Preparation and Characterization of Anti-Cancer Crystal Drugs Based on Erythrocyte Membrane Nanoplatform
Abstract
:1. Introduction
2. Materials and Methods
2.1. RBC Membrane Extraction
2.2. Preparation of RBC-NC(DOX)
2.3. Transmission Electron Microscopy Imaging
2.4. Potential and Particle Size
2.5. Stability Study
2.6. Aggregation
2.7. In Vitro DOX Release
2.8. Cell Culture
2.9. In Vitro Cell Uptake Experiment
2.10. Cytotoxicity Assay
2.11. In Vivo Safety Evaluation
2.12. In Vivo Antitumor Efficacy
2.13. Histological Analysis
2.14. Hemolysis Test
2.15. Statistical Analysist
3. Results and Discussion
3.1. Preparation of RBC-NC(DOX)
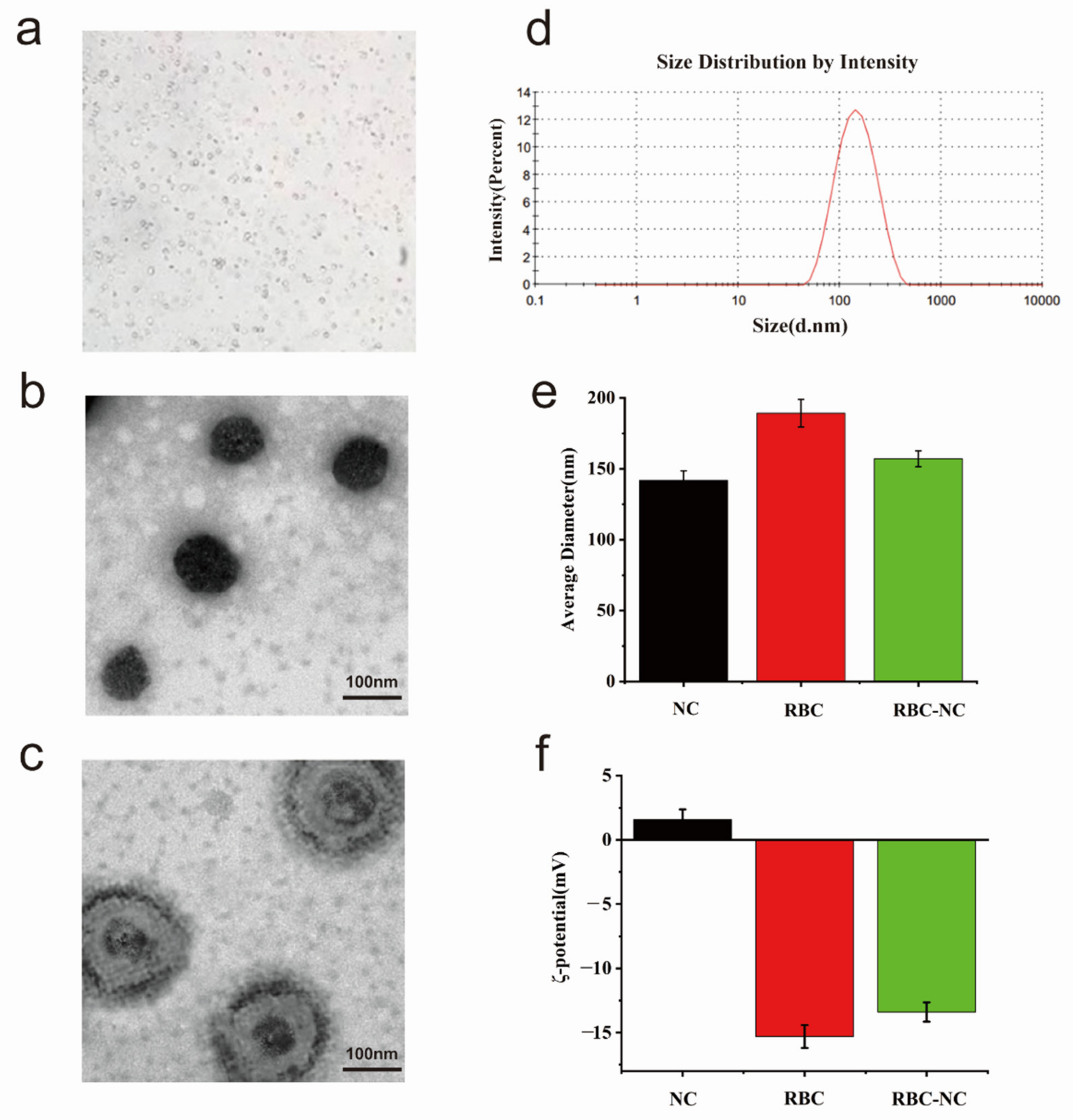
3.2. Characterization
3.3. Physical Properties In Vitro
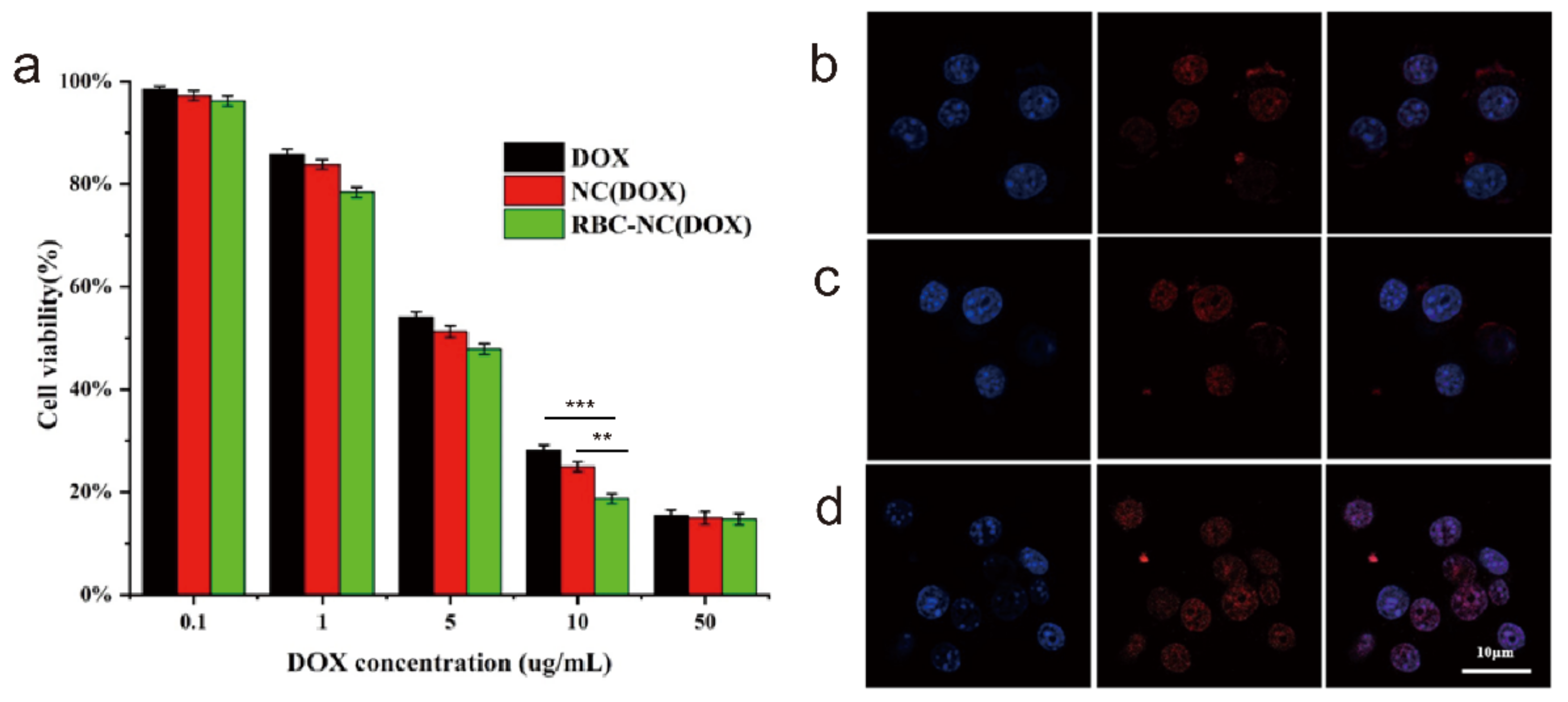
3.4. Cellular Uptake and In Vitro Cytotoxicity of RBC-NC(DOX)
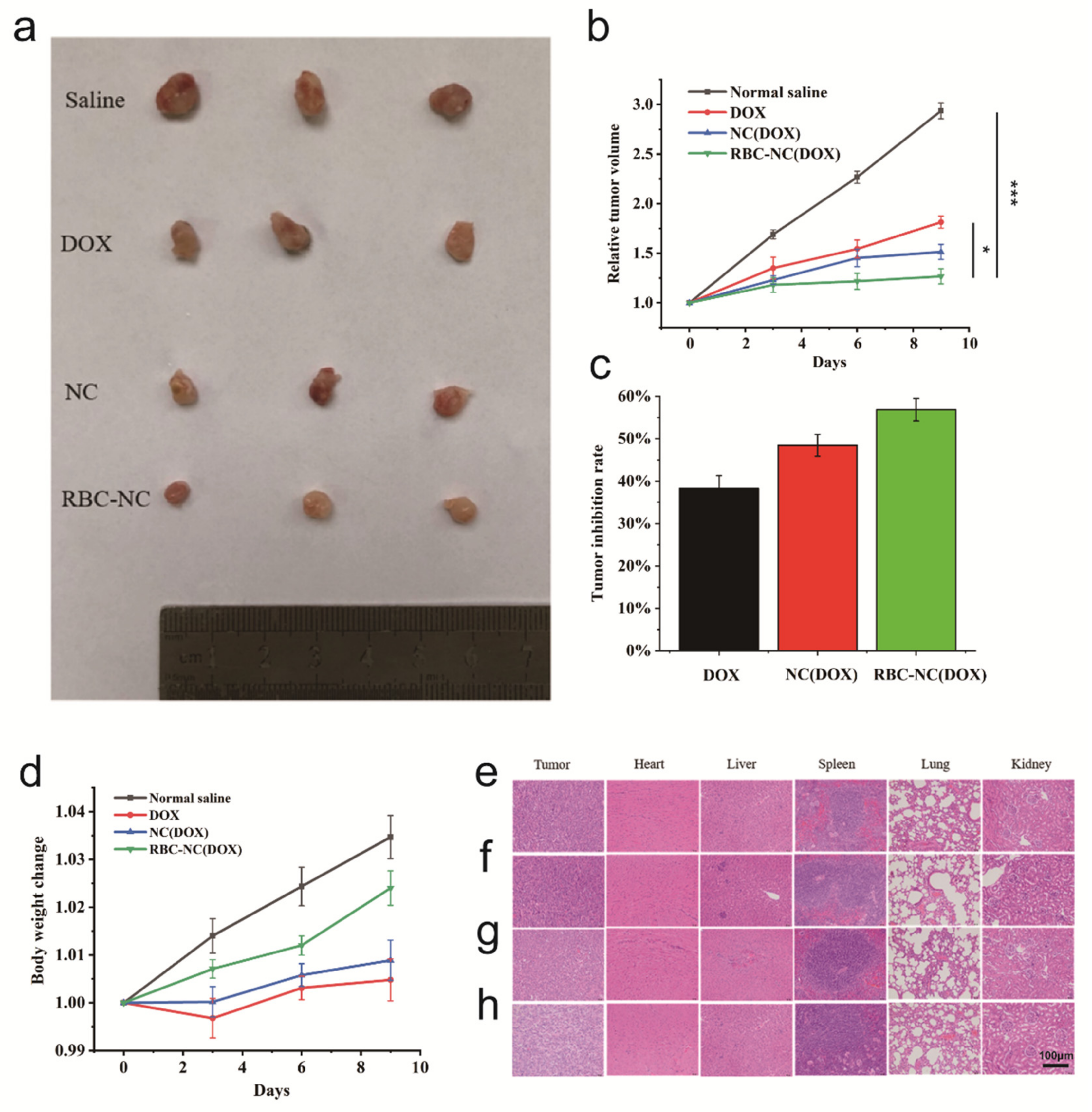
3.5. In Vivo Antitumor Effect on Subcutaneous Tumor Model
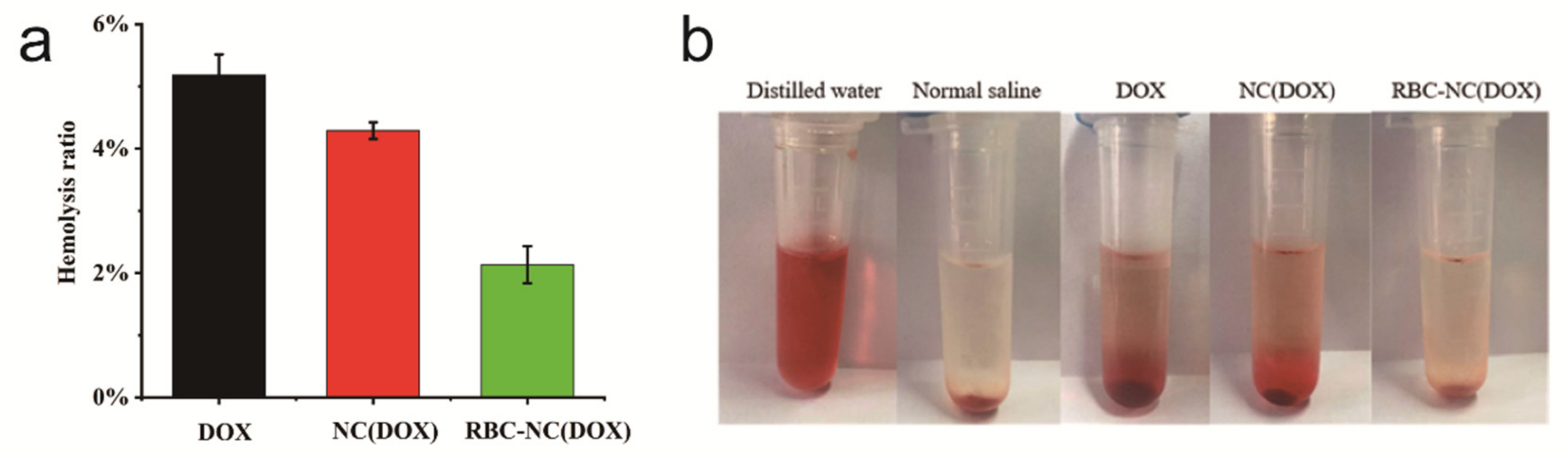
3.6. Blood Compatibility
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilczewska, A.Z.; Niemirowicz, K.; Markiewicz, K.H. Nanoparticles as drug delivery systems. Pharm. Rep. 2012, 64, 1020–1037. [Google Scholar] [CrossRef]
- Petros, R.A.; DeSimone, J.M. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 2010, 9, 615–627. [Google Scholar] [CrossRef]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef]
- Zhang, M.; Du, Y.; Wang, S.; Chen, B. A review of biomimetic nanoparticle drug delivery systems based on cell membranes. Drug Des. Dev. Ther. 2020, 14, 5495–5503. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-M.J.; Fang, R.H.; Luk, B.T.; Zhang, L. Polymeric nanotherapeutics: Clinical development and advances in stealth functionalization strategies. Nanoscale 2013, 6, 65–75. [Google Scholar] [CrossRef]
