Multifunctional Composite Coatings Based on Photoactive Metal-Oxide Nanopowders (MgO/TiO2) in Hydrophobic Polymer Matrix for Stone Heritage Conservation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Oxide Preparation and Characterization
2.2. Composite Coatings Preparation and Characterization
3. Results
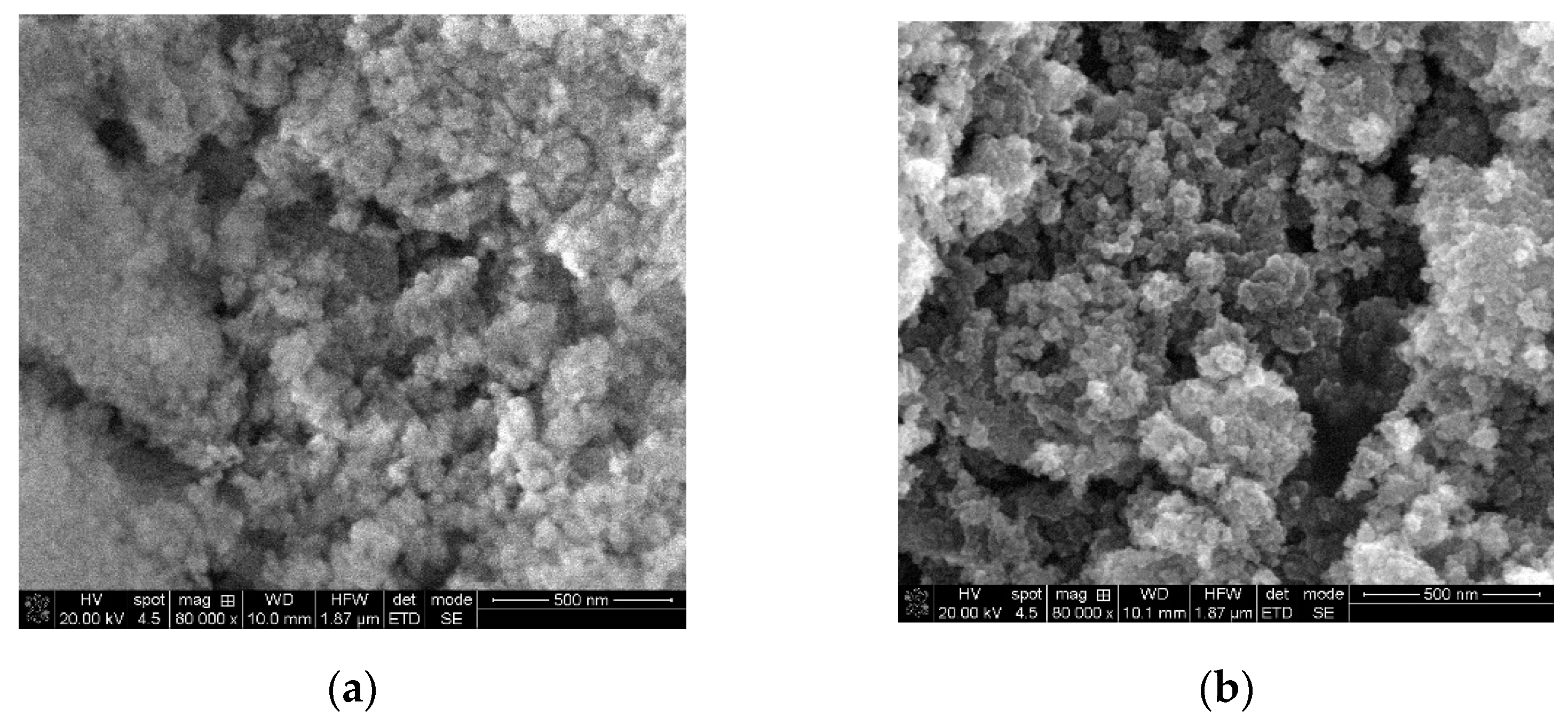
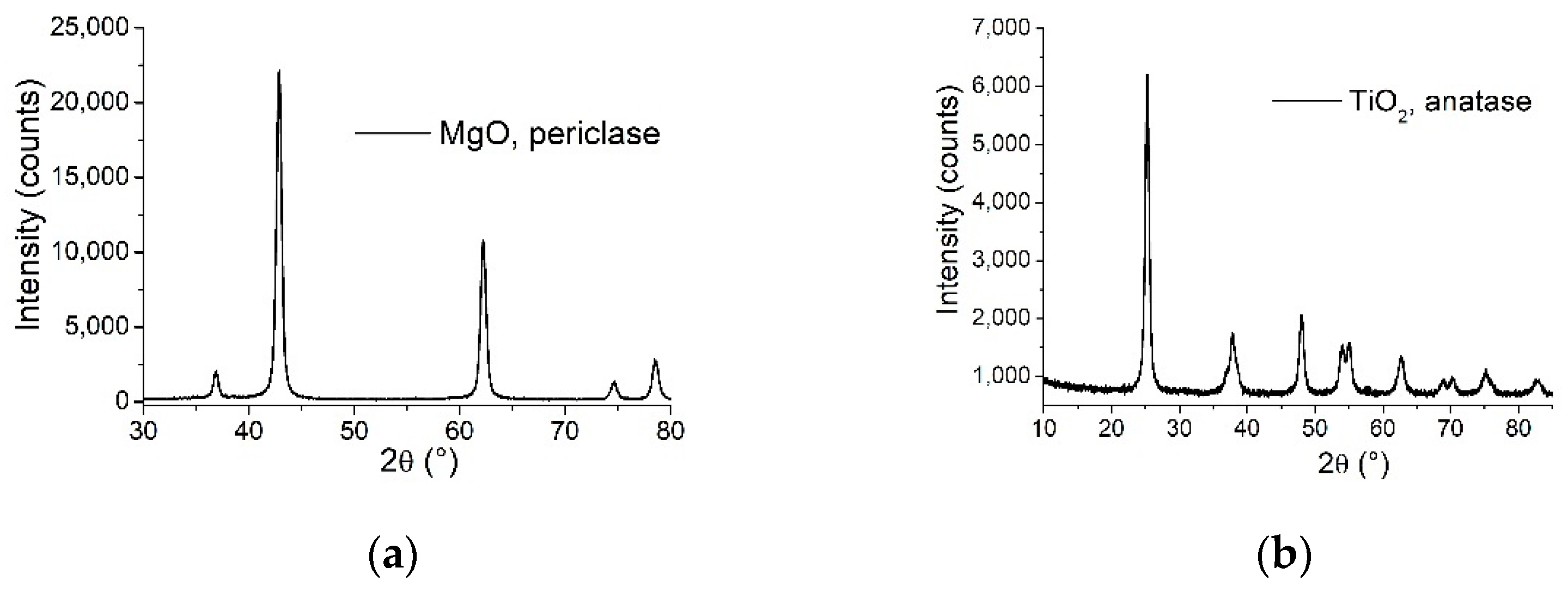
3.1. MgO and TiO2 Nano-Powders
3.2. Antimicrobial Activity of MgO- and TiO2-Based Suspensions and Polymer-Oxide Suspensions (NaPAC16-MgO and NaPAC16-TiO2)
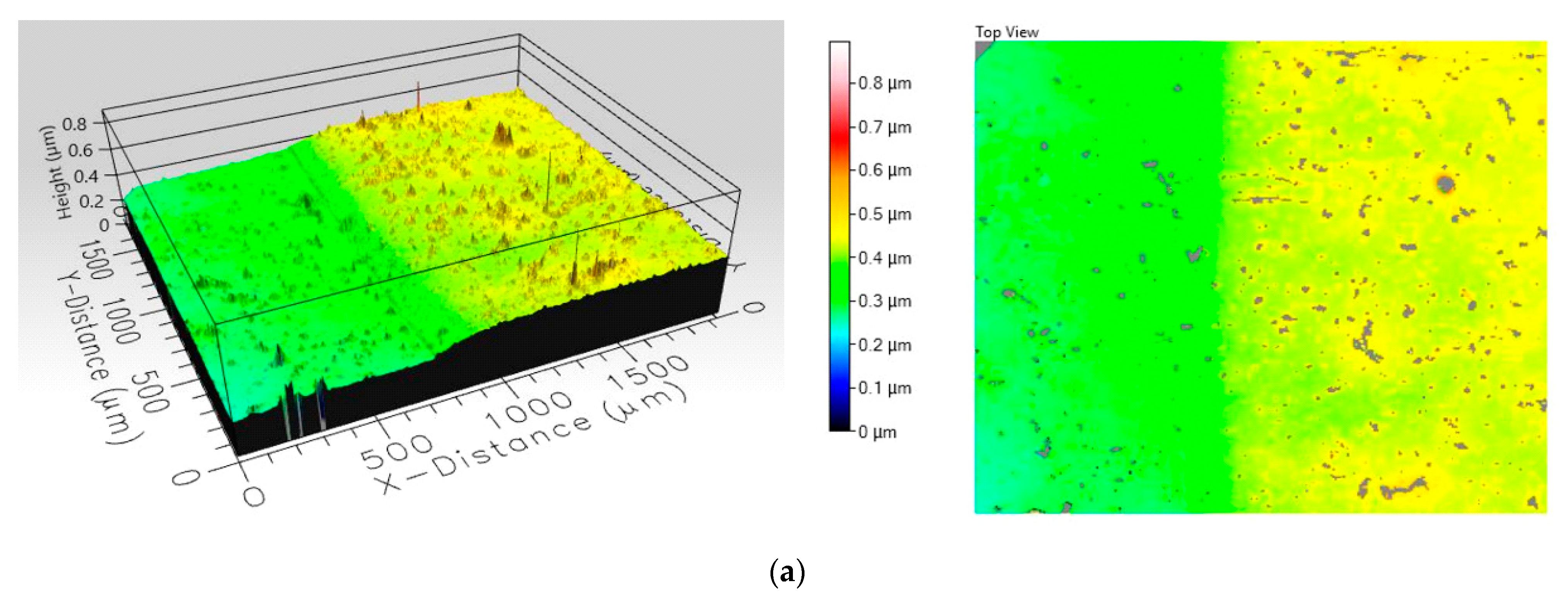
3.3. MgO- and TiO2-Based Films on Glass Substrates
3.4. MgO- and TiO2-Based Films on Stone Substrates
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sierra-Fernandez, A.; Gomez-Villalba, L.S.; Rabanal, M.E.; Fort, R. New Nanomaterials for Applications in Conservation and Restoration of Stony Materials: A Review. Mater. Construcc. 2017, 67, e107. [Google Scholar] [CrossRef]
- Aldoasri, M.A.; Darwish, S.S.; Adam, M.A.; Elmarzugi, N.A.; Ahmed, S.M. Protecting of Marble Stone Facades of Historic Buildings Using Multifunctional TiO2 Nanocoatings. Sustainability 2017, 9, 2002. [Google Scholar] [CrossRef]
- Aslanidou, D.; Karapanagiotis, I.; Lampakis, D. Waterborne Superhydrophobic and Superoleophobic Coatings for the Protection of Marble and Sandstone. Materials 2018, 11, 585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doehne, E.F.; Price, C.A. Stone Conservation: An Overview of Current Research, 2nd ed.; Getty Conservation Institute: Los Angeles, CA, USA, 2010. [Google Scholar]
- Hikku, G.S.; Jeyasubramanian, K.; Kumar, V.S. Nanoporous MgO as Self-Cleaning and Anti-Bacterial Pigment for Alkyd Based Coating. J. Ind. Eng. Chem. 2017, 52, 168–178. [Google Scholar] [CrossRef]
- Sassoni, E. Phosphate-Based Treatments for Conservation of Stone. RILEM Tech. Lett 2017, 2, 14–19. [Google Scholar] [CrossRef] [Green Version]
- Matsukevich, I.V.; Atkinson, I.; Basarab, S.V.; Petcu, G.; Petrescu, S.; Pârvulescu, V.; Fruth, V. Composite Materials Based on MgO and Metallic Nanoparticles for Catalytic Applications. Rom. J. Mater. 2019, 49, 483–490. [Google Scholar]
