Rational Constructed Ultra-Small Iron Oxide Nanoprobes Manifesting High Performance for T1-Weighted Magnetic Resonance Imaging of Glioblastoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cells and Animals
2.3. Characterization
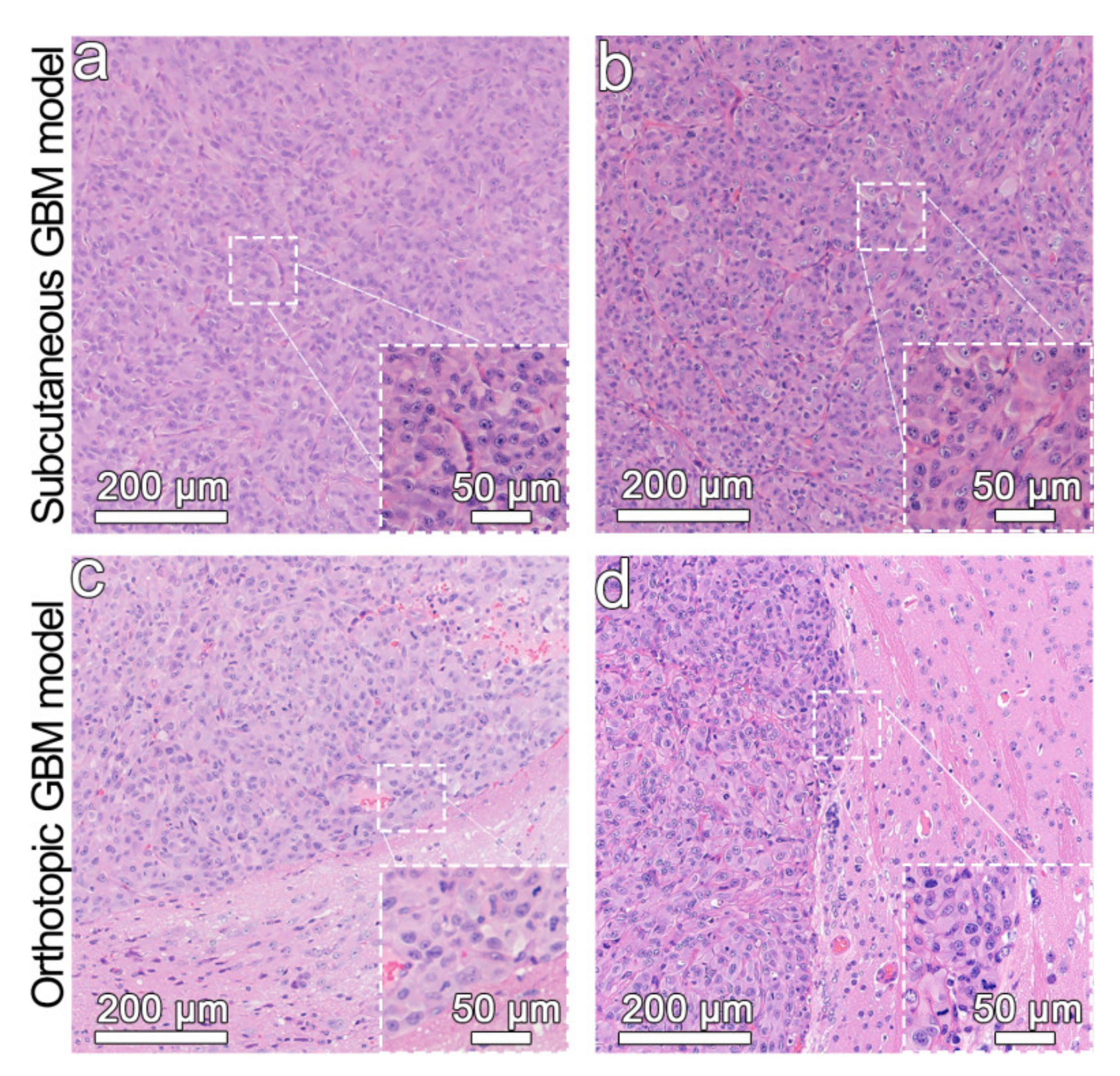
2.4. Establishment of Glioblastoma Models
2.5. Synthesis, Purification and Characterization of Fe3O4 NPs
2.6. Ligand Exchange with DP-PEG2000-Mal to Prepare Fe3O4-Mal NPs
2.7. Conjugation of ANG to Fe3O4-Mal Surface to Prepare Fe3O4-ANG NPs
2.8. Measurement of the Relaxivity of Fe3O4-Mal and Fe3O4-ANG NPs by MRI
2.9. Hydrodynamic Size, Zeta Potential and Stability of Fe3O4-Mal and Fe3O4-ANG NPs
2.10. Cytotoxicity Assessment In Vitro
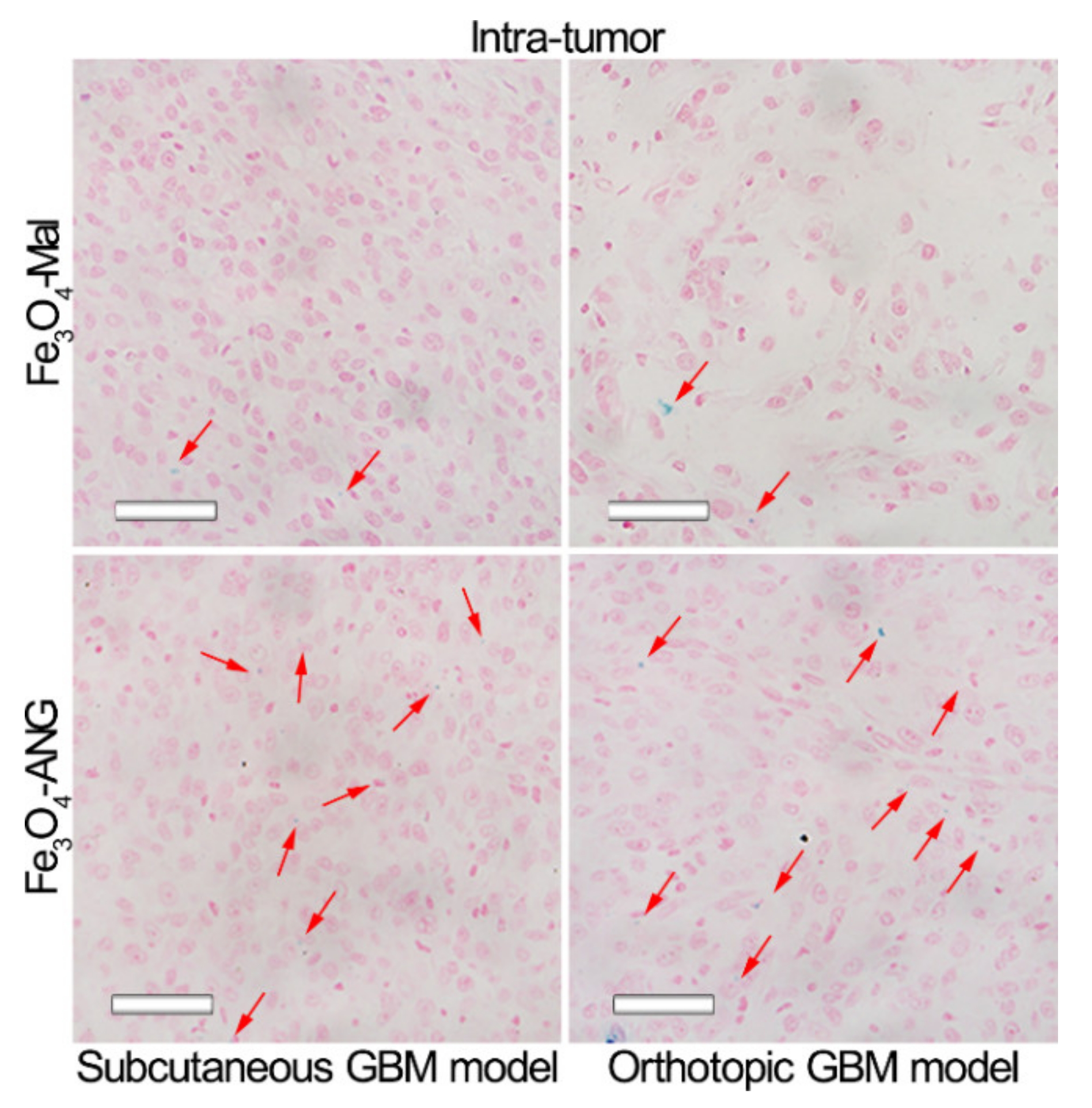
2.11. Prussian Blue Staining of Fe3O4-Mal and Fe3O4-ANG NPs In Vitro
2.12. In Vivo MRI Assessment
3. Results and Discussion
3.1. Synthesis and Characterization of Fe3O4, Fe3O4-Mal and Fe3O4-ANG NPs
3.2. Relaxation Performance and Stability of Fe3O4-Mal NPs, Fe3O4-ANG NPs and Gd-DTPA
3.3. Cytotoxicity of the Fe3O4-Mal and Fe3O4-ANG NPs
3.4. In Vitro Specificity Studies of MRI Nanoprobes
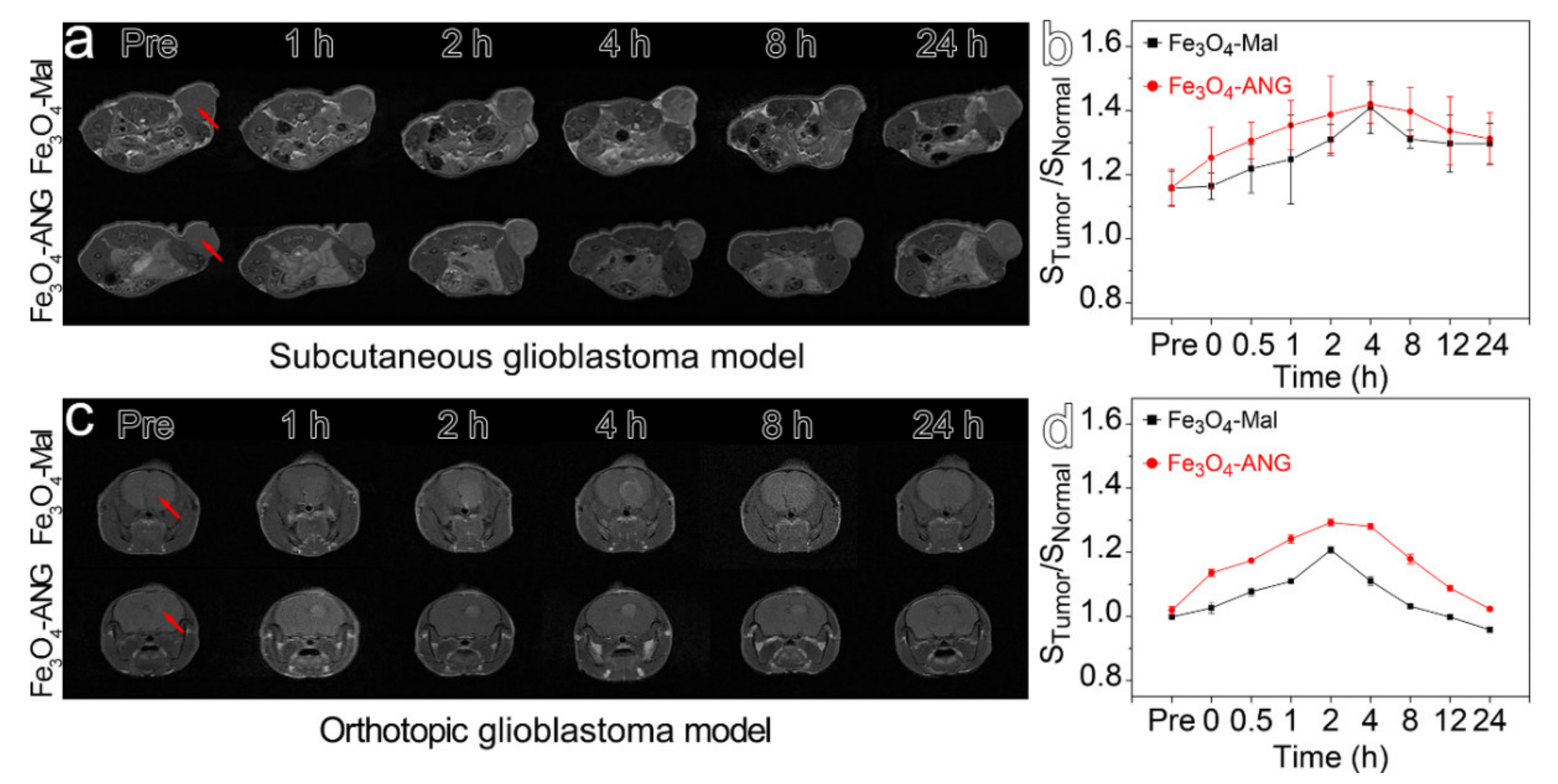
3.5. In Vivo MR Imaging of the Glioblastoma
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Shin, T.-H.; Kim, P.K.; Kang, S.; Cheong, J.; Kim, S.; Lim, Y.; Shin, W.; Jung, J.-Y.; Lah, J.D.; Choi, B.W.; et al. High-resolution T1 MRI via renally clearable dextran nanoparticles with an iron oxide shell. Nat. Biomed. Eng. 2021, 5, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Yang, L.; Gao, J.; Chen, X. Structure-relaxivity relationships of magnetic nanoparticles for magnetic resonance imaging. Adv. Mater. 2019, 31, e1804567. [Google Scholar] [CrossRef] [PubMed]
