Phytoproduct, Arabic Gum and Opophytum forsskalii Seeds for Bio-Fabrication of Silver Nanoparticles: Antimicrobial and Cytotoxic Capabilities
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Preparation of Aqueous Extracts for AgNP Fabrication
2.2.1. Extraction Method
2.2.2. Synthesis of AgNPs
2.3. Characterization of Biogenic AgNPs
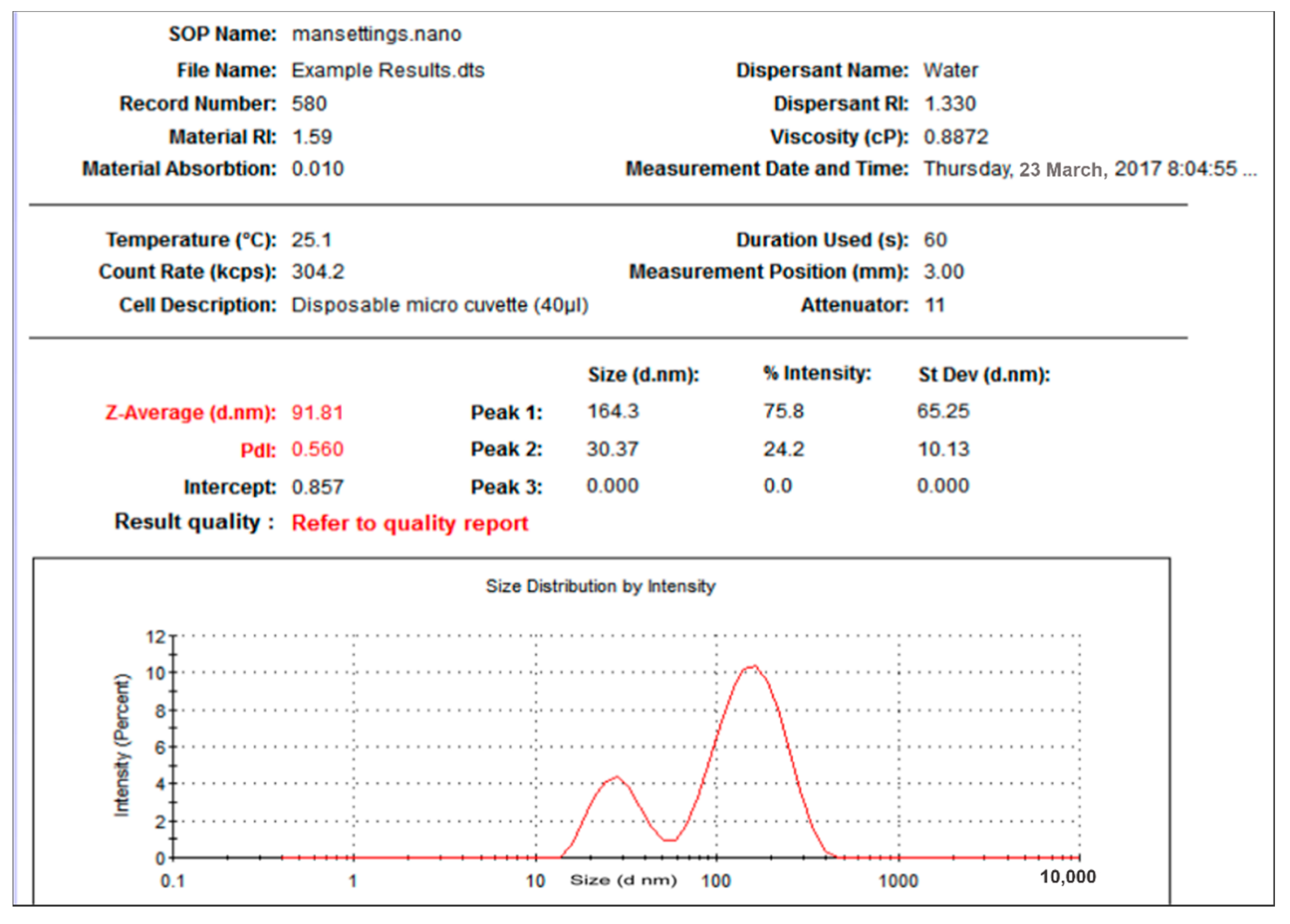
2.3.1. Dynamic Light Scattering (DLS) and Zeta Potential
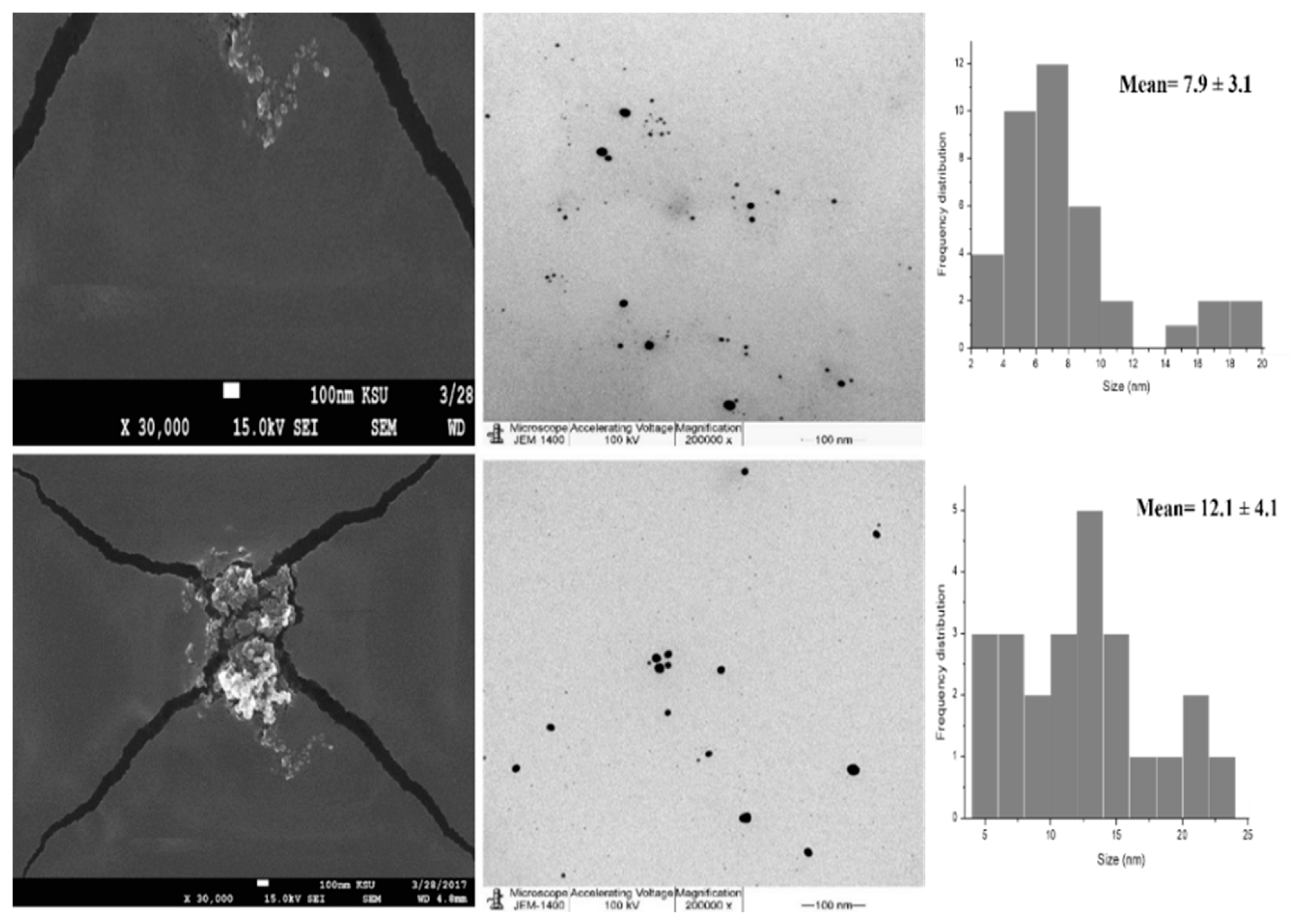
2.3.2. Transmission Electron Microscopy (TEM) and Scanning Electron Microscope (SEM)
2.3.3. Fourier Transform Infrared (FTIR) Spectroscopy
2.4. Antibacterial Susceptibility Testing (AST)
2.4.1. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
2.4.2. Tolerance Level
2.5. Synergistic Effect of AgNPs and Antibiotics
2.6. Field Emission Scanning Electron Microscope ((FESEM)
2.7. Cytotoxicity of AgNPs
Cell Lines and Culture
2.8. Chromatography/Mass Spectrometry (GC-MS) Techniques
2.9. Statistical Analysis
3. Results and Discussion
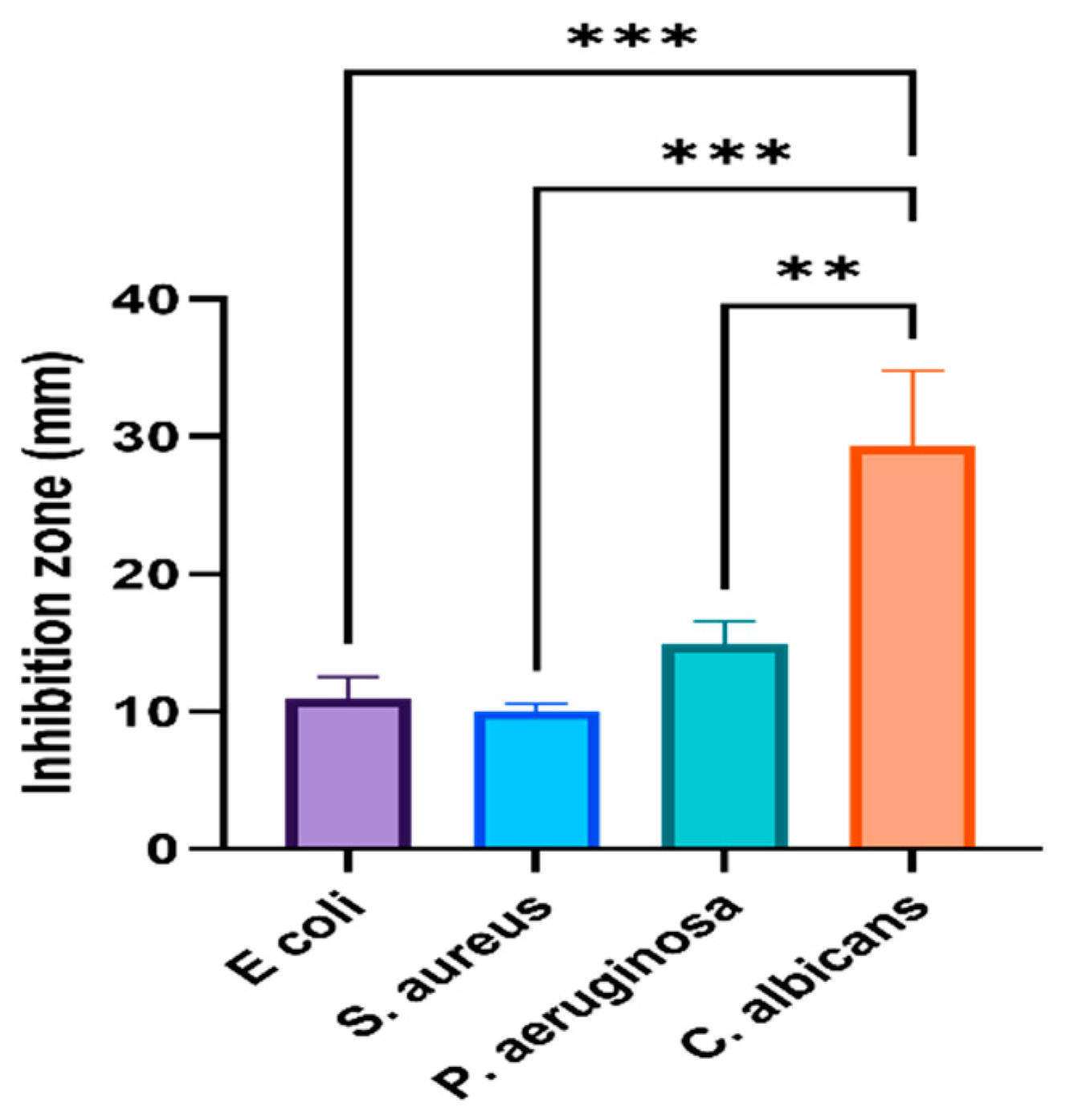
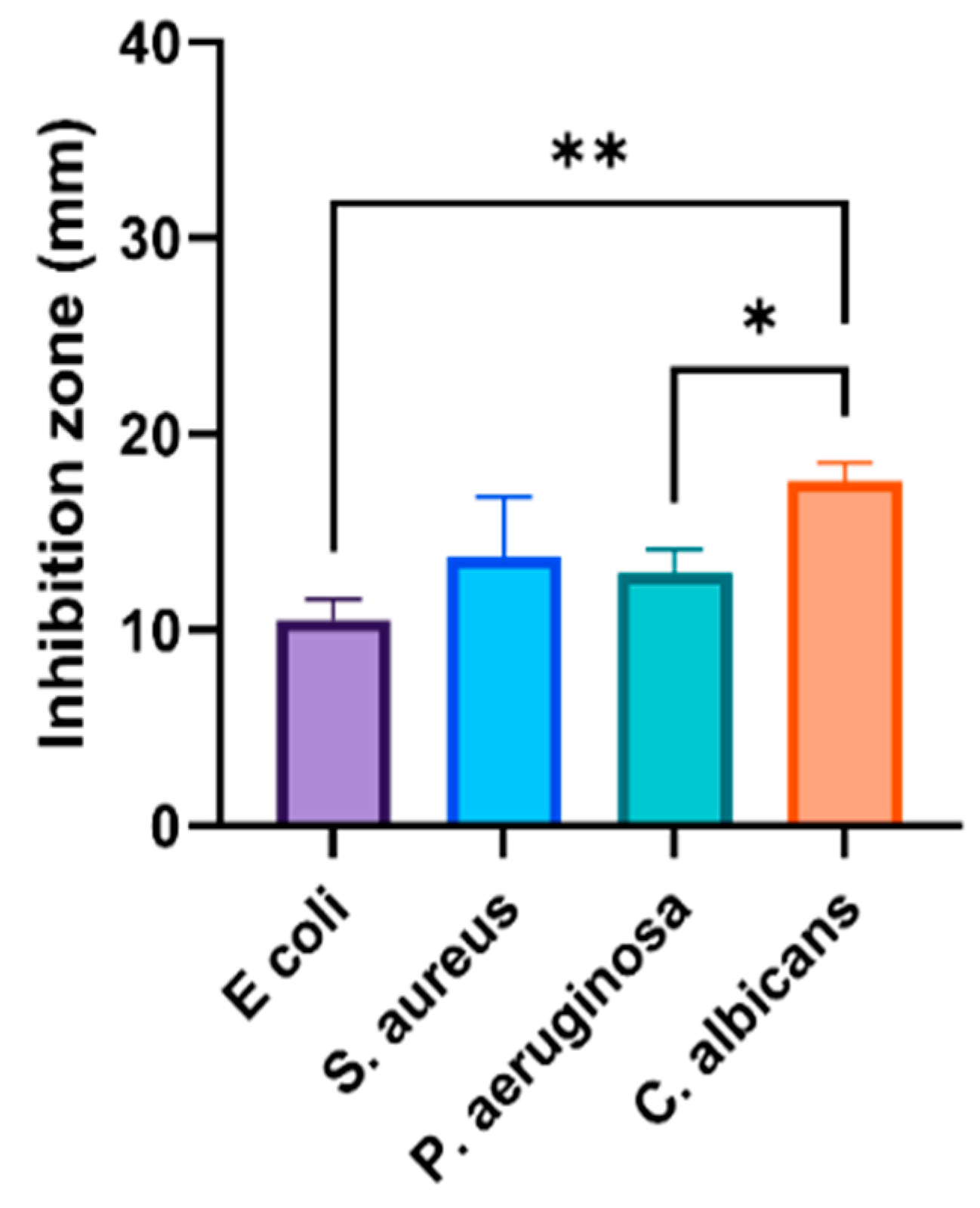
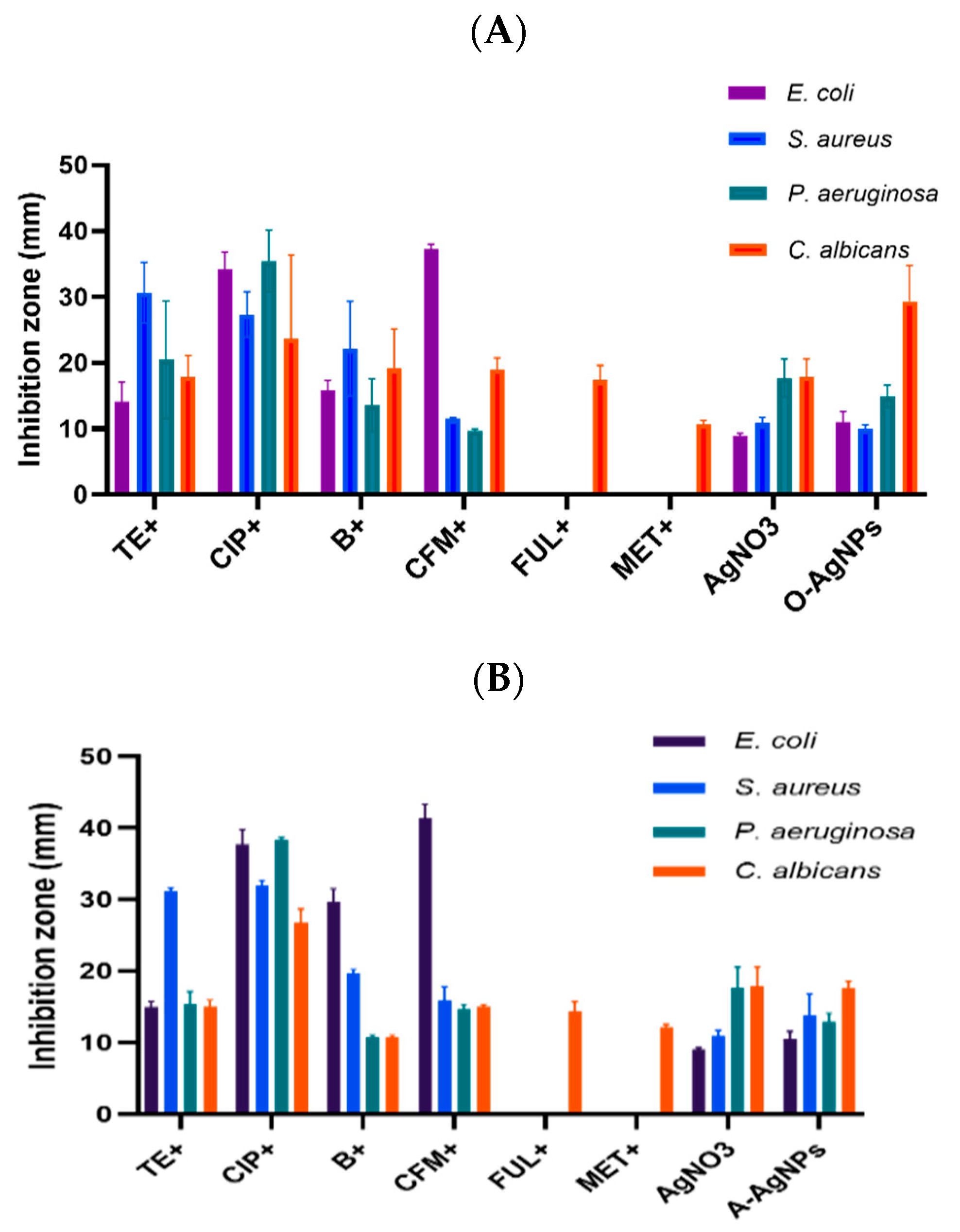
3.1. Antimicrobial Capability of Phyto-Fabricated AgNPs
3.2. Synergistic Effect of Phyto-Fabricated AgNPs
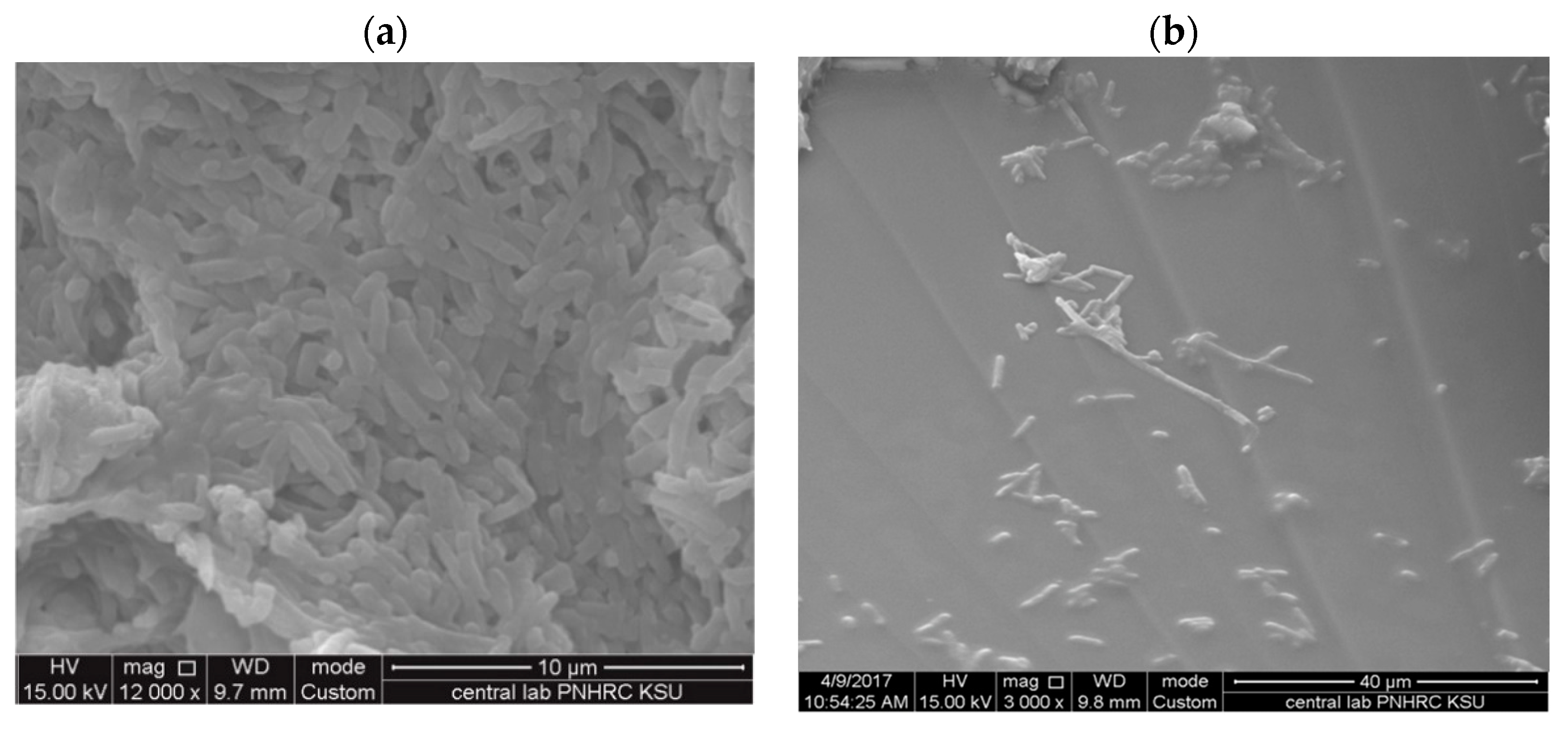
3.3. Morphological Studies on Treated Bacteria
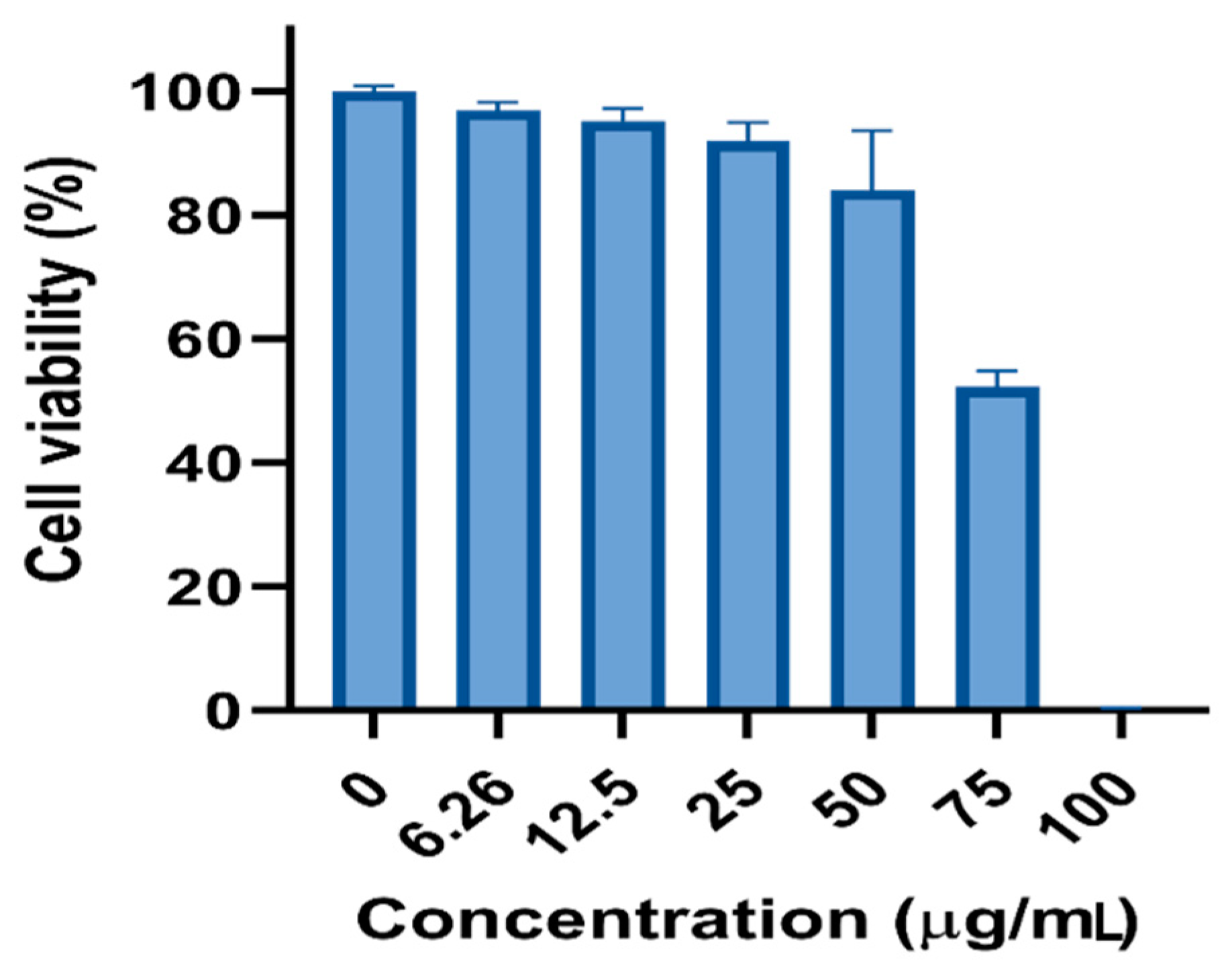
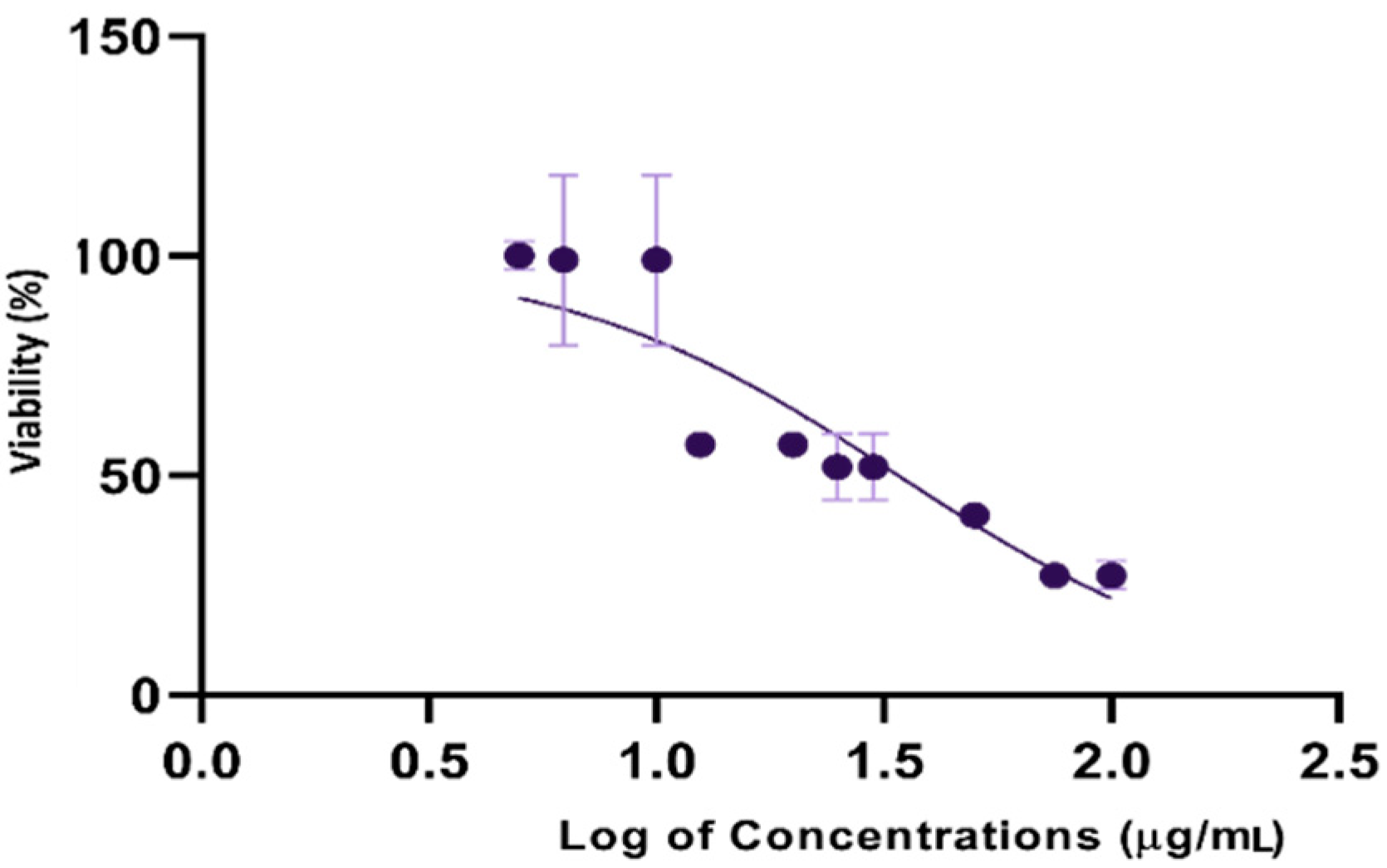
3.4. Cytotoxic Potential
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korbekandi, H.; Iravani, S. Silver Nanoparticles, the Delivery of Nanoparticles; Hashim Abbass, A., Ed.; IntechOpen: London, UK, 2012; ISBN 978-953-51-0615-9. [Google Scholar]
- Khalil, K.A.; Fouad, H.; Elsarnagawy, T.; Almajhdi, F.N. Preparation and characterization of electrospun PLGA/silver composite nanofibers for biomedical applications. Int. J. Electrochem. Sci. 2013, 8, 3483–3493. [Google Scholar]
- Prabha, S.; Lahtinen, M.; Sillanpää, M. Green synthesis and characterizations of silver and gold nanoparticles using leaf extract of Rosa rugosa. Colloids Surf. A Physicochem. Eng. Asp. 2010, 364, 34–41. [Google Scholar]
