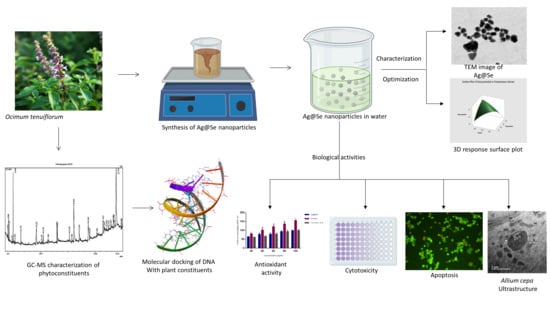
Biogenic Synthesis of Silver-Core Selenium-Shell Nanoparticles Using Ocimum tenuiflorum L.: Response Surface Methodology-Based Optimization and Biological Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plant Extraction and Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
2.3. Biogenic Synthesis of Ag@Se NPs
2.4. Generation of a NP Synthesis Model and Optimization of Physicochemical Parameters
2.5. Nanoparticle Characterization
2.6. Antioxidant Activity
2.7. Cytotoxicity
2.8. Apoptosis
2.9. Allium Cepa Toxicity
2.9.1. Cytogenotoxocity Studies
2.9.2. Electron Microscopy Studies
2.10. Molecular Modelling with DNA
2.11. Statistical Analysis of Data
3. Results
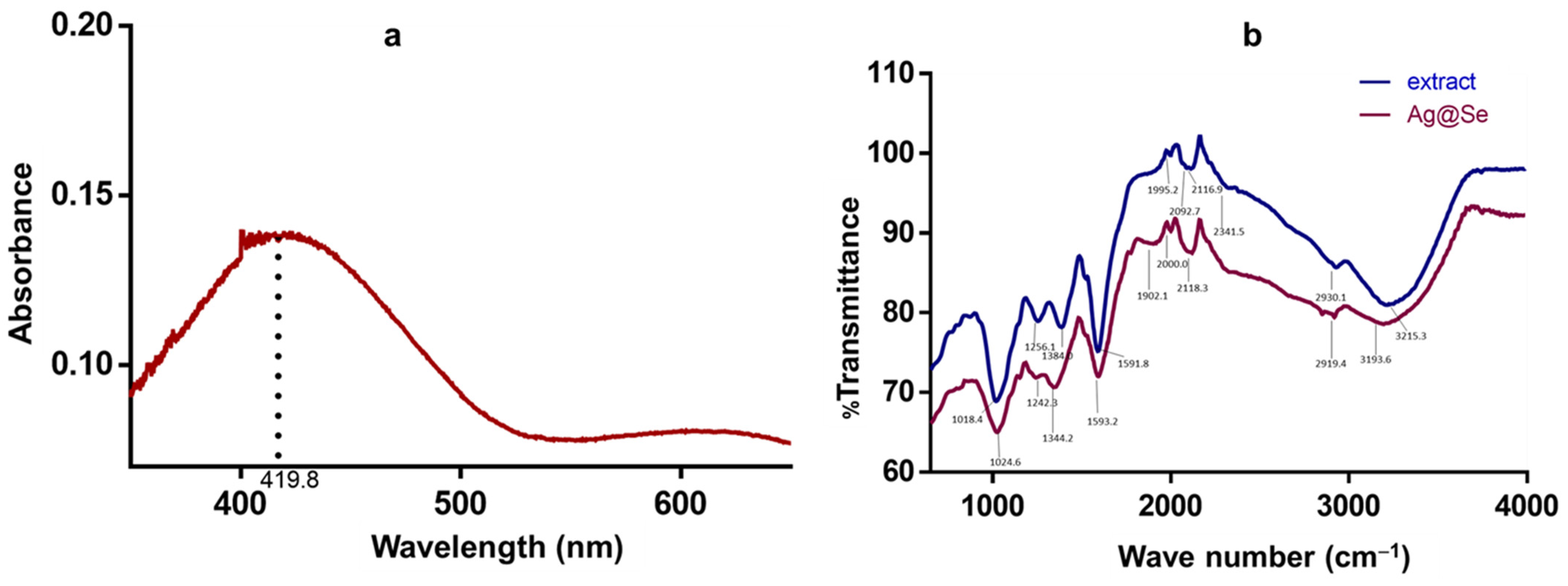
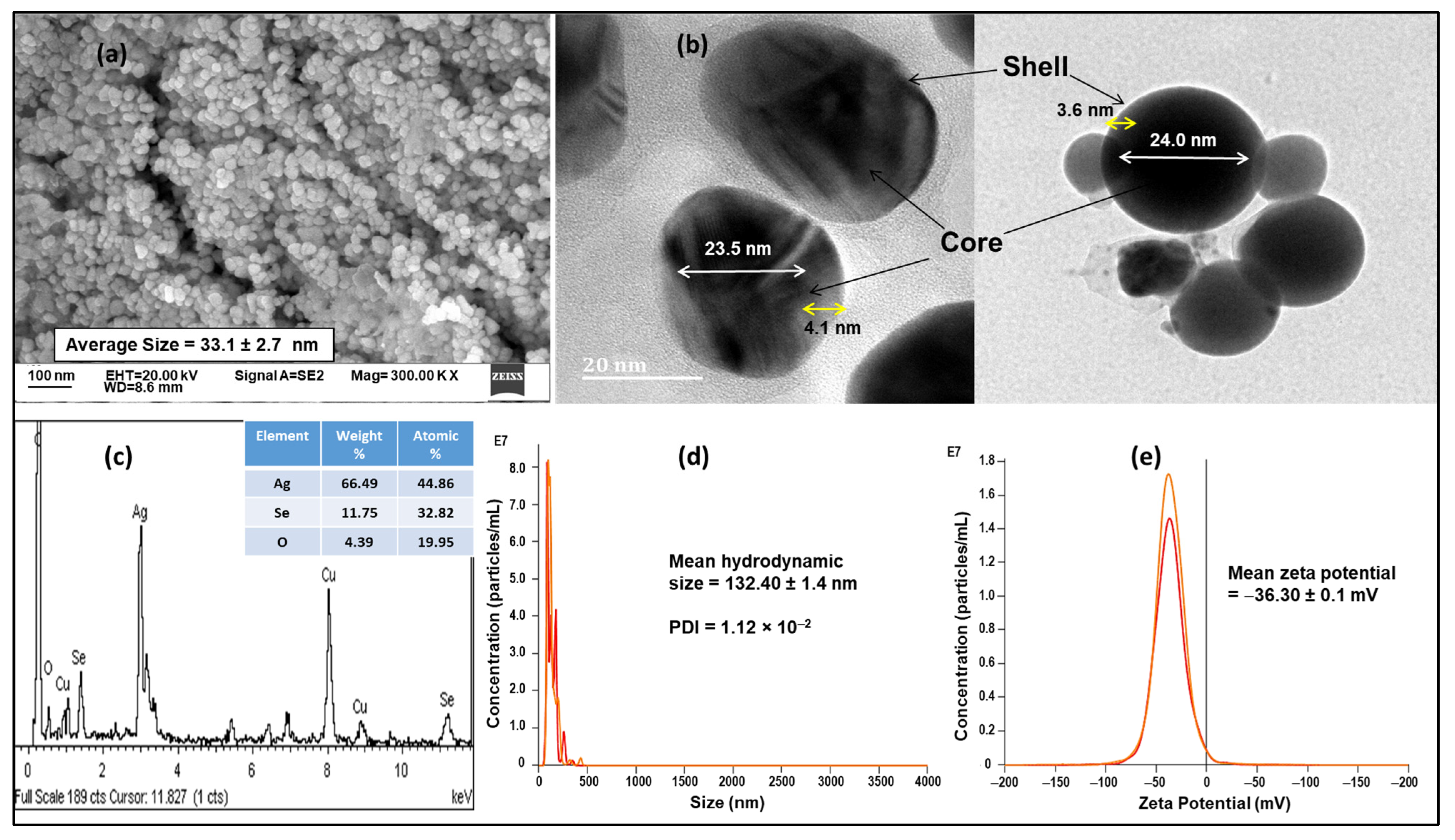
3.1. Nanoparticle Synthesis and Characterization
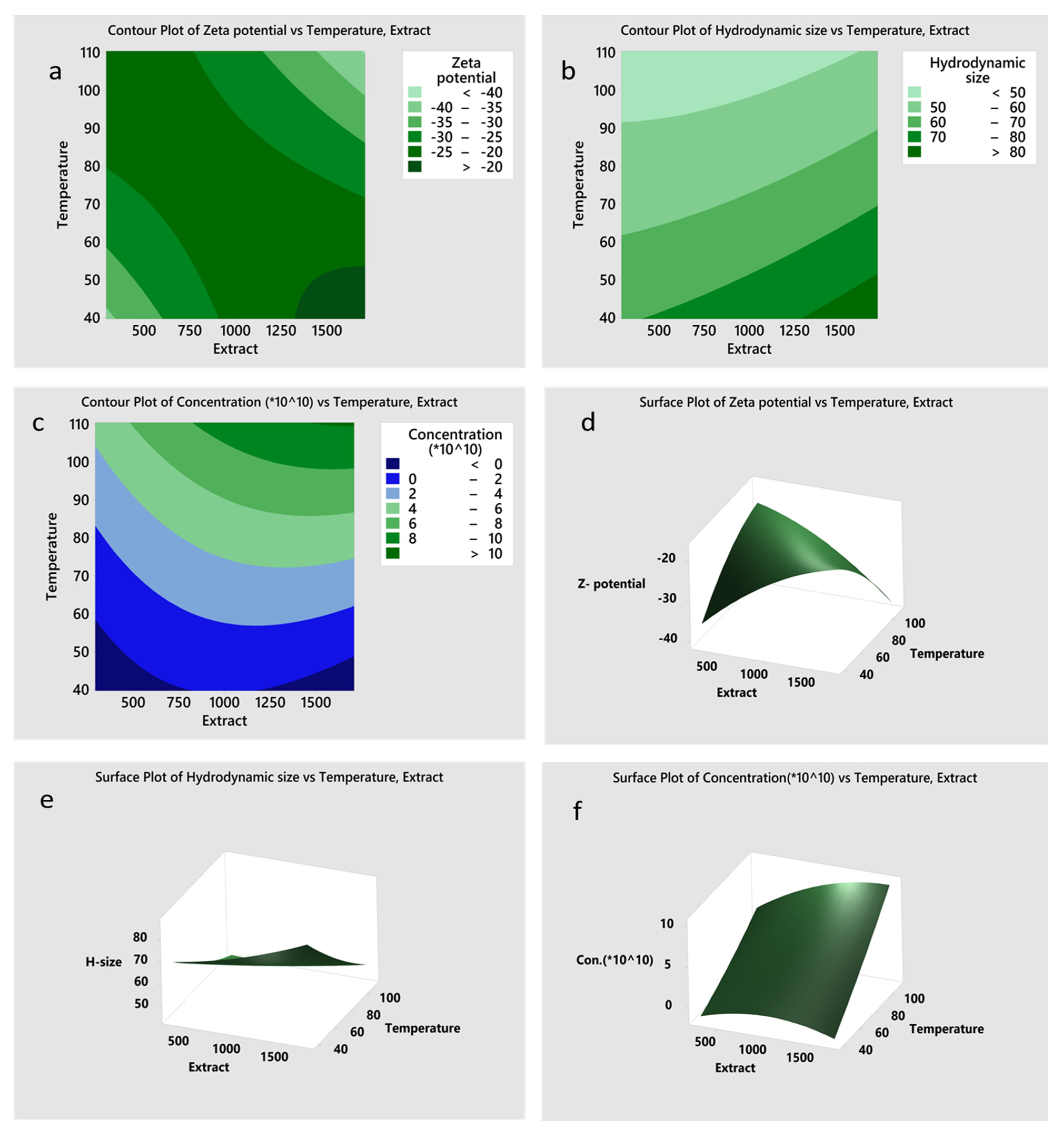
3.2. Model-Based Optimization of Physicochemical Conditions for NP Synthesis
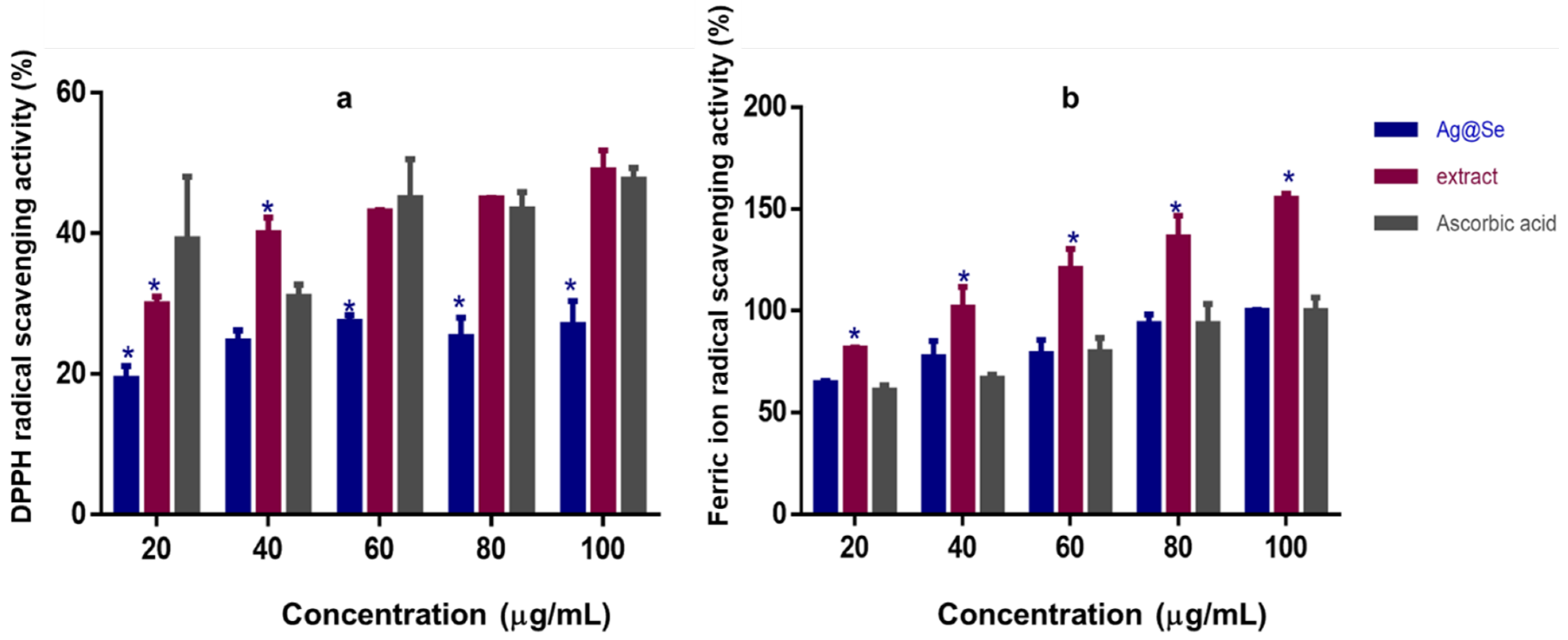
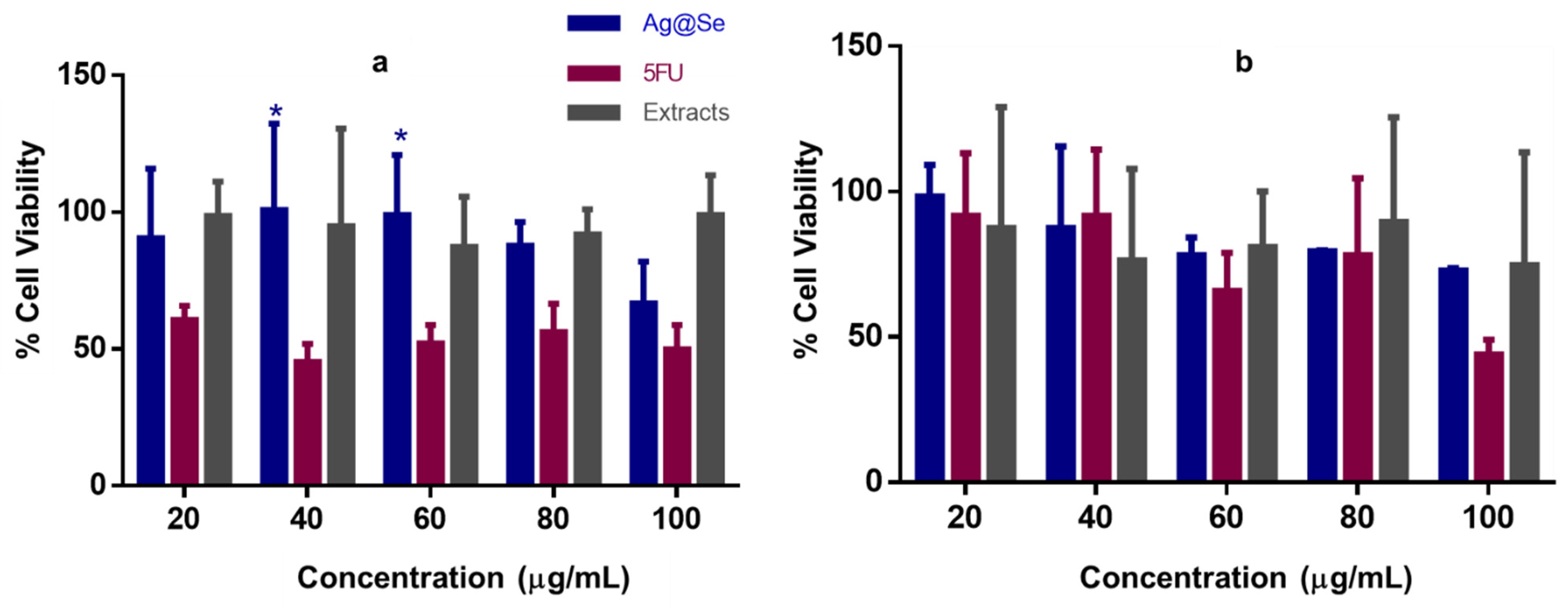
3.3. Antioxidant Activity and Cytotoxicity
3.4. Apoptosis
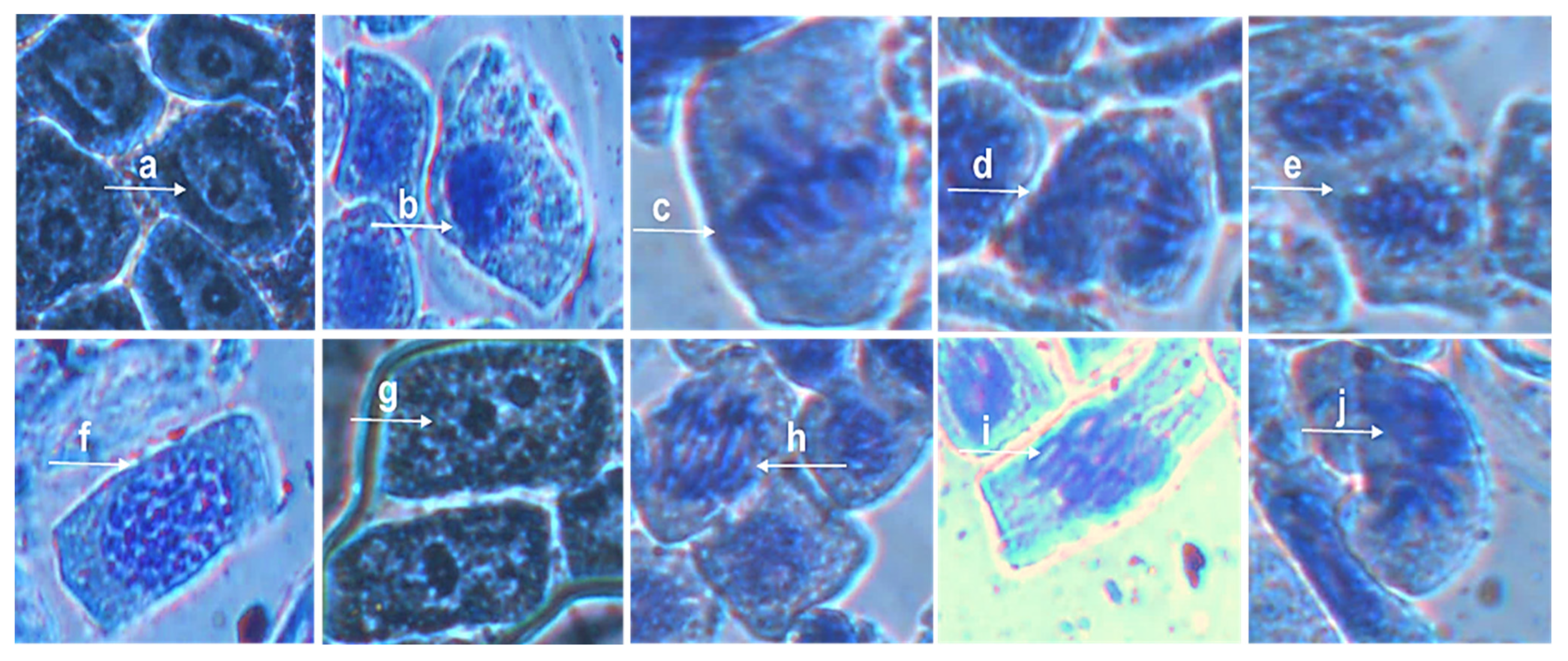
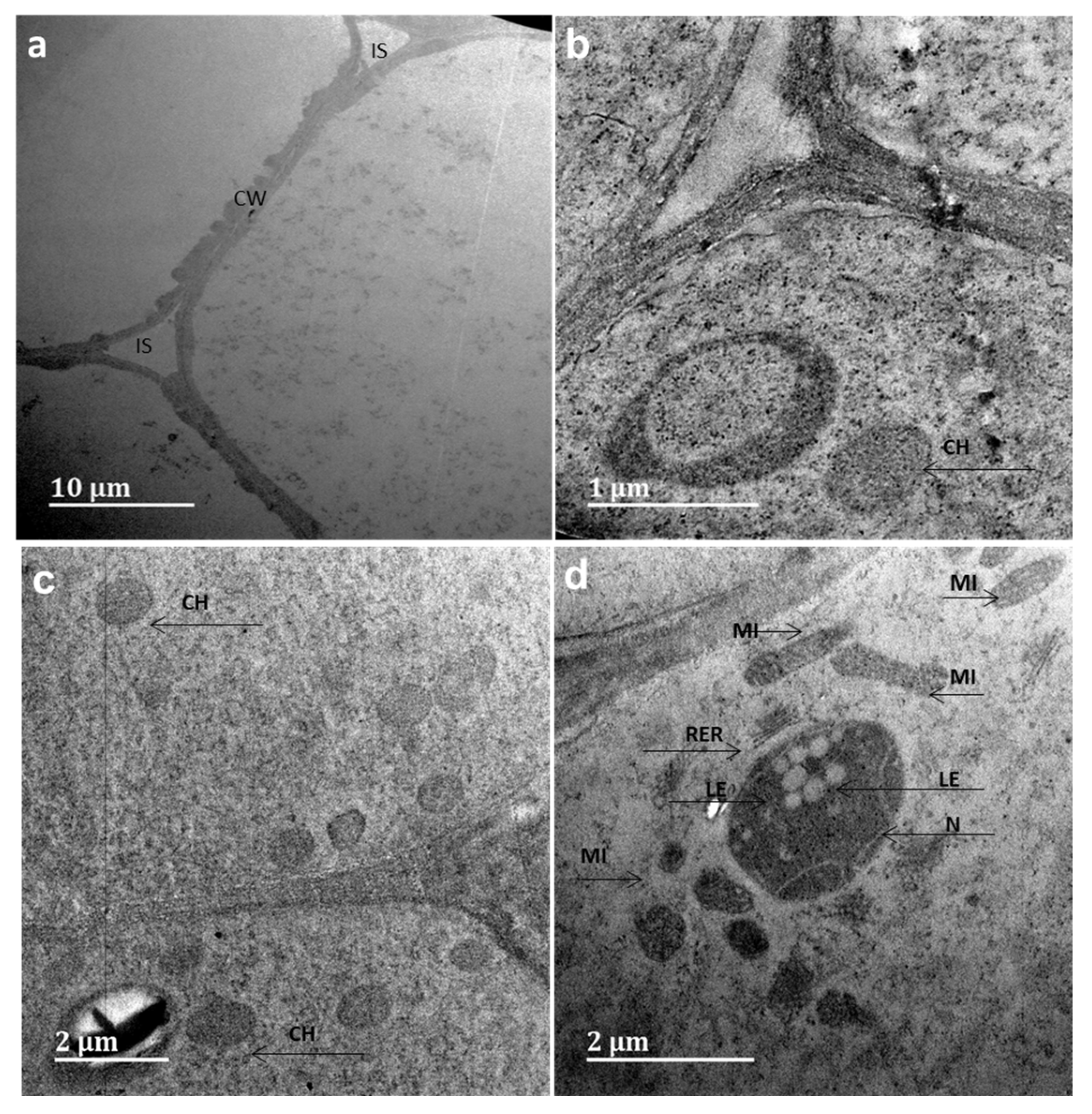
3.5. Allium cepa Assay
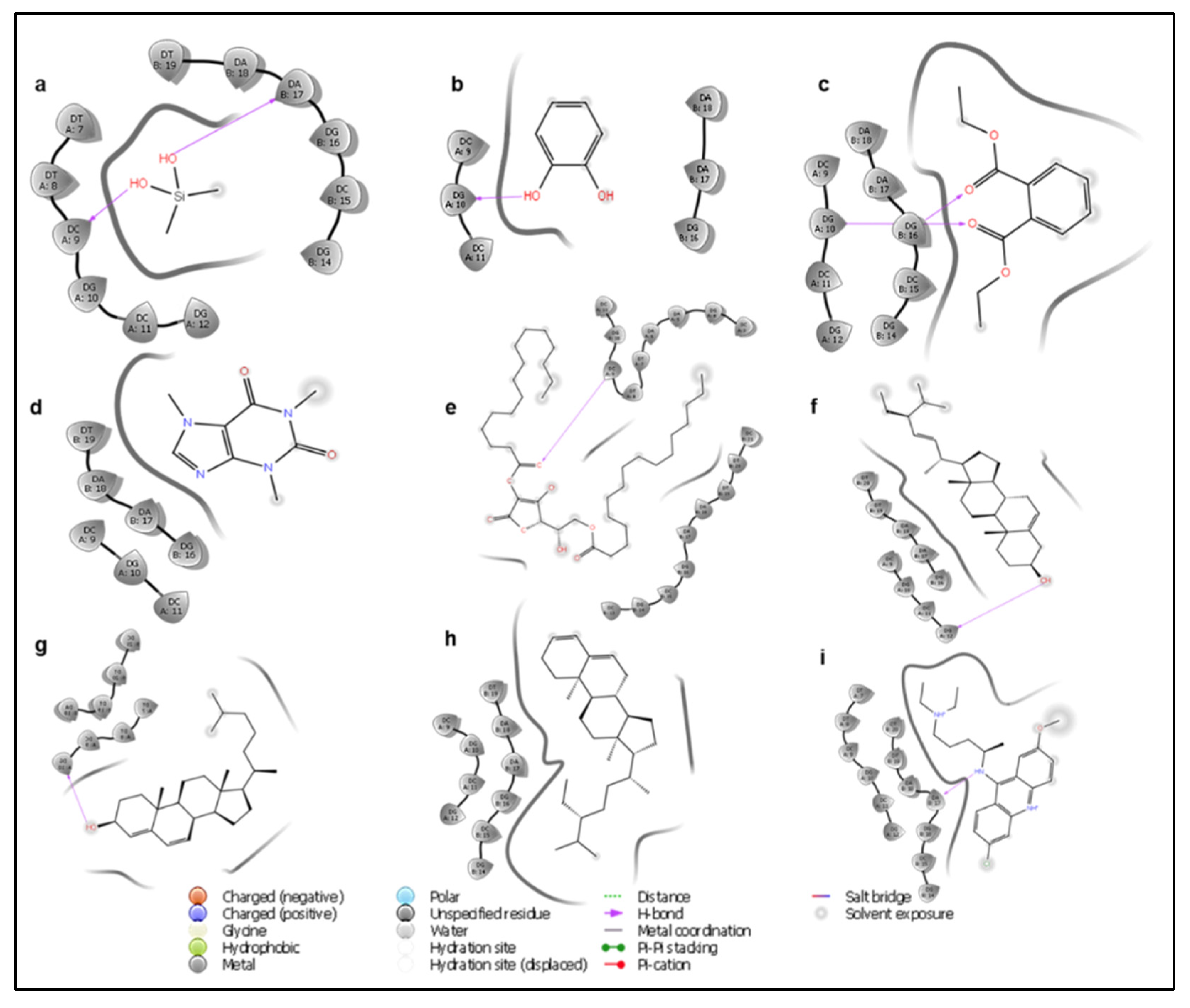
3.6. Molecular Docking Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, G.; Kumar, A.; Sharma, S.; Naushad, M.; Dwivedi, R.P.; ALOthman, Z.A.; Mola, G.T. Novel development of nanoparticles to bimetallic nanoparticles and their composites: A review. J. King Saud Univ. Sci. 2019, 31, 257–269. [Google Scholar] [CrossRef]
- Maney, V.; Singh, M. The synergism of Platinum-Gold bimetallic nanoconjugates enhance 5-Fluorouracil delivery in vitro. Pharmaceutics 2019, 11, 439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maney, V.; Singh, M. An in vitro assessment of Chitosan/ Bimetallic PtAu nanocomposites as delivery vehicles for Doxorubicin. Nanomedicine 2017, 12, 2625–2640. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, X.; Zhu, M.; Lin, G.; Liu, J.; Zhou, Z.; Tian, X.; Pan, Y. PEGylated Au@Pt Nanodendrites as Novel Theranostic Agents for Computed Tomography Imaging and Photothermal/Radiation Synergistic Therapy. ACS Appl. Mater. Interfaces 2016, 9, 279–285. [Google Scholar] [CrossRef]
- Yang, X.; Wang, C.; Zhang, X.; Wang, Y.; Gao, F.; Sun, L.; Xu, W.; Qiao, C.; Zhang, G. Photothermal and adsorption effects of silver selenide nanoparticles modified by different surfactants in nursing care of cancer patients. Sci. Technol. Adv. Mater. 2020, 21, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Maiyo, F.; Singh, M. Selenium Nanoparticles: Potential in Cancer Gene and Drug Delivery. Nanomedicine 2017, 12, 1075–1089. [Google Scholar] [CrossRef] [PubMed]
