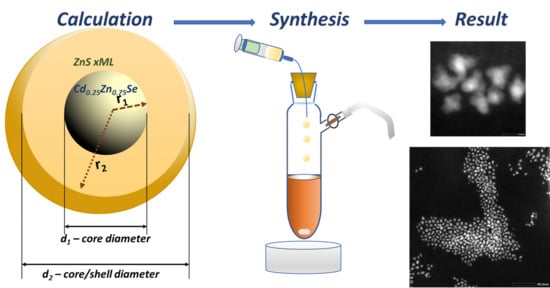
Highly Efficient and Controllable Methodology of the Cd0.25Zn0.75Se/ZnS Core/Shell Quantum Dots Synthesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
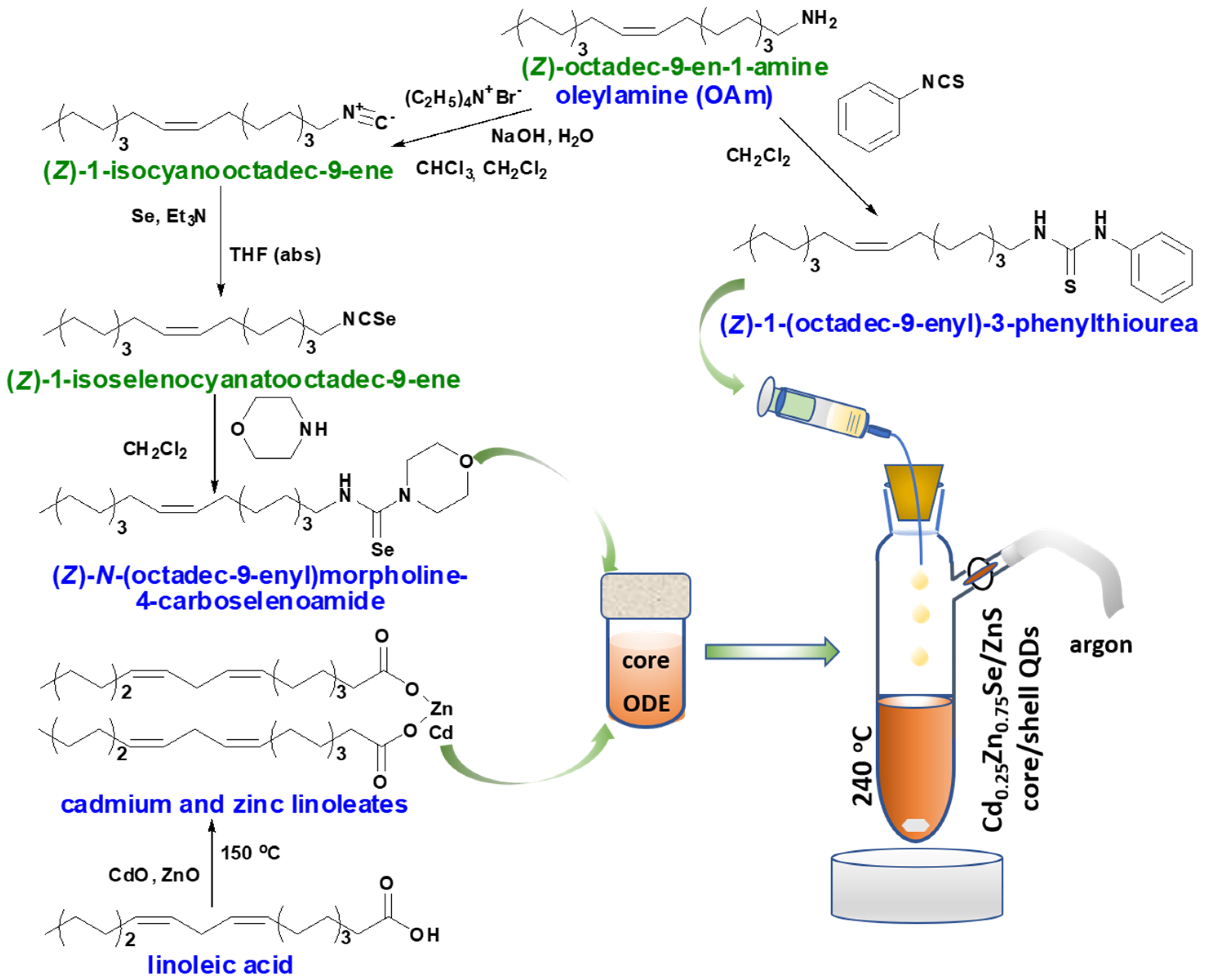
2.2. Synthesis of Selenium and Sulphur Precursors
2.2.1. Synthesis of (Z)-1-isocyanooctadec-9-ene
2.2.2. Synthesis of (Z)-1-isoselenocyanatooctadec-9-ene
2.2.3. Synthesis of (Z)-N-(octadec-9-enyl)morpholine-4-carboselenoamide
2.2.4. Synthesis (Z)-1-(octadec-9-enyl)-3-phenylthiourea
2.3. Preparation of Cd0.25Zn0.75Se/ZnS (1–5 MLs) QDs
2.3.1. Synthesis of Core Cd0.25Zn0.75Se
2.3.2. Synthesis of Core/Shell Cd0.25Zn0.75Se/ZnS 1 ML QDs
2.3.3. Synthesis of Core/Shell Cd0.25Zn0.75Se/ZnS 2 ML QDs
2.3.4. Synthesis of Core/Shell Cd0.25Zn0.75Se/ZnS 3 ML QDs
2.3.5. Synthesis of Core/Shell Cd0.25Zn0.75Se/ZnS 4 ML QDs
2.3.6. Synthesis of Core/Shell Cd0.25Zn0.75Se/ZnS 5 ML QDs
2.4. Characterization
3. Results and Discussion
3.1. Synthesis of Core/Shell Cd0.25Zn0.75Se/ZnS (1–5 MLs) QDs
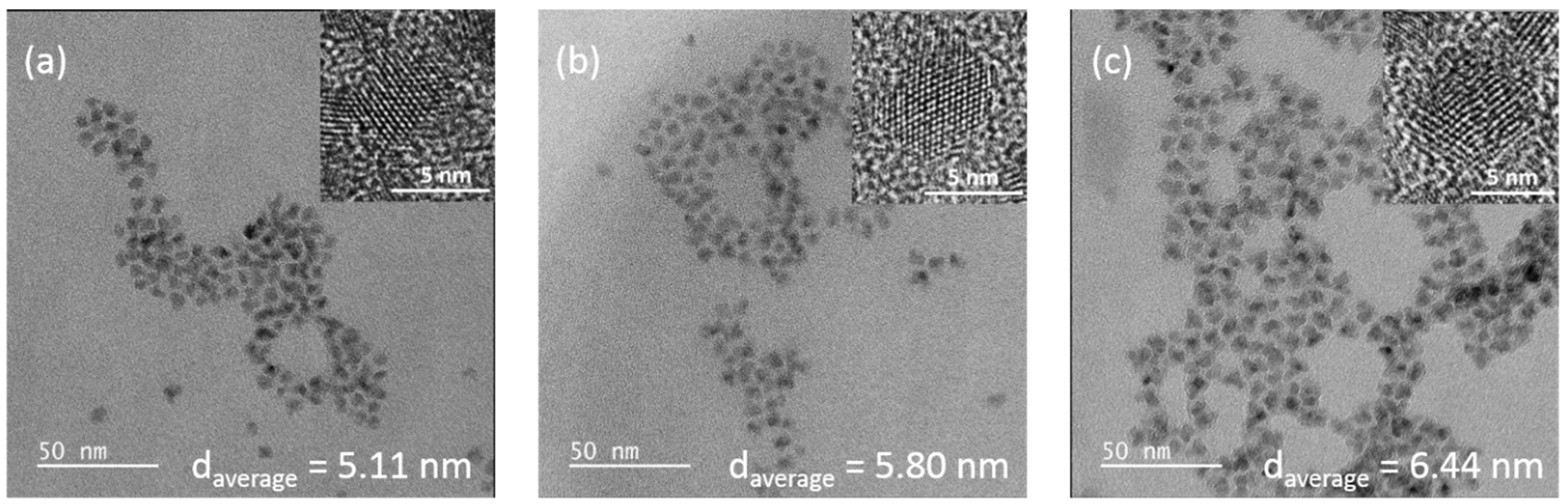
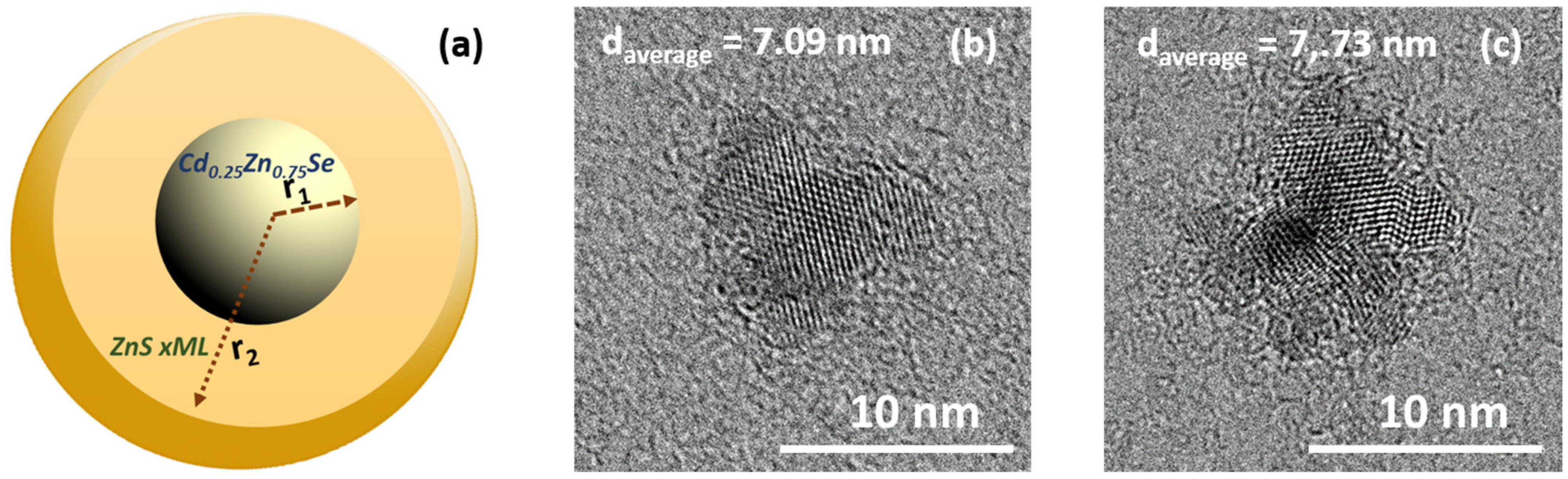
3.2. Morphology and Analytical Characterization of Cd0.25Zn0.75Se/ZnS (1–5 MLs) QDs
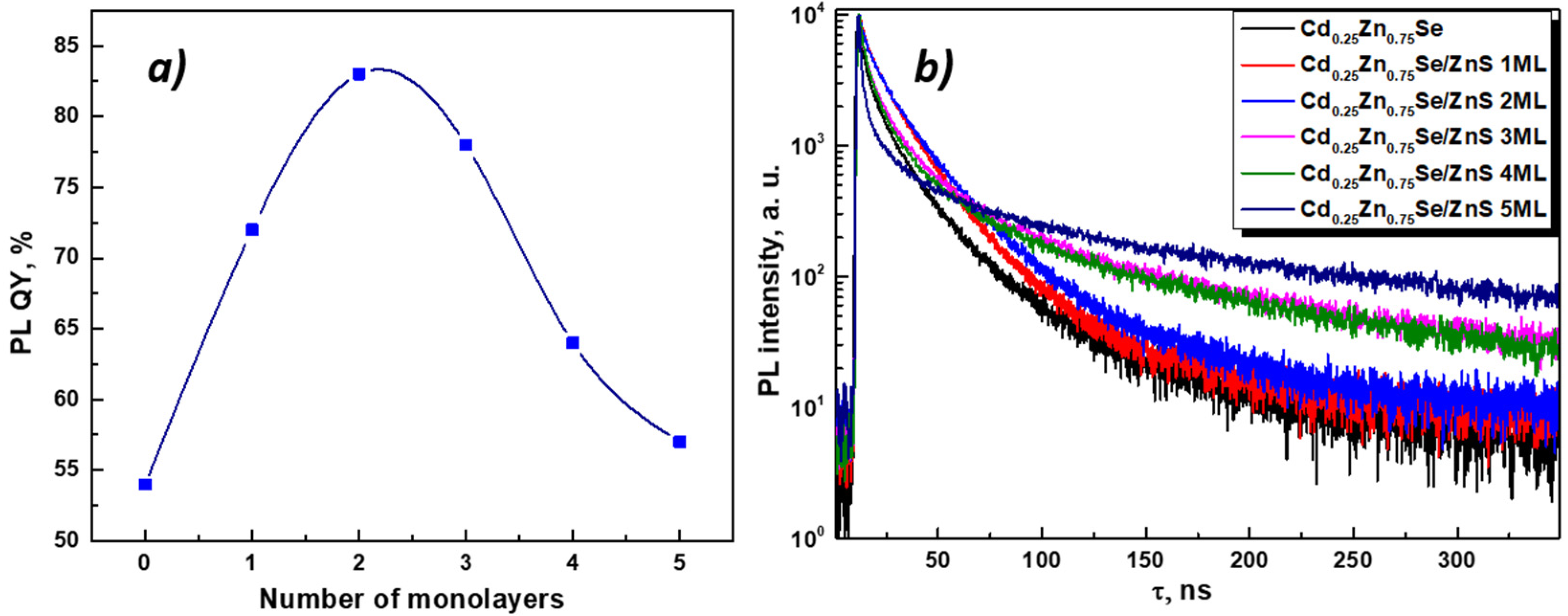
3.3. Optical Properties of Cd0.25Zn0.75Se/ZnS (1–5 MLs) QDs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Alivisatos, A.P. Semiconductor Clusters, Nanocrystals, and Quantum Dots. Science 1996, 271, 933–937. [Google Scholar] [CrossRef] [Green Version]
- Pietryga, J.M.; Park, Y.-S.; Lim, J.; Fidler, A.F.; Bae, W.K.; Brovelli, S.; Klimov, V.I. Spectroscopic and Device Aspects of Nanocrystal Quantum Dots. Chem. Rev. 2016, 116, 10513–10622. [Google Scholar] [CrossRef]