- Mageshwari, K.; Mali, S.S.; Sathyamoorthy, R.; Patil, P.S. Template-Free Synthesis of MgO Nanoparticles for Effective Photocatalytic Applications. Powder Technol. 2013, 249, 456–462. [Google Scholar] [CrossRef]
- Patil, K.C.; Hegde, M.S.; Rattan, T.; Aruna, S.T. Chemistry of Nanocrystalline Oxide Materials; World Scientific: Singapore, 2008. [Google Scholar] [CrossRef]
- Athar, T. Chapter 14—Metal oxide nanopowder. In Emerging Nanotechnologies for Manufacturing, 2nd ed.; Ahmed, W., Jackson, M.J., Eds.; William Andrew Publishing: Boston, MA, USA, 2015; pp. 343–401. [Google Scholar] [CrossRef]
- Byrappa, K.; Adschiri, T. Hydrothermal Technology for Nanotechnology. Prog. Cryst. Growth Charact. Mater. 2007, 53, 117–166. [Google Scholar] [CrossRef] [Green Version]
- Predoana, L.; Stanciu, I.; Zaharescu, M. Metal oxide nanomaterials obtained by sol-gel and microwave assisted sol-gel methods, chapter 7. In Advances in Microelectronics: Reviews; Yurish, S.Y., Ed.; Book Series; International Frequency Sensor Association (IFSA) Publishing, S. L: Barcelona, Spain, 2019; Volume 2. [Google Scholar]
- Mbarki, R.; Mnif, A.; Hamzaoui, A.H. Structural, Dielectric Relaxation and Electrical Conductivity Behavior in MgO Powders Synthesized by Sol–Gel. Mater. Sci. Semicond. Process. 2015, 29, 300–306. [Google Scholar] [CrossRef]
- Popov, A.I.; Kotomin, E.A.; Maier, J. Basic Properties of the F-Type Centers in Halides, Oxides and Perovskites. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2010, 268, 3084–3089. [Google Scholar] [CrossRef]
- Pacchioni, G.; Freund, H. Electron Transfer at Oxide Surfaces. The MgO Paradigm: From Defects to Ultrathin Films. Chem. Rev. 2013, 113, 4035–4072. [Google Scholar] [CrossRef]
- Popov, A.I.; Shirmane, L.; Pankratov, V.; Lushchik, A.; Kotlov, A.; Serga, V.E.; Kulikova, L.D.; Chikvaidze, G.; Zimmermann, J. Comparative Study of the Luminescence Properties of Macro- and Nanocrystalline MgO Using Synchrotron Radiation. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2013, 310, 23–26. [Google Scholar] [CrossRef]
- González, J.; Guinea, F.; Vozmediano, M.A.H. Marginal-Fermi-Liquid Behavior from Two-Dimensional Coulomb Interaction. Phys. Rev. B 1999, 59, R2474–R2477. [Google Scholar] [CrossRef] [Green Version]