- Luk, B.T.; Fang, R.H.; Hu, C.-M.J.; Copp, J.A.; Thamphiwatana, S.; Dehaini, D.; Gao, W.; Zhang, K.; Li, S.; Zhang, L. Safe and immunocompatible nanocarriers cloaked in RBC membranes for drug delivery to treat solid tumors. Theranostics 2016, 6, 1004–1011. [Google Scholar] [CrossRef] [Green Version]
- Jiang, S.; Win, K.Y.; Liu, S.; Teng, C.P.; Zheng, Y.; Han, M.-Y. Surface-functionalized nanoparticles for biosensing and imaging-guided therapeutics. Nanoscale 2013, 5, 3127–3148. [Google Scholar] [CrossRef]
- Luk, B.T.; Zhang, L. Cell membrane-camouflaged nanoparticles for drug delivery. J. Control. Release 2015, 220, 600–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.; He, Y.; Zhang, S.; Qin, J.; Wang, J. Cell membrane-based nanoparticles: A new biomimetic platform for tumor diagnosis and treatment. Acta Pharm. Sin. B 2018, 8, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-M.J.; Fang, R.H.; Luk, B.T.; Chen, K.N.H.; Carpenter, C.; Gao, W.; Zhang, K.; Zhang, L. “Marker-of-self” functionalization of nanoscale particles through a top-down cellular membrane coating approach. Nanoscale 2013, 5, 2664–2668. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.H.; Hu, C.-M.J.; Luk, B.T.; Gao, W.; Copp, J.A.; Tai, Y.; O’Connor, D.E.; Zhang, L. Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano Lett. 2014, 14, 2181–2188. [Google Scholar] [CrossRef]
- Zhao, Q.; Sun, X.; Wu, B.; Shang, Y.; Huang, X.; Dong, H.; Liu, H.; Chen, W.; Gui, R.; Li, J. Construction of homologous cancer cell membrane camouflage in a nano-drug delivery system for the treatment of lymphoma. J. Nanobiotechnol. 2021, 19, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, K.; Li, T.; Maruf, A.; Qin, X.; Luo, L.; Wang, G. Macrophage membrane functionalized biomimetic nanoparticles for targeted anti-atherosclerosis applications. Theranostics 2021, 11, 164. [Google Scholar] [CrossRef] [PubMed]