- Bin Na, H.; Song, I.-C.; Hyeon, T. Inorganic Nanoparticles for MRI Contrast Agents. Adv. Mater. 2009, 21, 2133–2148. [Google Scholar] [CrossRef]
- Zhou, J.; Zhu, X.; Chen, M.; Sun, Y.; Li, F. Water-stable NaLuF4-based upconversion nanophosphors with long-term validity for multimodal lymphatic imaging. Biomaterials 2012, 33, 6201–6210. [Google Scholar] [CrossRef]
- Delbeke, D.; Schöder, H.; Martin, W.H.; Wahl, R.L. Hybrid Imaging (SPECT/CT and PET/CT): Improving Therapeutic Decisions. Semin. Nucl. Med. 2009, 39, 308–340. [Google Scholar] [CrossRef] [PubMed]
- Chizhik, V.I.; Tagirov, M.S. Current Trends in Magnetic Resonance. Appl. Magn. Reson. 2020, 51, 103–106. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Gong, T.; Xu, N.; Cui, F.; Yuan, B.; Yuan, Q.; Sun, H.; Wang, L.; Liu, J. Improved Stability and Photothermal Performance of Polydopamine-Modified Fe 3 O 4 Nanocomposites for Highly Efficient Magnetic Resonance Imaging-Guided Photothermal Therapy. Small 2020, 16, e2003969. [Google Scholar] [CrossRef]
- Jeon, M.; Halbert, M.V.; Stephen, Z.R.; Zhang, M. Iron Oxide Nanoparticles as T 1 Contrast Agents for Magnetic Resonance Imaging: Fundamentals, Challenges, Applications, and Prospectives. Adv. Mater. 2020, 33, e1906539. [Google Scholar] [CrossRef] [PubMed]
- Busquets, M.A.; Estelrich, J.; Sánchez-Martín, M.J. Nanoparticles in magnetic resonance imaging: From simple to dual contrast agents. Int. J. Nanomed. 2015, 10, 1727–1741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, R.; Yang, C.; Gao, M. Superparamagnetic iron oxide nanoparticles: From preparations to in vivo MRI applications. J. Mater. Chem. 2009, 19, 6274–6293. [Google Scholar] [CrossRef]