- Bose, D.; Chatterjee, S. Biogenic synthesis of silver nanoparticles using guava (Psidium guajava) leaf extract and its antibacterial activity against Pseudomonas aeruginosa. Appl. Nanosci. 2015, 6, 895–901. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, M.; Turkdemir, H.; Kilic, M.A.; Bayram, E.; Cicek, A.; Mete, A.; Ulug, B. Biosynthesis of silver nanoparticles using leaves of Stevia rebaudiana. Mater. Chem. Phys. 2011, 130, 1195–1202. [Google Scholar] [CrossRef]
- De Matteis, V.; Rizzello, L.; Ingrosso, C.; Liatsi-Douvitsa, E.; De Giorgi, M.L.; De Matteis, G.; Rinaldi, R. Cultivar-dependent anticancer and antibacterial properties of silver nanoparticles synthesized using leaves of different Olea Europaea trees. Nanomaterials 2019, 9, 1544. [Google Scholar] [CrossRef] [Green Version]
- Gudimalla, A.; Jose, J.; Varghese, R.J.; Thomas, S. Green Synthesis of silver nanoparticles using nymphae odorata extract incorporated films and antimicrobial activity. J. Polym. Environ. 2020, 29, 1412–1423. [Google Scholar] [CrossRef]
- Okaiyeto, K.; Hoppe, H.; Okoh, A. Plant-based synthesis of silver nanoparticles using aqueous leaf extract of salvia officinalis: Characterization and its antiplasmodial activity. J. Clust. Sci. 2020, 32, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.; Patil, S.; Ahire, M.; Kitture, R.; Kale, S.; Pardesi, K.; Cameotra, S.S.; Bellare, J.; Dhavale, D.D.; Jabgunde, A.; et al. Synthesis of silver nanoparticles using Dioscorea bulbifera tuber extract and evaluation of its synergistic potential in combination with antimicrobial agents. Int. J. Nanomed. 2012, 7, 483–496. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.; Nagi, E.E.; Wang, E.L.M. Present state of juniper in Rodhmallazi Forest of Balochistan Pakistan. Pak. J. For. 1990, 40, 227–236. [Google Scholar]
- Dastur, J.F. Useful Plants of India and Pakistan; D.B. Taraporewala & Co., Pvt. Ltd.: Bombay, India, 1964. [Google Scholar]
- Ali, A.; Akhtar, N.; Khan, B.A.; Khan, M.S.; Rasul, A.; Zaman, S.-U.Z.; Khalid, N.; Waseem, K.; Mahmood, T.; Ali, L. Acacia nilotica: A plant of multipurpose medicinal uses. J. Med. Plants Res. 2012, 6, 1492–1496. [Google Scholar]
