.; Kosova, N.V.; Lin, J.Y. Improved high-temperature cyclability of AlF3 modified spinel LiNi0.5Mn1.5O4 cathode for lithium-ion batteries. Appl. Surf. Sci. 2020, 530, 147169. [Google Scholar] [CrossRef]
- Yu, R.; Wang, X.; Fu, Y.; Wang, L.; Cai, S.; Liu, M.; Lu, B.; Wang, G.; Wang, D.; Ren, Q.; et al. Effect of magnesium doping on properties of lithium-rich layered oxide cathodes based on a one-step co-precipitation strategy. J. Mater. Chem. A 2016, 4, 4941–4951. [Google Scholar] [CrossRef]
- Billaud, J.; Sheptyakov, D.; Sallard, S.; Leanza, D.; Talianker, M.; Grinblat, J.; Sclar, H.; Aurbach, D.; Novák, P.; Villevieille, C. Li/Fe substitution in Li-rich Ni, Co, Mn oxides for enhanced electrochemical performance as cathode materials. J. Mater. Chem. A 2019, 7, 15215–15224. [Google Scholar] [CrossRef]
- Otoyama, M.; Jacquet, Q.; Iadecola, A.; Saubanère, M.; Rousse, G.; Tarascon, J.M. Synthesis and Electrochemical Activity of Some Na(Li)-Rich Ruthenium Oxides with the Feasibility to Stabilize Ru6+. Adv. Energy Mater. 2019, 9, 1803674. [Google Scholar] [CrossRef]
- Xu, X.; Meng, Z.; Zhu, X.; Zhang, S.; Han, W.Q. Biomass carbon composited FeS2 as cathode materials for high-rate rechargeable lithium-ion battery. J. Power Sources 2018, 380, 12–17. [Google Scholar] [CrossRef]
- Sheil, R.; Butts, D.; Jungjohann, K.; Yoo, J.; Dunn, B.; Chang, J.P. Plasma enhanced atomic layer deposition of thin film Li1+xMn2−xO4 for realization of all solid-state 3D lithium-ion microbatteries. J. Vac. Sci. Technol. A Vacuum Surfaces Film. 2020, 39, 012408. [Google Scholar] [CrossRef]
- Mosquera, N.L.; Bedoya, F.E.; Calderon, J. Electrochemical Behaviour of High Specific Capacity Spinel LixMn2-YTiyO4 As Cathode Material for Li-Ion Batteries. ECS Meet. Abstr. 2020, MA2020-02, 82. [Google Scholar] [CrossRef]
- Cao, L.; Kou, L.; Li, J.; Huang, J.; Yang, J.; Wang, Y. Nitrogen-doped carbon-coated V2O5 nanocomposite as cathode materials for lithium-ion battery. J. Mater. Sci. 2018, 53, 10270–10279. [Google Scholar] [CrossRef]
- Jin, X.; Xu, Q.; Liu, H.; Yuan, X.; Xia, Y. Excellent rate capability of Mg doped Li[Li0.2Ni0.13Co0.13Mn0.54]O2 cathode material for lithium-ion battery. Electrochim. Acta 2014, 136, 19–26. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, J.; Peng, J.; Ou, M.; Wei, P.; Fang, C.; Li, Q.; Huang, J.; Han, J.; Huang, Y. A High Rate and Stable Hybrid Li/Na-Ion Battery Based on a Hydrated Molten Inorganic Salt Electrolyte. Small 2021, 2101650. [Google Scholar] [CrossRef]
- Liu, D.; Fan, X.; Li, Z.; Liu, T.; Sun, M.; Qian, C.; Ling, M.; Liu, Y.; Liang, C. A cation/anion co-doped Li1.12Na0.08Ni0.2Mn0.6O1.95F0.05 cathode for lithium ion batteries. Nano Energy 2019, 58, 786–796. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Ma, X.; Dahn, J.R. Synthesis of Single Crystal LiNi0.6Mn0.2Co0.2O2 with Enhanced Electrochemical Performance for Lithium Ion Batteries. J. Electrochem. Soc. 2018, 165, A1038. [Google Scholar] [CrossRef]
- Kaneko, Y.; Park, J.; Yokotsuji, H.; Odawara, M.; Takase, H.; Ue, M.; Lee, M.E. Cathode solid electrolyte interface’s function originated from salt type additives in lithium ion batteries. Electrochim. Acta 2016, 222, 271–279. [Google Scholar] [CrossRef]
- Wan, S.; Chen, S. A dithiol-based new electrolyte additive for improving electrochemical performance of NCM811 lithium ion batteries. Ionics 2020, 26, 6023–6033. [Google Scholar] [CrossRef]
- Diederichsen, K.M.; McCloskey, B.D. Electrolyte additives to enable nonaqueous polyelectrolyte solutions for lithium ion batteries. Mol. Syst. Des. Eng. 2020, 5, 91–96. [Google Scholar] [CrossRef]
- Al-Bonsrulah, H.A.Z.; Alshukri, M.J.; Mikhaeel, L.M.; Al-Sawaf, N.N.; Nesrine, K.; Reddy, M.V.; Zaghib, K. Design and simulation studies of hybrid power systems based on photovoltaic, wind, electrolyzer, and pem fuel cells. Energies 2021, 14, 2643. [Google Scholar] [CrossRef]
- Zeng, Z.; Liu, X.; Jiang, X.; Liu, Z.; Peng, Z.; Feng, X.; Chen, W.; Xia, D.; Ai, X.; Yang, H.; et al. Enabling an intrinsically safe and high-energy-density 4.5 V-class Li-ion battery with nonflammable electrolyte. InfoMat 2020, 2, 984–992. [Google Scholar] [CrossRef]
- Kim, S.; Choi, J.; Lee, H.; Jeong, Y.C.; Lee, Y.M.; Ryou, M.H. Suppression of dendrites and granules in surface-patterned Li metal anodes using CsPF6. J. Power Sources 2019, 413, 344–350. [Google Scholar] [CrossRef]
- Li, S.; Fang, S.; Dou, H.; Zhang, X. RbF as a Dendrite-Inhibiting Additive in Lithium Metal Batteries. ACS Appl. Mater. Interfaces 2019, 11, 20804–20811. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Yin, Y.X.; Zhang, S.F.; Shi, Y.; Liu, L.; Zeng, X.X.; Wen, R.; Guo, Y.G.; Wan, L.J. Synergism of Al-containing solid electrolyte interphase layer and Al-based colloidal particles for stable lithium anode. Nano Energy 2017, 36, 411–417. [Google Scholar] [CrossRef]
- Wang, D.; Liu, H.; Li, M.; Xia, D.; Holoubek, J.; Deng, Z.; Yu, M.; Tian, J.; Shan, Z.; Ong, S.P.; et al. A long-lasting dual-function electrolyte additive for stable lithium metal batteries. Nano Energy 2020, 75, 104889. [Google Scholar] [CrossRef]
- Liu, Q.C.; Xu, J.J.; Yuan, S.; Chang, Z.W.; Xu, D.; Yin, Y.B.; Li, L.; Zhong, H.X.; Jiang, Y.S.; Yan, J.M.; et al. Artificial Protection Film on Lithium Metal Anode toward Long-Cycle-Life Lithium-Oxygen Batteries. Adv. Mater. 2015, 27, 5241–5247. [Google Scholar] [CrossRef]
- Zheng, G.; Xiang, Y.; Chen, S.; Ganapathy, S.; Verhallen, T.W.; Liu, M.; Zhong, G.; Zhu, J.; Han, X.; Wang, W.; et al. Additives synergy for stable interface formation on rechargeable lithium metal anodes. Energy Storage Mater. 2020, 29, 377–385. [Google Scholar] [CrossRef]
- Zhao, Q.; Tu, Z.; Wei, S.; Zhang, K.; Choudhury, S.; Liu, X.; Archer, L.A. Building Organic/Inorganic Hybrid Interphases for Fast Interfacial Transport in Rechargeable Metal Batteries. Angew. Chemie—Int. Ed. 2018, 57, 992–996. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Wu, Y.; Chen, Y.; Jiang, C.; Zhu, S.; Zhuo, S.; Wang, C. An organic cathode with high capacities for fast-charge potassium-ion batteries. J. Mater. Chem. A 2019, 7, 486–492. [Google Scholar] [CrossRef]
- Electrodes, M.; Kuwata, H.; Sonoki, H.; Matsui, M.; Matsuda, Y.; Imanishi, N. Surface layer and morphology of lithium. Electrochemistry 2016, 84, 854–860. [Google Scholar] [CrossRef] [Green Version]
- Wan, G.; Guo, F.; Li, H.; Cao, Y.; Ai, X.; Qian, J.; Li, Y.; Yang, H. Suppression of Dendritic Lithium Growth by in Situ Formation of a Chemically Stable and Mechanically Strong Solid Electrolyte Interphase. ACS Appl. Mater. Interfaces 2018, 10, 593–601. [Google Scholar] [CrossRef]
- Yu, Y.; Yin, Y.B.; Ma, J.L.; Chang, Z.W.; Sun, T.; Zhu, Y.H.; Yang, X.Y.; Liu, T.; Zhang, X.B. Designing a self-healing protective film on a lithium metal anode for long-cycle-life lithium-oxygen batteries. Energy Storage Mater. 2019, 18, 382–388. [Google Scholar] [CrossRef]
- Ding, F.; Xu, W.; Graff, G.L.; Zhang, J.; Sushko, M.L.; Chen, X.; Shao, Y.; Engelhard, M.H.; Nie, Z.; Xiao, J.; et al. Dendrite-free lithium deposition via self-healing electrostatic shield mechanism. J. Am. Chem. Soc. 2013, 135, 4450–4456. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Wang, G.; Dang, D.; Liu, Q.; Xiong, X.; Wu, C. Sulfuryl chloride as a functional additive towards dendrite-free and long-life Li metal anodes. J. Mater. Chem. A 2019, 7, 25003–25009. [Google Scholar] [CrossRef]
- Hu, J.; Chen, K.; Li, C. Nanostructured Li-Rich Fluoride Coated by Ionic Liquid as High Ion-Conductivity Solid Electrolyte Additive to Suppress Dendrite Growth at Li Metal Anode. ACS Appl. Mater. Interfaces 2018, 10, 34322–34331. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Han, B.; Zeng, Y.; Chi, S.S.; Zeng, X.; Zheng, Z.; Xu, K.; Deng, Y. New Lithium Salt Forms Interphases Suppressing Both Li Dendrite and Polysulfide Shuttling. Adv. Energy Mater. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Chen, F.; Jing, M.; Yang, H.; Yuan, W.; Liu, M.; Ji, Y.; Hussain, S.; Shen, X. Improved ionic conductivity and Li dendrite suppression of PVDF-based solid electrolyte membrane by LLZO incorporation and mechanical reinforcement. Ionics 2021, 27, 1101–1111. [Google Scholar] [CrossRef]
- Xu, B.; Li, W.; Duan, H.; Wang, H.; Guo, Y.; Li, H.; Liu, H. Li3PO4-added garnet-type Li6.5La3Zr1.5Ta0.5O12 for Li-dendrite suppression. J. Power Sources 2017, 354, 68–73. [Google Scholar] [CrossRef]
- Gao, C.; Hong, B.; Sun, K.; Fan, H.; Zhang, K.; Zhang, Z.; Lai, Y. Self-Suppression of Lithium Dendrite with Aluminum Nitride Nanoflake Additive in 3D Carbon Paper for Lithium Metal Batteries. Energy Technol. 2020, 8, 1–7. [Google Scholar] [CrossRef]
- Shi, P.; Zhang, L.; Xiang, H.; Liang, X.; Sun, Y.; Xu, W. Lithium Difluorophosphate as a Dendrite-Suppressing Additive for Lithium Metal Batteries. ACS Appl. Mater. Interfaces 2018, 10, 22201–22209. [Google Scholar] [CrossRef]