- Pei, W.; Huang, B.; Chen, S.; Wang, L.; Xu, Y.; Niu, C. Platelet-mimicking drug delivery nanoparticles for enhanced chemo-photothermal therapy of breast cancer. Int. J. Nanomed. 2020, 15, 10151–10167. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xin, Y.; Cao, H.; Li, W.; Hua, Y.; Webster, T.J.; Zhang, C.; Tang, W.; Liu, Z. Recent advances in mesenchymal stem cell membrane-coated nanoparticles for enhanced drug delivery. Biomater. Sci. 2021, 9, 1088–1103. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Wang, Q.; Li, J.; Zhao, Q.; Huang, X.; Dong, H.; Nie, X. Platelet-membrane-camouflaged zirconia nanoparticles inhibit the invasion and metastasis of hela cells. Front. Chem. 2020, 8, 377. [Google Scholar] [CrossRef]
- Yang, Q.; Xiao, Y.; Yin, Y.; Li, G.; Peng, J. Erythrocyte membrane-camouflaged IR780 and DTX coloading polymeric nanoparticles for imaging-guided cancer photo–chemo combination therapy. Mol. Pharm. 2019, 16, 3208–3220. [Google Scholar] [CrossRef]
- Dehaini, D.; Wei, X.; Fang, R.H.; Masson, S.; Angsantikul, P.; Luk, B.T.; Zhang, L. Erythrocyte-platelet hybrid membrane coating for enhanced nanoparticle functionalization. Adv. Mater. 2017, 29, 1606209. [Google Scholar] [CrossRef] [Green Version]
- Hamidi, M.; Tajerzadeh, H. Carrier erythrocytes: An overview. Drug Deliv. 2003, 10, 9–20. [Google Scholar] [CrossRef]
- Barclay, A.N.; Van den Berg, T.K. The interaction between signal regulatory protein alpha (SIRPα) and CD47: Structure, function, and therapeutic target. Annu. Rev. Immunol. 2014, 32, 25–50. [Google Scholar] [CrossRef]
