Effect of Graphene Concentration on the Electrochemical Properties of Cobalt Ferrite Nanocomposite Materials
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Synthesis of Nanoparticles
2.2. Synthesis of Graphene Sheets
2.3. Fabrication of Nanocomposites
2.4. Preparation of Electrodes
2.5. Sample Characterization Techniques
2.5.1. X-ray Diffraction (XRD)
2.5.2. Fourier-Transform Infrared Spectroscopy (FTIR)
2.5.3. Thermogravimetric Analysis (TGA)
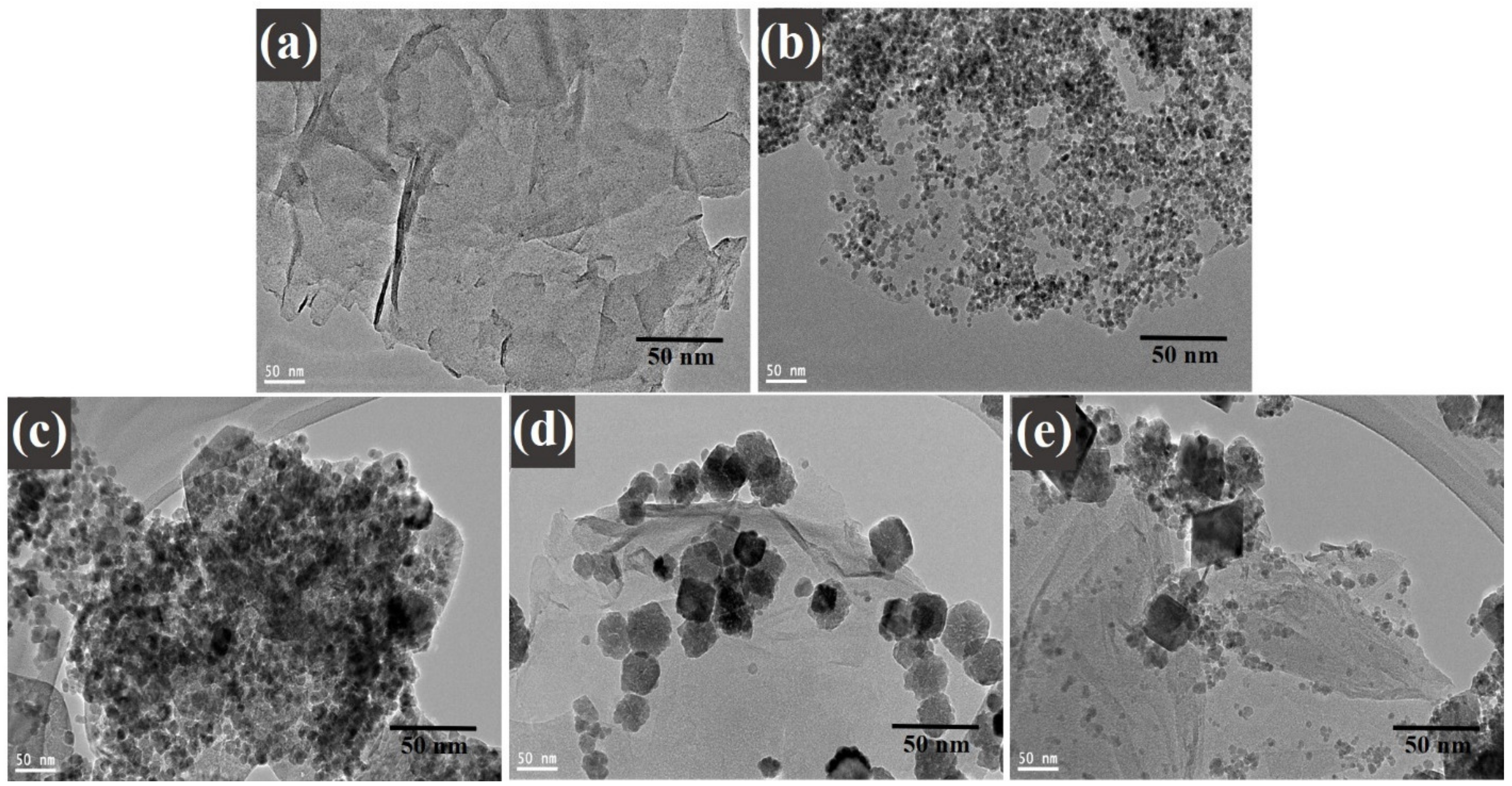
2.5.4. Scanning Electron Microscopy (SEM), Energy Dispersive X-ray Analysis (EDX), and Transmission Electron Microscopy (TEM)
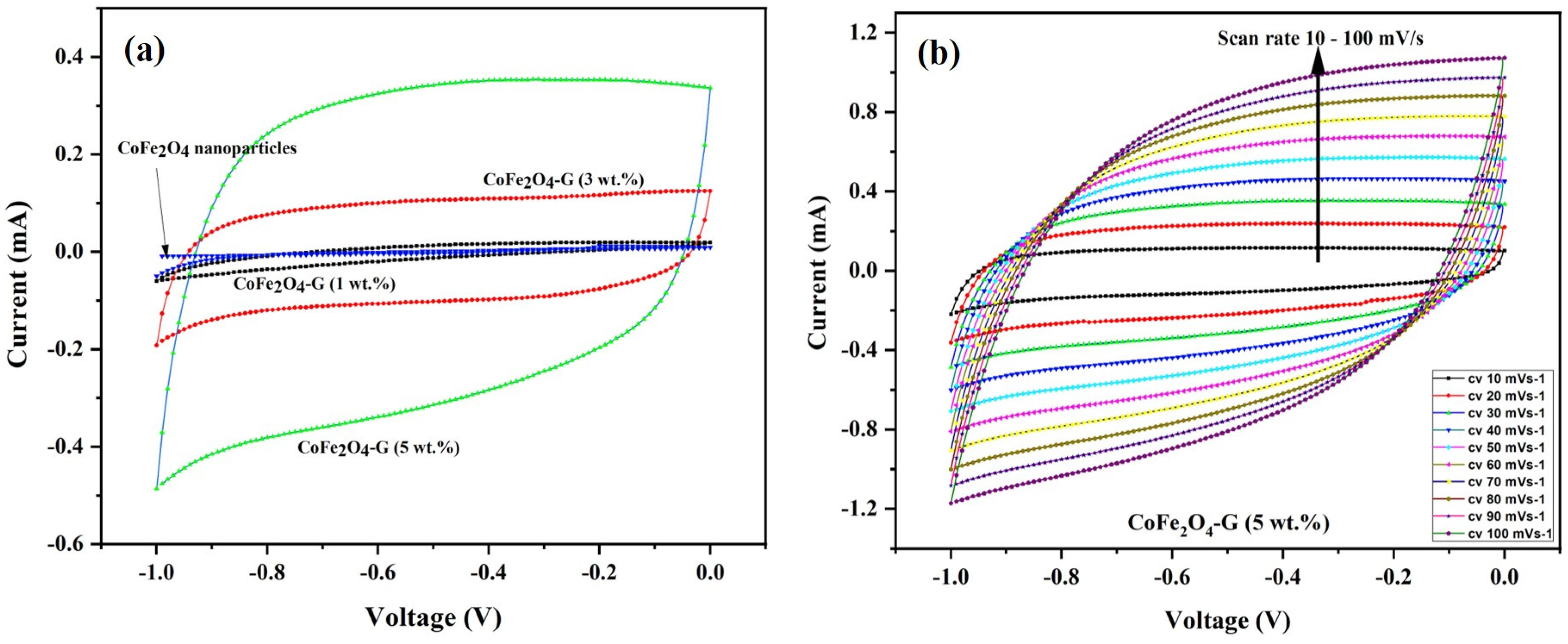
2.6. Electrochemical Measurements
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dar, A.M.; Nam, S.H.; Abdo, H.S.; Almajid, A.A.; Kim, D.W.; Qurashi, A.; Kim, W.B. Self-assembled Co3O4 nanoplatelets into micro-spheres via a simple solvothermal route: Structural and electrochemical properties. J. Alloys Compd. 2017, 695, 329–336. [Google Scholar] [CrossRef]
- Dar, A.M.; Kim, Y.; Kim, W.; Sohn, J.; Shin, H. Structural and magnetic properties of CuO nanoneedles synthesized by hydrothermal method. Appl. Surf. Sci. 2008, 254, 7477–7481. [Google Scholar] [CrossRef]
- Dar, M.; Ahsanulhaq, Q.; Kim, Y.; Sohn, J.; Kim, W.; Shin, H. Versatile synthesis of rectangular shaped nanobat-like CuO nanostructures by hydrothermal method; structural properties and growth mechanism. Appl. Surf. Sci. 2009, 255, 6279–6284. [Google Scholar] [CrossRef]
- Rana, S.; Philip, J.; Raj, B. Micelle based synthesis of cobalt ferrite nanoparticles and its characterization using Fourier Transform Infrared Transmission Spectrometry and Thermogravimetry. Mater. Chem. Phys. 2010, 124, 264–269. [Google Scholar] [CrossRef]