- Thiruvengadam, V.; Bansod, A.V. Characterization of Silver Nanoparticles Synthesized using Chemical Method and its Antibacterial Property. Biointerface Res. Appl. Chem. 2020, 10, 7257–7264. [Google Scholar]
- Kumar, N.; Krishnani, K.K.; Singh, N.P. Comparative study of selenium and selenium nanoparticles with reference to acute toxicity, biochemical attributes, and histopathological response in fish. Environ. Sci. Pollut. Res. Int. 2018, 25, 8914–8927. [Google Scholar] [CrossRef]
- Kumari, M.; Mukherjee, A.; Chandrasekaran, N. Genotoxicity of silver nanoparticles in Allium cepa. Sci. Total Environ. 2009, 407, 5243–5246. [Google Scholar] [CrossRef]
- Wen, H.; Dan, M.; Yang, Y.; Lyu, J.; Shao, A.; Cheng, X.; Chen, L.; Xu, L. Acute toxicity and genotoxicity of silver nanoparticle in rats. PLoS ONE 2017, 12, e0185554. [Google Scholar] [CrossRef] [Green Version]
- Chiang, W.H.; Sankaran, R.M. Synergistic effects in bimetallic nanoparticles for low temperature carbon nanotube growth. Adv. Mater. 2008, 20, 4857–4861. [Google Scholar] [CrossRef]
- Kumari, M.M.; Jacob, J.; Philip, D. Green synthesis and applications of Au–Ag bimetallic nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 137, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Ranno, L.; Dal Forno, S.; Lischner, J. Computational design of bimetallic core-shell nanoparticles for hot-carrier photocatalysis. Npj Comput. Mater. 2018, 4, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Garcia, D.A.; Mendoza, L.; Vizuete, K.; Debut, A.; Arias, M.T.; Gavilanes, A.; Terencio, T.; Avila, E.; Jeffryes, C.; Dahoumane, S.A. Sugar-Mediated Green Synthesis of Silver Selenide Semiconductor Nanocrystals under Ultrasound Irradiation. Molecules 2020, 25, 5193. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.K.; Kumar, S.; Banerjee, U.C. Quercetin and gallic acid mediated synthesis of bimetallic (silver and selenium) nanoparticles and their antitumor and antimicrobial potential. J. Colloid Interface Sci. 2014, 431, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Sibiya, P.N.; Moloto, M.J. Shape control of silver selenide nanoparticles using green capping molecules. Green Process. Synth. 2017, 6, 183–188. [Google Scholar] [CrossRef]
- Sibiya, P.N.; Moloto, M.J. Effect of precursor concentration and pH on the shape and size of starch capped silver selenide (Ag2Se) nanoparticles. Chalcogenide Lett. 2014, 11, 577–588. [Google Scholar]
- Daniel, S.; Kumar, R.; Sathish, V.; Sivakumar, M.; Sunitha, S.; Sironmani, T.A. Green synthesis (Ocimum tenuiflorum) of silver nanoparticles and toxicity studies in zebra fish (Danio rerio) model. Int. J. NanoSci. Nanotechnol. 2011, 2, 103–117. [Google Scholar]