- Ho, V.C.; Ngo, D.T.; Le, H.T.T.; Verma, R.; Kim, H.S.; Park, C.N.; Park, C.J. Effect of an organic additive in the electrolyte on suppressing the growth of Li dendrites in Li metal-based batteries. Electrochim. Acta 2018, 279, 213–223. [Google Scholar] [CrossRef]
- Chu, F.; Hu, J.; Tian, J.; Zhou, X.; Li, Z.; Li, C. In Situ Plating of Porous Mg Network Layer to Reinforce Anode Dendrite Suppression in Li-Metal Batteries. ACS Appl. Mater. Interfaces 2018, 10, 12678–12689. [Google Scholar] [CrossRef]
- Park, M.S.; Lee, J.W.; Choi, W.; Im, D.; Doo, S.G.; Park, K.S. On the surface modifications of high-voltage oxide cathodes for lithium-ion batteries: New insight and significant safety improvement. J. Mater. Chem. 2010, 20, 7208–7213. [Google Scholar] [CrossRef]
- Zheng, F.; Ou, X.; Pan, Q.; Xiong, X.; Yang, C.; Fu, Z.; Liu, M. Nanoscale gadolinium doped ceria (GDC) surface modification of Li-rich layered oxide as a high performance cathode material for lithium ion batteries. Chem. Eng. J. 2018, 334, 497–507. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, X.; Xiao, R.; Lu, X.; Li, Y.; Xu, T.; Pan, D.; Hu, Y.S.; Bai, Y. Electrochemical performance of Li-rich Li[Li0.2Mn0.56Ni0.17Co0.07]O2 cathode stabilized by metastable Li2SiO3 surface modification for advanced Li-ion batteries. Electrochim. Acta 2018, 265, 244–253. [Google Scholar] [CrossRef]
- Zheng, F.; Deng, Q.; Zhong, W.; Ou, X.; Pan, Q.; Liu, Y.; Xiong, X.; Yang, C.; Chen, Y.; Liu, M. Fluorine-Doped Carbon Surface Modification of Li-Rich Layered Oxide Composite Cathodes for High Performance Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2018, 6, 16399–16411. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Zhao, L.; Fan, Q.; Zeng, X.; Liu, S.; Pang, W.K.; He, Y.B.; Guo, Z. Lithium Metal Electrode with Increased Air Stability and Robust Solid Electrolyte Interphase Realized by Silane Coupling Agent Modification. Adv. Mater. 2021, 33, 2008133. [Google Scholar] [CrossRef]
- Chai, J.; Chen, B.; Xian, F.; Wang, P.; Du, H.; Zhang, J.; Liu, Z.; Zhang, H.; Dong, S.; Zhou, X.; et al. Dendrite-Free Lithium Deposition via Flexible-Rigid Coupling Composite Network for LiNi0.5Mn1.5O4/Li Metal Batteries. Small 2018, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhang, Y.; Han, S.; Wang, J.; Peng, Z.; Chen, L. Unlocking the Energy Capabilities of Lithium Metal Electrode with Solid-State Electrolytes. Joule 2018, 2, 1674–1689. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Hwang, S.; Jiang, M.; Liang, J.; Sun, Y.; Adair, K.; Zheng, M.; Mukherjee, S.; Li, X.; Li, R.; et al. Deciphering Interfacial Chemical and Electrochemical Reactions of Sulfide-Based All-Solid-State Batteries. Adv. Energy Mater. 2021, 11, 2100210. [Google Scholar] [CrossRef]
- Lou, S.; Zhang, F.; Fu, C.; Chen, M.; Ma, Y.; Yin, G.; Wang, J. Interface Issues and Challenges in All-Solid-State Batteries: Lithium, Sodium, and Beyond. Adv. Mater. 2021, 33, 2000721. [Google Scholar] [CrossRef]
- Li, C.; Wang, Z.-y.; He, Z.-j.; Li, Y.-j.; Mao, J.; Dai, K.-h.; Yan, C.; Zheng, J.-c. An advance review of solid-state battery: Challenges, progress and prospects. Sustain. Mater. Technol. 2021, 29, e00297. [Google Scholar] [CrossRef]
- Essoumhi, A.; Favotto, C.; Mansori, M.; Satre, P. Synthesis and characterization of a NaSICON series with general formula Na2.8Zr2−ySi1.8−4yP1.2+4yO12 (0 ≤ y ≤ 0.45). J. Solid State Chem. 2004, 177, 4475–4481. [Google Scholar] [CrossRef]
- Stramare, S.; Thangadurai, V.; Weppner, W. Lithium Lanthanum Titanates: A Review. Chem. Mater. 2003, 15, 3974–3990. [Google Scholar] [CrossRef]
- Inaguma, Y.; Chen, L.; Itoh, M.; Nakamura, T. Candidate compounds with perovskite structure for high lithium ionic conductivity. Solid State Ionics 1994, 70, 196–202. [Google Scholar] [CrossRef]
- Wang, J.; Sun, C.; Gong, Y.-D.; Zhang, H.; Alonso, J.A.; Fernández-Díaz, M.T.; Wang, Z.-L.; Goodenough, J.B. Imaging the diffusion pathway of Al3+ ion in NASICON-type (Al0.2Zr0.8)20/19Nb(PO4)3 as electrolyte for rechargeable solid-state Al batteries. Chin. Phys. B 2018, 27. [Google Scholar] [CrossRef]
- Tian, M.; Pei, F.; Yao, M.; Fu, Z.; Lin, L.; Wu, G.; Xu, G.; Kitagawa, H.; Fang, X. Ultrathin MOF nanosheet assembled highly oriented microporous membrane as an interlayer for lithium-sulfur batteries. Energy Storage Mater. 2019, 21, 14–21. [Google Scholar] [CrossRef]
- Tang, W.; Tang, S.; Zhang, C.; Ma, Q.; Xiang, Q.; Yang, Y.-W.; Luo, J. Simultaneously Enhancing the Thermal Stability, Mechanical Modulus, and Electrochemical Performance of Solid Polymer Electrolytes by Incorporating 2D Sheets. Adv. Energy Mater. 2018, 8, 1800866. [Google Scholar] [CrossRef]
- Chen, X.; Xie, J.; Lu, Y.; Zhao, X.; Zhu, T. Two-dimensional lithiophilic YFδ enabled lithium dendrite removal for quasi-solid-state lithium batteries. J. Mater. 2021, 7, 355–365. [Google Scholar] [CrossRef]
- Becking, J.; Gröbmeyer, A.; Kolek, M.; Rodehorst, U.; Schulze, S.; Winter, M.; Bieker, P.; Stan, M.C. Lithium-Metal Foil Surface Modification: An Effective Method to Improve the Cycling Performance of Lithium-Metal Batteries. Adv. Mater. Interfaces 2017, 4, 1–9. [Google Scholar] [CrossRef]
- Chen, D.; Huang, S.; Zhong, L.; Wang, S.; Xiao, M.; Han, D.; Meng, Y. In Situ Preparation of Thin and Rigid COF Film on Li Anode as Artificial Solid Electrolyte Interphase Layer Resisting Li Dendrite Puncture. Adv. Funct. Mater. 2020, 30, 1–9. [Google Scholar] [CrossRef]
- Zhai, P.; Wei, Y.; Xiao, J.; Liu, W.; Zuo, J.; Gu, X.; Yang, W.; Cui, S.; Li, B.; Yang, S.; et al. In Situ Generation of Artificial Solid-Electrolyte Interphases on 3D Conducting Scaffolds for High-Performance Lithium-Metal Anodes. Adv. Energy Mater. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Deng, K.; Han, D.; Ren, S.; Wang, S.; Xiao, M.; Meng, Y. Single-ion conducting artificial solid electrolyte interphase layers for dendrite-free and highly stable lithium metal anodes. J. Mater. Chem. A 2019, 7, 13113–13119. [Google Scholar] [CrossRef]
- Fan, L.; Guo, Z.; Zhang, Y.; Wu, X.; Zhao, C.; Sun, X.; Yang, G.; Feng, Y.; Zhang, N. Stable artificial solid electrolyte interphase films for lithium metal anode: Via metal-organic frameworks cemented by polyvinyl alcohol. J. Mater. Chem. A 2020, 8, 251–258. [Google Scholar] [CrossRef]
- Hou, G.; Ma, X.; Sun, Q.; Ai, Q.; Xu, X.; Chen, L.; Li, D.; Chen, J.; Zhong, H.; Li, Y.; et al. Lithium Dendrite Suppression and Enhanced Interfacial Compatibility Enabled by an Ex Situ SEI on Li Anode for LAGP-Based All-Solid-State Batteries. ACS Appl. Mater. Interfaces 2018, 10, 18610–18618. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, Z.; Wang, H.; Liu, W.; Lee, H.W.; Yan, K.; Zhuo, D.; Lin, D.; Liu, N.; Cui, Y. Artificial Solid Electrolyte Interphase-Protected LixSi Nanoparticles: An Efficient and Stable Prelithiation Reagent for Lithium-Ion Batteries. J. Am. Chem. Soc. 2015, 137, 8372–8375. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, D.; Yuen, P.Y.; Liu, K.; Xie, J.; Dauskardt, R.H.; Cui, Y. An Artificial Solid Electrolyte Interphase with High Li-Ion Conductivity, Mechanical Strength, and Flexibility for Stable Lithium Metal Anodes. Adv. Mater. 2017, 29, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Reyes Jiménez, A.; Nölle, R.; Wagner, R.; Hüsker, J.; Kolek, M.; Schmuch, R.; Winter, M.; Placke, T. A step towards understanding the beneficial influence of a LIPON-based artificial SEI on silicon thin film anodes in lithium-ion batteries. Nanoscale 2018, 10, 2128–2137. [Google Scholar] [CrossRef]
- Nan, Y.; Li, S.; Li, B.; Yang, S. An artificial TiO2/lithium: N-butoxide hybrid SEI layer with facilitated lithium-ion transportation ability for stable lithium anodes. Nanoscale 2019, 11, 2194–2201. [Google Scholar] [CrossRef]
- Zhong, Y.; Chen, Y.; Cheng, Y.; Fan, Q.; Zhao, H.; Shao, H.; Lai, Y.; Shi, Z.; Ke, X.; Guo, Z. Li Alginate-Based Artificial SEI Layer for Stable Lithium Metal Anodes. ACS Appl. Mater. Interfaces 2019, 11, 37726–37731. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Zhang, Z.; Chen, X.; Lie, W.; He, Y.B.; Zhou, Z.; Xia, G.; Guo, Z. Building Artificial Solid-Electrolyte Interphase with Uniform Intermolecular Ionic Bonds toward Dendrite-Free Lithium Metal Anodes. Adv. Funct. Mater. 2020, 30, 1–10. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, S.; Dong, S.; Li, W.; Li, H.; Cui, G.; Chen, L. Poly(ethyl α-cyanoacrylate)-Based Artificial Solid Electrolyte Interphase Layer for Enhanced Interface Stability of Li Metal Anodes. Chem. Mater. 2017, 29, 4682–4689. [Google Scholar] [CrossRef]
- Verma, P.; Novák, P. Formation of artificial solid electrolyte interphase by grafting for improving Li-ion intercalation and preventing exfoliation of graphite. Carbon 2012, 50, 2599–2614. [Google Scholar] [CrossRef]