- Anderson, D.M.W.; Weiping, W. Gum arabic (Acacia senegal) from Uganda: Characteristic NMR spectra, amino acid compositions, and gum/soil cationic relationships. Inter. Tree Crops J. 1992, 7, 167–179. [Google Scholar] [CrossRef]
- Mohammed, A.E. Green synthesis and antimicrobial activity of Eucalyptus camaldulensis mediated silver nanoparticles. Asian Pac. J. Trop. Biomed. 2015, 5, 930–934. [Google Scholar] [CrossRef]
- Das, P.; Xenopoulos, M.A.; Williams, C.J.; Hoque, E.; Metcalfe, C.D. Effects of silver nanoparticles on bacterial activity in natural waters. Environ. Toxicol. Chem. 2011, 31, 122–130. [Google Scholar] [CrossRef]
- Pankey, G.A.; Sabath, L.D. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of gram-positive bacterial infections. Clin. Infect. Dis. 2004, 38, 864–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, T.E. Repurposing Thiram and Disulfiram as Antibacterial Agents for Multidrug-Resistant Staphylococcus aureus Infections. Antimicrob. Agents Chemother. 2017, 61, e00898-17. [Google Scholar] [CrossRef] [Green Version]
- González, A.L.; Noguez, C.; Beránek, J.; Barnard, A. Size, shape, stability, and color of plasmonic silver nanoparticles. J. Phys. Chem. C 2014, 118, 9128–9136. [Google Scholar] [CrossRef]
- Jena, J.; Pradhan, N.; Dash, B.P.; Sukla, L.B.; Panda, P.K. Biosynthesis and characterization of silver nanoparticles using microalga Chlorococcum humicola and its antibacterial activity. Int. J. Nanomater. Biostruct. 2013, 3, 1–8. [Google Scholar]
- Hamouda, R.A.; Hussein, M.H.; Abo-Elmagd, R.A.; Bawazir, S.S. Synthesis and biological characterization of silver nanoparticles derived from the cyanobacterium Oscillatoria limnetica. Sci. Rep. 2019, 9, 13071. [Google Scholar] [CrossRef]
- Jalal, M.; Ansari, M.A.; A Alzohairy, M.; Ali, S.G.; Khan, H.M.; Almatroudi, A.; Siddiqui, M.I. Anticandidal activity of biosynthesized silver nanoparticles: Effect on growth, cell morphology, and key virulence attributes of Candida species. Int. J. Nanomed. 2019, 14, 4667–4679. [Google Scholar] [CrossRef] [Green Version]