- Asadpour-Zeynali, K.; Mollarasouli, F. Novel electrochemical biosensor based on PVP capped CoFe2O4 @CdSe core-shell nanoparticles modified electrode for ultra-trace level determination of rifampicin by square wave adsorptive stripping voltammetry. Biosens. Bioelectron. 2017, 92, 509–516. [Google Scholar] [CrossRef]
- Vemuri, R.; Raju, G.; Gnana Kiran, M.; Prasad, M.S.N.A.; Rajesh, E.; Pavan Kumar, G.; Murali, N. Effect on structural and magnetic properties of Mg2+ substituted cobalt nano ferrite. Results Phys. 2019, 12, 947–952. [Google Scholar] [CrossRef]
- Yang, X.; He, Y.; Wang, X.; Yuan, R. A SERS biosensor with magnetic substrate CoFe2O4 @Ag for sensitive detection of Hg2+. Appl. Surf. Sci. 2017, 416, 581–586. [Google Scholar] [CrossRef]
- Yanik, M.; Yigit, E.; Akansu, Y.; Sahmetlioglu, E. Magnetic conductive polymer-graphene nanocomposites based supercapacitors for energy storage. Energy 2017, 138, 883–889. [Google Scholar] [CrossRef]
- Ain, Q.; Haq, S.; Alshammari, A.; Al-Mutlaq, M.; Anjum, M. The systemic effect of PEG-nGO-induced oxidative stress in vivo in a rodent model. Beilstein J. Nanotechnol. 2019, 10, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Phiri, J.; Gane, P.; Maloney, T. General overview of graphene: Production, properties and application in polymer composites. Mater. Sci. Eng. B 2017, 215, 9–28. [Google Scholar] [CrossRef] [Green Version]
- Barstugan, R.; Barstugan, M.; Ozaytekin, I. PBO/graphene added β-PVDF piezoelectric composite nanofiber production. Compos. Part B Eng. 2019, 158, 141–148. [Google Scholar] [CrossRef]
- Li, N.; Jiang, H.-L.; Wang, X.; Wang, X.; Xu, G.; Zhang, B.; Wang, L.; Zhao, R.; Lin, J.-M. Recent advances in graphene-based magnetic composites for magnetic solid-phase extraction. TrAC Trends Anal. Chem. 2018, 102, 60–74. [Google Scholar] [CrossRef]