- Liang, T.; Qiu, X.; Ye, X.; Liu, Y.; Li, Z.; Tian, B.; Yan, D. Biosynthesis of selenium nanoparticles and their effect on changes in urinary nanocrystallites in calcium oxalate stone formation. 3 Biotech 2020, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.K.; Upadhyay, S.N.; Mishra, P.K. Light-induced synthesis of silver nanoparticles using Ocimum tenuiflorum extract: Characterization and application. J. Chem. Res. 2021, 45, 179–186. [Google Scholar] [CrossRef]
- Sankhalkar, S.; Vernekar, V. Quantitative and Qualitative analysis of Phenolic and Flavonoid content in Moringa oleifera Lam and Ocimum tenuiflorum L. Pharmacog. Res. 2016, 8, 16. [Google Scholar] [CrossRef] [Green Version]
- Öznur, K.; Bildi, C.O.; Nuri, S.O.; Bariş, S. Response surface methodology based desirability function approach to investigate optimal mixture ratio of silver nanoparticles synthesis process. Ind. Eng. Chem. Res. 2017, 56, 8180–8189. [Google Scholar]
- Patra, J.K.; Baek, K.-H. Green nanobiotechnology: Factors affecting synthesis and characterization techniques. J. Nanomater. 2014, 2014, 417305. [Google Scholar] [CrossRef] [Green Version]
- Barbalinardo, M.; Cavallini, M.; Gentili, D. Protein Corona Mediated Uptake and Cytotoxicity of Silver Nanoparticles in Mouse Embryonic Fibroblast. Small 2018, 14, 1801219. [Google Scholar] [CrossRef] [PubMed]
- Barbalinardo, M.; Bertacchini, J.; Bergamini, L.; Magarò, M.S.; Ortolani, L.; Sanson, A.; Palumbo, C.; Cavallini, M.; Gentili, D. Surface properties modulate protein corona formation and determine cellular uptake and cytotoxicity of silver nanoparticles. Nanoscale 2021, 13, 14119. [Google Scholar] [CrossRef] [PubMed]
- Moodley, J.S.; Krishna, S.B.N.; Pillay, K.; Govender, P. Green synthesis of silver nanoparticles from Moringa oleifera leaf extracts and its antimicrobial potential. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 015011. [Google Scholar] [CrossRef] [Green Version]
- Yousaf, H.; Mehmood, A.; Ahmad, K.S.; Raffi, M. Green synthesis of silver nanoparticles and their applications as an alternative antibacterial and antioxidant agents. Mater. Sci. Eng. C 2020, 112, 110901. [Google Scholar] [CrossRef] [PubMed]
- Saratale, R.G.; Benelli, G.; Kumar, G.; Kim, D.S.; Saratale, G.D. Bio-fabrication of silver nanoparticles using the leaf extract of an ancient herbal medicine, dandelion (Taraxacum officinale), evaluation of their antioxidant, anticancer potential, and antimicrobial activity against phytopathogens. Environ. Sci. Pollut. Res. 2018, 25, 10392–10406. [Google Scholar] [CrossRef]
- Elemike, E.E.; Onwudiwe, D.C.; Nundkumar, N.; Singh, M.; Iyekowa, O. Green synthesis of Ag, Au and Ag-Au bimetallic nanoparticles using Stigmaphyllon ovatum leaf extract and their in vitro anticancer potential. Mater. Lett. 2019, 243, 148–152. [Google Scholar] [CrossRef]