- Jin, Q.; Zhang, X.; Gao, H.; Li, L.; Zhang, Z. Novel Li: XSiSy/Nafion as an artificial SEI film to enable dendrite-free Li metal anodes and high stability Li-S batteries. J. Mater. Chem. A 2020, 8, 8979–8988. [Google Scholar] [CrossRef]
- Han, B.; Feng, D.; Li, S.; Zhang, Z.; Zou, Y.; Gu, M.; Meng, H.; Wang, C.; Xu, K.; Zhao, Y.; et al. Self-Regulated Phenomenon of Inorganic Artificial Solid Electrolyte Interphase for Lithium Metal Batteries. Nano Lett. 2020, 20, 4029–4037. [Google Scholar] [CrossRef] [PubMed]
- Mery, A.; Bernard, P.; Valero, A.; Alper, J.P.; Herlin-Boime, N.; Haon, C.; Duclairoir, F.; Sadki, S. A polyisoindigo derivative as novel n-type conductive binder inside Si@C nanoparticle electrodes for Li-ion battery applications. J. Power Sources 2019, 420, 9–14. [Google Scholar] [CrossRef]
- Wang, G.; Lu, C.; Zhang, X.; Wan, B.; Liu, H.; Xia, M.; Gou, H.; Xin, G.; Lian, J.; Zhang, Y. Toward ultrafast lithium ion capacitors: A novel atomic layer deposition seeded preparation of Li4Ti5O12/graphene anode. Nano Energy 2017, 36, 46–57. [Google Scholar] [CrossRef] [Green Version]
- Zheng, H.; Xie, Y.; Xiang, H.; Shi, P.; Liang, X.; Xu, W. A bifunctional electrolyte additive for separator wetting and dendrite suppression in lithium metal batteries. Electrochim. Acta 2018, 270, 62–69. [Google Scholar] [CrossRef]
- Wu, J.; Zeng, H.; Li, X.; Xiang, X.; Liao, Y.; Xue, Z.; Ye, Y.; Xie, X. Ultralight Layer-by-Layer Self-Assembled MoS2-Polymer Modified Separator for Simultaneously Trapping Polysulfides and Suppressing Lithium Dendrites. Adv. Energy Mater. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Huo, H.; Li, X.; Chen, Y.; Liang, J.; Deng, S.; Gao, X.; Doyle-Davis, K.; Li, R.; Guo, X.; Shen, Y.; et al. Bifunctional composite separator with a solid-state-battery strategy for dendrite-free lithium metal batteries. Energy Storage Mater. 2020, 29, 361–366. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, J.; Xu, Z.; Li, H.; Guo, Y.; Liang, C.; Wang, J. Suppressing Dendrite Growth of a Lithium Metal Anode by Modifying Conventional Polypropylene Separators with a Composite Layer. ACS Appl. Energy Mater. 2020, 3, 506–513. [Google Scholar] [CrossRef]
- Li, X.; Yang, X.; Ye, J.; Xia, G.; Fu, Z.; Hu, C. A trifunctional modified separator based on Fe tetraaminophthalocyanine@rGO for lithium-sulfur batteries. Chem. Eng. J. 2021, 405, 126947. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, K.; Shen, F.; Han, X. Dendrite-suppressing separator with high thermal stability modified by beaded-chain-like polyimide coating for a Li metal anode. Energy Fuels 2021, 35, 8417–8422. [Google Scholar] [CrossRef]
- Cui, X.; Chu, Y.; Qin, L.; Pan, Q. Stabilizing Li Metal Anodes through a Novel Self-Healing Strategy. ACS Sustain. Chem. Eng. 2018, 6, 11097–11104. [Google Scholar] [CrossRef]
- Chen, N.; Dai, Y.; Xing, Y.; Wang, L.; Guo, C.; Chen, R.; Guo, S.; Wu, F. Biomimetic ant-nest ionogel electrolyte boosts the performance of dendrite-free lithium batteries. Energy Environ. Sci. 2017, 10, 1660–1667. [Google Scholar] [CrossRef]
- Xia, J.; Zhang, X.; Yang, Y.; Wang, X.; Yao, J. Electrospinning fabrication of flexible, foldable, and twistable Sb2S3/TiO2/C nanofiber anode for lithium ion batteries. Chem. Eng. J. 2021, 413, 127400. [Google Scholar] [CrossRef]
- Gardecka, A.J.; Lübke, M.; Armer, C.F.; Ning, D.; Reddy, M.V.; Williams, A.S.; Lowe, A.; Liu, Z.; Parkin, I.P.; Darr, J.A. Nb-doped rutile titanium dioxide nanorods for lithium-ion batteries. Solid State Sci. 2018, 83, 115–121. [Google Scholar] [CrossRef]
- Zhu, P.; Wu, Y.; Reddy, M.V.; Sreekumaran Nair, A.; Chowdari, B.V.R.; Ramakrishna, S. Long term cycling studies of electrospun TiO2 nanostructures and their composites with MWCNTs for rechargeable Li-ion batteries. RSC Adv. 2012, 2, 531–537. [Google Scholar] [CrossRef]
- Reddy, M.V.; Jose, R.; Teng, T.H.; Chowdari, B.V.R.; Ramakrishna, S. Preparation and electrochemical studies of electrospun TiO2 nanofibers and molten salt method nanoparticles. Electrochim. Acta 2010, 55, 3109–3117. [Google Scholar] [CrossRef]
- Cherian, C.T.; Sundaramurthy, J.; Reddy, M.V.; Suresh Kumar, P.; Mani, K.; Pliszka, D.; Sow, C.H.; Ramakrishna, S.; Chowdari, B.V.R. Morphologically robust NiFe2O4 nanofibers as high capacity Li-Ion battery anode material. ACS Appl. Mater. Interfaces 2013, 5, 9957–9963. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Duan, X.; Xu, L.; Yang, M.; Yuan, S.; Meng, C.; Wu, Q.; Wang, Q. A novel layered birnessite-type sodium molybdate as dual-ion electrodes for high capacity battery. Electrochim. Acta 2020, 363, 137229. [Google Scholar] [CrossRef]
- Eshetu, G.G.; Elia, G.A.; Armand, M.; Forsyth, M.; Komaba, S.; Rojo, T.; Passerini, S. Electrolytes and Interphases in Sodium-Based Rechargeable Batteries: Recent Advances and Perspectives. Adv. Energy Mater. 2020, 10. [Google Scholar] [CrossRef] [Green Version]
- Oh, S.M.; Myung, S.T.; Yoon, C.S.; Lu, J.; Hassoun, J.; Scrosati, B.; Amine, K.; Sun, Y.K. Advanced Na[Ni0.25Fe0.5Mn0.25]O2/C-Fe3O4 sodium-ion batteries using EMS electrolyte for energy storage. Nano Lett. 2014, 14, 1620–1626. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhang, J.; Lin, D.; Wang, D.W.; Li, B.; Lv, W.; Sun, S.; He, Y.B.; Kang, F.; Yang, Q.H.; et al. Evolution of the electrochemical interface in sodium ion batteries with ether electrolytes. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ponrouch, A.; Marchante, E.; Courty, M.; Tarascon, J.M.; Palacín, M.R. In search of an optimized electrolyte for Na-ion batteries. Energy Environ. Sci. 2012, 5, 8572–8583. [Google Scholar] [CrossRef]
- Ren, X.; Wang, B.; Zhu, J.; Liu, J.; Zhang, W.; Wen, Z. The doping effect on the catalytic activity of graphene for oxygen evolution reaction in a lithium-air battery: A first-principles study. Phys. Chem. Chem. Phys. 2015, 17, 14605–14612. [Google Scholar] [CrossRef]
- Ma, J.-L.; Zhang, W.-C.; Wang, X.-D.; Tang, M.; Huang, Z.-Y.; Li, J.; Zhang, H.; Yang, X.-H.; Guo, Z.-P.; Wang, Y. Revealing the mechanism of saturated ether electrolyte for improving the long-cycling stability of Na-O2 batteries. Nano Energy 2021, 84, 105927. [Google Scholar] [CrossRef]
- Takeuchi, S.; Suzuki, K.; Hirayama, M.; Kanno, R. Sodium superionic conduction in tetragonal Na3PS4. J. Solid State Chem. 2018, 265, 353–358. [Google Scholar] [CrossRef]
- Liu, X.; Lei, X.; Wang, Y.-G.; Ding, Y. Prevention of Na Corrosion and Dendrite Growth for Long-Life Flexible Na−Air Batteries. ACS Cent. Sci. 2021, 7, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Hasa, I.; Dou, X.; Buchholz, D.; Shao-Horn, Y.; Hassoun, J.; Passerini, S.; Scrosati, B. A sodium-ion battery exploiting layered oxide cathode, graphite anode and glyme-based electrolyte. J. Power Sources 2016, 310, 26–31. [Google Scholar] [CrossRef]
- Zhao, W.; Zheng, J.; Zou, L.; Jia, H.; Liu, B.; Wang, H.; Engelhard, M.H.; Wang, C.; Xu, W.; Yang, Y.; et al. High Voltage Operation of Ni-Rich NMC Cathodes Enabled by Stable Electrode/Electrolyte Interphases. Adv. Energy Mater. 2018, 8, 1800297. [Google Scholar] [CrossRef]
- Guo, Y.J.; Niu, Y.B.; Wei, Z.; Zhang, S.Y.; Meng, Q.; Li, H.; Yin, Y.X.; Guo, Y.G. Insights on electrochemical behaviors of sodium peroxide as a sacrificial cathode additive for boosting energy density of Na-ion battery. ACS Appl. Mater. Interfaces 2021, 13, 2772–2778. [Google Scholar] [CrossRef] [PubMed]
- Fondard, J.; Irisarri, E.; Courrèges, C.; Palacin, M.R.; Ponrouch, A.; Dedryvère, R. SEI Composition on Hard Carbon in Na-Ion Batteries After Long Cycling: Influence of Salts (NaPF6, NaTFSI) and Additives (FEC, DMCF). J. Electrochem. Soc. 2020, 167, 070526. [Google Scholar] [CrossRef]
- Li, T.; Li, H.; Qin, A.; Wu, H.; Zhang, D.; Xu, F. Assembled NiS nanoneedles anode for Na-ion batteries: Enhanced the performance by organic hyperbranched polymer electrode additives. J. Power Sources 2020, 451, 227796. [Google Scholar] [CrossRef]
- Liu, X.; Ma, W.; Lei, X.; Zhang, S.; Ding, Y. Rechargeable Na–SO2 Battery with Ethylenediamine Additive in Ether-Based Electrolyte. Adv. Funct. Mater. 2020, 30, 1–8. [Google Scholar] [CrossRef]
- Kim, D.H.; Kang, B.; Lee, H. Comparative study of fluoroethylene carbonate and succinic anhydride as electrolyte additive for hard carbon anodes of Na-ion batteries. J. Power Sources 2019, 423, 137–143. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Z.; Li, X.; Guo, H.; Wang, J.; Yan, G. Unraveling the role of LiODFB salt as a SEI-forming additive for sodium-ion battery. Ionics 2021, 27, 683–691. [Google Scholar] [CrossRef]
- Jo, J.H.; Choi, J.U.; Park, Y.J.; Ko, J.K.; Yashiro, H.; Myung, S.-T. A new pre-sodiation additive for sodium-ion batteries. Energy Storage Mater. 2020, 32, 281–289. [Google Scholar] [CrossRef]