- Usha, C.; Rachel, G.A.D. Biogenic Synthesis of Silver Nanoparticles by Acacia nilotica and their Antibacterial Activity. Int. J. Sci. Res. 2014, 3, 1–8. [Google Scholar]
- Tripathy, A.R.; Raichur, A.; Chandrasekaran, N.; Prathna, T.C.; Mukherjee, A. Process variables in biomimetic synthesis of silver nanoparticles by aqueous extract of Azadirachta indica (Neem) leaves. J. Nanopart. Res. 2009, 12, 237–246. [Google Scholar] [CrossRef]
- Farhadi, S.; Ajerloo, B.; Mohammadi, A. Green biosynthesis of spherical silver nanoparticles by using date palm (Phoenix Dactylifera) fruit extract and study of their antibacterial and catalytic activities. Acta Chim. Slov. 2017, 64, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.; Daima, H.K.; Kachhwaha, S.; Kothari, S. Synthesis of plant-mediated silver nanoparticles using papaya fruit extract and evaluation of their antimicrobial activities. Dig. J. Nanomater. Biostruct. 2009, 4, 557–563. [Google Scholar]
- Song, J.Y.; Kim, B.S. Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess Biosyst. Eng. 2008, 32, 79–84. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, X.; Zhang, Y.; Xue, X.; Fu, Y. Biosynthesis of silver nanoparticles at room temperature using aqueous aloe leaf extract and antibacterial properties. Colloids Surf. A Physicochem. Eng. Asp. 2013, 423, 63–68. [Google Scholar] [CrossRef]
- Khatoon, N.; Mazumder, J.A.; Sardar, M. Biotechnological applications of green synthesized silver nanoparticles. J. Nanosci. Curr. Res. 2017, 2, 1–8. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Bharath, L.V. Mechanism of plant-mediated synthesis of silver nanoparticles—A review on biomolecules involved, characterization and antibacterial activity. Chem. Biol. Interact. 2017, 273, 219–227. [Google Scholar] [CrossRef]
- Saratale, R.G.; Saratale, G.D.; Si-Kyung, C.; Ghodake, G.; Kadam, A.; Kumar, S.; Mulla, S.I.; Dong-Su, K.; Byong-Hun, J.; Shu, C.J.; et al. Phyto-fabrication of silver nanoparticles by Acacia nilotica leaves: Investigating their antineoplastic, free radical scavenging potential and application in H2O2 sensing. J. Taiwan Inst. Chem. Eng. 2019, 99, 239–249. [Google Scholar] [CrossRef]