- Rabouw, F.T.; de Mello Donega, C. Excited-State Dynamics in Colloidal Semiconductor Nanocrystals. Top. Curr. Chem. 2016, 374, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, C.-Y.; Yang, H. Development of Colloidal Quantum Dots for Electrically Driven Light-Emitting Devices. J. Korean Ceram. Soc. 2017, 54, 449–469. [Google Scholar] [CrossRef] [Green Version]
- Chandrasekhar, S.; Martinuzzi, S.; Nataren, F.Z. Improved efficiency of CdZnS thin-film solar cells. Can. J. Phys. 1985, 63, 716–718. [Google Scholar] [CrossRef]
- Cao, D.; Yang, G. Quantum dot/polymer nanocomposite monolith for radiation detection. Mater. Today Commun. 2020, 24, 101246. [Google Scholar] [CrossRef]
- Liu, C.; Li, Z.; Hajagos, T.J.; Kishpaugh, D.; Chen, D.Y.; Pei, Q. Transparent Ultra-High-Loading Quantum Dot/Polymer Nanocomposite Monolith for Gamma Scintillation. ACS Nano 2017, 11, 6422–6430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, S.S.; Rondinone, A.J.; Pawel, M.D.; Dai, S. Ternary Cadmium Sulphide Selenide Quantum Dots as New Scintillation Materials. Mater. Technol. 2008, 23, 94–99. [Google Scholar] [CrossRef]
- Huang, W.; Ge, S.; Lei, Y.; Zheng, Z. Composition-dependent perfect band gap tuning of ZnS1−x Sex solid solutions for efficient photocatalysis. J. Phys. Chem. Solids 2019, 130, 41–45. [Google Scholar] [CrossRef]
- Eskandari, P.; Kazemi, F.; Zand, Z. Photocatalytic reduction of aromatic nitro compounds using CdS nanostructure under blue LED irradiation. J. Photochem. Photobiol. A Chem. 2014, 274, 7–12. [Google Scholar] [CrossRef]
- Bernt, C.M.; Burks, P.T.; DeMartino, A.W.; Pierri, A.E.; Levy, E.S.; Zigler, D.F.; Ford, P.C. Photocatalytic Carbon Disulfide Production via Charge Transfer Quenching of Quantum Dots. J. Am. Chem. Soc. 2014, 136, 2192–2195. [Google Scholar] [CrossRef] [PubMed]