- Hirschle, P.; Preiß, T.; Auras, F.; Pick, A.; Völkner, J.; Valdepérez, D.; Witte, G.; Parak, W.J.; Rädler, J.O.; Wuttke, S. Exploration of MOF nanoparticle sizes using various physical characterization methods–is what you measure what you get? CrystEngComm 2016, 18, 4359–4368. [Google Scholar] [CrossRef] [Green Version]
- Bukhari, S.M.; Simic, N.; Siddiqui, H.L.; Ahmad, V.U. Determination of antioxidant activity of Crambe cordifolia. World Appl. Sci. J. 2013, 22, 1561–1565. [Google Scholar]
- Oyaizu, M. Studies on products of browning reaction antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Ciniglia, C.; Pinto, G.; Sansone, C.; Pollio, A. Acridine orange/Ethidium bromide double staining test: A simple In-vitro assay to detect apoposis induced by phenolic compounds in plant cells. Allelopathy J. 2010, 26, 301–308. [Google Scholar]
- Constantin, M.J.; Owens, E.T. Introduction and perspectives of plant genetic and cytogenetic assays a report of the US environmental protection agency Gene-Tox program. Mutation Res./Rev. Genetic Toxicol. 1982, 99, 1–12. [Google Scholar] [CrossRef]
- Fiskesjo, G. The Allium Test as a Standard in Environmental Monitoring. Hereditas 1985, 102, 99–112. [Google Scholar] [CrossRef]
- Akwu, N.; Naidoo, Y.; Singh, M. Cytogenotoxic and biological evaluation of the aqueous extracts of Grewia lasiocarpa: An Allium cepa Assay. S. Afr. J. Bot. 2019, 125, 371–380. [Google Scholar] [CrossRef]
- Kumari, M.; Khan, S.S.; Pakrashi, S.; Mukherjee, A.; Chandrasekaran, N. Cytogenetic and genotoxic effects of zinc oxide nanoparticles on root cells of Allium cepa. J. Hazard. Mater. 2011, 190, 613–621. [Google Scholar] [CrossRef]
- Bell, J.; Cao, Y.; Gunn, J.; Day, T.; Gallicchio, E.; Zhou, Z.; Levy, R.; Farid, R. PrimeX and the Schrödinger computational chemistry suite of programs. In International Tables for Crystallography, 2nd ed.; John Wiley and Sons: Hoboken, NJ, USA, 2012; Volume 1, pp. 534–538. [Google Scholar]
- Alotaibi, S.H.; Momen, A.A. Anticancer drugs’ deoxyribonucleic acid (DNA) interactions. In Biophysical Chemistry-Advance Applications; Mohammed, A.A., Ed.; IntechOpen: London, UK, 2019; p. 836. [Google Scholar]
- Oien, D.B.; Pathoulas, C.L.; Ray, U.; Thirusangu, P.; Kalogera, E.; Shridhar, V. Repurposing quinacrine for treatment-refractory cancer. Sem. Cancer Biol. 2021, 68, 21–30. [Google Scholar] [CrossRef]
- Kadhim, M.J.; Sosa, A.A.; Hameed, I.H. Evaluation of antibacterial activity and bioactive chemical analysis of Ocimum basilicum using Fourier transform infrared (FT-IR) and gas chromatography-mass spectrometry (GC-MS) techniques. J. Pharmacog. Phytother. 2016, 8, 127–146. [Google Scholar]