- Xing, H.; Zhang, S.; Bu, W.; Zheng, X.; Wang, L.; Xiao, Q.; Ni, D.; Zhang, J.; Zhou, L.; Peng, W.; et al. Ultrasmall NaGdF4Nanodots for Efficient MR Angiography and Atherosclerotic Plaque Imaging. Adv. Mater. 2014, 26, 3867–3872. [Google Scholar] [CrossRef] [PubMed]
- Caschera, L.; Lazzara, A.; Piergallini, L.; Ricci, D.; Tuscano, B.; Vanzulli, A. Contrast agents in diagnostic imaging: Present and future. Pharmacol. Res. 2016, 110, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Caspani, S.; Magalhães, R.; Araújo, J.P.; Sousa, C.T. Magnetic Nanomaterials as Contrast Agents for MRI. Materials 2020, 13, 2586. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, Z.; Wang, Y.; Wang, Y.; Liu, C.; Cao, K.; Lu, Y.; Behboodpour, L.; Hou, Y.; Gao, M. An MRI contrast agent based on a zwitterionic metal-chelating polymer for hepatorenal angiography and tumor imaging. J. Mater. Chem. B 2020, 8, 6956–6963. [Google Scholar] [CrossRef]
- Hu, Y.; Mignani, S.; Majoral, J.-P.; Shen, M.; Shi, X. Construction of iron oxide nanoparticle-based hybrid platforms for tumor imaging and therapy. Chem. Soc. Rev. 2018, 47, 1874–1900. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; He, Q.; Jiang, C. Magnetic Iron Oxide Nanoparticles: Synthesis and Surface Functionalization Strategies. Nanoscale Res. Lett. 2008, 3, 397–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arami, H.; Khandhar, A.; Liggitt, D.; Krishnan, K.M. In Vivo delivery, pharmacokinetics, biodistribution and toxicity of iron oxide nanoparticles. Chem. Soc. Rev. 2015, 44, 8576–8607. [Google Scholar] [CrossRef]