- Yong, K.T.; Roy, I.; Ding, H.; Bergey, E.J.; Prasad, P.N. Biocompatible Near-Infrared Quantum Dots as Ultrasensitive Probes for Long-Term in Vivo Imaging Applications. Small 2009, 5, 1997–2004. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Feng, Y.; Zhang, Y.; Gu, Z.; Zou, L. A Facile Route to Violet- to Orange-Emitting CdxZn1− xSe Alloy Nanocrystals via Cation Exchange Reaction. Nanotechnology 2007, 18, 385606. [Google Scholar] [CrossRef]
- Liu, F.-C.; Cheng, T.-L.; Shen, C.-C.; Tseng, W.-L.; Chiang, M.Y. Synthesis of Cysteine-Capped ZnxCd1-xSe Alloyed Quantum Dots Emitting in the Blue−Green Spectral Range. Langmuir 2008, 24, 2162–2167. [Google Scholar] [CrossRef]
- Loghina, L.; Iakovleva, A.; Chylii, M.; Svec, P.; Houdek, J.; Slang, S.; Palka, K.; Michalicka, J.; Vlcek, M. Synthetic Development in Cd–Zn–Se Quantum Dots Chemistry. Opt. Mater. 2019, 97, 109385. [Google Scholar] [CrossRef]
- Shang, Y.; Ning, Z. Colloidal Quantum-Dots Surface and Device Structure Engineering for High-Performance Light-Emitting Diodes. Natl. Sci. Rev. 2017, 4, 170–183. [Google Scholar] [CrossRef]
- Xu, S.; Shen, H.; Zhou, C.; Yuan, H.; Liu, C.; Wang, H.; Ma, L.; Li, L.S. Effect of Shell Thickness on the Optical Properties in CdSe/CdS/Zn0.5Cd0.5S/ZnS and CdSe/CdS/ZnxCd1–XS/ZnS Core/Multishell Nanocrystals. J. Phys. Chem. C 2011, 115, 20876–20881. [Google Scholar] [CrossRef]
- Xie, H.Y.; Liang, J.G.; Liu, Y.; Zhang, Z.L.; Pang, D.W.; He, Z.K.; Lu, Z.X.; Huang, W.H. Preparation and Characterization of Overcoated II-VI Quantum Dots. J. Nanosci. Nanotechnol. 2005, 5, 880–886. [Google Scholar] [CrossRef]