- Xia, Q.; Zhang, Y.; Li, Z.; Hou, X.; Feng, N. Red blood cell membrane-camouflaged nanoparticles: A novel drug delivery system for antitumor application. Acta Pharm. Sin. B 2019, 9, 675–689. [Google Scholar] [CrossRef] [PubMed]
- Ihler, G.M.; Glew, R.H.; Schnure, F.W. Enzyme loading of erythrocytes. Proc. Natl. Acad. Sci. USA 1973, 70, 2663–2666. [Google Scholar] [CrossRef] [PubMed]
- Hirlekar, R.; Patel, P.; Dand, N.; Kadam, V. Drug loaded erythrocytes: As novel drug delivery system. Curr. Pharm. Des. 2008, 14, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Rabinow, B.E. Nanosuspensions in drug delivery. Nat. Rev. Drug Discov. 2004, 3, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Du, J.; Zhou, Y.; Wang, Y. Safety of nanosuspensions in drug delivery. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Leone, F.; Cavalli, R. Drug nanosuspensions: A ZIP tool between traditional and innovative pharmaceutical formulations. Expert Opin. Drug Deliv. 2015, 12, 1607–1625. [Google Scholar] [CrossRef]
- Matrai, A.A.; Varga, G.; Tanczos, B.; Barath, B.; Varga, A.; Horvath, L.; Bereczky, Z.; Deak, A.; Nemeth, N. In vitro effects of temperature on red blood cell deformability and membrane stability in human and various vertebrate species. Clin. Hemorheol. Microcirc. 2021, 78, 291–300. [Google Scholar] [CrossRef]
- Maudens, P.; Seemayer, C.A.; Pfefferlé, F.; Jordan, O.; Allémann, E. Nanocrystals of a potent p38 MAPK inhibitor embedded in microparticles: Therapeutic effects in inflammatory and mechanistic murine models of osteoarthritis. J. Control. Release 2018, 276, 102–112. [Google Scholar] [CrossRef]
- Banal, S.; Bansal, S.; Kumria, R. Nanocrystals: Current strategies and trends. Int. J. Res. Pharm. Biomed. Sci. 2012, 3, 406–417. [Google Scholar]