- Park, K.; Cho, J.H.; Shanmuganathan, K.; Song, J.; Peng, J.; Gobet, M.; Greenbaum, S.; Ellison, C.J.; Goodenough, J.B. New battery strategies with a polymer/Al2O3 separator. J. Power Sources 2014, 263, 52–58. [Google Scholar] [CrossRef]
- Janakiraman, S.; Surendran, A.; Ghosh, S.; Anandhan, S.; Venimadhav, A. Electroactive poly(vinylidene fluoride) fluoride separator for sodium ion battery with high coulombic efficiency. Solid State Ionics 2016, 292, 130–135. [Google Scholar] [CrossRef]
- Tu, N.D.K.; Park, J.; Na, S.; Kim, K.M.; Kwon, T.H.; Ko, H.; Kang, S.J. Co-solvent induced piezoelectric γ-phase nylon-11 separator for sodium metal battery. Nano Energy 2020, 70, 104501. [Google Scholar] [CrossRef]
- Zhang, L.; Feng, G.; Li, X.; Cui, S.; Ying, S.; Feng, X.; Mi, L.; Chen, W. Synergism of surface group transfer and in-situ growth of silica-aerogel induced high-performance modified polyacrylonitrile separator for lithium/sodium-ion batteries. J. Memb. Sci. 2019, 577, 137–144. [Google Scholar] [CrossRef]
- Casas, X.; Niederberger, M.; Lizundia, E. A Sodium-Ion Battery Separator with Reversible Voltage Response Based on Water-Soluble Cellulose Derivatives. ACS Appl. Mater. Interfaces 2020, 12, 29264–29274. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Singh, S.K.; Gupta, H.; Srivastava, N.; Meghnani, D.; Tiwari, R.K.; Patel, A.; Tiwari, A.; Tiwari, V.K.; Singh, R.K. Surface modification of nano Na[Ni0.60Mn0.35Co0.05]O2 cathode material by dextran functionalized RGO via hydrothermal treatment for high performance sodium batteries. Appl. Surf. Sci. 2021, 535, 147695. [Google Scholar] [CrossRef]
- Cao, C.; Wang, H.; Liu, W.; Liao, X.; Li, L. Nafion membranes as electrolyte and separator for sodium-ion battery. Int. J. Hydrogen Energy 2014, 39, 16110–16115. [Google Scholar] [CrossRef]
- Hayashi, K.; Shima, K.; Sugiyama, F. A Mixed Aqueous/Aprotic Sodium/Air Cell Using a NASICON Ceramic Separator. J. Electrochem. Soc. 2013, 160, A1467–A1472. [Google Scholar] [CrossRef]
- Yang, H.; Sun, J.; Wang, H.; Liang, J.; Li, H. A titanium dioxide nanoparticle sandwiched separator for Na-O2 batteries with suppressed dendrites and extended cycle life. Chem. Commun. 2018, 54, 4057–4060. [Google Scholar] [CrossRef]
- Mao, Q.; Zhang, C.; Yang, W.; Yang, J.; Sun, L.; Hao, Y.; Liu, X. Mitigating the voltage fading and lattice cell variations of O3-NaNi0.2Fe0.35Mn0.45O2 for high performance Na-ion battery cathode by Zn doping. J. Alloys Compd. 2019, 794, 509–517. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, X.; Zhang, A.; Shen, C.; Liu, Q.; Enaya, H.A.; Zhou, C. Layered P2-Na2/3[Ni1/3Mn2/3]O2 as high-voltage cathode for sodium-ion batteries: The capacity decay mechanism and Al2O3 surface modification. Nano Energy 2016, 27, 27–34. [Google Scholar] [CrossRef]
- Kong, D.; Wang, Y.; Huang, S.; Lim, Y.V.; Zhang, J.; Sun, L.; Liu, B.; Chen, T.; Valdivia Y Alvarado, P.; Yang, H.Y. Surface modification of Na2Ti3O7 nanofibre arrays using N-doped graphene quantum dots as advanced anodes for sodium-ion batteries with ultra-stable and high-rate capability. J. Mater. Chem. A 2019, 7, 12751–12762. [Google Scholar] [CrossRef]
- Xiao, N.; Zheng, J.; Gourdin, G.; Schkeryantz, L.; Wu, Y. Anchoring an Artificial Protective Layer to Stabilize Potassium Metal Anode in Rechargeable K-O2 Batteries. ACS Appl. Mater. Interfaces 2019, 11, 16571–16577. [Google Scholar] [CrossRef]
- Zhang, D.; Li, B.; Wang, S.; Yang, S. Simultaneous Formation of Artificial SEI Film and 3D Host for Stable Metallic Sodium Anodes. ACS Appl. Mater. Interfaces 2017, 9, 40265–40272. [Google Scholar] [CrossRef] [PubMed]
- Moeez, I.; Susanto, D.; Chang, W.; Lim, H.D.; Chung, K.Y. Artificial cathode electrolyte interphase by functional additives toward long-life sodium-ion batteries. Chem. Eng. J. 2021, 425, 130547. [Google Scholar] [CrossRef]
- Choi, M.J.; Kim, J.; Yoo, J.K.; Yim, S.; Jeon, J.; Jung, Y.S. Extremely Small Pyrrhotite Fe7S8 Nanocrystals with Simultaneous Carbon-Encapsulation for High-Performance Na–Ion Batteries. Small 2018, 14, 1–6. [Google Scholar] [CrossRef]
- Ren, X.; Ren, X.; Zhao, Y.; Li, Q.; Cheng, F.; Wen, W.; Zhang, L.; Huang, Y.; Xia, X.; Li, X.; et al. A novel multielement nanocomposite with ultrahigh rate capacity and durable performance for sodium-ion battery anodes. J. Mater. Chem. A 2020, 8, 11598–11606. [Google Scholar] [CrossRef]
- Wu, T.; Hou, H.; Zhang, C.; Ge, P.; Huang, Z.; Jing, M.; Qiu, X.; Ji, X. Antimony Anchored with Nitrogen-Doping Porous Carbon as a High-Performance Anode Material for Na-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 26118–26125. [Google Scholar] [CrossRef]
- Wang, G.; Yu, F.; Zhang, Y.; Zhang, Y.; Zhu, M.; Xu, G.; Wu, M.; Liu, H.K.; Dou, S.X.; Wu, C. 2D Sn/C freestanding frameworks as a robust nucleation layer for highly stable sodium metal anodes with a high utilization. Nano Energy 2021, 79, 105457. [Google Scholar] [CrossRef]
- Wang, H.; Matios, E.; Wang, C.; Luo, J.; Lu, X.; Hu, X.; Zhang, Y.; Li, W. Tin nanoparticles embedded in a carbon buffer layer as preferential nucleation sites for stable sodium metal anodes. J. Mater. Chem. A 2019, 7, 23747–23755. [Google Scholar] [CrossRef]
- Qin, G.; Liu, Y.; Han, P.; Wang, L.; Liu, F.; Ma, J. Self-Regulating Organic Polymer Coupled with Enlarged Inorganic SnS2 Interlamellar Composite for Potassium Ion Batteries. Adv. Funct. Mater. 2020, 30, 1–12. [Google Scholar] [CrossRef]
- Tsai, P.C.; Chung, S.C.; Lin, S.K.; Yamada, A. Ab initio study of sodium intercalation into disordered carbon. J. Mater. Chem. A 2015, 3, 9763–9768. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, L.; Chihara, K.; Okada, S.; Yamaki, J.I.; Matsumoto, S.; Kuze, S.; Nakane, K. Electrochemical and thermal properties of hard carbon-type anodes for Na-ion batteries. J. Power Sources 2013, 244, 752–757. [Google Scholar] [CrossRef]
- Alcántara, R.; Jiménez Mateos, J.M.; Tirado, J.L. Negative Electrodes for Lithium- and Sodium-Ion Batteries Obtained by Heat-Treatment of Petroleum Cokes below 1000 °C. J. Electrochem. Soc. 2002, 149, A201. [Google Scholar] [CrossRef]
- Jin, J.; Yu, B.J.; Shi, Z.Q.; Wang, C.Y.; Chong, C. Bin Lignin-based electrospun carbon nanofibrous webs as free-standing and binder-free electrodes for sodium ion batteries. J. Power Sources 2014, 272, 800–807. [Google Scholar] [CrossRef]
- Li, W.; Zeng, L.; Yang, Z.; Gu, L.; Wang, J.; Liu, X.; Cheng, J.; Yu, Y. Free-standing and binder-free sodium-ion electrodes with ultralong cycle life and high rate performance based on porous carbon nanofibers. Nanoscale 2014, 6, 693–698. [Google Scholar] [CrossRef]
- Luo, W.; Schardt, J.; Bommier, C.; Wang, B.; Razink, J.; Simonsen, J.; Ji, X. Carbon nanofibers derived from cellulose nanofibers as a long-life anode material for rechargeable sodium-ion batteries. J. Mater. Chem. A 2013, 1, 10662–10666. [Google Scholar] [CrossRef]
- Wang, Z.; Qie, L.; Yuan, L.; Zhang, W.; Hu, X.; Huang, Y. Functionalized N-doped interconnected carbon nanofibers as an anode material for sodium-ion storage with excellent performance. Carbon 2013, 55, 328–334. [Google Scholar] [CrossRef]
- Tang, K.; Fu, L.; White, R.J.; Yu, L.; Titirici, M.M.; Antonietti, M.; Maier, J. Hollow carbon nanospheres with superior rate capability for sodium-based batteries. Adv. Energy Mater. 2012, 2, 873–877. [Google Scholar] [CrossRef]
- Shao, Y.; Xiao, J.; Wang, W.; Engelhard, M.; Chen, X.; Nie, Z.; Gu, M.; Saraf, L.V.; Exarhos, G.; Zhang, J.G.; et al. Surface-driven sodium ion energy storage in nanocellular carbon foams. Nano Lett. 2013, 13, 3909–3914. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; He, K.; Zhu, Y.; Han, F.; Xu, Y.; Matsuda, I.; Ishii, Y.; Cumings, J.; Wang, C. Expanded graphite as superior anode for sodium-ion batteries. Nat. Commun. 2014, 5, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.M.; Abel, P.R.; Gupta, A.; Goodenough, J.B.; Heller, A.; Mullins, C.B. Sn-Cu nanocomposite anodes for rechargeable sodium-ion batteries. ACS Appl. Mater. Interfaces 2013, 5, 8273–8277. [Google Scholar] [CrossRef]
- Kim, I.T.; Allcorn, E.; Manthiram, A. Cu6Sn5-TiC-C nanocomposite anodes for high-performance sodium-ion batteries. J. Power Sources 2015, 281, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Darwiche, A.; Dugas, R.; Fraisse, B.; Monconduit, L. Reinstating lead for high-loaded efficient negative electrode for rechargeable sodium-ion battery. J. Power Sources 2016, 304, 1–8. [Google Scholar] [CrossRef]
- Su, D.; Dou, S.; Wang, G. Anatase TiO2: Better Anode Material Than Amorphous and Rutile Phases of TiO2 for Na-Ion Batteries. Chem. Mater. 2015, 27, 6022–6029. [Google Scholar] [CrossRef]