- Balakrishnan, G.; Velavan, R.; Mujasam Batoo, K.; Raslan, E.H. Microstructure, Optical and Photocatalytic Properties of MgO Nanoparticles. Results Phys. 2020, 16, 103013. [Google Scholar] [CrossRef]
- Tsebriienko, T.; Popov, A.I. Effect of Poly(Titanium Oxide) on the Viscoelastic and Thermophysical Properties of Interpenetrating Polymer Networks. Crystals 2021, 11, 794. [Google Scholar] [CrossRef]
- Sutapa, I.W.; Wahab, A.W.; Taba, P.; Nafie, N.L. Synthesis and Structural Profile Analysis of the MgO Nanoparticles Produced Through the Sol-Gel Method Followed by Annealing Process. Orient J. Chem. 2018, 34, 1016–1025. [Google Scholar] [CrossRef] [Green Version]
- Munafò, P.; Goffredo, G.B.; Quagliarini, E. TiO2-Based Nanocoatings for Preserving Architectural Stone Surfaces: An Overview. Constr. Build. Mater. 2015, 84, 201–218. [Google Scholar] [CrossRef]
- Akpan, U.G.; Hameed, B.H. The Advancements in Sol–Gel Method of Doped-TiO2 Photocatalysts. Appl. Catal. A Gen. 2010, 375, 1–11. [Google Scholar] [CrossRef]
- Lin, W.-C.; Yang, W.-D.; Jheng, S.-Y. Photocatalytic Degradation of Dyes in Water Using Porous Nanocrystalline Titanium Dioxide. J. Taiwan Inst. Chem. Eng. 2012, 43, 269–274. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Lu, X. Preparation of Silver-Modified TiO2 via Microwave-Assisted Method and Its Photocatalytic Activity for Toluene Degradation. J. Hazard. Mater. 2010, 177, 639–647. [Google Scholar] [CrossRef]
- Tongon, W.; Chawengkijwanich, C.; Chiarakorn, S. Visible Light Responsive Ag/TiO2/MCM-41 Nanocomposite Films Synthesized by a Microwave Assisted Sol–Gel Technique. Superlattices Microstruct. 2014, 69, 108–121. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, D.; Askari, S.; Patel, J.; Macias-Montero, M.; Mitra, S.; Zhang, R.; Lin, W.-F.; Mariotti, D.; Maguire, P. Enhanced Dispersion of TiO2 Nanoparticles in a TiO2/PEDOT:PSS Hybrid Nanocomposite via Plasma-Liquid Interactions. Sci. Rep. 2015, 5, 15765. [Google Scholar] [CrossRef] [Green Version]
- Tuncer, E.; Sauers, I.; James, D.R.; Ellis, A.R.; Paranthaman, M.P.; Aytuğ, T.; Sathyamurthy, S.; More, K.L.; Li, J.; Goyal, A. Electrical Properties of Epoxy Resin Based Nano-Composites. Nanotechnology 2006, 18, 025703. [Google Scholar] [CrossRef]