- Mitragotri, S.; Burke, P.A.; Langer, R. Overcoming the challenges in administering biopharmaceuticals: Formulation and delivery strategies. Nat. Rev. Drug Discov. 2014, 13, 655–672. [Google Scholar] [CrossRef] [Green Version]
- Sun, B.; Yeo, Y. Nanocrystals for the parenteral delivery of poorly water-soluble drugs. Curr. Opin. Solid State Mater. Sci. 2012, 16, 295–301. [Google Scholar] [CrossRef] [Green Version]
- Fan, M.; Geng, S.; Liu, Y.; Wang, J.; Wang, Y.; Zhong, J.; Yan, Z.; Yu, L. Nanocrystal technology as a strategy to improve drug bioavailability and antitumor efficacy for the cancer treatment. Curr. Pharm. Des. 2018, 24, 2416–2424. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.; Ma, Y.; Cao, G.; Wang, J.; Zhang, X.; Feng, J.; Wang, W. Improved oral bioavailability for lutein by nanocrystal technology: Formulation development, in vitro and in vivo evaluation. Artif. Cells Nanomed. Biotechnol. 2017, 46, 1018–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Du, J.; Wang, L.; Wang, Y. Nanocrystals technology for improving bioavailability of poorly soluble drugs: A mini-review. J. Nanosci. Nanotechnol. 2017, 17, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.; Xu, J.H.; Cai, B. Synthetic nanoparticles camouflaged with biomimetic erythrocyte membranes for reduced reticulo-endothelial system uptake. Nanotechnology 2016, 27, 085106. [Google Scholar] [CrossRef]
- Dodge, J.T.; Mitchell, C.; Hanahan, D.J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch. Biochem. Biophys. 1963, 100, 119–130. [Google Scholar] [CrossRef]
- Iversen, T.-G.; Skotland, T.; Sandvig, K. Endocytosis and intracellular transport of nanoparticles: Present knowledge and need for future studies. Nano Today 2011, 6, 176–185. [Google Scholar] [CrossRef]