- Kjeldgaard, S.; Dugulan, I.; Mamakhel, A.; Wagemaker, M.; Iversen, B.B.; Bentien, A. Strategies for synthesis of Prussian blue analogues. R. Soc. Open Sci. 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Pang, H. Recent advancements in Prussian blue analogues: Preparation and application in batteries. Energy Storage Mater. 2021, 36, 387–408. [Google Scholar] [CrossRef]
- Xie, B.; Wang, L.; Li, H.; Huo, H.; Cui, C.; Sun, B.; Ma, Y.; Wang, J.; Yin, G.; Zuo, P. An interface-reinforced rhombohedral Prussian blue analogue in semi-solid state electrolyte for sodium-ion battery. Energy Storage Mater. 2021, 36, 99–107. [Google Scholar] [CrossRef]
- Qiu, S.; Xu, Y.; Wu, X.; Ji, X. Prussian Blue Analogues as Electrodes for Aqueous Monovalent Ion Batteries. Electrochem. Energy Rev. 2021, 1, 3. [Google Scholar]
- Han, J.; Lin, Y.; Yang, Y.; Zuo, D.; Wang, C.; Liu, X. Dominant role of M element on the stability and properties of Prussian blue analogues NaxMFe(CN)6 (M = 3d transition metal) as cathode material for the sodium-ion batteries. J. Alloys Compd. 2021, 870, 159533. [Google Scholar] [CrossRef]
- Zhang, G.; Shu, J.; Xu, L.; Cai, X.; Zou, W.; Du, L.; Hu, S.; Mai, L. Pancake-Like MOF Solid-State Electrolytes with Fast Ion Migration for High-Performance Sodium Battery. Nano-Micro Lett. 2021, 13, 1–12. [Google Scholar] [CrossRef]
- Ran, L.; Li, M.; Cooper, E.; Luo, B.; Gentle, I.; Wang, L.; Knibbe, R. Enhanced Safety and Performance of High-Voltage Solid-State Sodium Battery through Trilayer, Multifunctional Electrolyte Design. Energy Storage Mater. 2021, 41, 8–13. [Google Scholar] [CrossRef]
- Zhang, T.; Ran, F. Design Strategies of 3D Carbon-Based Electrodes for Charge/Ion Transport in Lithium Ion Battery and Sodium Ion Battery. Adv. Funct. Mater. 2021, 31, 2010041. [Google Scholar] [CrossRef]
- Salehi, A.H.; Masoudpanah, S.M.; Hasheminiasari, M.; Yaghtin, A.; Safanama, D.; Ong, C.K.; Reddy, M.V.; Adams, S. A solution synthesis of Na3V2(PO4)3 cathode for sodium storage by using CTAB additive. Solid State Ionics 2020, 347. [Google Scholar] [CrossRef]
- Ma, J.-L.; Meng, F.-L.; Yu, Y.; Liu, D.-P.; Yan, J.-M.; Zhang, Y.; Zhang, X.-B.; Jiang, Q. Prevention of dendrite growth and volume expansion to give high-performance aprotic bimetallic Li-Na alloy–O2 batteries. Nat. Chem. 2018, 11, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Ao, X.; Wen, Z.; Hu, Y.; Wu, T.; Wu, X.; He, Q. Enhanced cycle performance of a Na/NiCl2 battery based on Ni particles encapsulated with Ni3S2 layer. J. Power Sources 2017, 340, 411–418. [Google Scholar] [CrossRef]
- Wang, J.; Hu, Y.; Li, Y.; Gao, X.; Wu, X.; Wen, Z. Reversible AlCl4−/Al2Cl7− conversion in a hybrid Na–Al battery. J. Power Sources 2020, 453. [Google Scholar] [CrossRef]
- Lee, Y.; Jo, C.H.; Yoo, J.K.; Choi, J.U.; Ko, W.; Park, H.; Jo, J.H.; Shin, D.O.; Myung, S.T.; Kim, J. New conversion chemistry of CuSO4 as ultra-high-energy cathode material for rechargeable sodium battery. Energy Storage Mater. 2020, 24, 458–466. [Google Scholar] [CrossRef]
- Zhang, T.; Feng, Y.; Zhang, J.; He, C.; Itkis, D.M.; Song, J. Ultrahigh-rate sodium-ion battery anode enabled by vertically aligned (1T-2H MoS2)/CoS2 heteronanosheets. Mater. Today Nano 2020, 12, 100089. [Google Scholar] [CrossRef]
- Jia, H.; Peng, L.; Zhang, Z.; An, T.; Xie, J. Na3.8[Sn0.67Si0.33]0.8Sb0.2S4: A quinary sodium fast ionic conductor for all-solid-state sodium battery. J. Energy Chem. 2020, 48, 102–106. [Google Scholar] [CrossRef]
- Lu, Q.; Wang, X.; Omar, A.; Mikhailova, D. 3D Ni/Na metal anode for improved sodium metal batteries. Mater. Lett. 2020, 275, 128206. [Google Scholar] [CrossRef]
- Lu, Y.; Lu, L.; Qiu, G.; Sun, C. Flexible Quasi-Solid-State Sodium Battery for Storing Pulse Electricity Harvested from Triboelectric Nanogenerators. ACS Appl. Mater. Interfaces 2020, 12, 39342–39351. [Google Scholar] [CrossRef]
- Pham, T.A.; Kweon, K.E.; Samanta, A.; Lordi, V.; Pask, J.E. Solvation and Dynamics of Sodium and Potassium in Ethylene Carbonate from ab Initio Molecular Dynamics Simulations. J. Phys. Chem. C 2017, 121, 21913–21920. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, C.; Dai, L.; Deng, Q.; Wang, L.; Luo, J.; Liu, S.; Hu, N. The Features and Progress of Electrolyte for Potassium Ion Batteries. Small 2020, 16, 1–13. [Google Scholar] [CrossRef]
- Jian, Z.; Luo, W.; Ji, X. Carbon Electrodes for K-Ion Batteries. J. Am. Chem. Soc. 2015, 137, 11566–11569. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, J.; Ma, L.; Yu, C.; Ji, Z.; Pan, L.; Mai, W. Graphite Anode for Potassium Ion batteries: Current Status and Perspective. Energy Environ. Mater. 2021. [Google Scholar] [CrossRef]
- Gao, X.; Dong, X.; Xing, Z.; Nie, C.; Zheng, G.; Ju, Z. Electrolyte Salt Chemistry Enables 3D Nitrogen and Phosphorus Dual-Doped Graphene Aerogels for High-Performance Potassium-Ion Batteries. Adv. Mater. Technol. 2021, 2100207. [Google Scholar] [CrossRef]
- Komaba, S.; Hasegawa, T.; Dahbi, M.; Kubota, K. Potassium intercalation into graphite to realize high-voltage/high-power potassium-ion batteries and potassium-ion capacitors. Electrochem. Commun. 2015, 60, 172–175. [Google Scholar] [CrossRef] [Green Version]
- Niu, X.; Li, L.; Qiu, J.; Yang, J.; Huang, J.; Wu, Z.; Zou, J.; Jiang, C.; Gao, J.; Wang, L. Salt-concentrated electrolytes for graphite anode in potassium ion battery. Solid State Ionics 2019, 341, 115050. [Google Scholar] [CrossRef]
- Liu, X.; Elia, G.A.; Gao, X.; Qin, B.; Zhang, H.; Passerini, S. Highly Concentrated KTFSI: Glyme Electrolytes for K/Bilayered-V2O5 Batteries. Batter. Supercaps 2020, 3, 261–267. [Google Scholar] [CrossRef] [Green Version]
- Jian, Z.; Liang, Y.; Pérez, I.A.R.; Yao, Y.; Ji, X. Poly(anthraquinonyl sulfide) cathode for potassium-ion batteries. Electrochem. Commun. 2016, 71, 5–8. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Kang, L.; Jun, S.C. Challenges and Strategies toward Cathode Materials for Rechargeable Potassium-Ion Batteries. Adv. Mater. 2021, 2004689. [Google Scholar] [CrossRef]
- Verma, R.; Didwal, P.N.; Hwang, J.; Park, C. Recent Progress in Electrolyte Development and Design Strategies for Next-Generation Potassium-Ion Batteries. Batter. Supercaps 2021. [Google Scholar] [CrossRef]
- Wang, L.; Yang, J.; Li, J.; Chen, T.; Chen, S.; Wu, Z.; Qiu, J.; Wang, B.; Gao, P.; Niu, X.; et al. Graphite as a potassium ion battery anode in carbonate-based electrolyte and ether-based electrolyte. J. Power Sources 2019, 409, 24–30. [Google Scholar] [CrossRef]
- Wang, L.; Zou, J.; Chen, S.; Zhou, G.; Bai, J.; Gao, P.; Wang, Y.; Yu, X.; Li, J.; Hu, Y.S.; et al. TiS2 as a high performance potassium ion battery cathode in ether-based electrolyte. Energy Storage Mater. 2018, 12, 216–222. [Google Scholar] [CrossRef]
- Yang, H.; Chen, C.Y.; Hwang, J.; Kubota, K.; Matsumoto, K.; Hagiwara, R. Potassium Difluorophosphate as an Electrolyte Additive for Potassium-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 36168–36176. [Google Scholar] [CrossRef]
- Wu, X.; Xing, Z.; Hu, Y.; Zhang, Y.; Sun, Y.; Ju, Z.; Liu, J.; Zhuang, Q. Effects of functional binders on electrochemical performance of graphite anode in potassium-ion batteries. Ionics 2019, 25, 2563–2574. [Google Scholar] [CrossRef]
- Liu, G.; Cao, Z.; Zhou, L.; Zhang, J.; Sun, Q.; Hwang, J.Y.; Cavallo, L.; Wang, L.; Sun, Y.K.; Ming, J. Additives Engineered Nonflammable Electrolyte for Safer Potassium Ion Batteries. Adv. Funct. Mater. 2020, 30, 1–8. [Google Scholar] [CrossRef]
- Zhou, L.; Cao, Z.; Zhang, J.; Cheng, H.; Liu, G.; Park, G.T.; Cavallo, L.; Wang, L.; Alshareef, H.N.; Sun, Y.K.; et al. Electrolyte-Mediated Stabilization of High-Capacity Micro-Sized Antimony Anodes for Potassium-Ion Batteries. Adv. Mater. 2021, 33, 2005993. [Google Scholar] [CrossRef]
- Zhang, C.; Qiao, Y.; Xiong, P.; Ma, W.; Bai, P.; Wang, X.; Li, Q.; Zhao, J.; Xu, Y.; Chen, Y.; et al. Conjugated Microporous Polymers with Tunable Electronic Structure for High-Performance Potassium-Ion Batteries. ACS Nano 2019, 13, 745–754. [Google Scholar] [CrossRef]
- Liu, Q.; Rao, A.M.; Han, X.; Lu, B. Artificial SEI for Superhigh-Performance K-Graphite Anode. Adv. Sci. 2021, 8, 2003639. [Google Scholar] [CrossRef]
- Wang, H.; Hu, J.; Dong, J.; Lau, K.C.; Qin, L.; Lei, Y.; Li, B.; Zhai, D.; Wu, Y.; Kang, F. Artificial Solid-Electrolyte Interphase Enabled High-Capacity and Stable Cycling Potassium Metal Batteries. Adv. Energy Mater. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Li, J.; Liu, S.; Cui, Y.; Zhang, S.; Wu, X.; Xiang, J.; Li, M.; Wang, X.; Xia, X.; Gu, C.; et al. Potassium Hexafluorophosphate Additive Enables Stable Lithium-Sulfur Batteries. ACS Appl. Mater. Interfaces 2020, 12, 56017–56026. [Google Scholar] [CrossRef]