- Decher, G.; Hong, J.D. Buildup of Ultrathin Multilayer Films by a Self-Assembly Process: II. Consecutive Adsorption of Anionic and Cationic Bipolar Amphiphiles and Polyelectrolytes on Charged Surfaces. Ber. Bunsenges. Phys. Chem. 1991, 95, 1430–1434. [Google Scholar] [CrossRef]
- Han, J.T.; Zheng, Y.; Cho, J.H.; Xu, X.; Cho, K. Stable Superhydrophobic Organic−Inorganic Hybrid Films by Electrostatic Self-Assembly. J. Phys. Chem. B 2005, 109, 20773–20778. [Google Scholar] [CrossRef] [PubMed]
- Manoudis, P.N.; Karapanagiotis, I.; Tsakalof, A.; Zuburtikudis, I.; Panayiotou, C. Superhydrophobic Composite Films Produced on Various Substrates. Langmuir 2008, 24, 11225–11232. [Google Scholar] [CrossRef] [PubMed]
- Manoudis, P.N.; Karapanagiotis, I.; Tsakalof, A.; Zuburtikudis, I.; Kolinkeová, B.; Panayiotou, C. Superhydrophobic Films for the Protection of Outdoor Cultural Heritage Assets. Appl. Phys. A 2009, 97, 351–360. [Google Scholar] [CrossRef]
- Aricov, L.; Băran, A.; Simion, E.L.; Gîfu, I.C.; Anghel, D.-F.; Jerca, V.V.; Vuluga, D.M. New Insights into the Self-Assembling of Some Hydrophobically Modified Polyacrylates in Aqueous Solution. Colloid Polym. Sci. 2016, 294, 667–679. [Google Scholar] [CrossRef]
- Aricov, L.; Petkova, H.; Arabadzhieva, D.; Iovescu, A.; Mileva, E.; Khristov, K.; Stinga, G.; Mihailescu, C.-F.; Anghel, D.F.; Todorov, R. Aqueous Solutions of Associative Poly(Acrylates): Bulk and Interfacial Properties. Colloids Surf. A Physicochem. Eng. Asp. 2016, 505, 138–149. [Google Scholar] [CrossRef]
- Băran, A.; Aricov, L.; Stîngă, G.; Iovescu, A.; Leontieş, A.-R.; Jerca, V.V. The Effect of C12E6 Nonionic Surfactant on the Solubilization of Eosin Y in Unmodified- and Hydrophobically Modified Poly(Acrylic Acid) Solutions. J. Mol. Liq. 2021, 117103. [Google Scholar] [CrossRef]
- Aricov, L.; Băran, A.; Stîngă, G.; Simion, E.L.; Gîfu, I.C.; Anghel, D.-F.; Rădiţoiu, V. Formation and Hosting Properties of Polyacrylate–Surfactant Complexes. Colloid Polym. Sci. 2017, 295, 1017–1038. [Google Scholar] [CrossRef]
- Branzoi, F.; Băran, A.; Ludmila, A.; Alexandrescu, E. The Inhibition Action of Some Organic Polymers on the Corrosion Carbon Steel in Acidic Media. Chem. Pap. 2020, 74, 4315–4335. [Google Scholar] [CrossRef]