- Ma, M.; Zhu, H.; Ling, J.; Gong, S.; Zhang, Y.; Xia, Y.; Tang, Z. Quasi-amorphous and Hierarchical Fe2O3 Supraparticles: Active T1-Weighted Magnetic Resonance Imaging in Vivo and Renal Clearance. ACS Nano 2020, 14, 4036–4044. [Google Scholar] [CrossRef]
- Bulte, J.W.M.; Kraitchman, D.L. Iron oxide MR contrast agents for molecular and cellular imaging. NMR Biomed. 2004, 17, 484–499. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yi, P.W.; Sun, Q.; Lei, H.; Zhao, H.L.; Zhu, Z.H.; Smith, S.C.; Lan, M.B.; Lu, G.Q. (Max) Ultrasmall Water-Soluble and Biocompatible Magnetic Iron Oxide Nanoparticles as Positive and Negative Dual Contrast Agents. Adv. Funct. Mater. 2012, 22, 2387–2393. [Google Scholar] [CrossRef]
- Kim, B.H.; Lee, N.; Kim, H.; An, K.; Park, Y.I.; Choi, Y.; Shin, K.; Lee, Y.; Kwon, S.G.; Bin Na, H.; et al. Large-Scale Synthesis of Uniform and Extremely Small-Sized Iron Oxide Nanoparticles for High-ResolutionT1Magnetic Resonance Imaging Contrast Agents. J. Am. Chem. Soc. 2011, 133, 12624–12631. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Wang, W.; Ashley, J.; Zhang, M.; Feng, X.; Shen, J.; Sun, Y. Self-Assembly Protein Superstructures as a Powerful Chemodynamic Therapy Nanoagent for Glioblastoma Treatment. Nano-Micro Lett. 2020, 12, 151. [Google Scholar] [CrossRef] [PubMed]
- Hua, D.; Tang, L.; Wang, W.; Tang, S.; Yu, L.; Zhou, X.; Wang, Q.; Sun, C.; Shi, C.; Luo, W.; et al. Improved Antiglioblastoma Activity and BBB Permeability by Conjugation of Paclitaxel to a Cell-Penetrative MMP-2-Cleavable Peptide. Adv. Sci. 2021, 8, 2001960. [Google Scholar] [CrossRef] [PubMed]