- Gu, M.; Fan, L.; Zhou, J.; Rao, A.M.; Lu, B. Regulating Solvent Molecule Coordination with KPF6for Superstable Graphite Potassium Anodes. ACS Nano 2021. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Q.; Yuan, Y.; Liu, H.; Yang, C.; Lin, Z.; Lu, J. Surface Amorphization of Vanadium Dioxide (B) for K-Ion Battery. Adv. Energy Mater. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Zhang, W.; Jin, H.; Du, Y.; Zhang, J. Semi-liquid anode for dendrite-free K-ion and Na-ion batteries. Chem. Eng. J. 2021, 412, 128597. [Google Scholar] [CrossRef]
- Kandeeban, R.; Brindha, R.; Manojkumar, K.; Batoo, K.M.; Raslan, E.H.; Hadi, M.; Imran, A.; Saminathan, K. Revealing the synergetic electrocatalyst behaviour of Kish graphite recovered from polyethylene plastics. Mater. Lett. 2021, 297, 129740. [Google Scholar] [CrossRef]
- Tao, L.; Liu, L.; Chang, R.; He, H.; Zhao, P.; Liu, J. Structural and interface design of hierarchical porous carbon derived from soybeans as anode materials for potassium-ion batteries. J. Power Sources 2020, 463, 228172. [Google Scholar] [CrossRef]
- Ramakrishna, S. Circular economy and sustainability pathways to build a new-modern society. Dry. Technol. 2021, 39, 711–712. [Google Scholar] [CrossRef]
- Rezakazemi, M.; Arabi Shamsabadi, A.; Lin, H.; Luis, P.; Ramakrishna, S.; Aminabhavi, T.M. Sustainable MXenes-based membranes for highly energy-efficient separations. Renew. Sustain. Energy Rev. 2021, 143, 110878. [Google Scholar] [CrossRef]
- Lau, H.C.; Ramakrishna, S.; Zhang, K.; Radhamani, A.V. The role of carbon capture and storage in the energy transition. Energy Fuels 2021, 35, 7364–7386. [Google Scholar] [CrossRef]
- Das, S.K.; Eshkalak, S.K.; Chinnappan, A.; Ghosh, R.; Jayathilaka, W.A.D.M.; Baskar, C.; Ramakrishna, S. Plastic Recycling of Polyethylene Terephthalate (PET) and Polyhydroxybutyrate (PHB)—A Comprehensive Review. Mater. Circ. Econ. 2021, 3, 3. [Google Scholar] [CrossRef]
- Lu, X.; Bowden, M.E.; Sprenkle, V.L.; Liu, J. A Low Cost, High Energy Density, and Long Cycle Life Potassium-Sulfur Battery for Grid-Scale Energy Storage. Adv. Mater. 2015, 27, 5915–5922. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.K.; Brindha, R.; Nandhini, M.; Selvam, M.; Saminathan, K.; Sakthipandi, K. Water-suspended graphene as electrolyte additive in zinc-air alkaline battery system. Ionics 2019, 25, 1699–1706. [Google Scholar] [CrossRef]
- Brindha, R.; Ajith, S.S.R.; Nandhini, M.; Selvam, M.; Subannajui, K.; Khotmungkhun, K.; Sakthipandi, K. Evaluation of anticorrosive behaviour of ZnO nanotetra-pods on a AZ91-grade Mg alloy. Bull. Mater. Sci. 2019, 42, 221. [Google Scholar] [CrossRef] [Green Version]
- Gnanaraj, J.S.; Levi, M.D.; Gofer, Y.; Aurbach, D.; Schmidt, M. LiPF3(CF2CF3)3: A Salt for Rechargeable Lithium Ion Batteries. J. Electrochem. Soc. 2003, 150, A445. [Google Scholar] [CrossRef]
- Lee, B.; Cho, J.H.; Seo, H.R.; Na, S.B.; Kim, J.H.; Cho, B.W.; Yim, T.; Oh, S.H. Strategic combination of Grignard reagents and allyl-functionalized ionic liquids as an advanced electrolyte for rechargeable magnesium batteries. J. Mater. Chem. A 2018, 6, 3126–3133. [Google Scholar] [CrossRef]
- Carter, T.J.; Mohtadi, R.; Arthur, T.S.; Mizuno, F.; Zhang, R.; Shirai, S.; Kampf, J.W. Boron Clusters as Highly Stable Magnesium-Battery Electrolytes. Angew. Chemie 2014, 126, 3237–3241. [Google Scholar] [CrossRef] [Green Version]
- Brindha, R.; Mohanraj, R.; Manojkumar, P.; Selvam, M.; Sakthipandi, K. Hybrid Electrochemical Behaviour of La1-xCaxMnO3 Nano Perovskites and Recycled Polar Interspersed Graphene for Metal-Air Battery System. J. Electrochem. Soc. 2020, 167, 120539. [Google Scholar] [CrossRef]
- Long, Y.; Li, H.; Ye, M.; Chen, Z.; Wang, Z.; Tao, Y.; Weng, Z.; Qiao, S.Z.; Yang, Q.H. Suppressing Al dendrite growth towards a long-life Al-metal battery. Energy Storage Mater. 2021, 34, 194–202. [Google Scholar] [CrossRef]
- Guan, Q.; Li, Y.; Bi, X.; Yang, J.; Zhou, J.; Li, X.; Cheng, J.; Wang, Z.; Wang, B.; Lu, J. Dendrite-Free Flexible Fiber-Shaped Zn Battery with Long Cycle Life in Water and Air. Adv. Energy Mater. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Pu, S.D.; Gong, C.; Gao, X.; Ning, Z.; Yang, S.; Marie, J.J.; Liu, B.; House, R.A.; Hartley, G.O.; Luo, J.; et al. Current-Density-Dependent Electroplating in Ca Electrolytes: From Globules to Dendrites. ACS Energy Lett. 2020, 5, 2283–2290. [Google Scholar] [CrossRef]
- Er, D.; Li, J.; Naguib, M.; Gogotsi, Y.; Shenoy, V.B. Ti3C2 MXene as a high capacity electrode material for metal (Li, Na, K, Ca) ion batteries. ACS Appl. Mater. Interfaces 2014, 6, 11173–11179. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.G.; Arulraj, A.; Khalid, M.; Reddy, M.V.; Jose, R. Energy storage in metal cobaltite electrodes: Opportunities & challenges in magnesium cobalt oxide. Renew. Sustain. Energy Rev. 2021, 141, 110798. [Google Scholar]
- Lyu, Z.; Lim, G.J.H.; Guo, R.; Pan, Z.; Zhang, X.; Zhang, H.; He, Z.; Adams, S.; Chen, W.; Ding, J.; et al. 3D-printed electrodes for lithium metal batteries with high areal capacity and high-rate capability. Energy Storage Mater. 2020, 24, 336–342. [Google Scholar] [CrossRef]
- Kong, D.; Wang, Y.; Huang, S.; Zhang, B.; Lim, Y.V.; Sim, G.J.; Valdivia Y Alvarado, P.; Ge, Q.; Yang, H.Y. 3D Printed Compressible Quasi-Solid-State Nickel-Iron Battery. ACS Nano 2020, 14, 9675–9686. [Google Scholar] [CrossRef]
- Bao, Y.; Liu, Y.; Kuang, Y.; Fang, D.; Li, T. 3D-printed highly deformable electrodes for flexible lithium ion batteries. Energy Storage Mater. 2020, 33, 55–61. [Google Scholar] [CrossRef]
- Lee, J.; Kim, H.C.; Choi, J.W.; Lee, I.H. A review on 3D printed smart devices for 4D printing. Int. J. Precis. Eng. Manuf. Green Technol. 2017, 4, 373–383. [Google Scholar] [CrossRef]
- Li, X.; Shang, J.; Wang, Z. Intelligent materials: A review of applications in 4D printing. Assem. Autom. 2017, 37, 170–185. [Google Scholar] [CrossRef]
- Agarwala, S.; Goh, G.L.; Goh, G.D.; Dikshit, V.; Yeong, W.Y. 3D and 4D printing of polymer/CNTs-based conductive composites. In 3D and 4D Printing of Polymer Nanocomposite Materials: Processes, Applications, and Challenges; Elsevier: Amsterdam, The Netherlands, 2019; pp. 297–324. ISBN 9780128168059. [Google Scholar]
- Zuo, W.; Li, R.; Zhou, C.; Li, Y.; Xia, J.; Liu, J. Battery-Supercapacitor Hybrid Devices: Recent Progress and Future Prospects. Adv. Sci. 2017, 4, 1–21. [Google Scholar] [CrossRef]
- Thakur, A.K.; Majumder, M.; Patole, S.P.; Zaghib, K.; Reddy, M.V. Metal-organic framework-based materials: Advances, exploits, and challenges in promoting post Li-ion battery technologies. Mater. Adv. 2021, 2, 2457–2482. [Google Scholar] [CrossRef]
- Ma, S.; Lin, M.; Lin, T.E.; Lan, T.; Liao, X.; Maréchal, F.; Van herle, J.; Yang, Y.; Dong, C.; Wang, L. Fuel cell-battery hybrid systems for mobility and off-grid applications: A review. Renew. Sustain. Energy Rev. 2021, 135. [Google Scholar] [CrossRef]
- Zhou, Y.; Ravey, A.; Péra, M.C. Real-time cost-minimization power-allocating strategy via model predictive control for fuel cell hybrid electric vehicles. Energy Convers. Manag. 2021, 229. [Google Scholar] [CrossRef]
- Sancarlos, A.; Cameron, M.; Abel, A.; Cueto, E.; Duval, J.L.; Chinesta, F. From ROM of Electrochemistry to AI-Based Battery Digital and Hybrid Twin; Springer: Berlin/Heidelberg, Germany, 2021; Volume 28, ISBN 0123456789. [Google Scholar]
- Rivera, F.F.; Miranda-Alcántara, B.; Orozco, G.; Ponce de León, C.; Arenas, L.F. Pressure drop analysis on the positive half-cell of a cerium redox flow battery using computational fluid dynamics: Mathematical and modelling aspects of porous media. Front. Chem. Sci. Eng. 2021, 15, 399–409. [Google Scholar] [CrossRef]
- Dhillon, S.; Hernández, G.; Wagner, N.P.; Svensson, A.M.; Brandell, D. Modelling capacity fade in silicon-graphite composite electrodes for lithium-ion batteries. Electrochim. Acta 2021, 377, 138067. [Google Scholar] [CrossRef]
- Bugryniec, P.J.; Davidson, J.N.; Brown, S.F. Computational modelling of thermal runaway propagation potential in lithium iron phosphate battery packs. In Proceedings of the Energy Reports; Elsevier: Amsterdam, The Netherlands, 2020; Volume 6, pp. 189–197. [Google Scholar]
- Xiao, Y.; Wang, Y.; Bo, S.H.; Kim, J.C.; Miara, L.J.; Ceder, G. Understanding interface stability in solid-state batteries. Nat. Rev. Mater. 2020, 5, 105–126. [Google Scholar] [CrossRef]
- Deringer, V.L. Modelling and understanding battery materials with machine-learning-driven atomistic simulations. J. Phys. Energy 2020, 2, 41003. [Google Scholar] [CrossRef]
- Craig, B.; Skylaris, C.K.; Schoetz, T.; de León, C.P. A computational chemistry approach to modelling conducting polymers in ionic liquids for next generation batteries. In Proceedings of the Energy Reports; Elsevier: Amsterdam, The Netherlands, 2020; Volume 6, pp. 198–208. [Google Scholar]