- Gîfu, I.C.; Maxim, M.E.; Iovescu, A.; Simion, E.L.; Aricov, L.; Anastasescu, M.; Munteanu, C.; Anghel, D.-F. Surface Hydrophobization by Electrostatic Deposition of Hydrophobically Modified Poly(Acrylates) and Their Complexes with Surfactants. Appl. Surf. Sci. 2016, 371, 519–529. [Google Scholar] [CrossRef]
- Gîfu, I.C.; Maxim, M.E.; Iovescu, A.; Aricov, L.; Simion, E.L.; Leontieş, A.R.; Anastasescu, M.; Munteanu, C.; Anghel, D.-F. Natural Aging of Multilayer Films Containing Hydrophobically Modified Poly(Acrylate)s or Their Complexes with Surfactants. Appl. Surf. Sci. 2017, 412, 489–496. [Google Scholar] [CrossRef]
- Gîfu, I.C.; Maxim, M.E.; Cinteza, L.O.; Popa, M.; Aricov, L.; Leontieș, A.R.; Anastasescu, M.; Anghel, D.-F.; Ianchis, R.; Ninciuleanu, C.M.; et al. Antimicrobial Activities of Hydrophobically Modified Poly(Acrylate) Films and Their Complexes with Different Chain Length Cationic Surfactants. Coatings 2019, 9, 244. [Google Scholar] [CrossRef] [Green Version]
- National Patent Application with Title Process for Obtaining Nanocomposite Films Intended to Protect the Lithic Architec-tural Components of the Cultural heritage (Procedeu de Obținere a Unor Pelicule Nanocompozite Destinate Protejării Com-ponentelor Arhitecturale Litice Ale Patrimoniului Cultural). Patent 134390, August 2021. OSIM Nr. A/00350/10.06.2019, Grant of Invention.
- Tang, Z.-X.; Lv, B.-F. MgO Nanoparticles as Antibacterial Agent: Preparation and Activity. Braz. J. Chem. Eng. 2014, 31, 591–601. [Google Scholar] [CrossRef]
- Hussein, E.M.; Ahmed, S.A.; Mokhtar, A.B.; Elzagawy, S.M.; Yahi, S.H.; Hussein, A.M.; El-Tantawey, F. Antiprotozoal Activity of Magnesium Oxide (MgO) Nanoparticles against Cyclospora Cayetanensis Oocysts. Parasitol. Int. 2018, 67, 666–674. [Google Scholar] [CrossRef]
- Hossain, F.; Perales-Perez, O.J.; Hwang, S.; Román, F. Antimicrobial Nanomaterials as Water Disinfectant: Applications, Limitations and Future Perspectives. Sci. Total Environ. 2014, 466, 1047–1059. [Google Scholar] [CrossRef]