- Leela, K.; Anchana, D.C. A study on the applications of silver nanoparticle synthesized using the aqueous extract and the purified secondary metabolites of lichen parmelia perlata. Int. J. Pharmac. Sci. Invent. 2017, 6, 42–59. [Google Scholar]
- Dasari, S.; Suresh, K.A.; Rajesh, M.; Reddy, C.S.S.; Hemalatha, C.S.; Wudayagiri, R.; Valluru, L. Biosynthesis, characterization, antibacterial and antioxidant activity of silver nanoparticles produced by lichens. J. Bionanosci. 2013, 7, 237–244. [Google Scholar] [CrossRef]
- Khandel, P.; Kumar Shahi, S.; Kanwar, L.; Kumar Yadaw, R.; Kumar Soni, D. Biochemical profiling of microbes inhibiting Silver nanoparticles using symbiotic organisms. Int. J. Nano Dimens. 2018, 9, 273–285. [Google Scholar]
- Macdonald, I.D.G.; Smith, W.E. Orientation of cytochrome C adsorbed on a citrate-reduced silver colloid surface. Langmuir 1996, 12, 706–713. [Google Scholar] [CrossRef]
- Sathishkumar, M.; Sneha, K.; Yun, Y.-S. Immobilization of silver nanoparticles synthesized using Curcuma longa tuber powder and extract on cotton cloth for bactericidal activity. Bioresour. Technol. 2010, 101, 7958–7965. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Farid, I.B.; Mahalel, U.A.; Jahangir, M. Metabolomic profiling and antioxidant activity of Opophytum forsskalii. Aljouf Sci. Eng. J. 2016, 3, 19–24. [Google Scholar] [CrossRef]
- Mahdieh, M.; Zolanvari, A.; Azimee, A. Green biosynthesis of silver nanoparticles by Spirulina platensis. Sci. Iran. 2012, 19, 926–929. [Google Scholar] [CrossRef] [Green Version]
- Sadiq, M.B.; Tarning, J.; Cho, T.Z.A.; Anal, A.K. Antibacterial activities and possible modes of action of Acacia nilotica (L.) Del. against multidrug-resistant Escherichia coli and Salmonella. Molecules 2017, 22, 47. [Google Scholar] [CrossRef]
- Shrivastava, S.; Bera, T.; Roy, A.; Singh, G.; Ramachandrarao, P.; Dash, D. Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology 2007, 18, 22. [Google Scholar] [CrossRef]
- Din, L.B.; Mie, R.; Samsudin, M.W.; Ahmad, A.; Ibrahim, N. Biomimetic synthesis of silver nanoparticles using the lichen Ramalina dumeticola and the antibacterial activity. Malays. J. Analyt. Sci. 2015, 19, 369–376. [Google Scholar]