- Kadyk, T.; Xiao, J.; Ooka, H.; Huang, J.; Exner, K.S. Editorial: Material and Composition Screening Approaches in Electrocatalysis and Battery Research. Front. Energy Res. 2021, 9, 227. [Google Scholar] [CrossRef]
- Novčić, K.A.; Dobrota, A.S.; Petković, M.; Johansson, B.; Skorodumova, N.V.; Mentus, S.V.; Pašti, I.A. Theoretical analysis of doped graphene as cathode catalyst in Li-O2 and Na-O2 batteries—The impact of the computational scheme. Electrochim. Acta 2020, 354, 136735. [Google Scholar] [CrossRef]
- Carlstedt, D.; Runesson, K.; Larsson, F.; Xu, J.; Asp, L.E. Electro-chemo-mechanically coupled computational modelling of structural batteries. Multifunct. Mater. 2020, 3, 045002. [Google Scholar] [CrossRef]
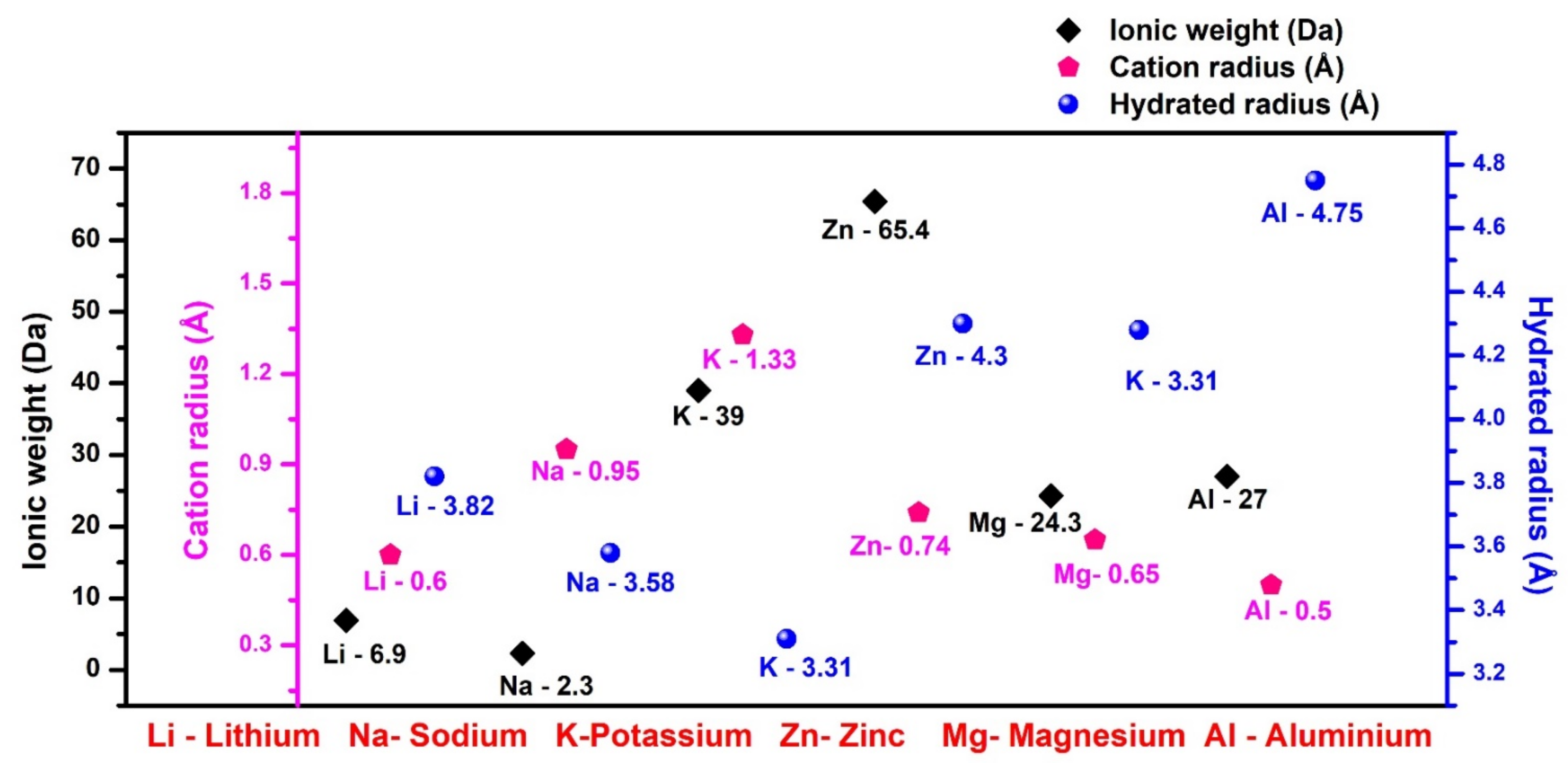
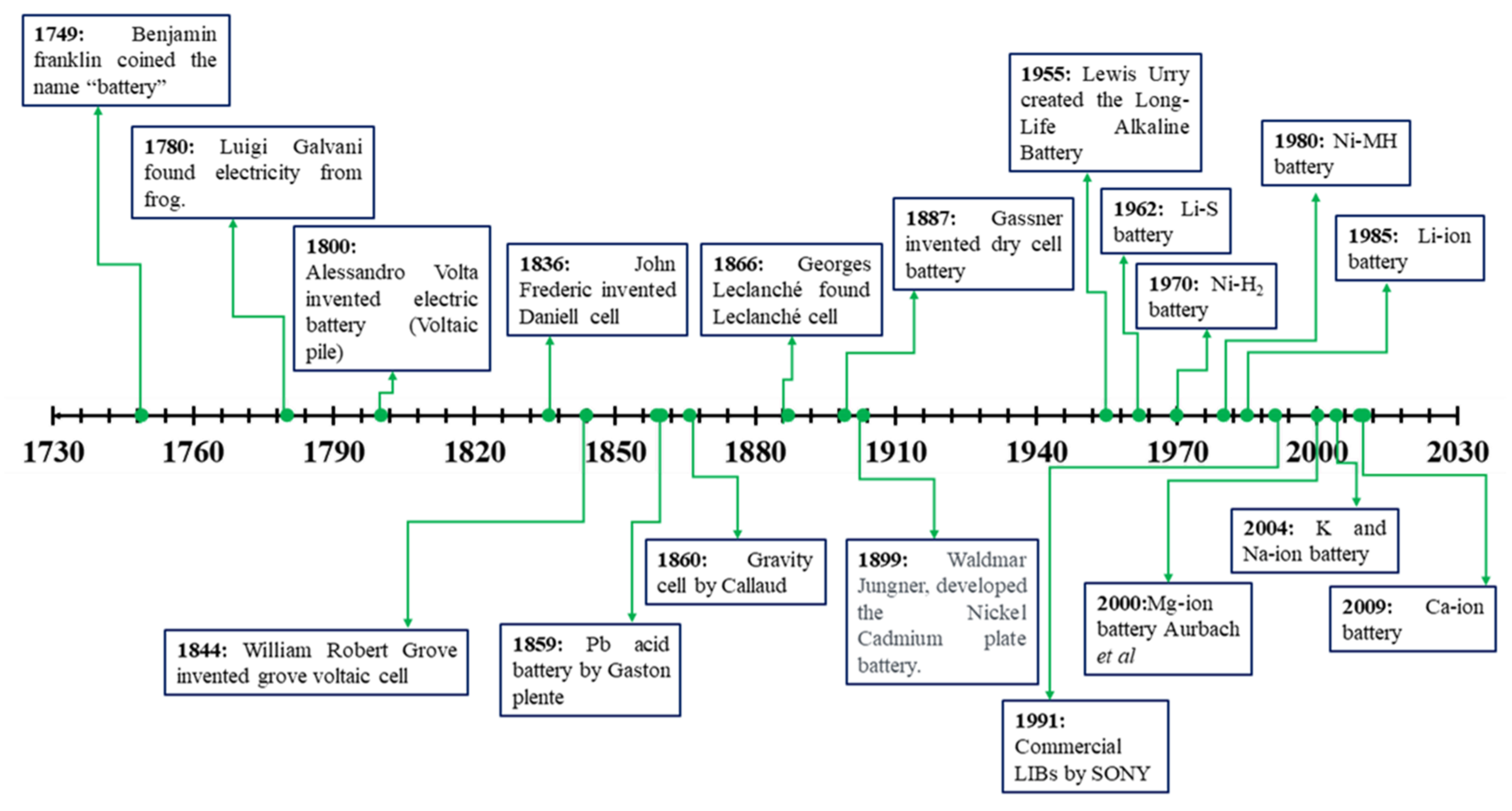
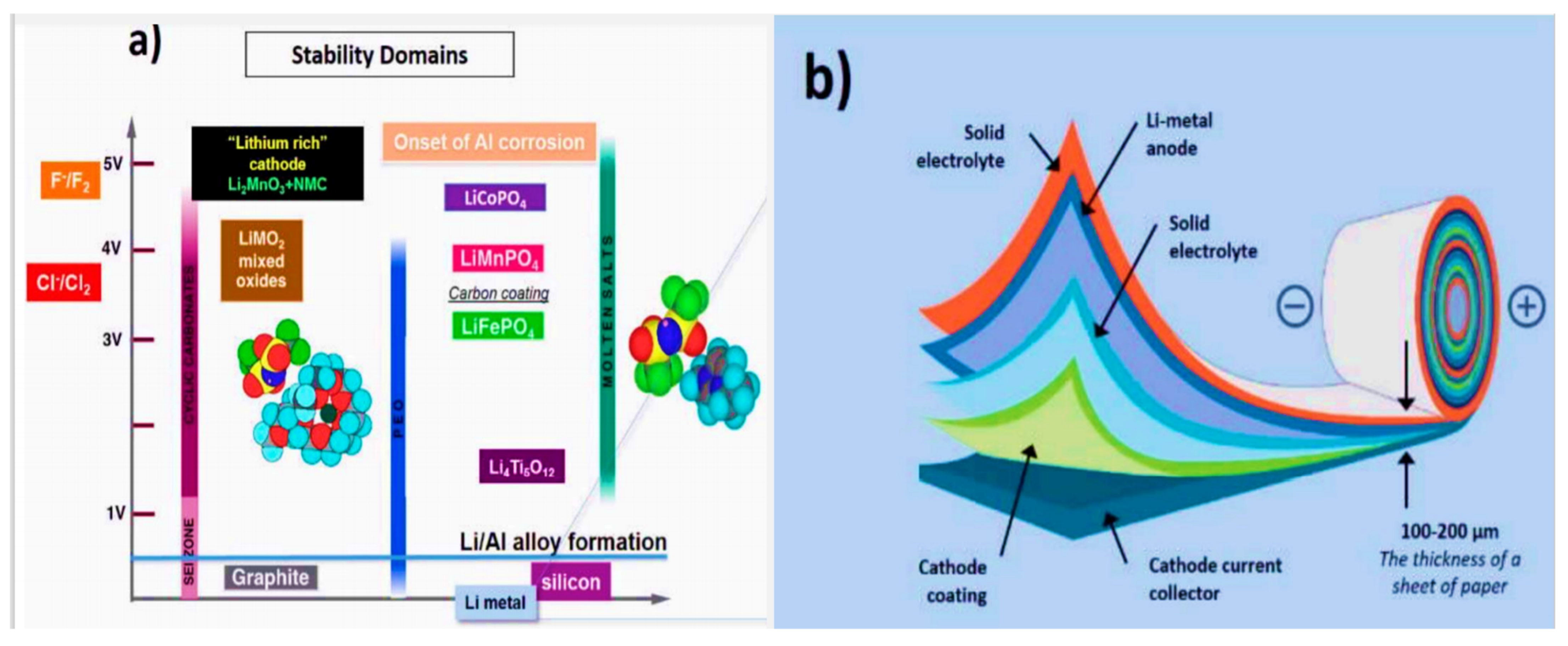

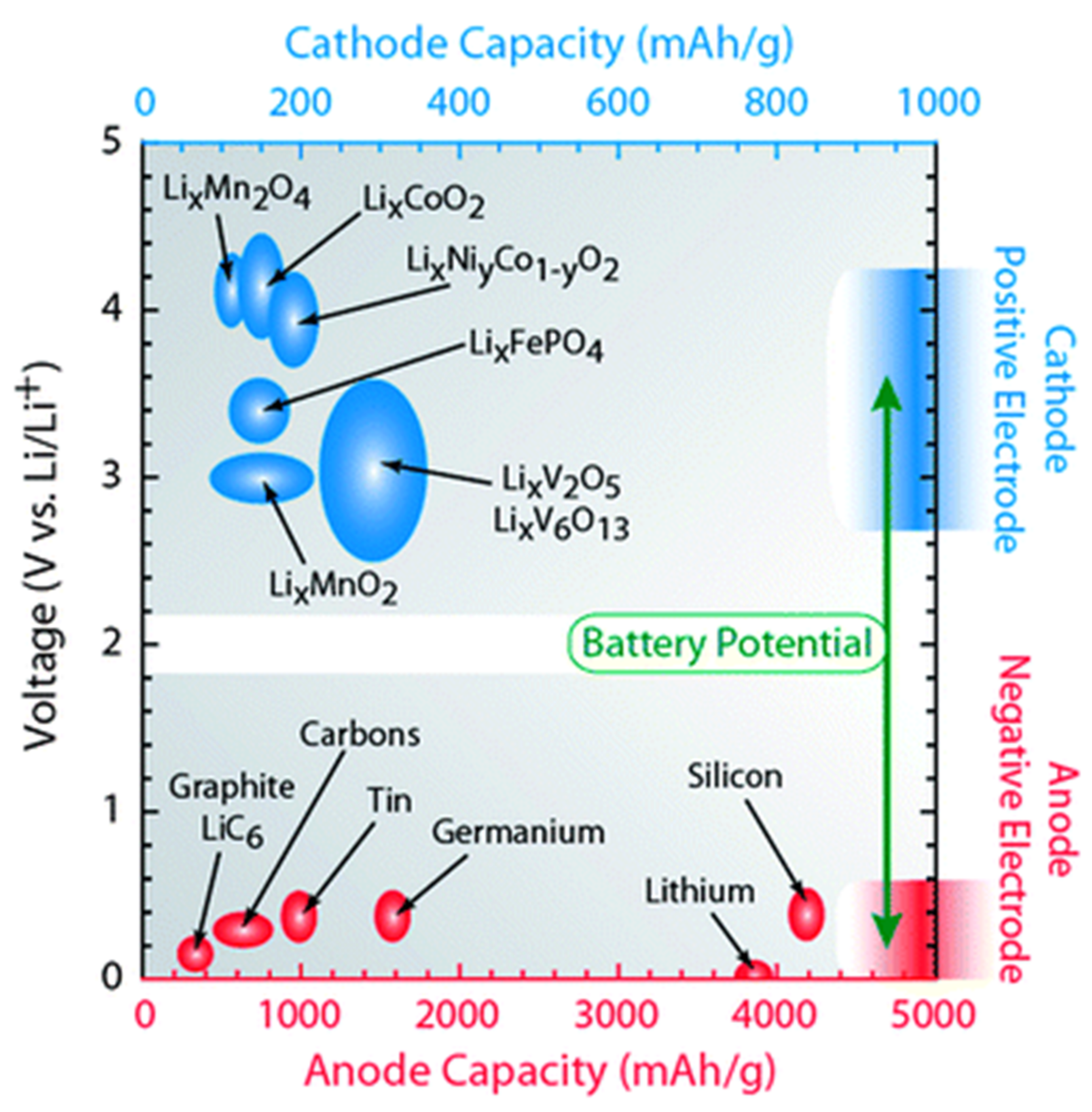
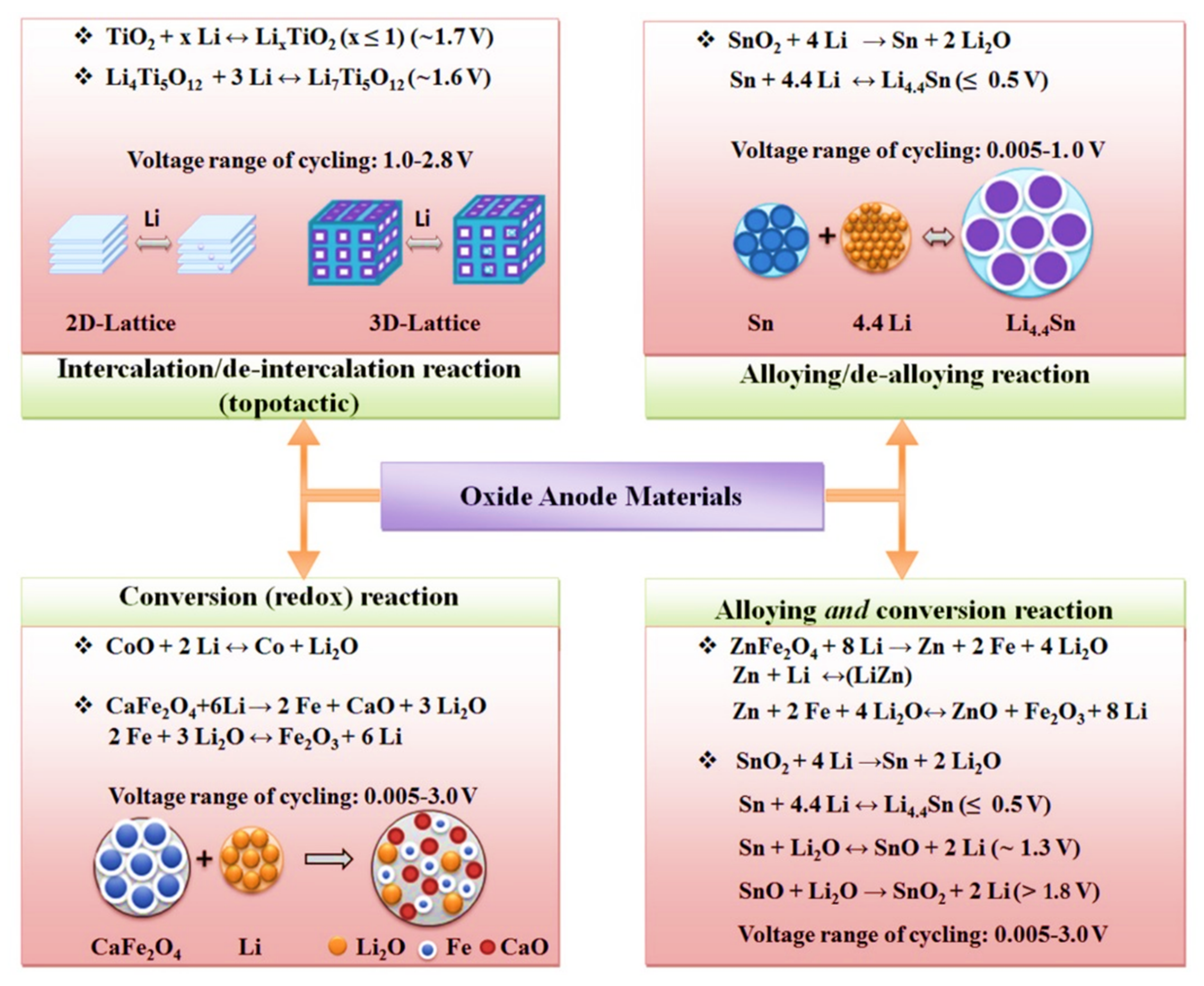

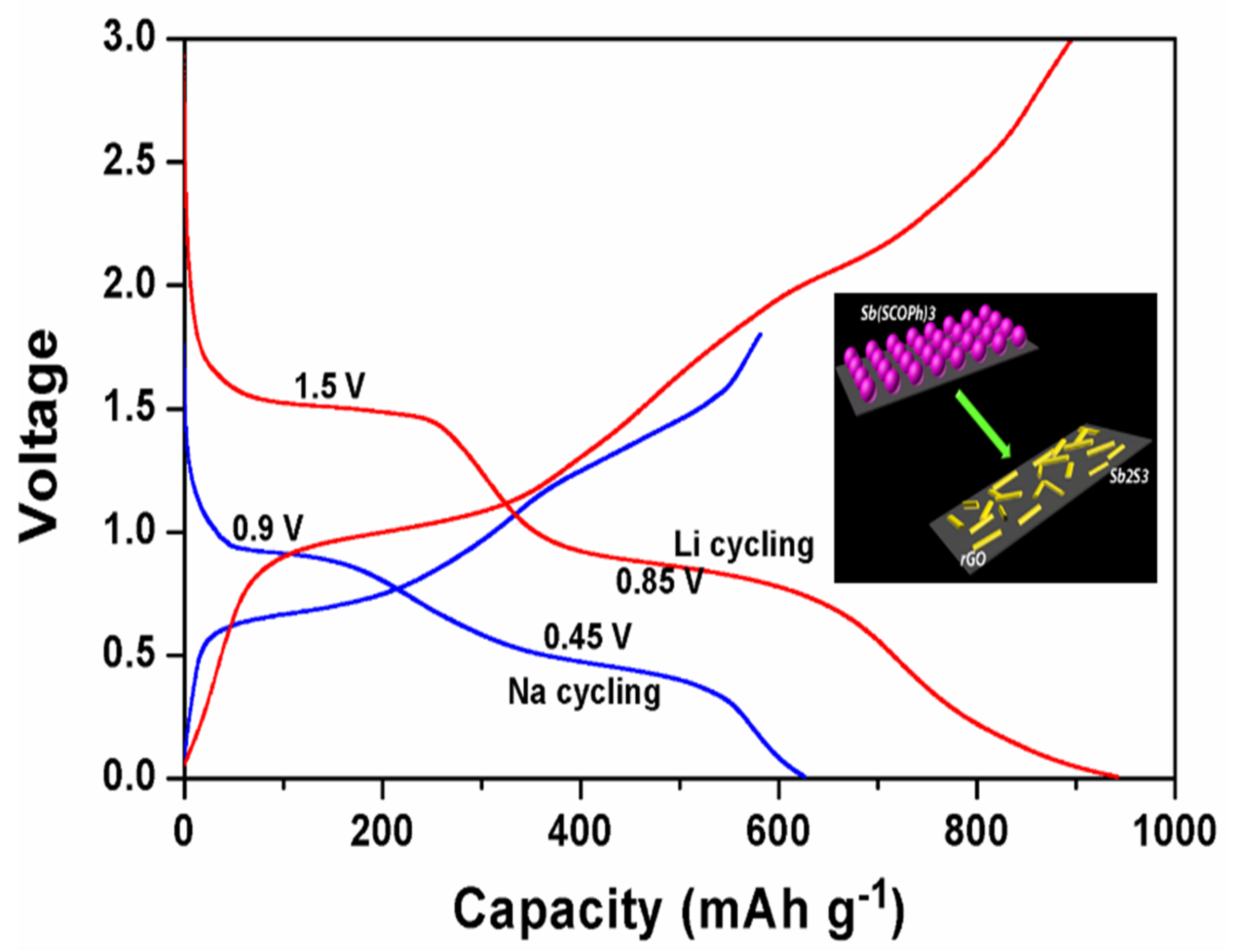
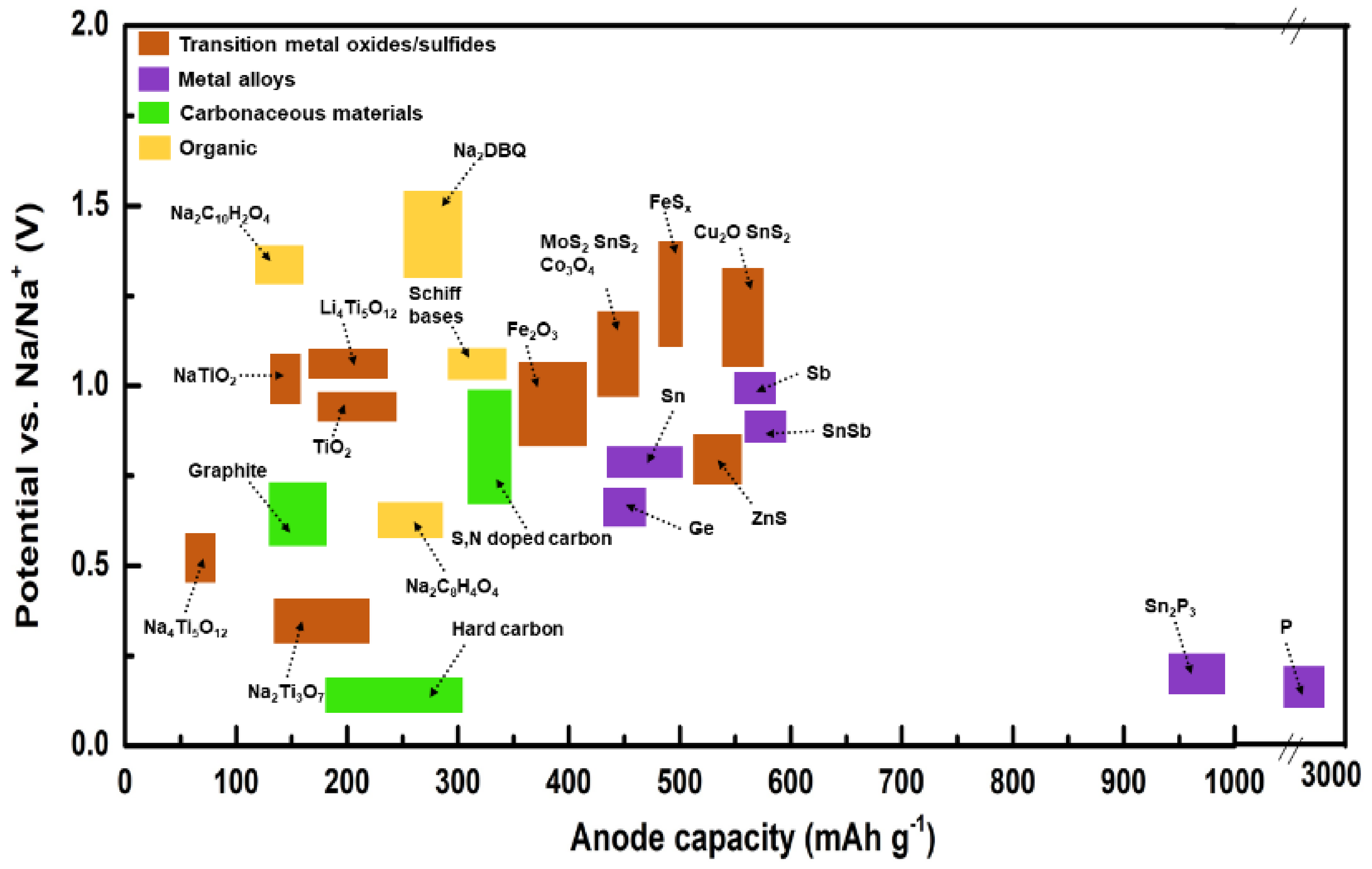
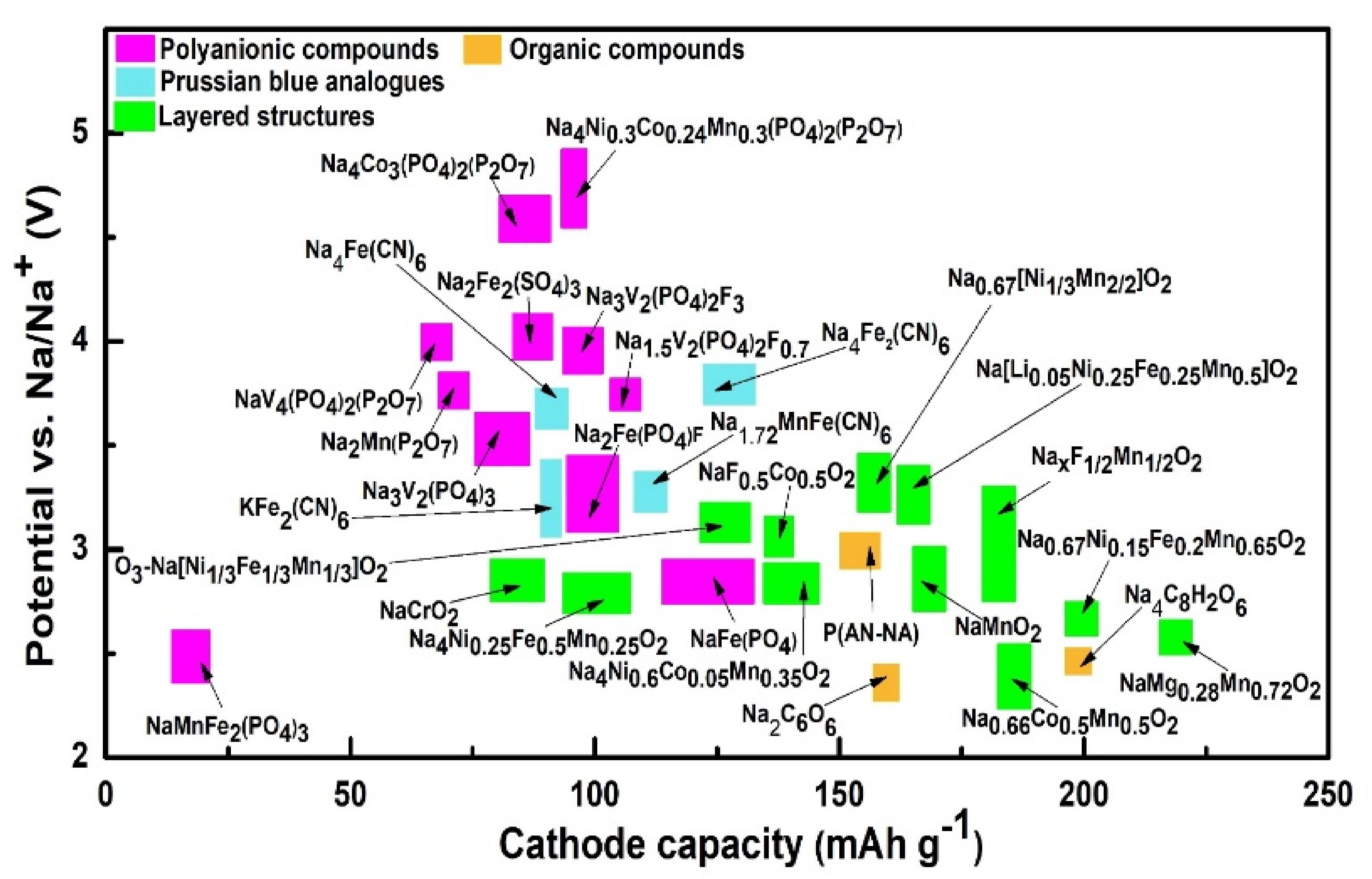
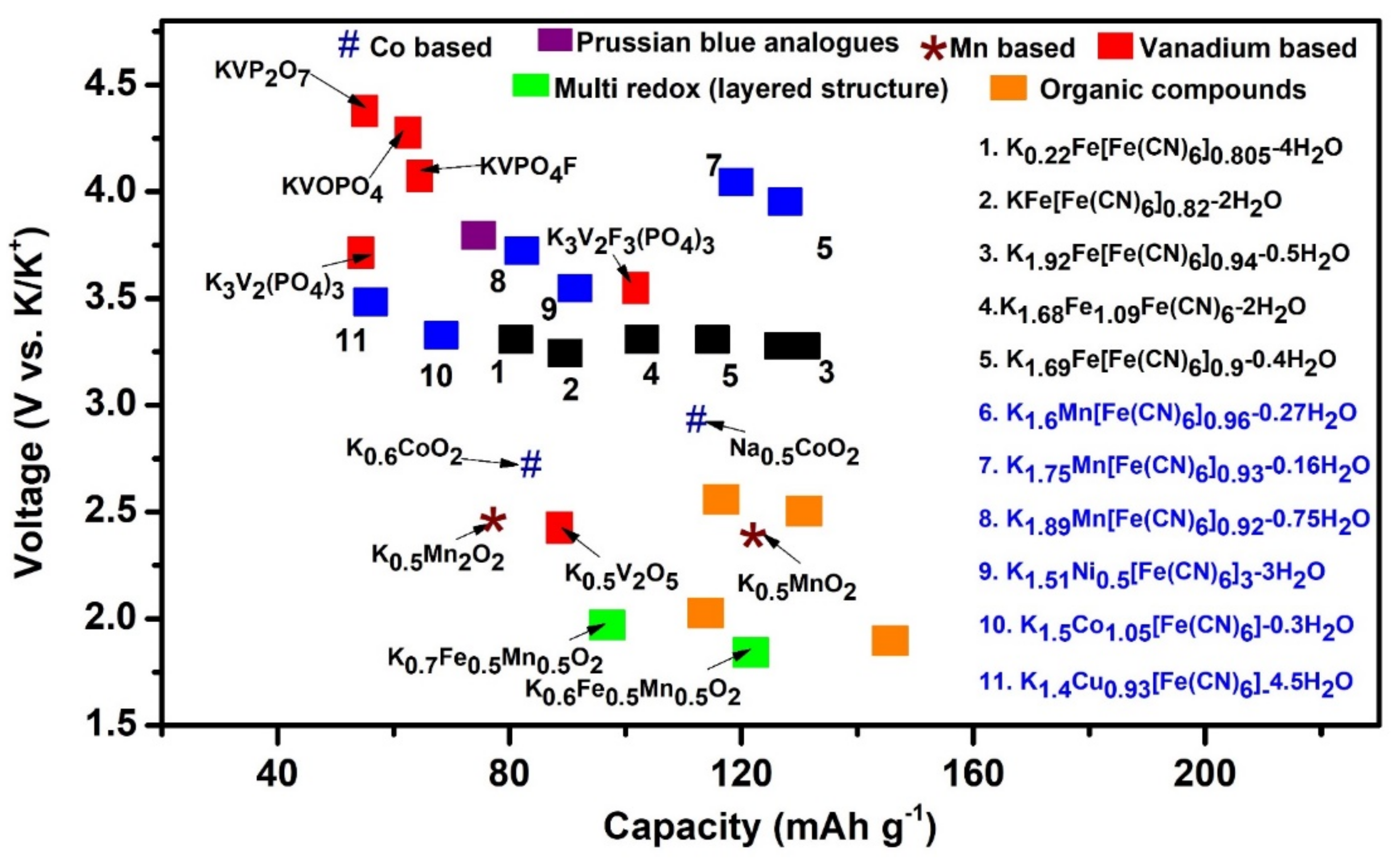

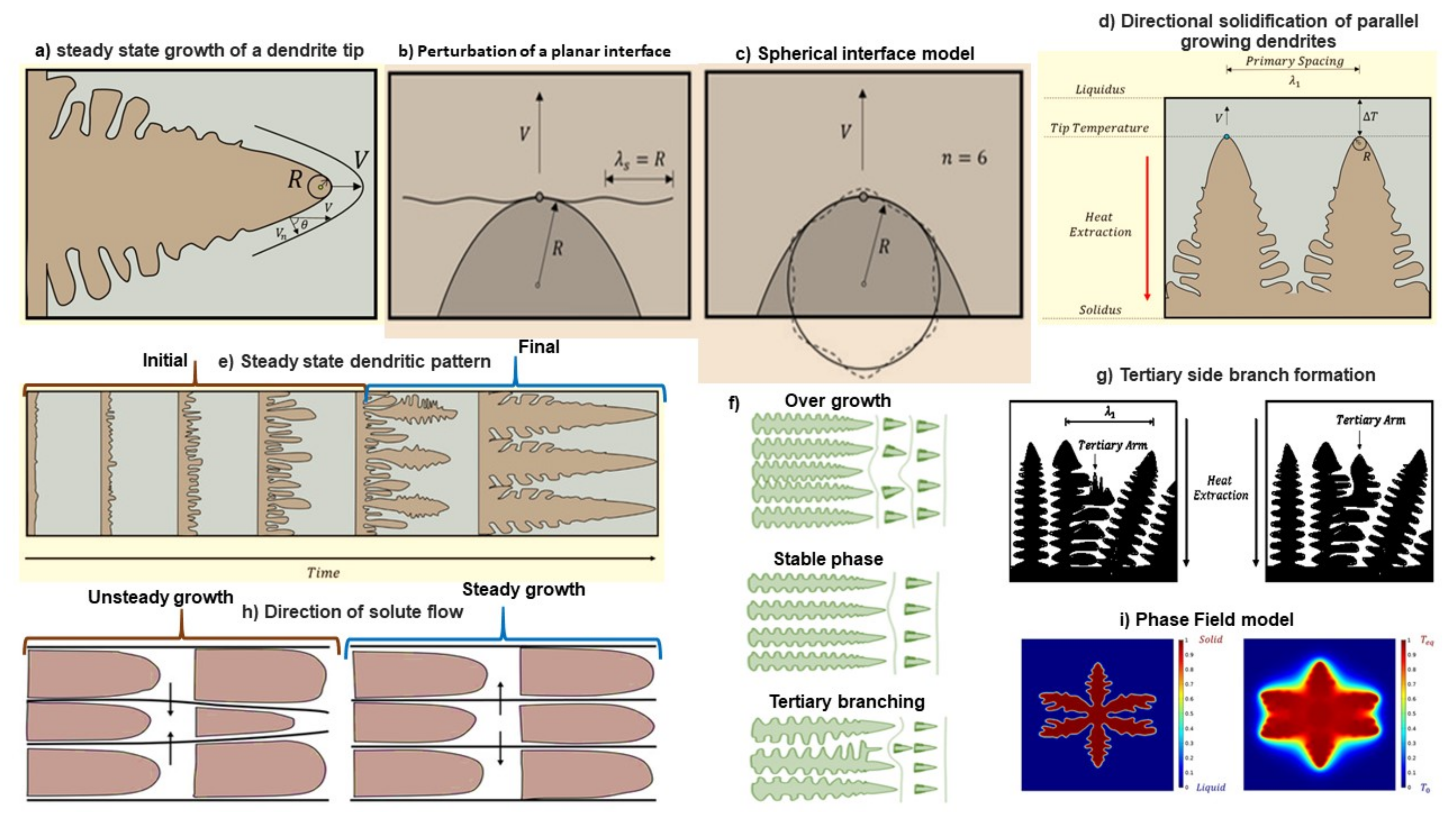
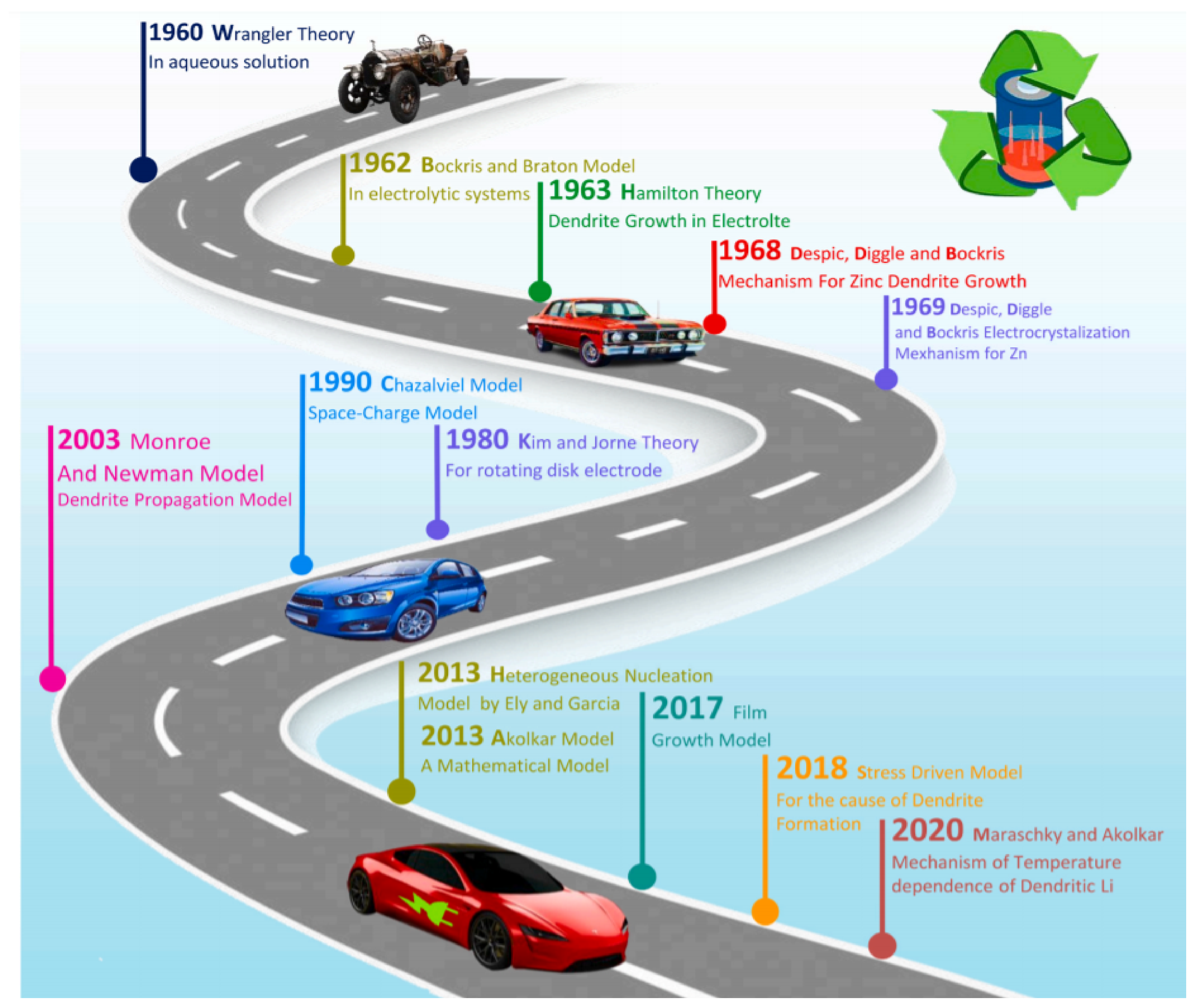

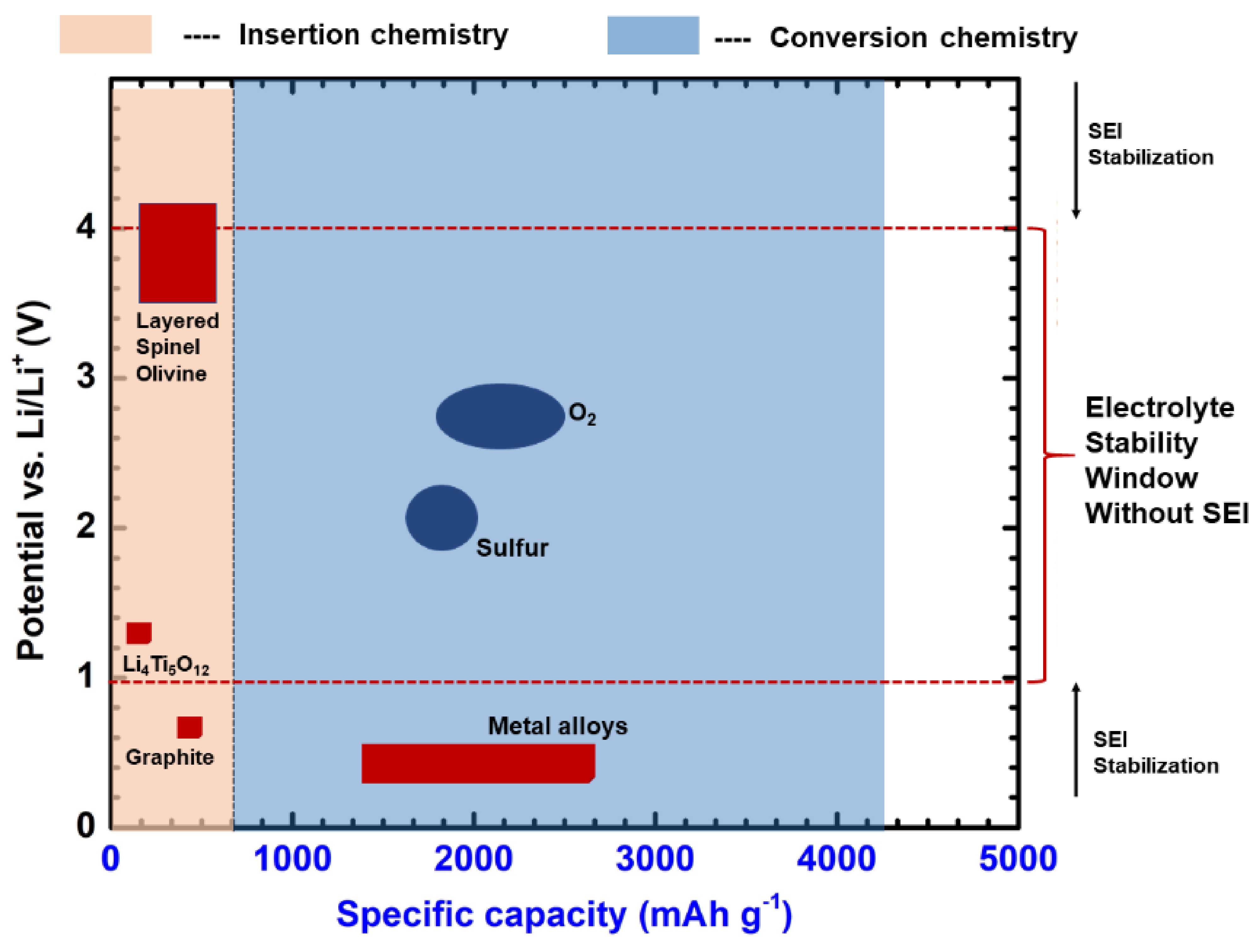
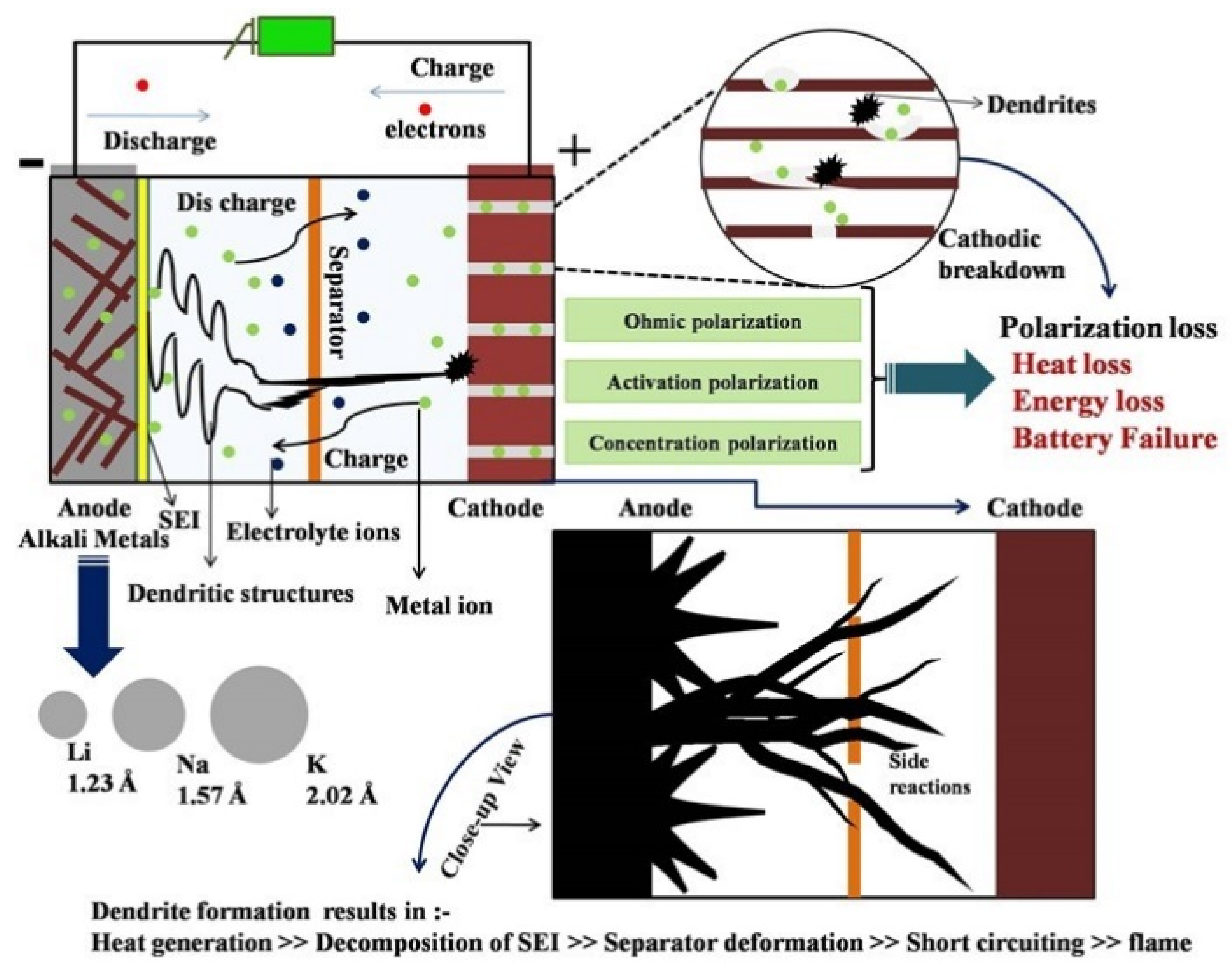
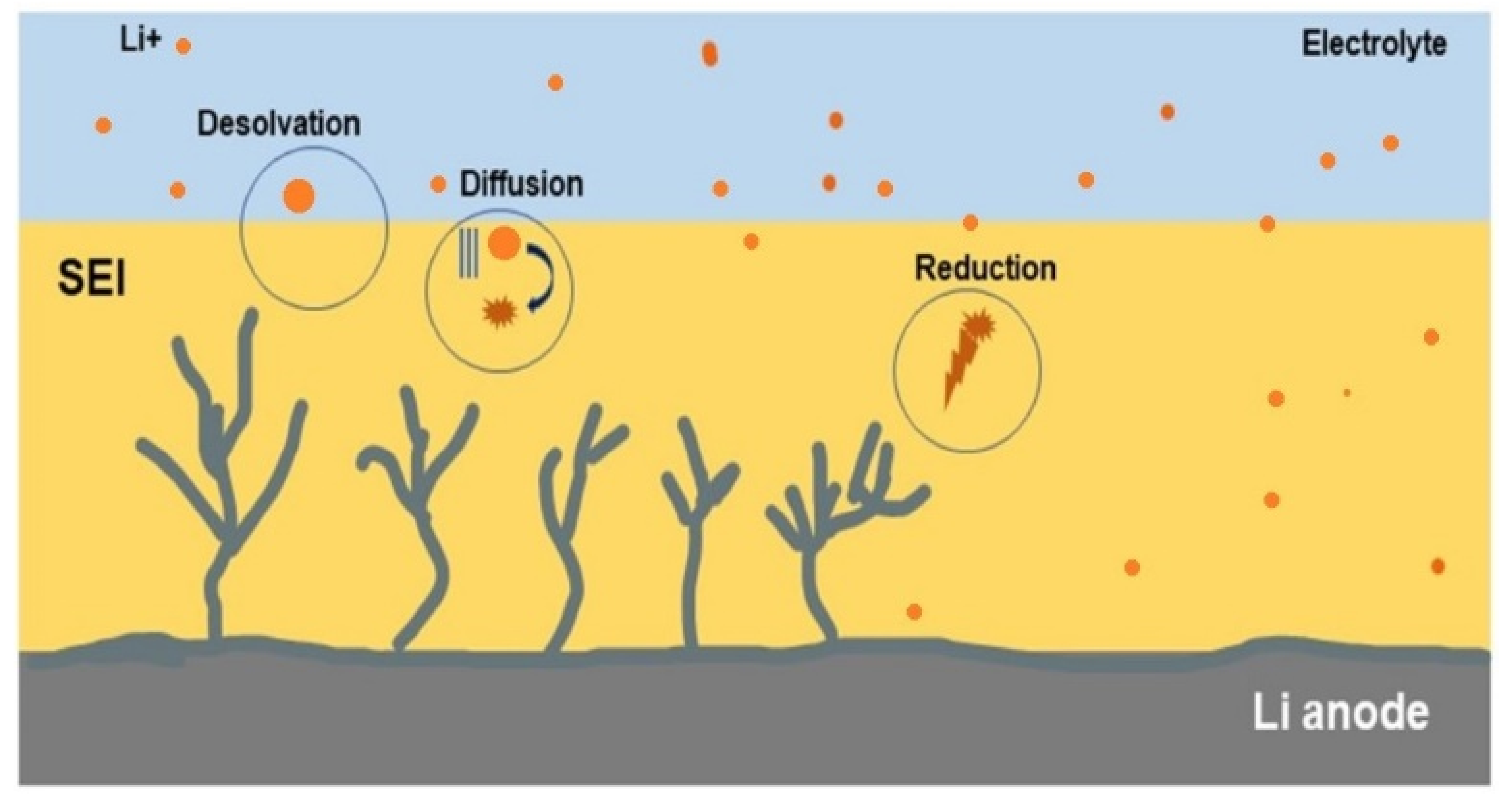
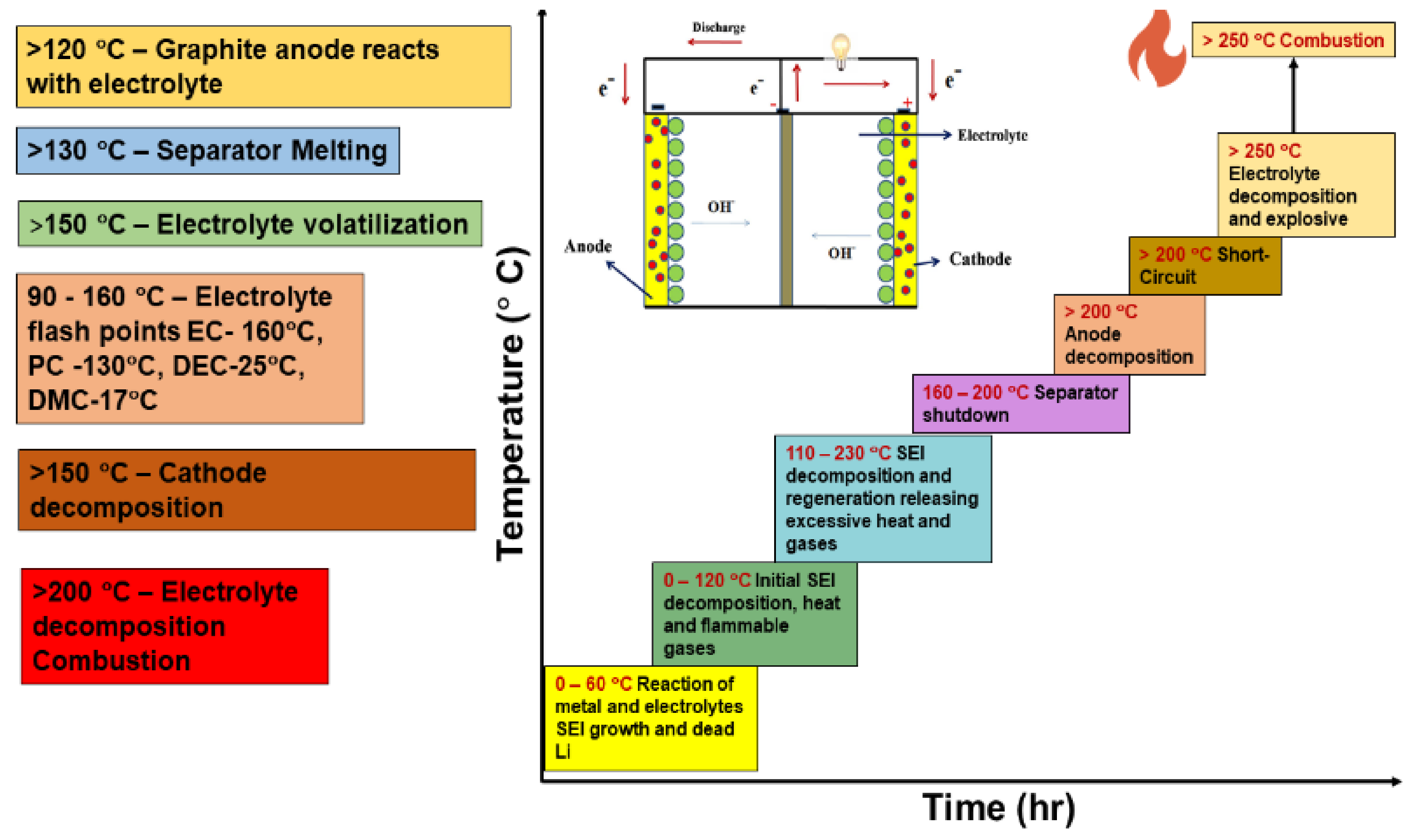
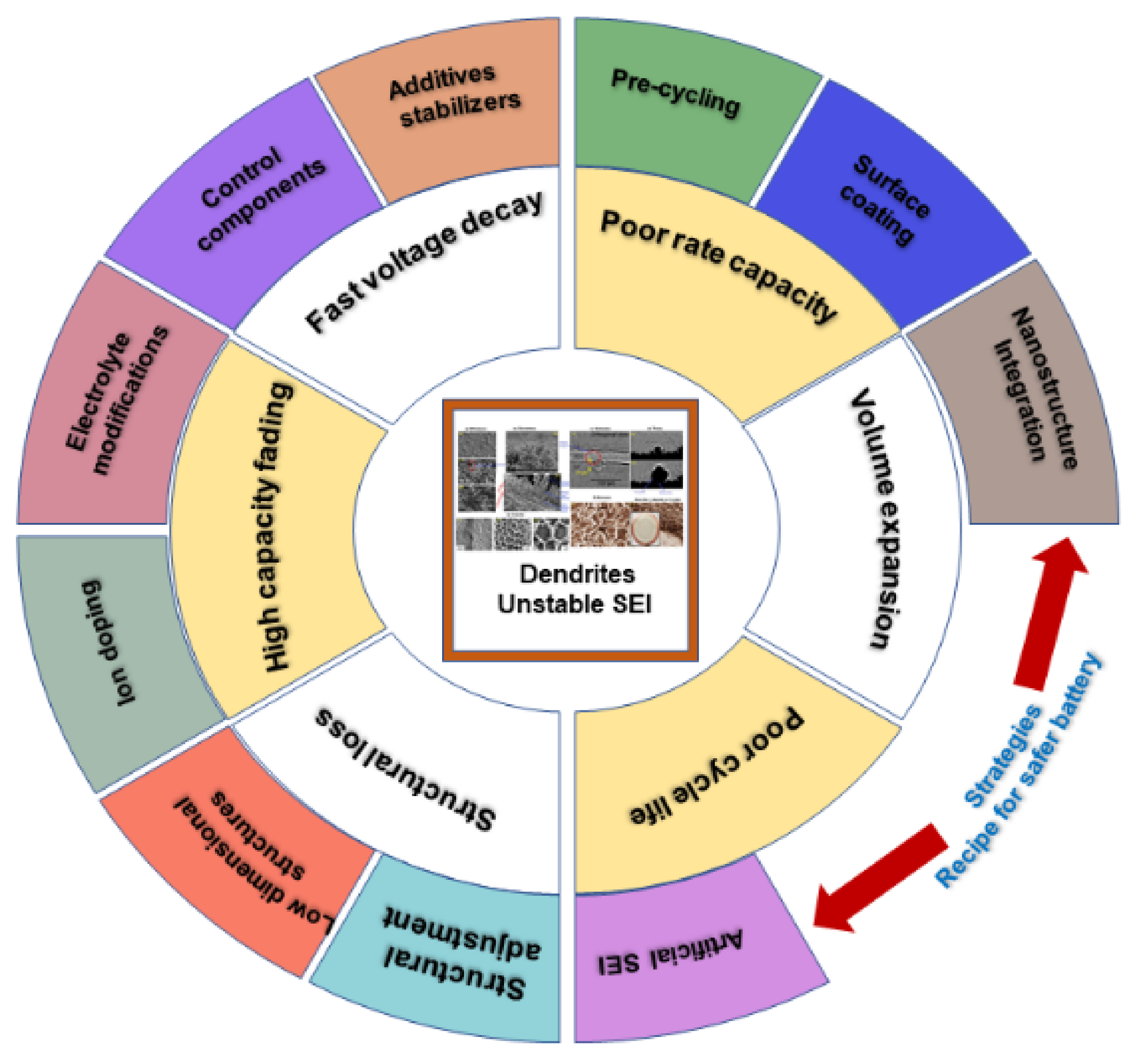
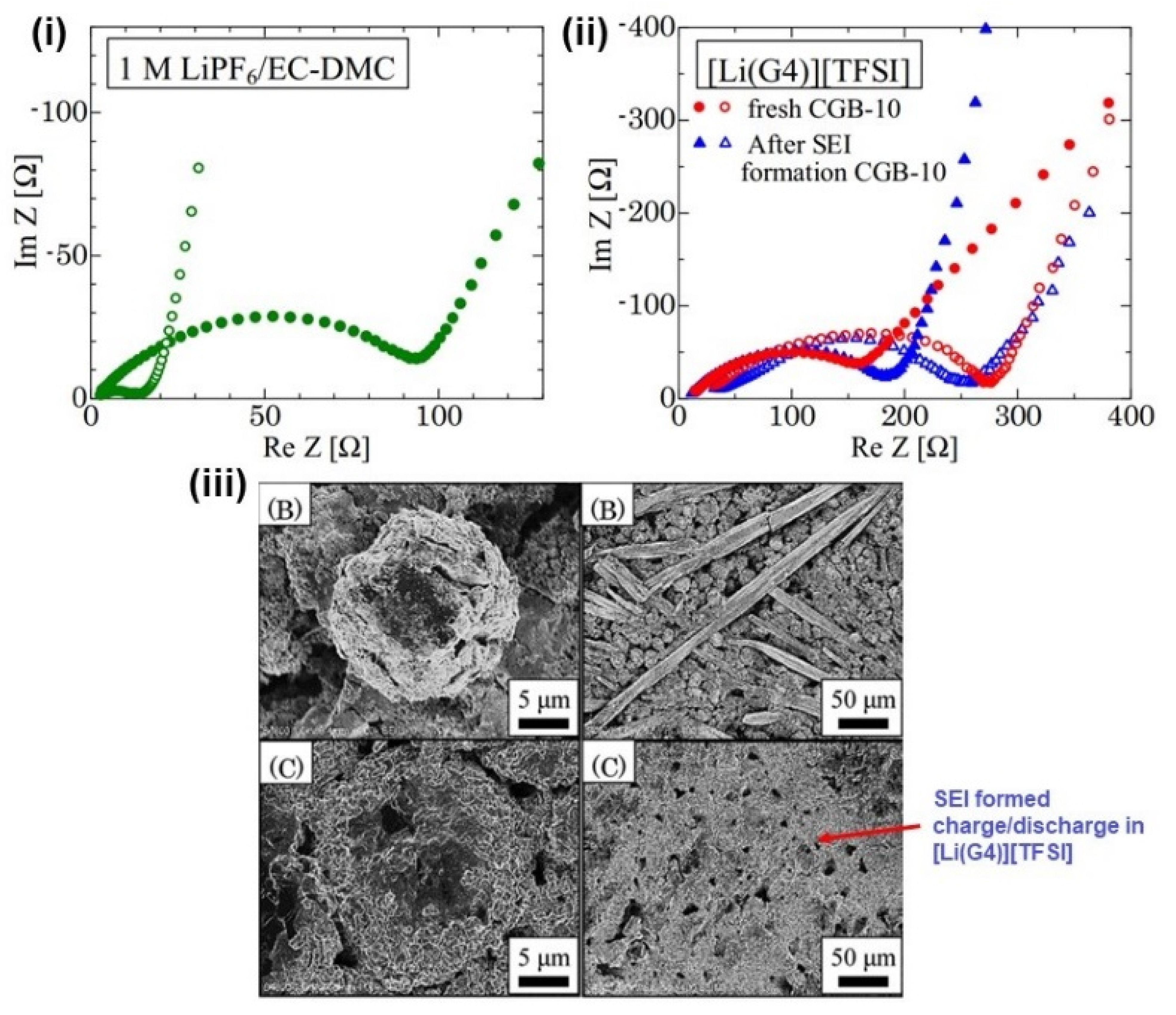
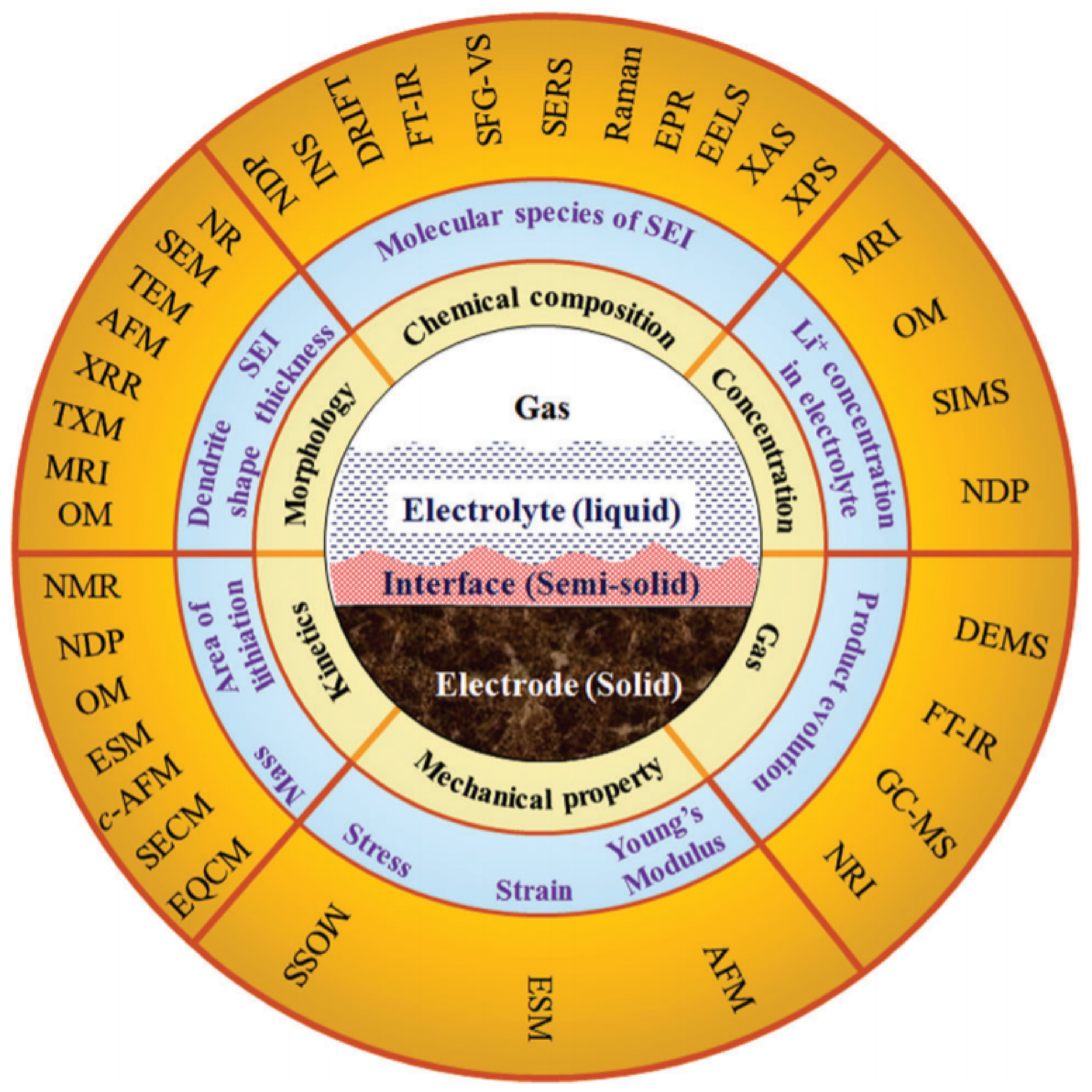

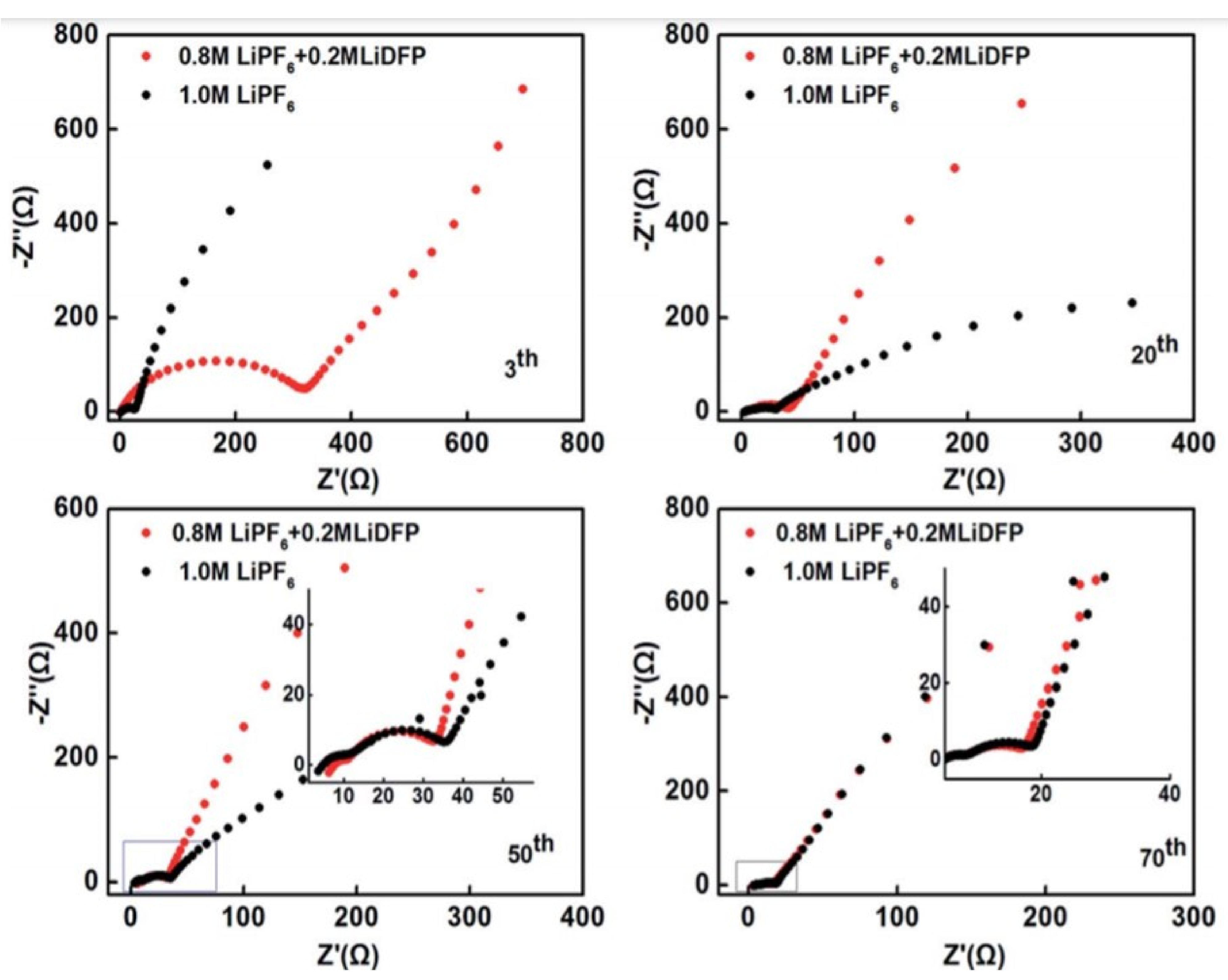
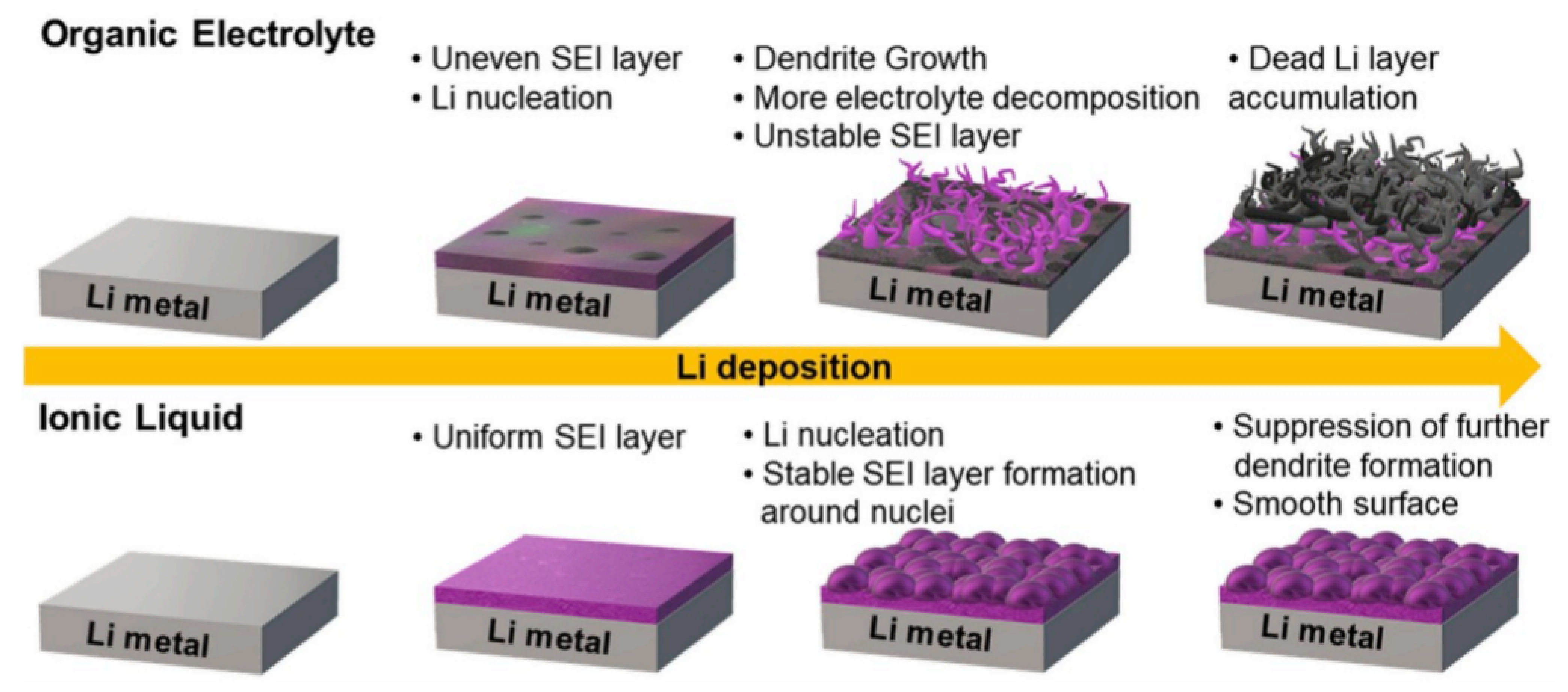
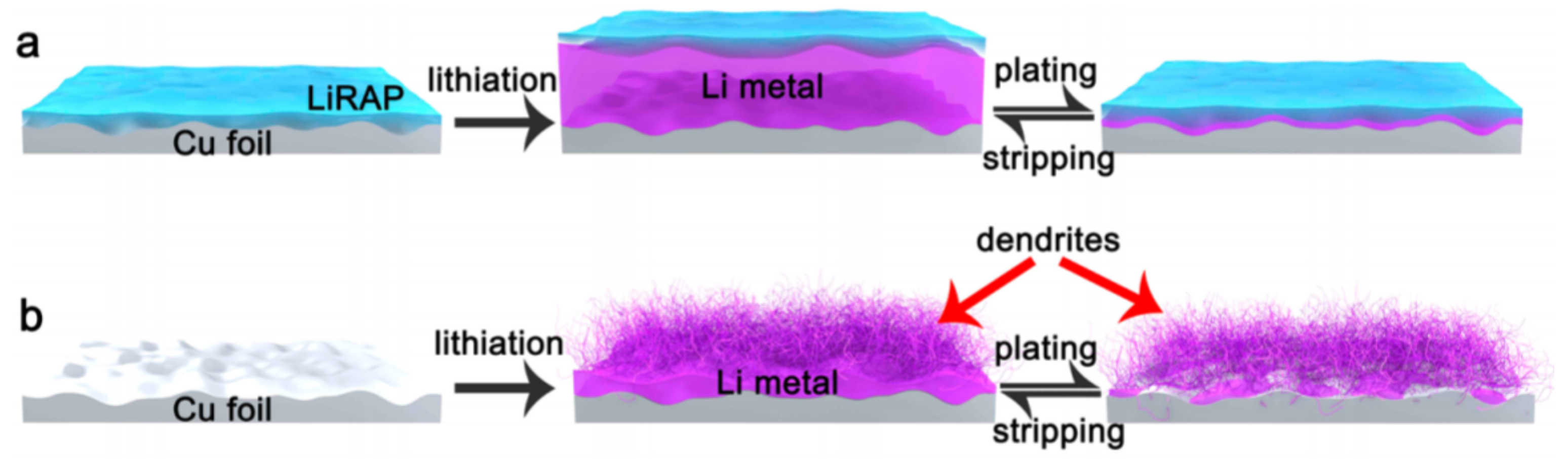
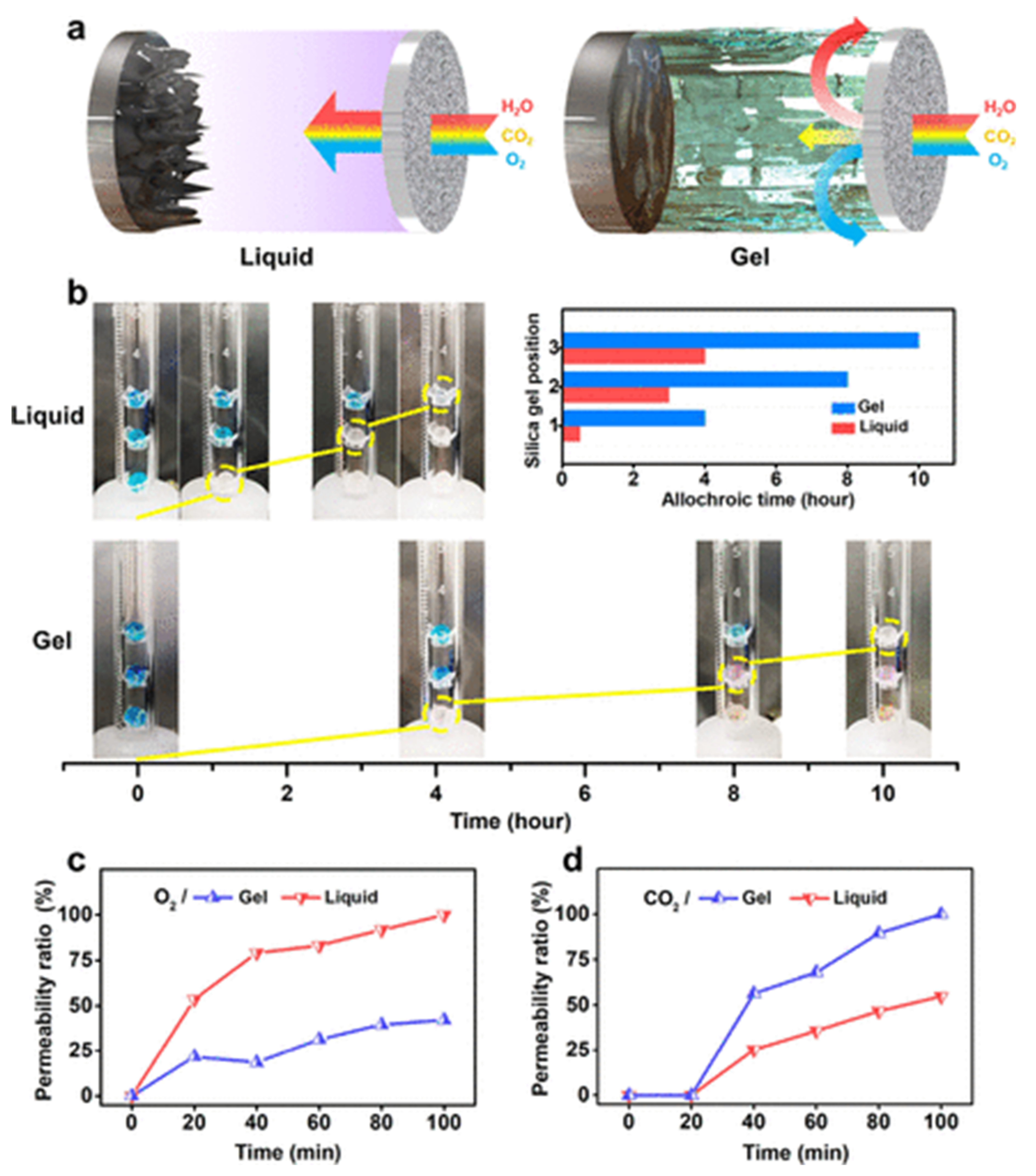
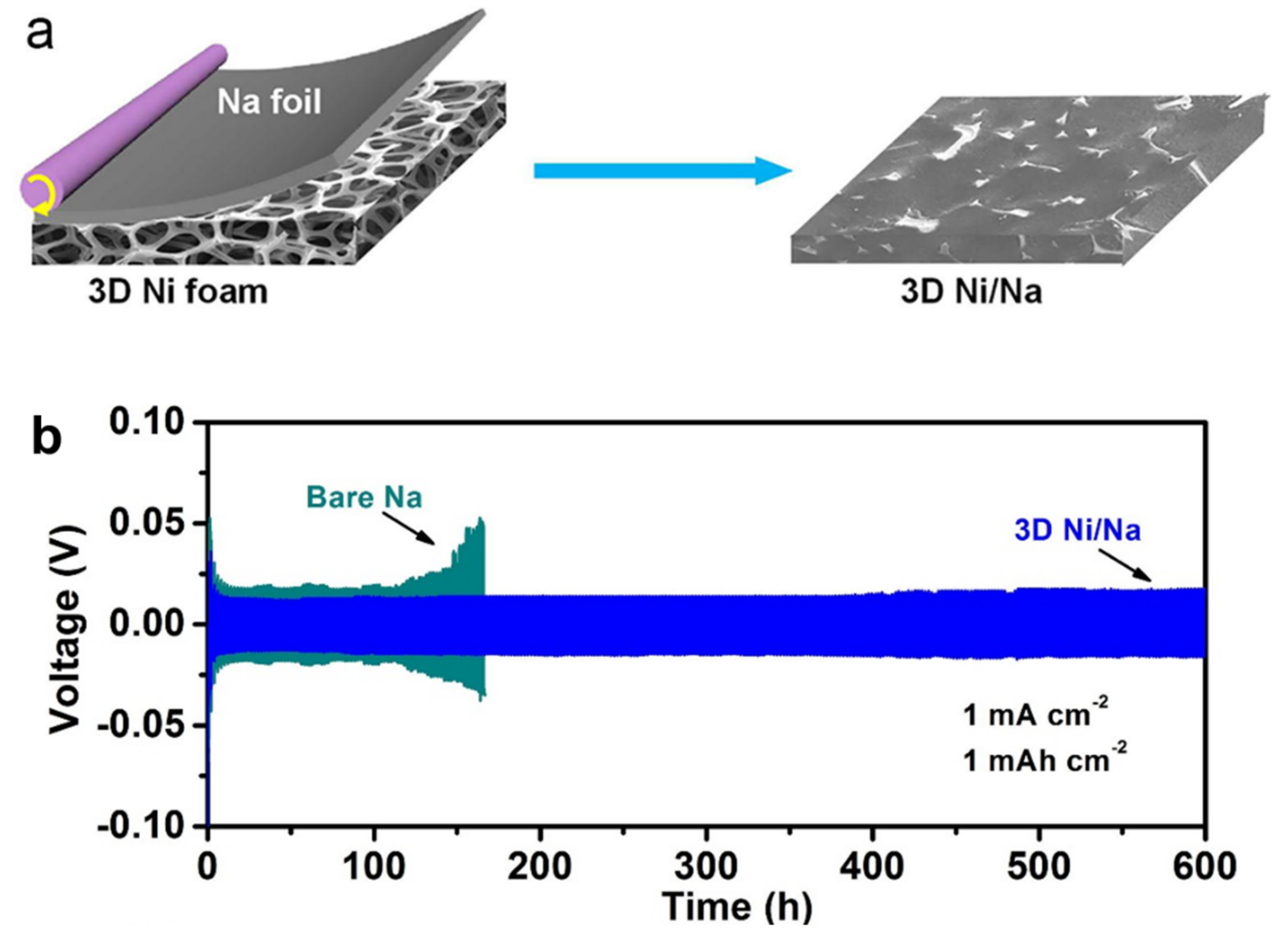
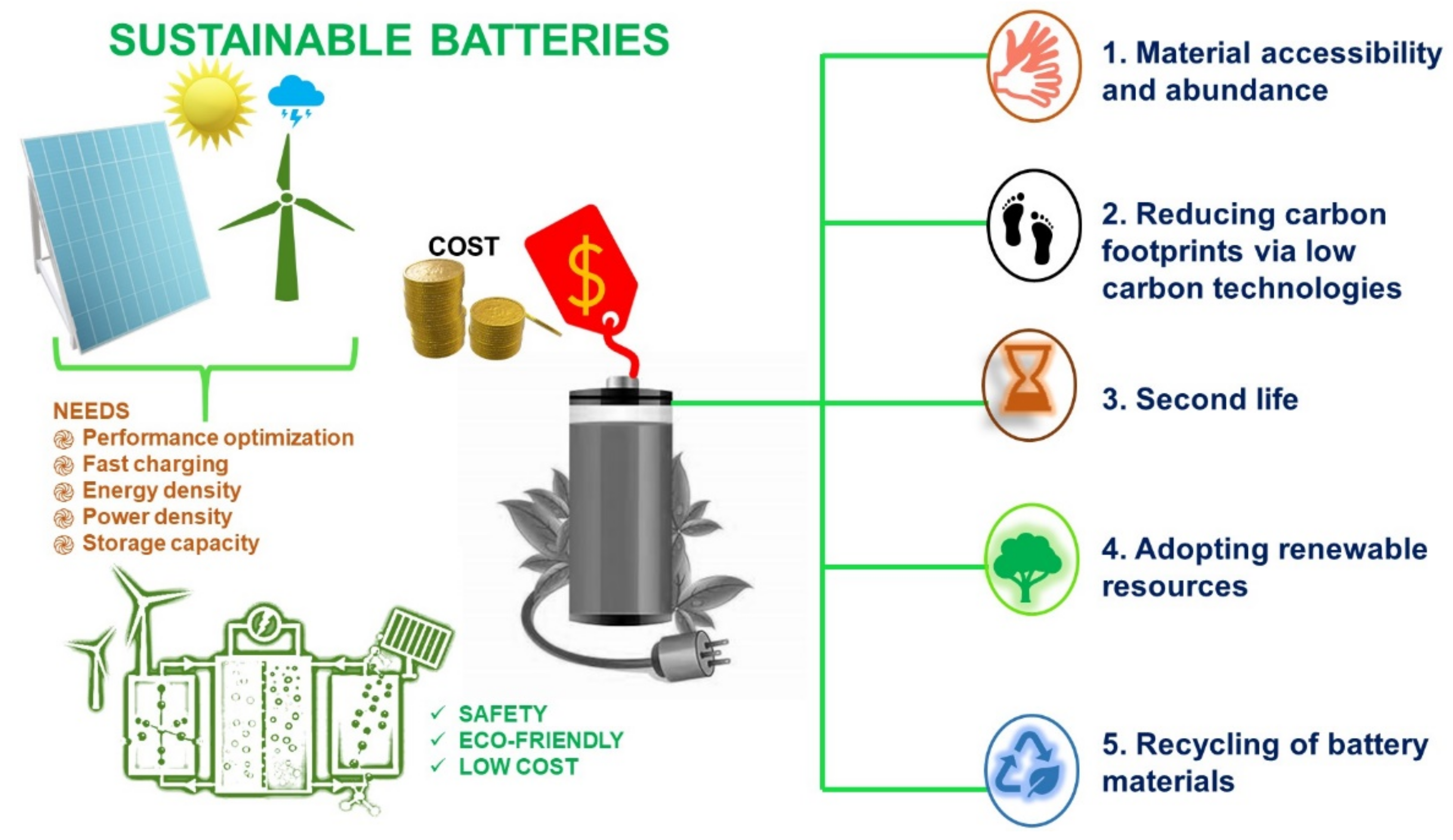
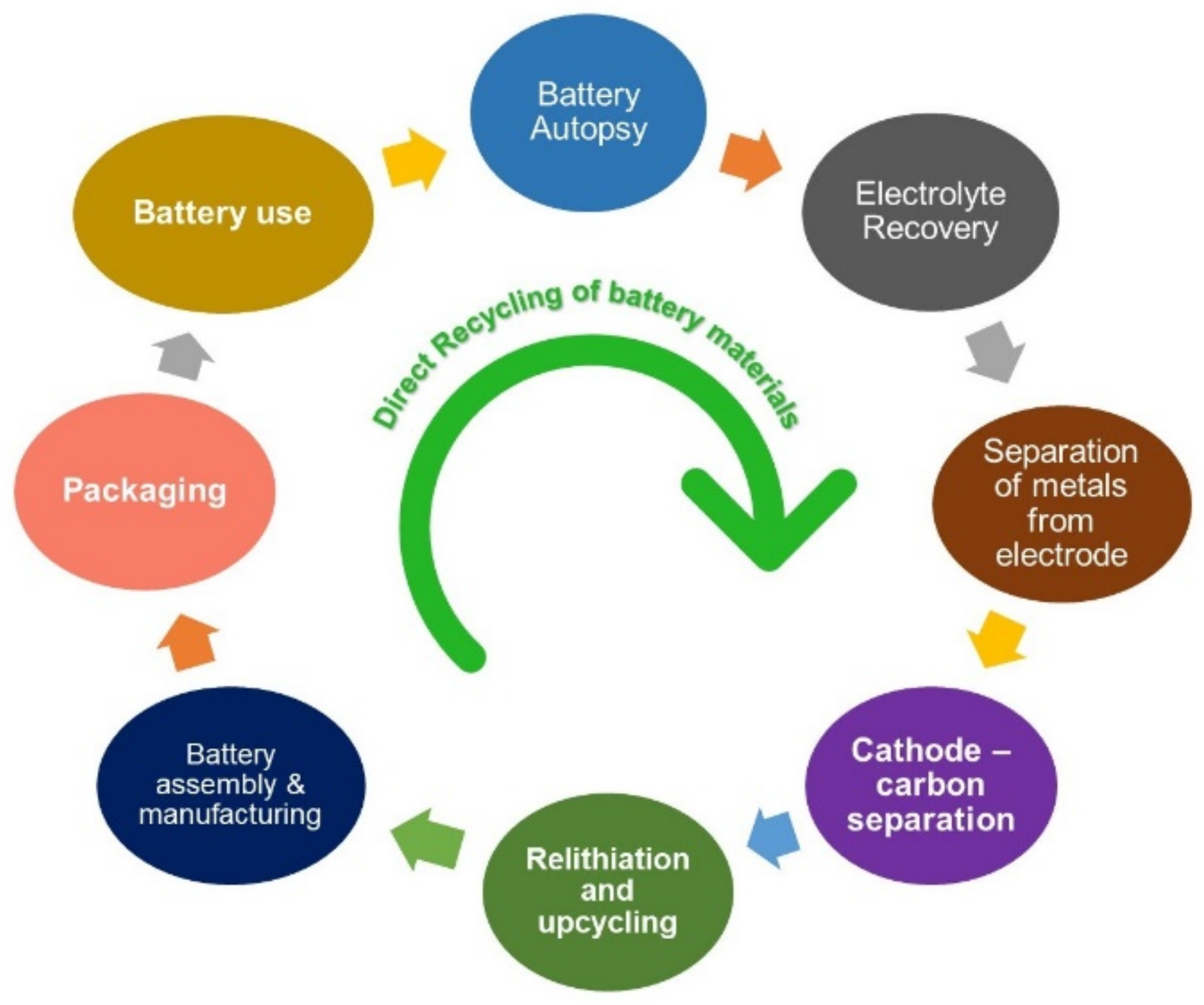
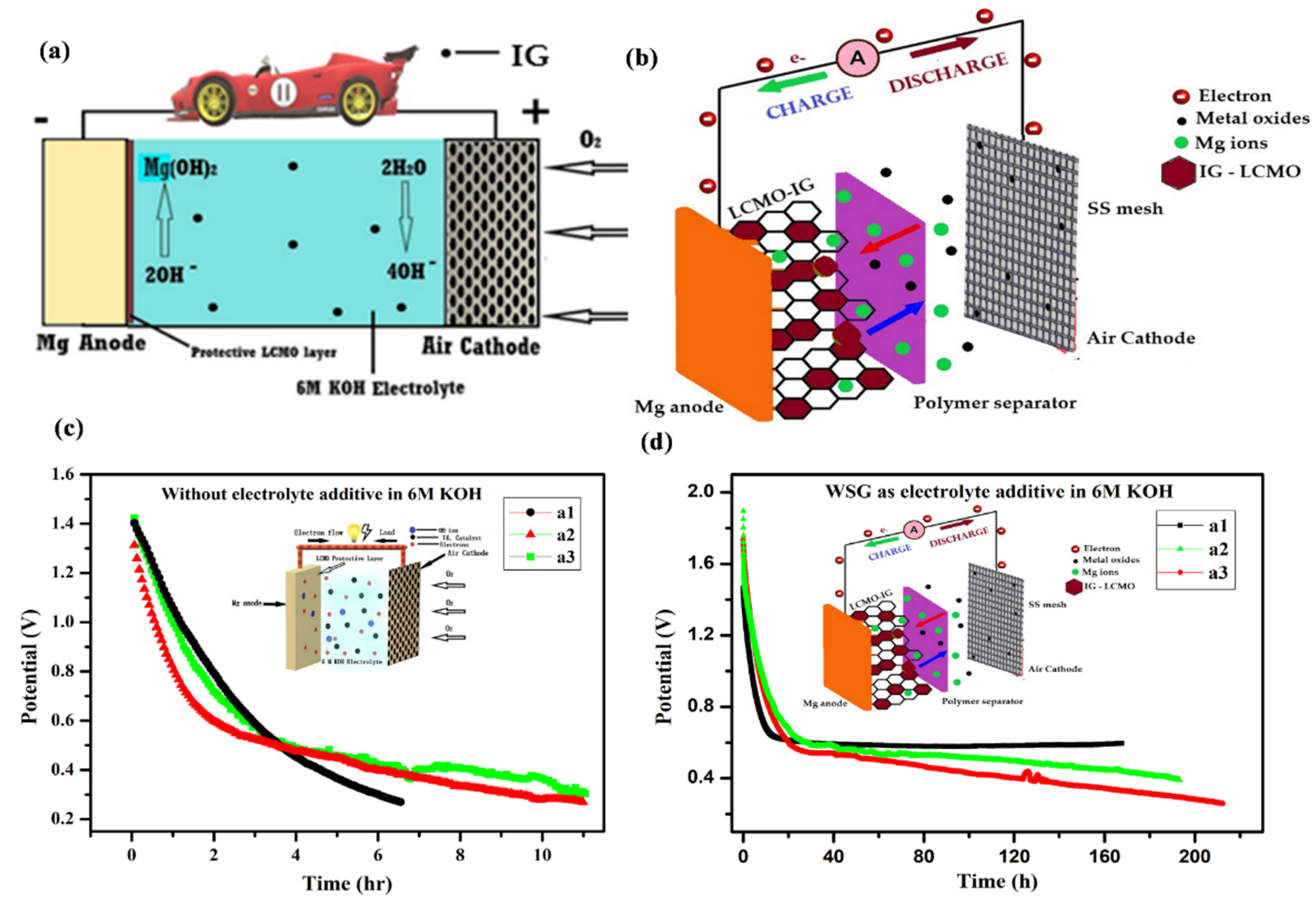
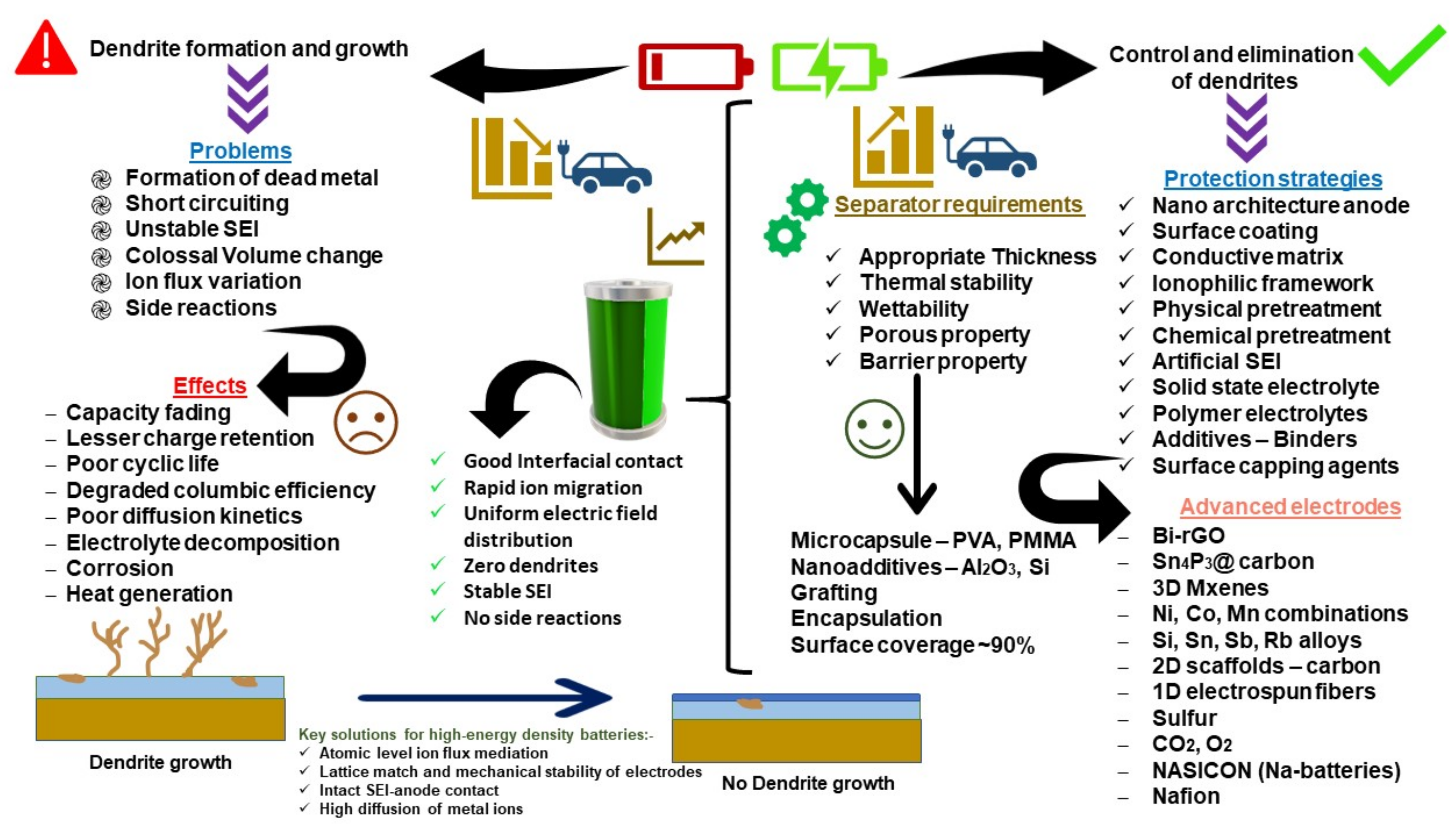
| Parameters | Li | Na | Mg | Al | K | Ca | Zn | |
|---|---|---|---|---|---|---|---|---|
| Chemical Property | Atomic No | 3 | 11 | 12 | 13 | 19 | 20 | 30 |
| Atomic mass (g·mol−1) | 6.941 | 22.98 | 24.3 | 26.98 | 39.09 | 40.08 | 65.38 | |
| Electronic configuration | [He]2s1 | [Ne]3s1 | [Ne]3s2 | [Ne]3s23p1 | [Ar]4s1 | [Ar]4s2 | [Ar]3d104s2 | |
| Metallic radius (pm) | 152 | 186 | 160 | 143 | 220 | 197 | 134 | |
| Ionic radius (pm) | 76 (Li+) | 102 (Na+) | 72 (Mg2+) | 53 (Al3+) | 138 (K+) | 100 (Ca2+) | 74 (Zn2+) | |
| Covalent radius (pm) | 133 | 155 | 139 | 126 | 203 | 171 | 118 | |
| Van der Waals radius (pm) | 182 | 227 | 173 | 184 | 280 | 231 | 139 | |
| Ionization energy (KJ/mol) | 520 | 496.9 | 738.1 | 577.9 | 418.7 | 590.2 | 908 | |
| Pauling electronegativity | 0.98 | 0.93 | 1.31 | 1.61 | 0.82 | 1 | 1.65 | |
| Standard potential E° (V) | −3.04 | −2.71 | −2.38 | −1.66 | −2.92 | −2.76 | −0.76 | |
| No. of isotopes | 2 | 13 | 3 | 9 | 3 | 5 | 5 | |
| Physical Property | Melting point (°C) | 180 | 98 | 650 | 660 | 63.5 | 842 | 420 |
| Boiling point (°C) | 1330 | 883 | 1091 | 2470 | 759 | 1484 | 907 | |
| Density (g/cm3) | 0.535 | 0.968 | 1.731 | 2.701 | 0.862 | 1.554 | 7.133 | |
| Earth crust abundance (%) | 0.002 | 2.6 | 2.5 | 8.1 | 2.1 | 4.1 | 0.005 | |
| Color | Silvery white | Silvery white | Gray–white | Silvery gray | Silvery white | Gray–yellow tint | Silvery–blue tint | |
| Mechanical Property | Tensile strength (Mpa) | 1.5 | -- | 175–250 | 90 | -- | 45 | 37 |
| Modulus of elasticity (Gpa) | 4.6 | 10 | 65–100 | 70 | 29.6–38.1 | 18 | 96.5 | |
| Mohs hardness | 0.6 | 0.5 | 2.5 | 2.75 | 0.4 | 1.75 | 2.5 | |
| Electrochemical Property | Voltage vs. SHE (V) | Li - 3.04 | Na - 2.71 | Mg - 2.38 | Al - 1.66 | K - 2.92 | Ca - 2.76 | Zn - 0.76 |
| Theoretical capacity (mAh g−1) | 3860 | 1166 | 903 | 8045 | 684 | 820 | ||
| Theoretical gravimetric energy density of M–O2 batteries (Wh kg−1) | 3458 | 1605 | 3925 | 4312 | 935 | 2643 | 552 | |
| Theoretical volumetric energy density of M–O2 batteries (Wh L−1) | 7983 | 4492 | 4042 | 8056 | 2001 | 8829 | 3071 | |
| Theoretical gravimetric energy density of | 2615 | 1273 | 1684 | 1319 | 915 | -- | 1850 | |
| M–S batteries (Wh kg−1) | ||||||||
| Theoretical volumetric energy density of | 4289 | 2362 | 3221 | 2981 | 1589 | -- | 3628 | |
| M–S batteries (Wh L−1) | ||||||||
| Desolvation energy in PC (kJ mol−1) | 215.8 | 158.2 | 148.2 | 182.6 | 119.4 | -- | 200 | |
| Desolvation energy in EC (kJ mol−1) | 208.9 | 151.3 | 202.4 | -- | 112.8 | -- | -- |
| Anode | Specific Capacity (mAh g−1) [108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128] | Advantages | Challenges |
|---|---|---|---|
| Insertion Compounds | |||
| Graphite | 100–400 | • Small volume change in layered structure | • More side reactions |
| Hard carbon | 200–600 | • Decent operating potential | • Less coulombic and cell efficiency |
| CNTs | 1116 | • High performance | • High cost |
| Graphene | 780/1116 | • Safety and low cost | • Low reversible capacity |
| LiTi2O4 | 161 | • Experiences two phase reactions • Good cyclability | • Difficult to synthesize • Preparation of mixed valence is not easy |
| LiTi4O5 | 175 | • Extreme safety | • Very low capacitance |
| Li4Ti5O12 | 175 | • 3 Li could be reversibly inserted • No SEI formation • Flat operating potential ~1.55 V | • Inherent electrical conductivity limits Li diffusion |
| LiCrTiO4 | 157 | • Unit cell volume variation is observed at low voltage | • Electrochemical stability is inferior |
| TiNbO7 | 280 | • No SEI formation • High reversible capacity • Eco-friendliness | • Moderate capacity fading upon cycling |
| SrLi2Ti6O16 | 262 | • High diffusion coefficient • Excellent high drain performance | -- |
| LiTi2(PO4)3 | 137 | • Excellent cyclic performance | • Lack of electrical conductivity so carbon coating is necessary |
| TiO2 | 330 | • High power capability | • Low energy density |
| TiO2-anatase | 413 | • Stable cyclability | • Unit cell volume variation • High insertion potential |
| TiO2-rutile | 150 | • Good stability | • Inferior electrochemical activity towards Li |
| Alloys and Materials | |||
| Silicon | 4212 | • Strong bonding, stable SEI | • High irreversibility of charge • Colossal volume expansion |
| Germanium | 1624 | • Lower working potential | • Cost of material is high |
| Tin | 993 | • High power capability | • Huge volume variation |
| Antimony | 660 | • High-rate performance | • High cost and less abundance |
| Metal Oxides | |||
| Tin oxide | 790 | • High storage capacity | • Suffers from volume changes |
| SiO | 1600 | • Good temperature stability | • Needs additives or alloy elements for high energy density |
| Fe2O3 | 1008 | • Low cost • Eco-friendly | • Inherent electrical conductivity |
| CuO | 674 | • Good cyclability | • Volume changes, high voltage hysteresis |
| MnO | 756 | • Lower redox potential | • Inherent electrical conductivity |
| Mn2O3 | 1018 | • Lower operating potential | • Huge ICL, high voltage hysteresis |
| CoO/Co3O4 | 715 | • Favorable electrochemical properties | • Toxic and high-cost |
| Metal Sulfides | |||
| FeS | 610 | • Very flat operating potential | • Low electrochemical stability |
| MoS2 | 167 | • Very small ICL | • Inferior operating potential to TiS2 |
| Type | Features | Width (μm) | Height (μm) | Aspect Ratio | Reference |
|---|---|---|---|---|---|
| Whiskers | Minimally branched and kinks surrounded by excess electrolyte | 0.1–5 | 10–100 | 100 | [175] |
| Dendrites | Branched fractal structures with pores | 1–20 | 100–600 | 10 | [176] |
| Globules | Interconnected globules nucleated on impurity sites | 20–150 | 20–150 | 1–2 | [177] |
| Trees | Narrow stem and branched top | 10–500 | 100–500 | 1–3 | [178] |
| Cracks | Developed through grains and structural instability in inorganic solid electrolyte | 1–5 | -- | 5 | [179] |
| Moss | Pebble-shaped interconnected object with gaps and pores | 10–50 | -- | 1 | [180] |
| Needle | Spiky, thorny thin structures with small gaps | 0.5–10 | 5–200 | 1–2 | [176] |
| SEI Components | Electrode | Electrolyte | In Situ | Ref. |
|---|---|---|---|---|
| LiF | Graphene-Li | LiPF6/EC:DMC | XRD | [222] |
| LiF | Glassy carbon/Li | LiPF6/EC:DMC | EELS | [223] |
| LiF | Cu/Li | LiPF6/EC:DMC | SERS | [224] |
| LiF | MoS2/Ti–Li | LiPF6/EC:DEC | SAED | [225] |
| LiEDC | Si/Li | LiPF6/EC:DEC | SFG-VS | [226] |
| LiEDC | Graphite/Li | LiClO4/EC:THF | SFG-VS | [227] |
| LixPF | Graphite/Li | LiPF6/EC:DEC | FT-IR | [228] |
| Li2CO3 | Carbon/Li F | LiPF6/EC:DMC | FT-IR | [199] |
| Li2CO3 | Carbon/Li | LiClO4/EC:DMC | FT-IR | [229] |
| Li2CO3 | Cu/Li | LiPF6/EC:DMC | SERS | [224] |
| Li2CO3 | Si/Li | LiPF6/EC:DMC | FT-IR | [230] |
| Li2CO3 | Graphite/Li | LiPF6/EC:DEC | DRIFT | [231] |
| Li2CO3 | CVD (artificial SEI) | LiNi0.6Co0.1Mn0.3O2 | DRIFT | [232] |
| ROCO2Li | Sn/Li | LiPF6/EC:DMC | MFT-IRS | [233] |
| LiOH | Glassy carbon | LiPF6/EC:DMC | EELS | [223] |
| Li2O | Li/ITO | PEO-LiN(CF3SO2)2 | Ellipsometry | [234] |
| Li3N | Li | LiPON | XPS | [235] |