- Kuilla, T.; Bhadra, S.; Yao, D.; Kim, N.; Bose, S.; Lee, J. Recent advances in graphene based polymer composites. Prog. Polym. Sci. 2010, 35, 1350–1375. [Google Scholar] [CrossRef]
- Shu, R.; Zhang, G.; Zhang, J.; Wang, X. Fabrication of reduced graphene oxide/multi-walled carbon nanotubes/zinc ferrite hybrid composites as high-performance microwave absorbers. J. Alloys Compd. 2018, 736, 1–11. [Google Scholar] [CrossRef]
- Zhang, H.; Kuila, T.; Kim, N.; Yu, D.; Lee, J. Simultaneous reduction, exfoliation, and nitrogen doping of graphene oxide via a hydrothermal reaction for energy storage electrode materials. Carbon 2014, 69, 66–78. [Google Scholar] [CrossRef]
- Bosch-Navarro, C.; Coronado, E.; Martí-Gastaldo, C.; Sánchez-Royo, J.; Gómez, M. Influence of the pH on the synthesis of reduced graphene oxide under hydrothermal conditions. Nanoscale 2012, 4, 3977. [Google Scholar] [CrossRef] [PubMed]
- Sanpo, N.; Berndt, C.; Wen, C.; Wang, J. Transition metal-substituted cobalt ferrite nanoparticles for biomedical applications. Acta Biomater. 2013, 9, 5830–5837. [Google Scholar] [CrossRef]
- Feng, X.; Huang, Y.; Chen, X.; Wei, C.; Zhang, X.; Chen, M. Hierarchical CoFe2O4/NiFe2O4 nanocomposites with enhanced electrochemical capacitive properties. J. Mater. Sci. 2017, 53, 2648–2657. [Google Scholar] [CrossRef]
- Siburian, R.; Sihotang, H.; Raja, S.L.; Supeno, M.; Simanjuntak, C. New Route to Synthesize of Graphene Nano Sheets. Orient. J. Chem. 2018, 34, 182–187. [Google Scholar] [CrossRef] [Green Version]
- Jabbar, A.; Yasin, G.; Khan, W.; Anwar, M.; Korai, R.; Nizam, M.; Muhyodin, G. Electrochemical deposition of nickel graphene composite coatings: Effect of deposition temperature on its surface morphology and corrosion resistance. RSC Adv. 2017, 7, 31100–31109. [Google Scholar] [CrossRef] [Green Version]