- Planchon, M.; Ferrari, R.; Guyot, F.; Gélabert, A.; Menguy, N.; Chanéac, C.; Thill, A.; Benedetti, M.F.; Spalla, O. Interaction between Escherichia Coli and TiO2 Nanoparticles in Natural and Artificial Waters. Colloids Surf. B Biointerfaces 2013, 102, 158–164. [Google Scholar] [CrossRef]
- Xu, C.; Zheng, J.; Wu, A. Antibacterial Applications of TiO 2 Nanoparticles. In TiO2 Nanoparticles; Wu, A., W. Ren, W., Eds.; Wiley Online Library: Hoboken, NJ, USA, 2020; pp. 105–132. [Google Scholar] [CrossRef]
- Azizi-Lalabadi, M.; Ehsani, A.; Divband, B.; Alizadeh-Sani, M. Antimicrobial Activity of Titanium Dioxide and Zinc Oxide Nanoparticles Supported in 4A Zeolite and Evaluation the Morphological Characteristic. Sci. Rep. 2019, 9, 17439. [Google Scholar] [CrossRef] [Green Version]
- Jin, T.; He, Y. Antibacterial Activities of Magnesium Oxide (MgO) Nanoparticles against Foodborne Pathogens. J. Nanoparticle Res. 2011, 13, 6877–6885. [Google Scholar] [CrossRef]
- Vasiliu, C.; Grigorescu, C.; Pavelescu, G.; Predoana, L.; Todan, L.; Gartner, M.; Anastasescu, M.; Negrila, C.; Logofatu, C.; Moldovan, A.; et al. Influence of the Phosphorous Precursors on the Structure and Properties of the SiO2-P2O5 Sol-Gel Films. J. Optoelectron. Adv. Mater. 2007, 9, 1407–1410. [Google Scholar]
- Todan, L.; Dascalescu, T.; Preda, S.; Andronescu, C.; Munteanu, C.; Culita, D.C.; Rusu, A.; State, R.; Zaharescu, M. Porous Nanosized Oxide Powders in the MgO-TiO2 Binary System Obtained by Sol-Gel Method. Ceram. Int. 2014, 40, 15693–15701. [Google Scholar] [CrossRef]
- Hunt, R.W.G.; Pointer, M.R. Relations Between Colour Stimuli. In Measuring Colour; Kriss, M.A., Hunt., R.W.G., Pointer, M.R., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011. [Google Scholar]
- Nourozi, B.; Aminian, A.; Fili, N.; Zangeneh, Y.; Boochani, A.; Darabi, P. The Electronic and Optical Properties of MgO Mono-Layer: Based on GGA-MBJ. Results Phys. 2019, 12, 2038–2043. [Google Scholar] [CrossRef]
- Shabbir, M.; Kaushik, M. Chapter 10—Engineered Nanomaterials: Scope in Today’s Textile Industry. In Handbook of Nanomaterials for Manufacturing Applications; Hussain, C.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 249–263. [Google Scholar] [CrossRef]


| Powder Sample | BET Surface Area (m2/g) | Total Pore Volume (cm2/g) | Pore Diameter (nm) |
|---|---|---|---|