- Qing, Y.; Cheng, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 2018, 13, 3311–3327. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, A.E.; Al-Qahtani, A.; Al-Mutairi, A.; Al-Shamri, B.; Aabed, K.F. Antibacterial and cytotoxic potential of biosynthesized silver nanoparticles by some plant extracts. Nanomaterials 2018, 8, 382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Algebaly, A.S.; Mohammed, A.E.; Abutaha, N.; Elobeid, M.M. Biogenic synthesis of silver nanoparticles: Antibacterial and cytotoxic potential. Saudi J. Biol. Sci. 2019, 27, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Jamaran, S.; Zarif, B.R. Synergistic effect of silver nanoparticles with neomycin or gentamicin antibiotics on mastitis-causing Staphylococcus aureus. Open J. Ecol. 2016, 6, 452–459. [Google Scholar] [CrossRef] [Green Version]
- Tawfeeq, S.M.; Maaroof, M.N.; Al-Ogaidi, I. Synergistic effect of biosynthesized silver nanoparticles with antibiotics against multi-drug resistance bacteria isolated from children with diarrhoea under five years. Iraqi J. Sci. 2017, 58, 41–52. [Google Scholar]
- Krychowiak-Masnicka, M.; Kawiak, A.; Narajczyk, M.; Borowik, A.; Królicka, A. Silver nanoparticles combined with naphthoquinones as an effective synergistic strategy against Staphylococcus aureus. Front. Pharmacol. 2018, 9, 816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopinath, V.; Priyadarshini, S.; Loke, M.F.; Arunkumar, J.; Marsili, E.; MubarakAli, D.; Velusamy, P.; Vadivelu, J. Biogenic synthesis, characterization of antibacterial silver nanoparticles and its cell cytotoxicity. Arab. J. Chem. 2015, 10, 1107–1117. [Google Scholar] [CrossRef] [Green Version]
- El-Rafie, M.; El-Naggar, M.; Ramadan, M.; Fouda, M.M.; Al-Deyab, S.S.; Hebeish, A. Environmental synthesis of silver nanoparticles using hydroxypropyl starch and their characterization. Carbohydr. Polym. 2011, 86, 630–635. [Google Scholar] [CrossRef]
- Yan, J.; Abdelgawad, A.; El-Naggar, M.E.; Rojas, O. Antibacterial activity of silver nanoparticles synthesized In-situ by solution spraying onto cellulose. Carbohydr. Polym. 2016, 147, 500–508. [Google Scholar] [CrossRef]