- Anasdass, J.; Kannaiyan, P.; Raghavachary, R.; Gopinath, S.; Chen, Y. Palladium nanoparticle-decorated reduced graphene oxide sheets synthesized using Ficus carica fruit extract: A catalyst for Suzuki cross-coupling reactions. PLoS ONE 2018, 13, e0193281. [Google Scholar] [CrossRef] [Green Version]
- Siburian, R.; Sari, D.; Gultom, J.; Sihotang, H.; Raja, S.; Gultom, J.; Supeno, M. Performance of graphite and graphene as electrodes in primary cell battery. J. Phys. Conf. Ser. 2018, 1116, 042034. [Google Scholar] [CrossRef]
- Strankowski, M.; Włodarczyk, D.; Piszczyk, Ł.; Strankowska, J. Polyurethane Nanocomposites Containing Reduced Graphene Oxide, FTIR, Raman, and XRD Studies. J. Spectrosc. 2016, 2016, 7520741. [Google Scholar] [CrossRef] [Green Version]
- Selvaraj, M.; Venkatachalapathy, V.; Mayandi, J.; Karazhanov, S.; Pearce, J. Preparation of meta-stable phases of barium titanate by Sol-hydrothermal method. AIP Adv. 2015, 5, 117119. [Google Scholar] [CrossRef] [Green Version]
- Yuliantika, D.; Taufiq, A.; Hidayat, A.; Sunaryono; Hidayat, N.; Soontaranon, S. Exploring Structural Properties of Cobalt Ferrite Nanoparticles from Natural Sand. IOP Conf. Ser. Mater. Sci. Eng. 2019, 515, 012047. [Google Scholar] [CrossRef]
- Yao, Y.; Yang, Z.; Zhang, D.; Peng, W.; Sun, H.; Wang, S. Magnetic CoFe2O4–Graphene Hybrids: Facile Synthesis, Characterization, and Catalytic Properties. Ind. Eng. Chem. Res. 2012, 51, 6044–6051. [Google Scholar] [CrossRef]
- Rouhani, A.; Esmaeil-Khanian, A.; Davar, F.; Hasani, S. The effect of agarose content on the morphology, phase evolution, and magnetic properties of CoFe2O4 nanoparticles prepared by sol-gel autocombustion method. Int. J. Appl. Ceram. Technol. 2017, 15, 758–765. [Google Scholar] [CrossRef]