- Chaudhuri, R.G.; Paria, S. Core/Shell Nanoparticles: Classes, Properties, Synthesis Mechanisms, Characterization, and Applications. Chem. Rev. 2011, 112, 2373–2433. [Google Scholar] [CrossRef]
- Huang, C.-H.; Yang, C.-H.; Shieh, Y.-T.; Wang, T.-L. Synthesis and Properties of Alloyed CdxZn1−xSe Core and Manganese-Doped CdxZn1−xSe/ZnS Core/Shell Nanocrystals. J. Alloys Compd. 2018, 748, 265–272. [Google Scholar] [CrossRef]
- Yang, H.; Holloway, P.H. Enhanced Photoluminescence from CdS:Mn/ZnS Core/Shell Quantum Dots. Appl. Phys. Lett. 2003, 82, 1965–1967. [Google Scholar] [CrossRef]
- Qin, W.; Shah, R.A.; Guyot-Sionnest, P. CdSeS/ZnS Alloyed Nanocrystal Lifetime and Blinking Studies under Electrochemical Control. ACS Nano 2012, 6, 912–918. [Google Scholar] [CrossRef]
- Rana, M.; Jain, A.; Rani, V.; Chowdhury, P. Glutathione Capped Core/Shell CdSeS/ZnS Quantum Dots as a Medical Imaging Tool for Cancer Cells. Inorg. Chem. Commun. 2020, 112, 107723. [Google Scholar] [CrossRef]
- Jin, X.; Xie, K.; Zhang, T.; Lian, H.; Zhang, Z.; Xu, B.; Li, D.; Li, Q. Cation Exchange Assisted Synthesis of ZnCdSe/ZnSe Quantum Dots with Narrow Emission Line Widths and near-Unity Photoluminescence Quantum Yields. Chem. Commun. 2020, 56, 6130–6133. [Google Scholar] [CrossRef]
- Li, Z.; Chen, F.; Wang, L.; Shen, H.; Guo, L.; Kuang, Y.; Wang, H.; Li, N.; Li, L.S. Synthesis and Evaluation of Ideal Core/Shell Quantum Dots with Precisely Controlled Shell Growth: Nonblinking, Single Photoluminescence Decay Channel, and Suppressed FRET. Chem. Mater. 2018, 30, 3668–3676. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, X.; Yang, P.; Bai, J.; Chang, C.; Jin, X.; Zhao, F.; Huang, Y.; Li, F. Bright Alloy CdZnSe/ZnSe QDs with Nonquenching Photoluminescence at High Temperature and Their Application to Light-Emitting Diodes. J. Nanomater. 2019, 2019, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Hung, L.X.; Thang, P.N.; Van Nong, H.; Yen, N.H.; Chinh, V.Đ.; Van Vu, L.; Hien, N.T.T.; de Marcillac, W.D.; Hong, P.N.; Loan, N.T.; et al. Synthesis, Structural and Optical Characterization of CdTeSe/ZnSe and CdTeSe/ZnTe Core/Shell Ternary Quantum Dots for Potential Application in Solar Cells. J. Electron. Mater. 2016, 45, 4425–4431. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.; Singhal, A.; Zheng, J.; Wang, C.; Chan, W.C.W. Optimizing the Synthesis of Red- to near-IR-Emitting CdS-Capped CdTexSe1-x Alloyed Quantum Dots for Biomedical Imaging. Chem. Mater. 2006, 18, 4845–4854. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, T.; Lin, X.; Yang, H.; Jin, X.; Huang, Z.; Zhang, Z.; Li, D.; Li, Q. One Pot Synthesis of Thick Shell Blue Emitting CdZnS/ZnS Quantum Dots with Narrow Emission Line Width. Opt. Mater. Express 2020, 10, 1232. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, S.; Kim, H.S.; Shin, J.B.; Choi, W.; Cho, H.; Kim, K.; Lee, T.; Kim, J.; Kang, I.B.; et al. Highly Luminescent Blue-Emitting CdZnS/ZnS Nanorods Having Electric-Field-Induced Fluorescence Switching Properties. J. Mater. Chem. C 2017, 5, 2098–2106. [Google Scholar] [CrossRef]
- Fitzmorris, B.C.; Pu, Y.C.; Cooper, J.K.; Lin, Y.F.; Hsu, Y.J.; Li, Y.; Zhang, J.Z. Optical Properties and Exciton Dynamics of Alloyed Core/Shell/Shell Cd1-xZnxSe/ZnSe/ZnS Quantum Dots. ACS Appl. Mater. Interfaces 2013, 5, 2893–2900. [Google Scholar] [CrossRef]