- Hemmalakshmi, S.; Priyanga, S.; Devaki, K. Fourier Transform Infra-Red Spectroscopy Analysis of Erythrina variegata L. J. Pharm. Sci. Res. 2017, 9, 2062–2067. [Google Scholar]
- Jafari, M.; Salavati-Niasari, M.; Mohandes, F. Synthesis and characterization of silver selenide nanoparticles via a facile sonochemical route starting from a novel inorganic precursor. J. Inorg. Organomet. Polym. Mater. 2013, 23, 357–364. [Google Scholar] [CrossRef]
- Khan, M.S.; Talib, A.; Pandey, S.; Bhaisare, M.L.; Gedda, G.; Wu, H.-F. Folic Acid navigated Silver Selenide nanoparticles for photothermal ablation of cancer cells. Colloids Surf. B 2017, 159, 564–570. [Google Scholar] [CrossRef]
- More, D.; Moloto, M.; Moloto, N. Hexadecylamine–capped silver selenide nanoparticles: Temperature study. Chalcogenide Lett. 2016, 13, 233–238. [Google Scholar]
- Alagesan, V.; Venugopal, S. Green synthesis of selenium nanoparticle using leaves extract of withania somnifera and its biological applications and photocatalytic activities. Bionanoscience 2019, 9, 105–116. [Google Scholar] [CrossRef] [Green Version]
- Anandan, M.; Poorani, G.; Boomi, P.; Varunkumar, K.; Anand, K.; Chuturgoon, A.A.; Saravanan, M.; Prabu, H.G. Green synthesis of anisotropic silver nanoparticles from the aqueous leaf extract of Dodonaea viscosa with their antibacterial and anticancer activities. Process Biochem. 2019, 80, 80–88. [Google Scholar] [CrossRef]
- Jagaran, K.; Singh, M. Nanomedicine for Neurodegenerative Disorders: Focus on Alzheimer’s and Parkinson’s Diseases. Int. J. Mol. Sci. 2021, 22, 9082. [Google Scholar] [CrossRef] [PubMed]
- Oladimeji, O.; Akinyelu, J.; Daniels, A.; Singh, M. Modified Gold Nanoparticles for efficient Delivery of Betulinic Acid to Cancer Cell Mitochondria. Int. J. Mol. Sci. 2021, 22, 5072. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, S.Z.; Lashgarian, H.E.; Karkhane, M.; Shahzamani, K.; Alhameedawi, A.K.; Marzban, A. Bio-inspired silver selenide nano-chalcogens using aqueous extract of Melilotus officinalis with biological activities. Bioresour. Bioprocess 2021, 8, 1–11. [Google Scholar] [CrossRef]
- Ayele, D.W. A facile one-pot synthesis and characterization of Ag2Se nanoparticles at low temperature. Egypt. J. Basic Appl. Sci. 2016, 3, 149–154. [Google Scholar] [CrossRef] [Green Version]
- Mal, J.; Nancharaiah, Y.V.; Van Hullebusch, E.D.; Lens, P.N.L. Metal chalcogenide quantum dots: Biotechnological synthesis and applications. RSC Adv. 2016, 6, 47. [Google Scholar] [CrossRef]
- Chauhan, V.; Stylianopoulos, T.; Martin, J.D.; Popović, Z.; Chen, O.; Kamoun, W.S.; Bawendi, M.G.; Fukumura, D.; Jain, R.K. Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nat. Nanotechnol. 2012, 7, 383–388. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.; Kim, B.Y.; Rutka, J.T.; Chan, W.C. Nanoparticle-mediated cellular response is size-dependent. Nat. Nanotechnol. 2008, 3, 145–150. [Google Scholar] [CrossRef]