- Chen, T.; Du, P.; Jiang, W.; Liu, J.; Hao, G.; Gao, H.; Xiao, L.; Ke, X.; Zhao, F.; Xuan, C. A facile one-pot solvothermal synthesis of CoFe2O4/RGO and its excellent catalytic activity on thermal decomposition of ammonium perchlorate. RSC Adv. 2016, 6, 83838–83847. [Google Scholar] [CrossRef]
- Ji, Z.; Shen, X.; Song, Y.; Zhu, G. In situ synthesis of graphene/cobalt nanocomposites and their magnetic properties. Mater. Sci. Eng. B 2011, 176, 711–715. [Google Scholar] [CrossRef]
- Zong, M.; Huang, Y.; Zhang, N. Reduced graphene oxide-CoFe2O4 composite: Synthesis and electromagnetic absorption properties. Appl. Surf. Sci. 2015, 345, 272–278. [Google Scholar] [CrossRef]
- Hu, L.; Li, M.; Cheng, L.; Jiang, B.; Ai, J. Solvothermal synthesis of octahedral and magnetic CoFe2O4–reduced graphene oxide hybrids and their photo-Fenton-like behavior under visible-light irradiation. RSC Adv. 2021, 11, 22250–22263. [Google Scholar] [CrossRef]
- Najafi, F.; Rajabi, M. Thermal gravity analysis for the study of stability of graphene oxide–glycine nanocomposites. Int. Nano Lett. 2015, 5, 187–190. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Kumar, M.; Kumar, A.; Singh, R.; Kashyap, R.; Rani, S.; Kumar, D. Surface modification of Graphene Oxide using Esterification. Mater. Today Proc. 2019, 18, 1556–1561. [Google Scholar] [CrossRef]
- Kuila, T.; Mishra, A.; Khanra, P.; Kim, N.; Lee, J. Recent advances in the efficient reduction of graphene oxide and its application as energy storage electrode materials. Nanoscale 2013, 5, 52–71. [Google Scholar] [CrossRef]
- Liu, H.; Huang, W.; Yang, X.; Dai, K.; Zheng, G.; Liu, C.; Shen, C.; Yan, C.; Yan, X.; Guo, J.; et al. Organic vapor sensing behaviors of conductive thermoplastic polyurethane–graphene nanocomposites. J. Mater. Chem. C 2016, 4, 4459–4469. [Google Scholar] [CrossRef]