- Janjua, T.I.; Rewatkar, P.; Ahmed-Cox, A.; Saeed, I.; Mansfeld, F.M.; Kulshreshtha, R.; Kumeria, T.; Ziegler, D.S.; Kavallaris, M.; Mazzieri, R.; et al. Frontiers in the treatment of glioblastoma: Past, present and emerging. Adv. Drug Deliv. Rev. 2021, 171, 108–138. [Google Scholar] [CrossRef]
- Zhao, M.; Van Straten, D.; Broekman, M.L.; Préat, V.; Schiffelers, R.M. Nanocarrier-based drug combination therapy for glioblastoma. Theranostics 2020, 10, 1355–1372. [Google Scholar] [CrossRef]
- Saraiva, C.; Praça, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernardino, L. Nanoparticle-mediated brain drug delivery: Overcoming blood–brain barrier to treat neurodegenerative diseases. J. Control. Release 2016, 235, 34–47. [Google Scholar] [CrossRef] [Green Version]
- Winkler, A.; Wrzos, C.; Haberl, M.; Weil, M.-T.; Gao, M.; Möbius, W.; Odoardi, F.; Thal, D.R.; Chang, M.; Opdenakker, G.; et al. Blood-brain barrier resealing in neuromyelitis optica occurs independently of astrocyte regeneration. J. Clin. Investig. 2021, 131, e141694. [Google Scholar] [CrossRef]
- Shi, X.-X.; Miao, W.-M.; Pang, D.-W.; Wu, J.-S.; Tong, Q.-S.; Li, J.-X.; Luo, J.-Q.; Li, W.-Y.; Du, J.-Z.; Wang, J. Angiopep-2 conjugated nanoparticles loaded with doxorubicin for the treatment of primary central nervous system lymphoma. Biomater. Sci. 2020, 8, 1290–1297. [Google Scholar] [CrossRef]
- Wei, L. Adsorptive-mediated brain delivery systems. Curr. Pharm. Biotechnol. 2012, 13, 2340–2348. [Google Scholar]
- Cisternino, S.; Chapy, H.; André, P.; Smirnova, M.; Debray, M.; Scherrmann, J.-M. Coexistence of Passive and Proton Antiporter-Mediated Processes in Nicotine Transport at the Mouse Blood–Brain Barrier. AAPS J. 2012, 15, 299–307. [Google Scholar] [CrossRef] [Green Version]