- Zhang, Q.; Nie, C.; Chang, C.; Guo, C.; Jin, X.; Qin, Y.; Li, F.; Li, Q. Highly Luminescent Red Emitting CdZnSe/ZnSe Quantum Dots Synthesis and Application for Quantum Dot Light Emitting Diodes. Opt. Mater. Express 2017, 7, 3875. [Google Scholar] [CrossRef]
- Susumu, K.; Field, L.D.; Oh, E.; Hunt, M.; Delehanty, J.B.; Palomo, V.; Dawson, P.E.; Huston, A.L.; Medintz, I.L. Purple-, Blue-, and Green-Emitting Multishell Alloyed Quantum Dots: Synthesis, Characterization, and Application for Ratiometric Extracellular PH Sensing. Chem. Mater. 2017, 29, 7330–7344. [Google Scholar] [CrossRef]
- Iakovleva, A.; Loghina, L.; Zmrhalova, Z.O.; Mistrik, J.; Svec, P.; Slang, S.; Palka, K.; Vlcek, M. Environmentally friendly approach to the synthesis of monodisperse and bright blue emitting Cd0.15Zn0.85S quantum dots. J. Alloy. Compd. 2019, 812, 152159. [Google Scholar] [CrossRef]
- Kaderavkova, A.; Loghina, L.; Chylii, M.; Slang, S.; Placek, P.; Frumarova, B.; Vlcek, M. N,N′,N′-Trisubstituted Thiourea as a Novel Sulfur Source for the Synthesis of Mn-Doped ZnS QDs. J. Alloys Compd. 2020, 831, 154814. [Google Scholar] [CrossRef]
- Loghina, L.; Grinco, M.; Iakovleva, A.; Slang, S.; Palka, K.; Vlcek, M. Mechanistic Investigation of the Sulfur Precursor Evolution in the Synthesis of Highly Photoluminescent Cd0.15Zn0.85S Quantum Dots. New J. Chem. 2018, 42, 14779–14788. [Google Scholar] [CrossRef]
- Aharoni, A.; Mokari, T.; Popov, I.; Banin, U. Synthesis of InAs/CdSe/ZnSe Core/Shell1/Shell2 Structures with Bright and Stable Near-Infrared Fluorescence. J. Am. Chem. Soc. 2006, 128, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Wang, S.; Wang, H.; Niu, J.; Qian, L.; Yang, Y.; Titov, A.; Hyvonen, J.; Zheng, Y.; Li, L.S. Highly Efficient Blue–Green Quantum Dot Light-Emitting Diodes Using Stable Low-Cadmium Quaternary-Alloy ZnCdSSe/ZnS Core/Shell Nanocrystals. ACS Appl. Mater. Interfaces 2013, 5, 4260–4265. [Google Scholar] [CrossRef] [PubMed]