- Montoya, E.d.; Guinel, M.; Rinaldi, C. Preparation of magnetic polymer colloids with Brownian magnetic relaxation. Colloid Polym. Sci. 2014, 292, 1191–1198. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, N.; Sharma, Y.; Leu, J.; Tseng, T. Synthesis of Free-Standing Flexible rGO/MWCNT Films for Symmetric Supercapacitor Application. Nanoscale Res. Lett. 2019, 14, 266. [Google Scholar] [CrossRef]
- Darwish, M.S.A.; Kim, H.; Lee, H.; Ryu, C.; Lee, J.Y.; Yoon, J. Synthesis of Magnetic Ferrite Nanoparticles with High Hyperthermia Performance via a Controlled Co-Precipitation Method. Nanomaterials 2019, 9, 1176. [Google Scholar] [CrossRef] [Green Version]
- Shaterian, M.; Aghaei, A.; Koohi, M.; Teymouri, M.; Mohammadi-Ganjgah, A. Synthesis, characterization and electrochemical sensing application of CoFe2O4/graphene magnetic nanocomposite for analysis of atenolol. Polyhedron 2020, 182, 114479. [Google Scholar] [CrossRef]
- Mousa, M.; Khairy, M.; Shehab, M. Nanostructured ferrite/graphene/polyaniline using for supercapacitor to enhance the capacitive behavior. J. Solid State Electrochem. 2016, 21, 995–1005. [Google Scholar] [CrossRef]
- Rather, J.; Pilehvar, S.; de Wael, K. A biosensor fabricated by incorporation of a redox mediator into a carbon nanotube/nafion composite for tyrosinase immobilization: Detection of matairesinol, an endocrine disruptor. Analyst 2013, 138, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Li, G.; Xiong, F.; Gao, X. Synergistic effect of molybdenum nitride and carbon nanotubes on electrocatalysis for dye-sensitized solar cells. J. Mater. Chem. 2012, 22, 20580. [Google Scholar] [CrossRef]