- Israel, L.L.; Braubach, O.; Galstyan, A.; Chiechi, A.; Shatalova, E.S.; Grodzinski, Z.; Ding, H.; Black, K.L.; Ljubimova, J.Y.; Holler, E. A Combination of Tri-Leucine and Angiopep-2 Drives a Polyanionic Polymalic Acid Nanodrug Platform Across the Blood–Brain Barrier. ACS Nano 2019, 13, 1253–1271. [Google Scholar] [CrossRef]
- Du, C.; Liu, X.; Hu, H.; Li, H.; Yu, L.; Geng, D.; Chen, Y.; Zhang, J. Dual-targeting and excretable ultrasmall SPIONs for T1-weighted positive MR imaging of intracranial glioblastoma cells by targeting the lipoprotein receptor-related protein. J. Mater. Chem. B 2020, 8, 2296–2306. [Google Scholar] [CrossRef]
- Ren, J.; Shen, S.; Wang, D.; Xi, Z.; Guo, L.; Pang, Z.; Qian, Y.; Sun, X.; Jiang, X. The targeted delivery of anticancer drugs to brain glioma by PEGylated oxidized multi-walled carbon nanotubes modified with angiopep-2. Biomaterials 2012, 33, 3324–3333. [Google Scholar] [CrossRef]
- Heggannavar, G.B.; Vijeth, S.; Kariduraganavar, M.Y. Development of dual drug loaded PLGA based mesoporous silica nanoparticles and their conjugation with Angiopep-2 to treat glioma. J. Drug Deliv. Sci. Technol. 2019, 53, 101157. [Google Scholar] [CrossRef]
- Tao, J.; Fei, W.; Tang, H.; Li, C.; Mu, C.; Zheng, H.; Li, F.; Zhu, Z. Angiopep-2-Conjugated "Core-shell" hybrid nanovehicles for targeted and pH-triggered delivery of arsenic trioxide into glioma. Mol. Pharm. 2019, 16, 786–797. [Google Scholar] [CrossRef]
- Jiao, M.; Zeng, J.; Jing, L.; Liu, C.; Gao, M. Flow Synthesis of Biocompatible Fe3O4 Nanoparticles: Insight into the Effects of Residence Time, Fluid Velocity, and Tube Reactor Dimension on Particle Size Distribution. Chem. Mater. 2015, 27, 1299–1305. [Google Scholar] [CrossRef]