- Faraji, A.H.; Wipf, P. Nanoparticles in cellular drug delivery. Bioorg. Med. Chem. 2009, 15, 2950–2962. [Google Scholar] [CrossRef]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Madras, G.; McCoy, B.J. Temperature effects on the transition from nucleation and growth to Ostwald ripening. Chem. Eng. Sci. 2004, 59, 2753–2765. [Google Scholar] [CrossRef]
- Xue, X.; Penn, R.L.; Leite, E.R.; Huang, F.; Lin, Z. Crystal growth by oriented attachment: Kinetic models and control factors. CrystEngComm 2014, 16, 1419–1429. [Google Scholar] [CrossRef]
- Madras, G.; McCoy, B.J. Temperature effects during Ostwald ripening. J. Chem. Phys. 2003, 119, 1683–1693. [Google Scholar] [CrossRef] [Green Version]
- Pala, F.S.; Gürkan, H. The role of free radicals in ethiopathogenesis of diseases. Adv. Mol. Biol. 2008, 1, 1–9. [Google Scholar]
- Gounden, S.; Daniels, A.; Singh, M. Chitosan-modified Silver Nanoparticles Enhance Cisplatin activity in Breast Cancer Cells. Biointerface Res. Appl. Chem. 2021, 11, 10572–10584. [Google Scholar]
- Singh, D.; Singh, M. Hepatocellular-Targeted mRNA Delivery using functionalized Selenium Nanoparticles in vitro. Pharmaceutics 2021, 13, 298. [Google Scholar] [CrossRef] [PubMed]
- Shakibaie, M.; Shahverdi, A.R.; Faramarzi, M.A.; Hassanzadeh, G.R.; Rahimi, H.R.; Sabzevari, O. Acute and subacute toxicity of novel biogenic selenium nanoparticles in mice. Pharm. Biol. 2013, 51, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Vijaya, P.; Rekha, B.; Mathew, A.T.; Ali, M.S.; Yogananth, N.; Anuradha, V.; Kalitha, P.P. Antigenotoxic effect of green-synthesized silver nanoparticles from Ocimum sanctum leaf extract against cyclophosphamide induced genotoxicity in human lymphocytes—In vitro. Appl. Nanosci. 2014, 4, 415–420. [Google Scholar] [CrossRef] [Green Version]

| Retention Time | Peak Area (mm2) | Peak Area (%) | Peak Height (mm) | Peak Height (%) | A/H | Molecular Formula | Tentative Compound | NIST LIB Database (CAS) ID | NIST Similarity Index (%) |
|---|---|---|---|---|---|---|---|---|---|
| 4.225 | 152.678 | 1.13 | 127.942 | 4.02 | 1.19 | C2H8O2Si | Dimethyl silanediol | 1066-42-8 | 95 |
| 4.775 | 76.412 | 0.57 | 16.309 | 0.51 | 4.69 | C6H6O2 | Catechol | 120-80-9 | 72 |
| 9.232 | 103.742 | 4 7.69 | 149.341 | 4.69 | 6.95 | C12H14O4 | Diethyl Phthalate | 84-66-2 | 97 |
| 16.049 | 1.831.956 | 13.58 | 316.649 | 9.94 | 5.79 | C8H10N4O2 | Caffeine | 58-08-2 | 98 |
| 17.049 | 356.096 | 2.64 | 85.134 | 2.67 | 4.18 | C38H68O8 | l-(+)-Ascorbic acid 2,6-dihexadecanoate | 28474-90-0 | 90 |