- Sangili, A.; Kalyani, T.; Chen, S.; Nanda, A.; Jana, S. Label-Free Electrochemical Immunosensor Based on One-Step Electrochemical Deposition of AuNP-RGO Nanocomposites for Detection of Endometriosis Marker CA 125. ACS Appl. Bio Mater. 2020, 3, 7620–7630. [Google Scholar] [CrossRef]
- Fu, Y.; Chen, H.; Sun, X.; Wang, X. Combination of cobalt ferrite and graphene: High-performance and recyclable visible-light photocatalysis. Appl. Catal. B Environ. 2012, 111–112, 280–287. [Google Scholar] [CrossRef]
- Razalli, R.; Abdi, M.; Tahir, P.; Moradbak, A.; Sulaiman, Y.; Heng, L. Polyaniline-modified nanocellulose prepared from Semantan bamboo by chemical polymerization: Preparation and characterization. RSC Adv. 2017, 7, 25191–25198. [Google Scholar] [CrossRef] [Green Version]
- Xia, H.; Zhu, D.; Fu, Y.; Wang, X. CoFe2O4-graphene nanocomposite as a high-capacity anode material for lithium-ion batteries. Electrochim. Acta 2012, 83, 166–174. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, B.; Wu, S.; Liu, Y.; Suo, X.; Li, H. Enhanced lubricant property of flame-sprayed aluminum coatings additivated by reduced graphene oxide nanosheets. J. Therm. Spray Technol. 2018, 27, 1643–1651. [Google Scholar] [CrossRef]
- Amin, M.; Khaled, K.; Fadl-Allah, S. Testing validity of the Tafel extrapolation method for monitoring corrosion of cold rolled steel in HCl solutions—Experimental and theoretical studies. Corros. Sci. 2010, 52, 140–151. [Google Scholar] [CrossRef]
- Rashad, M.; Pan, F.; Yu, Z.; Asif, M.; Lin, H.; Pan, R. Investigation on microstructural, mechanical and electrochemical properties of aluminum composites reinforced with graphene nanoplatelets. Prog. Nat. Sci. Mater. Int. 2015, 25, 460–470. [Google Scholar] [CrossRef] [Green Version]