| ROCO2Li | Graphite/Li | LiPF6/EC:DMC | FT-IR | [236] |
| ROCO2Li | Graphite/Li | LiClO4/EC:DMC | ATR-FT-IR | [229] |
| PEO | C-Coated ZnFe2O4/Li | LiPF6/EC:DMC | Raman | [237] |
| Recent Cathodes | Electrolyte | Attained Voltage (V) | Capacity (mAh g−1) | Ref. |
|---|---|---|---|---|
| K-doped LiMn2O4−ySy | 1 M LiPF6 | 3.01–4.5 V | 116 mAh/g | [268] |
| Layered Li(Ni,Mn)O2-coated LiCoO2 | LiPF6 in ethylene carbonate | 4.47 V | 112 mAh g−1 at 10 C | [269] |
| Biomass-carbon@FeS2 | 1 M lithium bis(trifluoromethanesulfonyl)-imide (LiTFSI) in a mixed solvent of 1,3-dioxolane and 1,2-dimethoxyethane | 3.06 V | 850 mAh g−1 after 80 cycles at 0.5 C | [270] |
| Nitrogen–carbon-doped V2O5 | LiPF6 in ethylene carbonate (EC) | 3.9 V | 440 mAh g−1 | [271] |
| Li[Li0.2Ni0.13-x + y/3Co0.13-x + y/3Mn0.54-x + y/3]AlxZryO2 | Standard electrolyte | 4.4 V | 245.5 mAh g−1 at 25 mA g−1 | [272] |
| Li1.2Mn0.6Ni0.2O2 | LiFP6 electrolyte in EC | 2.0–4.8 V | 266 mAh g−1 | [273] |
| LiFeSO4F | LiFP6 electrolyte in EC | 3.9 V | 60 mA h g−1 even at 5 C | [274] |
| Poly-(1,4-anthraquinone)/carbon nanotube | Standard electrolyte | 3.8 | 233 mAh g−1 | [275] |
| LiVPO4F/C LiNi0.5Mn1.5O4 LiVPO4F/graphene | 1.3 mol L1 LiPF6 LiPF6 1 mol L1 LiPF6 in EC, EMC, DMC | 4.2 V 3.6–4.2 V 3.1 V | 116.5 mA h g−1 118.1 mAh g−1 168 mAh g−1 | [75,276,277,278,279,280] |
| Bismuth oxyfluoride @ CMK-3 nanocomposite | LiPF6 (1 M) in dimethyl carbonate | 4.0 V | 148 mA h g−1 after 40 cycles | [281] |
| High-entropy ceramics | 4.2 V | 307 mAh g−1 | [282] | |
| Li2(Ir0.1Mn0.9)O3 | 1 M LiPF6 dissolved in ethylenecarbonate and dimethyl carbonate | 192 mA h·g−1 | [283] | |
| LiNi0.91Co0.07Y0.02O2 | 1 M LiPF6 | 3.6–4.2 V | 225 mAh g−1 | [284] |
| Li1+xMn2-xO4 | LiPF6 | 3 V | 300 mAh g−1 | [285] |
| LiMn1.8Ti0.2O4 | 1.2 mol dm−3 LiPF6 in ethylene carbonate | 2.0–4.6 V | 215 mA h g−1 | [286] |
| LiNi0.4V0.1Mn1.5O4 | LiPF6 | 3.5 V | 99.5 mAh g−1 | [287] |
| Polyphenyl film-coated LiNi0.5Mn1.5O4 | LiPF6 | 3–4 V | 136.7 mAh g−1 | [276] |
| Mg-doped Li1.5[Mn0.75Ni0.25]O2+δ | 1 M LiPF6 | 3. 5 V | 248.6 (20 mA g−1) | [288] |
| Li1.16(Ni0.18Co0.10Mn0.52Fe0.02)O2 | 1 M LiPF6 in 1:1 wt% EC:DMC | 3.8 V | >100 mA h g−1 | [282] |
| NaCoHCF | molten salt | 3–3.8 V | 90 mAh g−1 at 20 C | [289] |
| Li1.12Na0.08Ni0.2Mn0.6O1.95F0.05 | I M LiPF6 | 3.6 V | 167 mAh g−1 at 5 C | [290] |
| LiNi0.6Mn0.2Co0.2O2 | 1.0 M LiPF6 in EC/EMC | 3.0–4.6 V | 180 mAh g−1 | [291] |
| Efficient Additives | Purpose |
|---|---|
| Cs+ | Dendrite growth tip softens and becomes dome-shaped rather than needle-shaped (before additive) |
| CsPF6 | Diminishes mossy protrusions through electrostatic shield mechanism |
| RuF | Works even at low concentrations by actively lowering the electric field |
| AlCl3 | Forms nanosized Al-hydroxide layer covering the anode surface, improves storage capacity |
| LiF | Increases Li transport with trace-controlled water molecules |
| Vinylene carbonate | Readily breaks the P-F bonds in LiPO2F2, improves ion migration |
| SO2Cl2 | Increases ion migration |
| Propylene carbonate | Accelerates Li reactivity by ion diffusion |
| Fluro ethylene carbonate | Forms LiF-rich, stable SEI |
| Dimethylsulfate | Sulfur layer passivation reduces dendrite growth |
| N,N-dimethylethanolamine (DMEA) | Organic moieties forming stable SEI |
| Tetraethylorthosilicate in Li-O2 battery | Protects from anodic corrosion by forming stable film over anode |
| SiCl4 in Li-S battery | Increases coulombic efficiency |
| Electrolyte | SEI Components |
|---|---|
| LiBF4 in propylene carbonate | LiF, Li2CO3, LiOH, carbonate, hydrocarbons |
| LiBF4 in r-BL | LiF, LiOH |
| LiBF4 in THF | LiF, hydrocarbons |
| LiClO4 in PC | LiF, LiOH, Li2O, LiOCO2R |
| LiAsF6 in DMC:EC | ROCO2Li, Li2CO3 |
| LiPF6 in VC | Li3N, LiNO2, ROCO2Li (CH2CH2O), C-F, LiF |
| LiNO3 in EC | LiNaOb |
| LiNO3 in DME | Li2S2O3, LiNxOy, Li2S2 |
| Li2S6 in DME/DOL | Li2S2, Li2S |
| LiTFSI in DME | Li2S2O3, Li3N, Li2S |
| LiTFSI in DOL | Li2NSO2CF3, LiF, Li2S2O4, Li2S |
| Refs. [405,406,407,408,409,410,411,412,413,414,415,416,417,418,419,420,421,422,423,424,425,426,427] | ||
| 1. Anode Materials | ||
| Carbon-Based | ||
| Compound | Specific Capacity (mAh g−1) | Operating Voltage and Performance Characteristics |
| Hard carbon | ~300 | Closed nanopore structures, 40% irreversible capacity |
| Biomass-derived hard carbon | 430 | Capacity fading of 2.5% after 200 cycles |
| Aromatic structure-derived carbon | 321 | Strong pyrolization involved, high thermal stability |
| Petroleum coke | 80–100 | Less stable SEI |
| Electrospun carbon fibers | 233 | Capacity fading of 2.3% after 200 cycles |
| Lignin derived electrospun fibers | 293 | Less than 10% of capacity fading |
| Polyacrylonitrile fibers–carbon | 140 | Current density of 500 mA g−1 |
| Cellulose-derived carbon fibers | 255 | Current density of 40 mA g−1 |
| N-doped carbon fibers | 134 | Current density of 200 mA g−1 Uniform nitrogenation leads to improved stability |
| Nanocarbon spheres | ~240 | >400 cycles at 5 C |
| Nanocelular foam | 152 | >1600 cycles, less than 10% capacity fading |
| Expanded graphite | 280 | Current density 20 mA g−1 |
| Carbon, CaC2-doped Mxene | 430–582 | >50 cycles at 30 mA g−1 |
| Metal Alloys | ||
| Na-Sn | 776 | >100 cycles, free volume and elongated contacts |
| Sn-Sb-Carbon | 400 | >80 cycles at 30 °C |
| Cu-Sn | 400 | Nanosized SEI, 100 cycles |
| Cu-Sn-TiC | 150–180 | >100 cycles at 100 mA g−1 |
| Sn-Sb | 600 | 160 cycles, formation of intermetallides |
| Sb-C | 610 | Sb ~ 30 nm in size, >300 cycles |
| Sb-rGO | 400 | Current density 20 mA/g, >30 cycles |
| SiC-Sb-Carbon | 440–500 | 100 cycles, current density 100 mA g−1 |
| Pb-C | 464 | >50 cycles, high capacity retention |
| Oxide Materials | ||
| SnO | 550 | Flower-like morphology of SnO favored high stability |
| SnO2–carbon | 500 | >50 cycles, less capacity fading |
| TiO2 | 335 | 3.5–4 V, >100 cycles |
| SnO2-Fe2O3 | 300 | Current density 25 mA g−1 |
| Na2Ti3O7 | 93 | Current density of 5 C after 50 cycles |
| NaVO2 | 120 | ~1.6 V |
| Na–vanadium-based oxides, NaFeTiO4 | 150–300 | 0.8–2.6 V |
| Other Anodes | ||
| Sn4P3 | 700 | Current density of 50 mA g−1 |
| SnSe | 700 | >50 cycles at 50 mA g−1 |
| MoSe2 | 350 | High charge retention |
| NASICON, sodium vanadium complexes | 67–112 | 1000 cycles at 10 C |
| 2. Cathode Materials | ||
| NaxMnO2 | 308 | Structural changes lead to capacity fading |
| NaxCoO2 | 107 | ~3.6 V |
| NaFeO2 | 100 | ~3.3 V, degradation after 3.8 V |
| NaCrO2, NaxVO2 | 115 | Ability for sodium reversibility |
| Na2/3[Ni1/3Mn2/3]O2 | 173 | 3.3–4.5 V |
| Na2/3[Fe1/3Mn2/3]O2 | 190 | 3. 4 V |
| Na0.6Li0.6[Mn0.72Ni0.18Co0.10]O2 | 200 | 2–4 V at 20 mA g−1 |
| Phosphate-Based Materials | ||
| NaFePO4 | 90 | Current density of 90 mA/g, ~3.3 V |
| Na2FeP2O7 | 97 | >150 cycles, ~3.2 V |
| Na2MnP2O7 | 78–70 | 3.6 V |
| Na3MnPO4CO3 | 125 | 2–4 V, thermal stability |
| Na4Co3(PO4)2P2O7 | 95 | Low volume expansion |
| Na3V2(PO4)3 | 100 | ~3.3 V |
| NaVPO4F | 97–110 | 2–3 V |
| Other Cathodes | ||
| NaV6O15 | 80–140 | 3–3.5 V |
| NaFeF3 | 237 | 2–3.3 V |
| Chalcogenides | 300–600 | 2–3.4 V |
| Prussian blue analogues | 80 | 3.5–4 V |
| Electrolyte | Electrode | Current Density (mA g−1) | ICE (%) | Ref. |
|---|---|---|---|---|
| KPF6-EC/PC | Graphite | 20 | 66.5 | [439] |
| KPF6-EC/DEC | Bi-rGO | 50 | 47 | [440] |
| KFSI-EC/DEC | Sn-Carbon fiber | 50 | 64.17 | [441] |
| KFSI-DME | K-Cu | 0.05 | 99 | [442] |
| KTFSI | Bi@C | 10 | 90 | [443] |
| KTFSI-DOL/DME | PAQS-K | 20 | 90.1 | [444] |
| KPF6-EC/EMC | 3D SnSb | 500 | 58.5 | [445] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramasubramanian, B.; Reddy, M.V.; Zaghib, K.; Armand, M.; Ramakrishna, S. Growth Mechanism of Micro/Nano Metal Dendrites and Cumulative Strategies for Countering Its Impacts in Metal Ion Batteries: A Review. Nanomaterials 2021, 11, 2476. https://doi.org/10.3390/nano11102476
Ramasubramanian B, Reddy MV, Zaghib K, Armand M, Ramakrishna S. Growth Mechanism of Micro/Nano Metal Dendrites and Cumulative Strategies for Countering Its Impacts in Metal Ion Batteries: A Review. Nanomaterials. 2021; 11(10):2476. https://doi.org/10.3390/nano11102476
Chicago/Turabian StyleRamasubramanian, Brindha, M. V. Reddy, Karim Zaghib, Michel Armand, and Seeram Ramakrishna. 2021. "Growth Mechanism of Micro/Nano Metal Dendrites and Cumulative Strategies for Countering Its Impacts in Metal Ion Batteries: A Review" Nanomaterials 11, no. 10: 2476. https://doi.org/10.3390/nano11102476
APA StyleRamasubramanian, B., Reddy, M. V., Zaghib, K., Armand, M., & Ramakrishna, S. (2021). Growth Mechanism of Micro/Nano Metal Dendrites and Cumulative Strategies for Countering Its Impacts in Metal Ion Batteries: A Review. Nanomaterials, 11(10), 2476. https://doi.org/10.3390/nano11102476