| 21.403 | 880.775 | 6.53 | 100.591 | 3.16 | 8.76 | C29H48O | Stigmasterol | 83-48-7 | 90 |
| 22.837 | 134.922 | 1.00 | 19.079 | 0.60 | 7.07 | C27H44O | Cholesta-4,6-dien-3beta-ol | 14214-69-8 | 60 |
| 23.290 | 253.161 | 1.88 | 46.322 | 1.45 | 5.47 | C29H48 | Stigmastan-3,5-diene | 0-00-0 | 65 |
| Sample | Extract (µL) | Temperature (°C) | Zeta Potential (mV) | Hydrodynamic Size (nm) | Concentration Particles/mL (×1010) |
|---|---|---|---|---|---|
| 1 | 500.0 | 100.00 | −22.8 | 48.0 | 6.090 |
| 2 | 1500.0 | 50.00 | −17.8 | 77.4 | 0.249 |
| 3 | 1000.0 | 39.65 | −24.6 | 83.9 | 0.296 |
| 4 | 1000.0 | 75.00 | −20.1 | 62.4 | 4.095 |
| 5 | 1000.0 | 75.00 | −20.8 | 55.9 | 4.100 |
| 6 | 1000.0 | 75.00 | −24.0 | 59.5 | 3.950 |
| 7 | 292.9 | 75.00 | −25.2 | 62.7 | 0.354 |
| 8 | 1000.0 | 75.00 | −26.9 | 58.8 | 3.900 |
| 9 | 1500.0 | 100.0 | −30.4 | 64.1 | 8.550 |
| 10 | 1707.1 | 75.00 | −28.4 | 42.1 | 4.380 |
| 11 | 500.0 | 50.00 | −29.4 | 55.2 | 0.584 |
| 12 | 1000.0 | 110.36 | −28.6 | 38.0 | 7.950 |
| 13 | 1000.0 | 75.00 | −24.0 | 55.9 | 4.250 |
| Parameter | Response Variable | Extract (µL) | Temperature (°C) | Response Value | Composite Desirability |
|---|---|---|---|---|---|
| 1 | Zeta potential fit | 1707.110 | 110.355 | −40.394 mV | 1.000 |
| 2 | Hydrodynamic size | 507.168 | 110.355 | 44.908 nm | 0.849 |
| 3 | Concentration | 1707.110 | 110.355 | 1.019 × 1011 particles/mL | 1.000 |
| Nanoparticle/Drug | DPPH (µg/mL) | FRAP (µg/mL) | HEK293 (µg/mL) | MCF-7 (µg/mL) |
|---|---|---|---|---|
| Ag@Se | 13966.93 | 11.02 | 1649.65 | 456.38 |
| Ascorbic acid | 205.64 | 15.66 | - | - |
| 5FU | - | - | 111.29 | 136.71 |
| Extract | 112.34 | 10.98 | - | - |
| Treatment | Sample | I a (%) | P b (%) | M c (%) | A d (%) | T e (%) | MI f (%) | Ave MI (%) | MD g (%) | Ave MD (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Distilled H2O | Sample 1 | 61.49 | 32.76 | 1.72 | 1.15 | 2.87 | 38.51 | 38.03 ± 1.44 | −1.25 | 0.00 ± 3.79 |
| Sample 2 | 60.82 | 25.26 | 6.70 | 2.06 | 5.15 | 39.18 | −3.01 | |||
| Sample 3 | 63.59 | 26.15 | 7.69 | 1.54 | 1.03 | 36.41 | 4.26 | |||
| Ag@Se (1 µg/mL) | Sample 1 | 68.26 | 27.54 | 1.80 | 1.20 | 1.20 | 31.74 | 33.77 ± 4.26 | 16.55 | 11.19 ± 11.19 |
| Sample 2 | 69.08 | 27.63 | 1.97 | 0.66 | 0.66 | 30.92 | 18.69 | |||
| Sample 3 | 61.33 | 34.67 | 0 | 2.67 | 1.33 | 38.67 | −1.67 | |||
| Ag@Se (10 µg/mL) | Sample 1 | 77.33 | 20.89 | 0.44 | 0.44 | 0.89 | 22.67 | 28.19 ± 5.53 | 40.40 | 25.87 ± 14.53 |
| Sample 2 | 71.81 | 22.55 | 1.48 | 2.97 | 1.19 | 28.19 | 25.87 | |||
| Sample 3 | 66.28 | 31.39 | 1.16 | 0.58 | 0.58 | 33.72 | 11.33 | |||
| Ag@Se (100 µg/mL) | Sample 1 | 69.85 | 25.19 | 3.44 | 0.76 | 0.76 | 30.15 | 28.03 ± 3.43 | 20.71 | 26.30 ± 9.01 |
| Sample 2 | 75.93 | 17.28 | 1.23 | 3.70 | 1.85 | 24.07 | 36.70 | |||