- Guler, O.; Say, Y.; Dikici, B. The effect of graphene nano-sheet (GNS) weight percentage on mechanical and corrosion properties of AZ61 and AZ91 based magnesium matrix composites. J. Compos. Mater. 2020, 54, 4473–4485. [Google Scholar] [CrossRef]
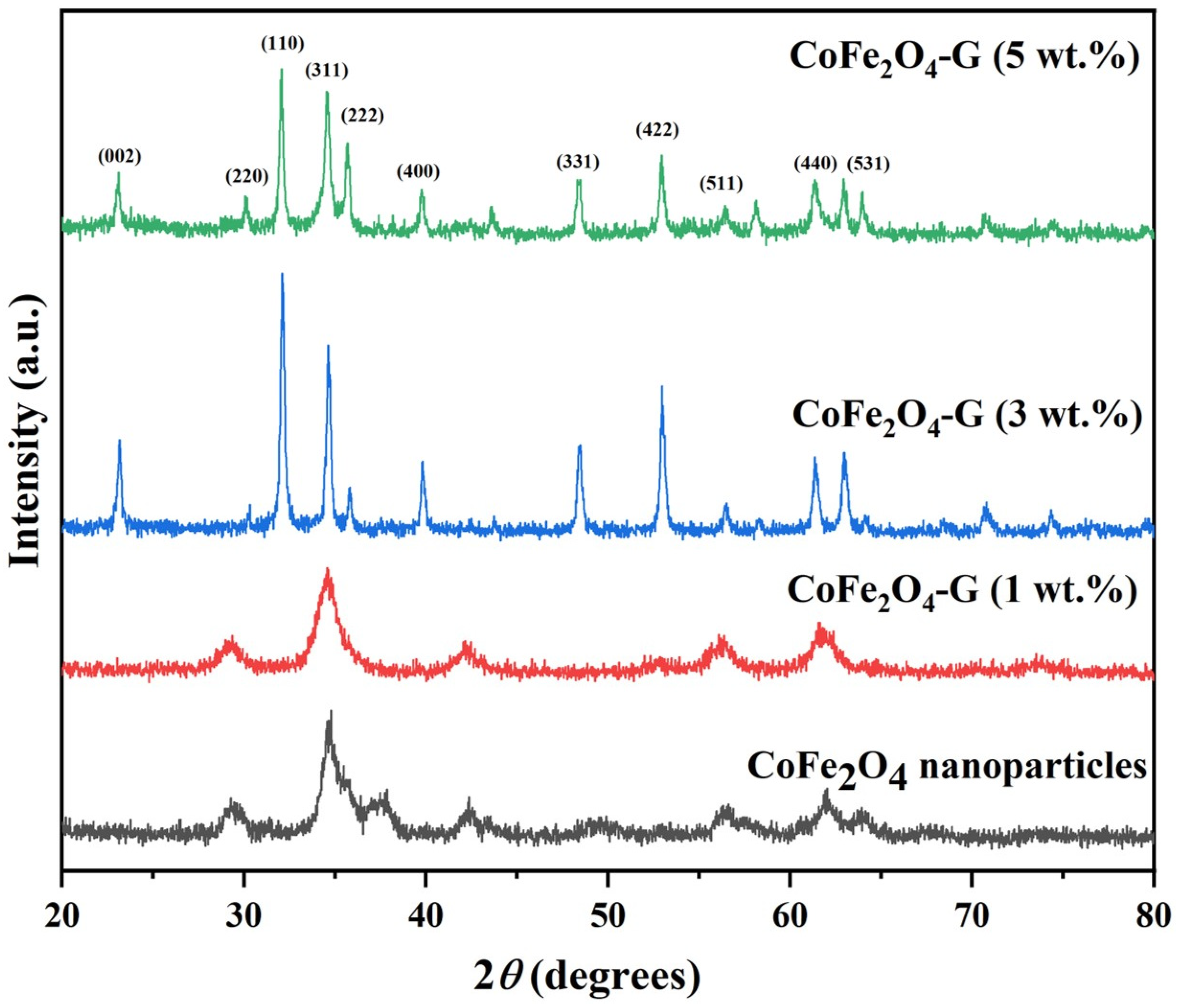
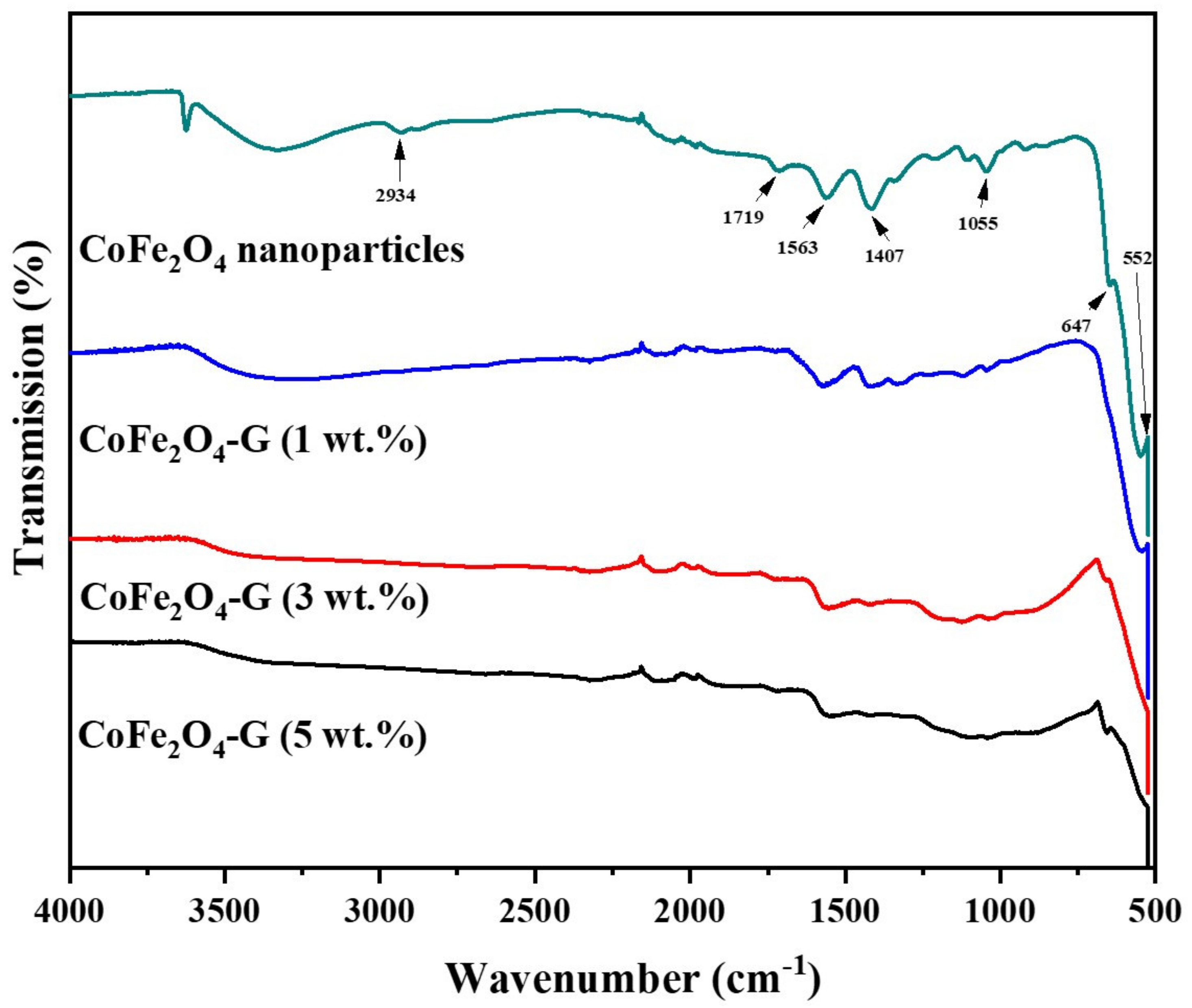
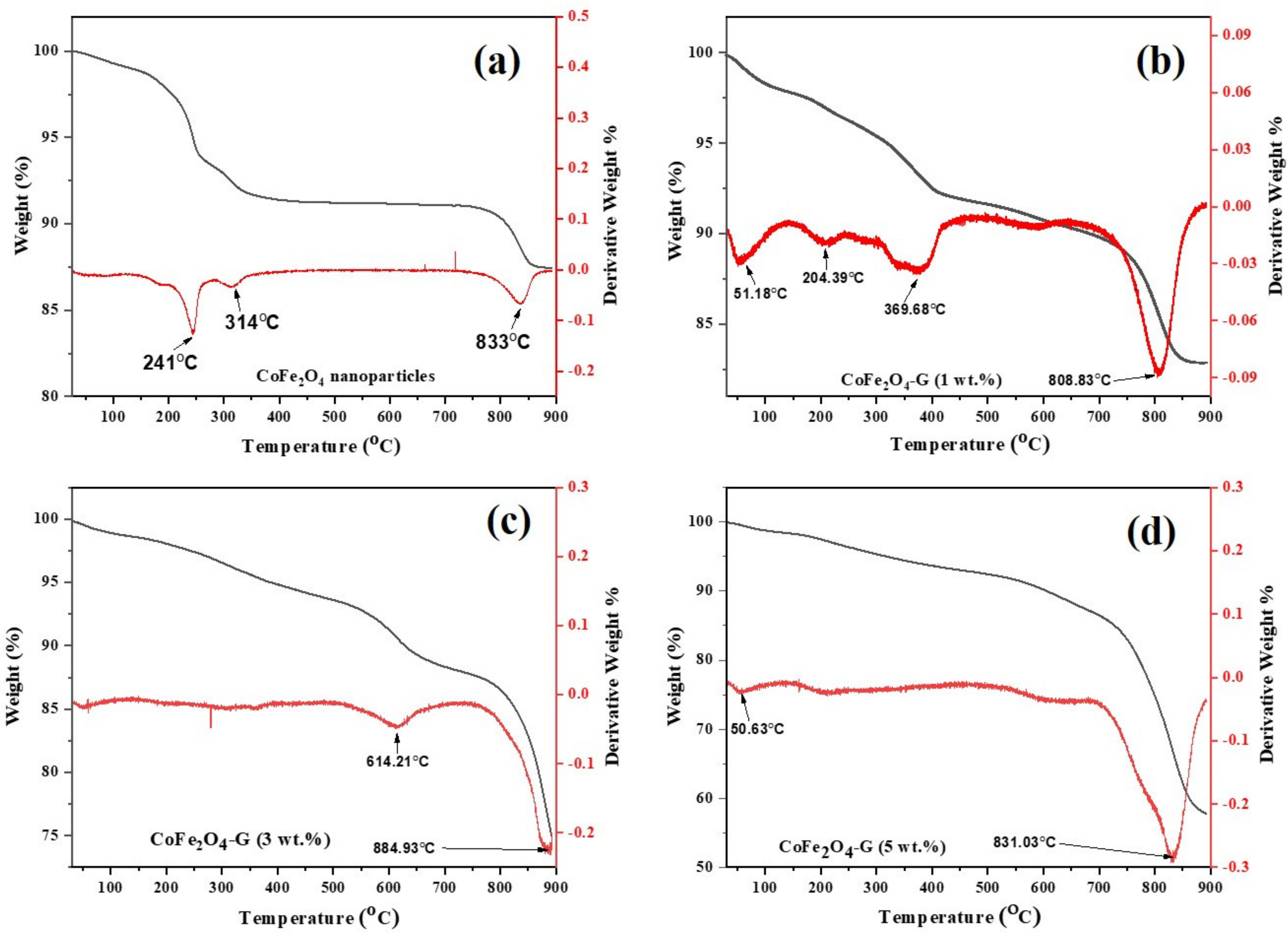
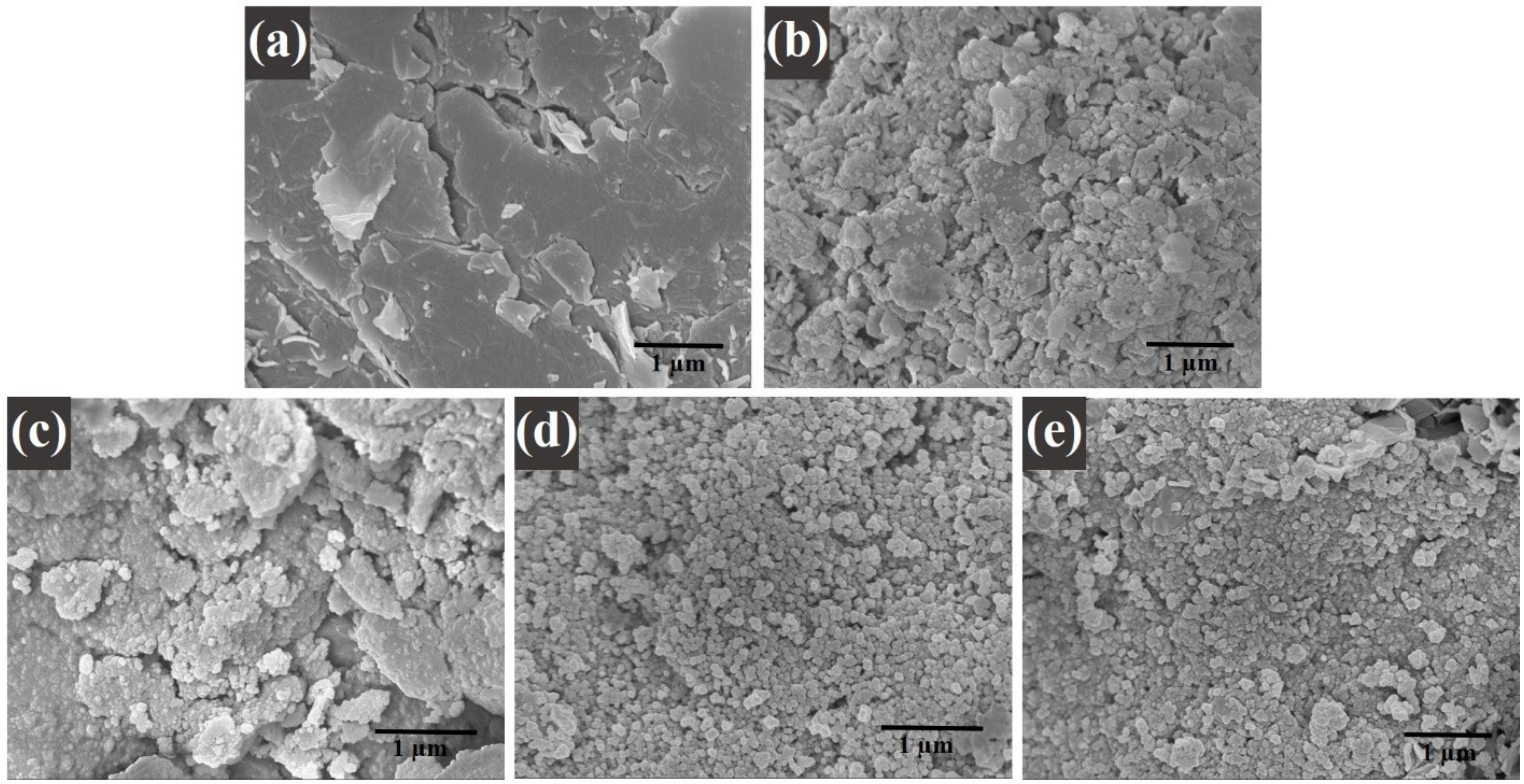


| Sample | (Co(NO3)2•6H2O) | (Fe(NO3)3•9H2O) | Graphene |
|---|---|---|---|
| CoFe2O4 (reference) | 4.005 g | 1.995 g | 0 g |
| CoFe2O4/graphene%1 | 3.96 g | 1.98 g | 0.06 g |
| CoFe2O4/graphene%3 | 3.88 g | 1.94 g | 0.18 g |
| CoFe2O4/graphene%5 | 3.8 g | 1.9 g | 0.3 g |
| Sample Name | Parameter | |||||
|---|---|---|---|---|---|---|
| βa/V·dec−1 | βc/V·dec−1 | ECorr/V | jCorr/µA·cm−2 | Rp/kΩ | RCorr/mmpy | |
| (a) | 0.368 | 0.237 | −0.6858 | 0.2844 | 312.7 | 0.01046 |
| (b) | 0.515 | 0.182 | −0.8295 | 3.528 | 23.52 | 0.1297 |
| (c) | 0.160 | 0.133 | −0.7763 | 3.844 | 11.69 | 0.1413 |
| (d) | 0.304 | 0.108 | −0.8686 | 5.539 | 8.864 | 0.2036 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alruwashid, F.S.; Dar, M.A.; Alharthi, N.H.; Abdo, H.S. Effect of Graphene Concentration on the Electrochemical Properties of Cobalt Ferrite Nanocomposite Materials. Nanomaterials 2021, 11, 2523. https://doi.org/10.3390/nano11102523
Alruwashid FS, Dar MA, Alharthi NH, Abdo HS. Effect of Graphene Concentration on the Electrochemical Properties of Cobalt Ferrite Nanocomposite Materials. Nanomaterials. 2021; 11(10):2523. https://doi.org/10.3390/nano11102523
Chicago/Turabian StyleAlruwashid, Firas S., Mushtaq A. Dar, Nabeel H. Alharthi, and Hany S. Abdo. 2021. "Effect of Graphene Concentration on the Electrochemical Properties of Cobalt Ferrite Nanocomposite Materials" Nanomaterials 11, no. 10: 2523. https://doi.org/10.3390/nano11102523