- Rashidi, M.; Khalilnezhad, A.; Amani, D.; Jamshidi, H.; Muhammadnejad, A.; Bazi, A.; Ziai, S.A. Umbelliprenin shows antitumor, antiangiogenesis, antimetastatic, anti-inflammatory, and immunostimulatory activities in 4T1 tumor-bearing Balb/c mice. J. Cell. Physiol. 2018, 233, 8908–8918. [Google Scholar] [CrossRef]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Maeda, H. Macromulecular therapeutics in cancer treatment: The EPR effect and beyond. J. Control. Release 2012, 164, 138–144. [Google Scholar] [CrossRef]
- Sharma, P.; Narwal, A.; Kamboj, M. Detection of apoptosis in leukoplakia and oral squamous cell carcinoma using methyl green pyronin and hematoxylin and eosin. Iran. J. Pathol. 2020, 15, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Raikar, A.; Nayak, A.; Kotrashetti, V.; Nayak, R.; Shree, S.; Kambali, S. Histochemical detection and comparison of apoptotic cells in the gingival epithelium using hematoxylin and eosin and methyl green-pyronin: A pilot study. J. Indian Soc. Periodontol. 2016, 20, 294–298. [Google Scholar] [CrossRef]
- Luk, B.T.; Zhang, L. Current advances in polymer-based nanotheranostics for cancer treatment and diagnosis. ACS Appl. Mater. Interfaces 2014, 6, 21859–21873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, L.; Liu, G.; Ma, J.; Wang, X.; Zhou, L.; Li, X. Drug nanocrystals: In vivo performances. J. Control. Release 2012, 160, 418–430. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Nakamura, S.; Sugimoto, N.; Shigemori, T.; Kato, Y.; Ohno, M.; Sakuma, S.; Ito, K.; Kumon, H.; Hirose, H.; et al. Turbulence activates platelet biogenesis to enable clinical scale ex vivo production. Cell 2018, 174, 