- Hsu, S.P.; Dhawan, U.; Tseng, Y.-Y.; Lin, C.-P.; Kuo, C.-Y.; Wang, L.-F.; Chung, R.-J. Glioma-sensitive delivery of Angiopep-2 conjugated iron gold alloy nanoparticles ensuring simultaneous tumor imaging and hyperthermia mediated cancer theranostics. Appl. Mater. Today 2020, 18, 100510. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Chen, L.; Ge, J.; Afshari, M.J.; Yang, L.; Miao, Q.; Duan, R.; Cui, J.; Liu, C.; Zeng, J.; et al. Rational Constructed Ultra-Small Iron Oxide Nanoprobes Manifesting High Performance for T1-Weighted Magnetic Resonance Imaging of Glioblastoma. Nanomaterials 2021, 11, 2601. https://doi.org/10.3390/nano11102601
Wang X, Chen L, Ge J, Afshari MJ, Yang L, Miao Q, Duan R, Cui J, Liu C, Zeng J, et al. Rational Constructed Ultra-Small Iron Oxide Nanoprobes Manifesting High Performance for T1-Weighted Magnetic Resonance Imaging of Glioblastoma. Nanomaterials. 2021; 11(10):2601. https://doi.org/10.3390/nano11102601
Chicago/Turabian StyleWang, Xiangyan, Lei Chen, Jianxian Ge, Mohammad Javad Afshari, Lei Yang, Qingqing Miao, Ruixue Duan, Jiabin Cui, Chunyi Liu, Jianfeng Zeng, and et al. 2021. "Rational Constructed Ultra-Small Iron Oxide Nanoprobes Manifesting High Performance for T1-Weighted Magnetic Resonance Imaging of Glioblastoma" Nanomaterials 11, no. 10: 2601. https://doi.org/10.3390/nano11102601
APA StyleWang, X., Chen, L., Ge, J., Afshari, M. J., Yang, L., Miao, Q., Duan, R., Cui, J., Liu, C., Zeng, J., Zhong, J., & Gao, M. (2021). Rational Constructed Ultra-Small Iron Oxide Nanoprobes Manifesting High Performance for T1-Weighted Magnetic Resonance Imaging of Glioblastoma. Nanomaterials, 11(10), 2601. https://doi.org/10.3390/nano11102601