| Sample 3 | 70.14 | 27.49 | 0.95 | 0.47 | 0.95 | 29.86 | 21.49 |
| Treatment | Sample | Vagrant | Binuclei | Sticky | C-metaphase | Laggard | Chromosomal Aberration | CAI * (%) | Average CAI |
|---|---|---|---|---|---|---|---|---|---|
| Distilled H2O | Sample 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0.1 | 0.13 ± 0.06 |
| Sample 2 | 0 | 1 | 0 | 0 | 0 | 1 | 0.1 | ||
| Sample 3 | 0 | 2 | 0 | 0 | 0 | 2 | 0.2 | ||
| Ag@Se (1 µg/mL) | Sample 1 | 1 | 1 | 2 | 0 | 1 | 5 | 0.5 | 0.60 ± 0.10 |
| Sample 2 | 1 | 3 | 1 | 0 | 2 | 7 | 0.7 | ||
| Sample 3 | 2 | 2 | 0 | 1 | 1 | 6 | 0.6 | ||
| Ag@Se (10 µg/mL) | Sample 1 | 2 | 1 | 0 | 1 | 2 | 6 | 0.6 | 0.63 ± 0.06 |
| Sample 2 | 1 | 2 | 1 | 2 | 1 | 7 | 0.7 | ||
| Sample 3 | 2 | 1 | 0 | 1 | 2 | 6 | 0.6 | ||
| Ag@Se (100 µg/mL) | Sample 1 | 3 | 6 | 5 | 1 | 4 | 19 | 1.9 | 1.67 ± 0.25 |
| Sample 2 | 2 | 5 | 2 | 3 | 2 | 14 | 1.4 | ||
| Sample 3 | 3 | 3 | 5 | 2 | 4 | 17 | 1.7 |
| Ligand | Standard Precision Docking Score | XP Docking Score | Interacting Residues |
|---|---|---|---|
| Dimethyl silanediol | −4.624 | −4.224 | DA (B: 17), DC (A: 9) |
| Catechol | −4.900 | −4.093 | DG (A: 10) |
| Diethyl Phthalate | −5.201 | −1.928 | DG (A: 10), DG (B: 16) |
| Caffeine | −6.282 | −2.065 | - |
| l-(+)-Ascorbic acid 2,6-dihexadecanoate | −1.972 | 4.987 | DC (A: 9) |
| Stigmasterol | −2.339 | −0.663 | DG (A: 12) |
| Cholesta-4,6-dien-3beta-ol | −3.918 | 1.045 | DG (A: 10) |
| Stigmastan-3,5-diene | −2.683 | 1.704 | - |
| Quinacrine (standard) | −8.130 | −8.983 | DA (B: 17) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olawale, F.; Ariatti, M.; Singh, M. Biogenic Synthesis of Silver-Core Selenium-Shell Nanoparticles Using Ocimum tenuiflorum L.: Response Surface Methodology-Based Optimization and Biological Activity. Nanomaterials 2021, 11, 2516. https://doi.org/10.3390/nano11102516
Olawale F, Ariatti M, Singh M. Biogenic Synthesis of Silver-Core Selenium-Shell Nanoparticles Using Ocimum tenuiflorum L.: Response Surface Methodology-Based Optimization and Biological Activity. Nanomaterials. 2021; 11(10):2516. https://doi.org/10.3390/nano11102516
Chicago/Turabian StyleOlawale, Femi, Mario Ariatti, and Moganavelli Singh. 2021. "Biogenic Synthesis of Silver-Core Selenium-Shell Nanoparticles Using Ocimum tenuiflorum L.: Response Surface Methodology-Based Optimization and Biological Activity" Nanomaterials 11, no. 10: 2516. https://doi.org/10.3390/nano11102516
APA StyleOlawale, F., Ariatti, M., & Singh, M. (2021). Biogenic Synthesis of Silver-Core Selenium-Shell Nanoparticles Using Ocimum tenuiflorum L.: Response Surface Methodology-Based Optimization and Biological Activity. Nanomaterials, 11(10), 2516. https://doi.org/10.3390/nano11102516