- Jun, F.; Jing, S.; Sirimanne, S.R.; Mounier-Lee, C.E. Kinetic and stereochemical studies on novel inactivators of C-terminal amidation. Biochem. J. 2000, 350, 521–530. [Google Scholar]
- Kim, K.-J.; Sung, W.S.; Suh, B.K.; Moon, S.-K.; Choi, J.-S.; Kim, J.G.; Lee, D.G. Antifungal activity and mode of action of silver nano-particles on Candida albicans. BioMetals 2008, 22, 235–242. [Google Scholar] [CrossRef]
- Hwang, I.-S.; Lee, J.; Hwang, J.H.; Kim, K.-J.; Lee, D.G. Silver nanoparticles induce apoptotic cell death in Candida albicans through the increase of hydroxyl radicals. FEBS J. 2012, 279, 1327–1338. [Google Scholar] [CrossRef]
- Yuan, Y.G.; Zhang, S.; Hwang, J.Y.; Kong, I.K. Silver Nanoparticles Potentiates Cytotoxicity and Apoptotic Potential of Camptothecin in Human Cervical Cancer Cells. Oxidative Med. Cell. Longev. 2018, 2018, 6121328. [Google Scholar] [CrossRef] [PubMed]
- Sankar, R.; Karthik, A.; Prabu, A.; Karthik, S.; Shivashangari, K.S.; Ravikumar, V. Origanum vulgare mediated biosynthesis of silver nanoparticles for its antibacterial and anticancer activity. Colloids Surf. B Biointerfaces 2013, 108, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Kovochich, M.; Brant, J.; Hotze, M.; Sempf, J.; Oberley, T.; Sioutas, C.; Yeh, J.I.; Wiesner, M.R.; Nel, A.E. Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett. 2006, 6, 1794–1807. [Google Scholar] [CrossRef] [PubMed]
- Ott, M.; Gogvadze, V.; Orrenius, S.; Zhivotovsky, B. Mitochondria, oxidative stress and cell death. Apoptosis 2007, 12, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Wang, J.; Patterson, T.; Saini, U.; Robinson, B.; Newport, G.; Murdock, R.; Schlager, J.; Hussain, S.; Ali, S. Expression of genes related to oxidative stress in the mouse brain after exposure to silver-25 nanoparticles. Toxicol. Lett. 2009, 187, 15–21. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, N.E.-A.; Hussein, M.H.; El-Sawah, A.A. Phycobiliprotein-mediated synthesis of biogenic silver nanoparticles, characterization, in vitro and in vivo assessment of anticancer activities. Sci. Rep. 2018, 8, 8925. [Google Scholar] [CrossRef]