- Ospina, R.; Rincón-Ortiz, S.A.; Rodriguez-Pereira, J. Cadmium Selenide by XPS. Surf. Sci. Spectra 2020, 27, 014021. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, F.; Zhao, R.; Huai, X.; Han, J.; Wang, L. Aqueous Synthesis of Core/Shell/Shell CdSe/CdS/ZnS Quantum Dots for Photocatalytic Hydrogen Generation. J. Mater. Sci. 2019, 54, 8571–8580. [Google Scholar] [CrossRef]
- Zimdars, J.; Pilger, J.; Entrup, M.; Deiting, D.; Schäfer, A.H.; Bredol, M. A Facile Synthesis of Alloyed Mn-Doped ZnSeS Nanoparticles Using a Modified Selenium/Sulfur Precursor in a One-Pot Approach. New J. Chem. 2016, 40, 8465–8470. [Google Scholar] [CrossRef]
- Barreca, D.; Gasparotto, A.; Maragno, C.; Tondello, E.; Spalding, T.R. Analysis of Nanocrystalline ZnS Thin Films by XPS. Surf. Sci. Spectra 2002, 9, 54–61. [Google Scholar] [CrossRef]
- La Porta, F.A.; Ferrer, M.M.; de Santana, Y.V.B.; Raubach, C.W.; Longo, V.M.; Sambrano, J.R.; Longo, E.; Andrés, J.; Li, M.S.; Varela, J.A. Synthesis of Wurtzite ZnS Nanoparticles Using the Microwave Assisted Solvothermal Method. J. Alloy. Compd. 2013, 556, 153–159. [Google Scholar] [CrossRef]
- Alonso, R.G.; Suh, E.-K.; Ramdas, A.K.; Samarth, N.; Luo, H.; Furdyna, J.K. Raman Spectroscopy of Two Novel Semiconductors and Related Superlattices: Cubic Cubic Cd1−xMnxSe and Cd1−xZnxSe. Phys. Rev. B 1989, 40, 3720–3728. [Google Scholar] [CrossRef] [PubMed]