| MgO | 72.22 | 0.68 | 33.18 |
| TiO2 | 80.68 | 0.16 | 6.04 |
| Powder Sample | Methyl Orange UV Photodegradation (%) | Band Gap (eV) | |
|---|---|---|---|
| 1 h | 3 h | ||
| MgO | 56 | 80 | 4.7 |
| TiO2 | 87 | 93.7 | 3.15 |
| Tested Nanopowder Concentration (0.5 mg/mL) | OD of Sample with S. aureus | OD of Sample without S. aureus | Real OD Caused by Microbial Growth |
|---|---|---|---|
| MgO | 0.043 | 0.038 | 0.005 |
| TiO2 | 0.220 | 0.101 | 0.119 |
| Biological control of S. aureus | - | - | 0.243 |
| Polymer-Oxide Suspensions | Microorganism Used for Testing/Results in mm | ||
|---|---|---|---|
| Staphylococcus aureus | Aspergillus niger | Candida albicans | |
| NaPAC16-MgO | 11 | 9 | 7 |
| NaPAC16-TiO2 | 14 | 6 | 4 |
| Film on Glass Slide Sample | Root Mean Square Height (µm) |
|---|---|
| Glass/5 layers NaPAC16 + MgO NPs (0.5%) | 0.02125 |
| Glass/5 layers NaPAC16 + TiO2 NPs (0.5%) | 0.01107 |
| Sample | Glass | Glass/5 Layers NaPAC16 | Glass/5 Layers NaPAC16 + MgO | Glass/5 Layers NaPAC16 + TiO2 |
|---|---|---|---|---|
| Contact angle θ (°) | 41.26 | 74.50 | 98.79 | 78.50 |
| Film on Glass Slide Sample | Methyl Orange UV Photodegradation (%) | ||
|---|---|---|---|
| 1 h | 3 h | 5 h | |
| Glass/1-layer NaPAC16 | 0% | 0% | 1% |
| Glass/5 layers NaPAC16 + TiO2 NPs (0.5%) | 3.9% | 13.62% | 17.10% |
| Glass/5 layers NaPAC16 + MgO NPs (0.5%) | 18.86% | 36.9% | 49.13% |
| MgO NPs | 56% | 80% | - |
| TiO2 NPs | 87% | 93.7% | - |
| Sample | L* | a* | b* | ΔL* | Δa* | Δb* | ΔEx | ΔE |
|---|---|---|---|---|---|---|---|---|
| Limestone 1 | 83.24 | 1.02 | 1.03 | −10.18 | 1.57 | −2.28 | 10.54 | |
| Limestone 1 coated with MgO-NaPAC16 | 82.86 | 1.19 | 0.99 | −10.56 | 1.73 | −2.31 | 10.95 | 0.41 |
| Limestone 2 | 84.39 | 0.99 | 3.74 | −9.03 | 1.54 | 0.44 | 9.17 | |
| Limestone 2 coated with TiO2—NaPAC16 | 84.14 | 1.14 | 3.97 | −9.28 | 1.69 | 0.67 | 9.46 | 0.29 |
| Sample | L* | a* | b* | ΔL* | Δa* | Δb* | ǀΔb*ǀ | Δbx | ΔEx | ΔEx’ |
|---|---|---|---|---|---|---|---|---|---|---|
| M1(a) reference | 85.85 | 0.46 | 1.58 | −7.57 | 1.01 | −1.72 | 1.72 | 7.83 | ||
| M1(a) MgO—NaPAC16 | 86.06 | 0.62 | 1.49 | −7.36 | 1.17 | −1.81 | 1.81 | 0.09 | 7.67 | 0.16 |
| M1(a) TiO2—NaPAC16 | 86.19 | 0.70 | 1.63 | −7.23 | 1.25 | −1.68 | 1.68 | −0.03 | 7.53 | 0.30 |