| Compounds Identified in Opophytum forsskalii Seed Extract | Formula | Molecular Weight | R Time (min) |
|---|---|---|---|
| 2,5-Cyclohexadiene-1,4-dione,2,6-bis (1,1-dimethylethyl)- | C14H20O2 | 220 | 66.573 |
| Carboxin, carbathiin | C12H13NO2S | 235 | 70.715 |
| Prednisone | C21H26O5 | 358 | 115.640 |
| Hydrocortisone acetate | C23H32O6 | 404 | 116.292 |
| Compounds Identified in Arabic gum extract | |||
| 3,5-cyclohexadiene-1,2-dione,3,5-bis (1,1-dimethylethyl)- | C14H20O2 | 220 | 45.076 |
| Phenol,2,4-bis (1,1-dimethylethyl)- | C14H22O | 206 | 45.491 |
| Pentadecane | C15H32 | 212 | 51.166 |
| Eicosane | C20H42 | 282 | 51.166 |
| 2,5-Cyclohexadiene-1,4-dione,2,6-bis (1,1-dimethylethyl)- | C14H20O2 | 220 | 66.582 |
| 9,10-anthracenedione, 2-ethyle- | C16H12O2 | 236 | 69.515 |
| Carboxin, carbathiin | C12H13NO2S | 235 | 70.743 |
| 9-octadecenoic acid (z)-, methyl ester | C19H36O2 | 296 | 93.004 |
| Phenol, 4-(2-aminoproyl)- | C9H13NO | 151 | 97.475 |
| Hydrocortisone acetate | C23H32O6 | 404 | 116.801 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aabed, K.; Mohammed, A.E. Phytoproduct, Arabic Gum and Opophytum forsskalii Seeds for Bio-Fabrication of Silver Nanoparticles: Antimicrobial and Cytotoxic Capabilities. Nanomaterials 2021, 11, 2573. https://doi.org/10.3390/nano11102573
Aabed K, Mohammed AE. Phytoproduct, Arabic Gum and Opophytum forsskalii Seeds for Bio-Fabrication of Silver Nanoparticles: Antimicrobial and Cytotoxic Capabilities. Nanomaterials. 2021; 11(10):2573. https://doi.org/10.3390/nano11102573
Chicago/Turabian StyleAabed, Kawther, and Afrah E. Mohammed. 2021. "Phytoproduct, Arabic Gum and Opophytum forsskalii Seeds for Bio-Fabrication of Silver Nanoparticles: Antimicrobial and Cytotoxic Capabilities" Nanomaterials 11, no. 10: 2573. https://doi.org/10.3390/nano11102573
APA StyleAabed, K., & Mohammed, A. E. (2021). Phytoproduct, Arabic Gum and Opophytum forsskalii Seeds for Bio-Fabrication of Silver Nanoparticles: Antimicrobial and Cytotoxic Capabilities. Nanomaterials, 11(10), 2573. https://doi.org/10.3390/nano11102573