636–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Z.; Gao, W.; Hu, H.; Ma, K.; He, B.; Dai, W.; Wang, X.; Wang, J.; Zhang, X.; Zhang, Q. Novel thermo-sensitive hydrogel system with paclitaxel nanocrystals: High drug-loading, sustained drug release and extended local retention guaranteeing better efficacy and lower toxicity. J. Control. Release 2014, 174, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, Z.-H.; Li, T.; McNally, H.; Park, K.; Sturek, M. Development and evaluation of transferrin-stabilized paclitaxel nanocrystal formulation. J. Control. Release 2014, 176, 76–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Formulation | IC50 (μg/mL) |
|---|---|
| DOX | 5.587 |
| NC(DOX) | 4.812 |
| RBC-NC(DOX) | 3.805 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, L.; Qiu, L.; Huang, B.; Yin, J.; Li, Y.; Yang, X.; Chen, G. Preparation and Characterization of Anti-Cancer Crystal Drugs Based on Erythrocyte Membrane Nanoplatform. Nanomaterials 2021, 11, 2513. https://doi.org/10.3390/nano11102513
Ren L, Qiu L, Huang B, Yin J, Li Y, Yang X, Chen G. Preparation and Characterization of Anti-Cancer Crystal Drugs Based on Erythrocyte Membrane Nanoplatform. Nanomaterials. 2021; 11(10):2513. https://doi.org/10.3390/nano11102513
Chicago/Turabian StyleRen, Lili, Lirong Qiu, Binbin Huang, Jun Yin, Yaning Li, Xiaolong Yang, and Guoguang Chen. 2021. "Preparation and Characterization of Anti-Cancer Crystal Drugs Based on Erythrocyte Membrane Nanoplatform" Nanomaterials 11, no. 10: 2513. https://doi.org/10.3390/nano11102513
APA StyleRen, L., Qiu, L., Huang, B., Yin, J., Li, Y., Yang, X., & Chen, G. (2021). Preparation and Characterization of Anti-Cancer Crystal Drugs Based on Erythrocyte Membrane Nanoplatform. Nanomaterials, 11(10), 2513. https://doi.org/10.3390/nano11102513