| M1(b) reference | 77.85 | 2.12 | 1.13 | −15.57 | 2.67 | −2.17 | 2.17 | 15.95 | ||
| M1(b) MgO—NaPAC16 | 78.13 | 2.29 | 0.82 | −15.29 | 2.84 | −2.48 | 2.48 | 0.11 | 15.74 | 0.21 |
| M1(b) TiO2—NaPAC16 | 78.57 | 2.28 | 0.92 | −14.85 | 2.83 | −2.39 | 2.39 | 0.22 | 15.31 | 0.64 |
| Sample | L* | a* | b* | ΔL* | Δa* | Δb* | ǀΔb*ǀ | Δbx | ΔEx | ΔE |
|---|---|---|---|---|---|---|---|---|---|---|
| Reference specimen | 76.44 | 4.35 | 1.31 | −16.98 | 4.90 | −2.00 | 2.00 | - | 17.79 | - |
| MgO + NaPAC16 | 76.28 | 4.52 | 1.48 | −17.14 | 5.07 | −1.83 | 1.83 | −0.18 | 17.97 | 0.18 |
| TiO2 + NaPAC16 | 76.06 | 4.45 | 1.25 | −17.36 | 5.01 | −2.05 | 2.05 | 0.05 | 18.18 | 0.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fruth, V.; Todan, L.; Codrea, C.I.; Poenaru, I.; Petrescu, S.; Aricov, L.; Ciobanu, M.; Jecu, L.; Ion, R.M.; Predoana, L. Multifunctional Composite Coatings Based on Photoactive Metal-Oxide Nanopowders (MgO/TiO2) in Hydrophobic Polymer Matrix for Stone Heritage Conservation. Nanomaterials 2021, 11, 2586. https://doi.org/10.3390/nano11102586
Fruth V, Todan L, Codrea CI, Poenaru I, Petrescu S, Aricov L, Ciobanu M, Jecu L, Ion RM, Predoana L. Multifunctional Composite Coatings Based on Photoactive Metal-Oxide Nanopowders (MgO/TiO2) in Hydrophobic Polymer Matrix for Stone Heritage Conservation. Nanomaterials. 2021; 11(10):2586. https://doi.org/10.3390/nano11102586
Chicago/Turabian StyleFruth, Victor, Ligia Todan, Cosmin Iulian Codrea, Iuliana Poenaru, Simona Petrescu, Ludmila Aricov, Madalina Ciobanu, Luiza Jecu, Rodica Mariana Ion, and Luminita Predoana. 2021. "Multifunctional Composite Coatings Based on Photoactive Metal-Oxide Nanopowders (MgO/TiO2) in Hydrophobic Polymer Matrix for Stone Heritage Conservation" Nanomaterials 11, no. 10: 2586. https://doi.org/10.3390/nano11102586
APA StyleFruth, V., Todan, L., Codrea, C. I., Poenaru, I., Petrescu, S., Aricov, L., Ciobanu, M., Jecu, L., Ion, R. M., & Predoana, L. (2021). Multifunctional Composite Coatings Based on Photoactive Metal-Oxide Nanopowders (MgO/TiO2) in Hydrophobic Polymer Matrix for Stone Heritage Conservation. Nanomaterials, 11(10), 2586. https://doi.org/10.3390/nano11102586