- Valakh, M.Y.; Lisitsa, M.P.; Pekar, G.S.; Polysskii, G.N.; Sidorenko, V.I.; Yaremko, A.M. Anharmonic Coupling of Phonon Modes in Mixed ZnxCd1−xSe Crystals. Phys. Status Solidi 1982, 113, 635–645. [Google Scholar] [CrossRef]
- Nilsen, W.G. Raman Spectrum of Cubic ZnS. Phys. Rev. 1969, 182, 838–850. [Google Scholar] [CrossRef]
- Vodopyanov, L.K.; Vinogradov, E.A.; Vinogradov, V.S.; Kucherenko, I.V.; Mavrin, B.N.; Novikova, N.N.; Shapkin, P.V. Optical Phonons in Zn1–xCdxSe Alloys. Phys. Status Solidi 2004, 1, 3162–3165. [Google Scholar] [CrossRef]
- Srivastava, S. Infrared Active Two-Phonon Processes in Cubic ZnS. Aust. J. Phys. 1973, 26, 111. [Google Scholar] [CrossRef] [Green Version]
- Klein, C.A.; Donadio, R.N. Infrared-active Phonons in Cubic Zinc Sulfide. J. Appl. Phys. 1980, 51, 797–800. [Google Scholar] [CrossRef]
- Yadav, A.N.; Singh, A.K.; Singh, K. Synthesis, Properties, and Applications of II–VI Semiconductor Core/Shell Quantum Dots. In Core/Shell Quantum Dots; Springer: Cham, Switzerland, 2020; pp. 1–28. [Google Scholar] [CrossRef]
- Tauc, J.; Menth, A. States in the gap. J. Non-Cryst. Solids 1972, 8–10, 569–585. [Google Scholar] [CrossRef]
- Gaikwad, A.P.; Tyagi, D.; Betty, C.A.; Sasikala, R. Photocatalytic and Photo Electrochemical Properties of Cadmium Zinc Sulfide Solid Solution in the Presence of Pt and RuS2 Dual Co-Catalysts. Appl. Catal. A Gen. 2016, 517, 91–99. [Google Scholar] [CrossRef]
- Wojtowicz, A.J.; Glodo, J.; Drozdowski, W.; Przegietka, K.R. Electron Traps and Scintillation Mechanism in YAlO3:Ce and LuAlO3:Ce Scintillators. J. Lumin. 1998, 79, 275–291. [Google Scholar] [CrossRef]
- Morgan, D.P.; Kelley, D.F. Exciton Localization and Radiative Lifetimes in CdSe Nanoplatelets. J. Phys. Chem. C 2019, 123, 18665–18675. [Google Scholar] [CrossRef]

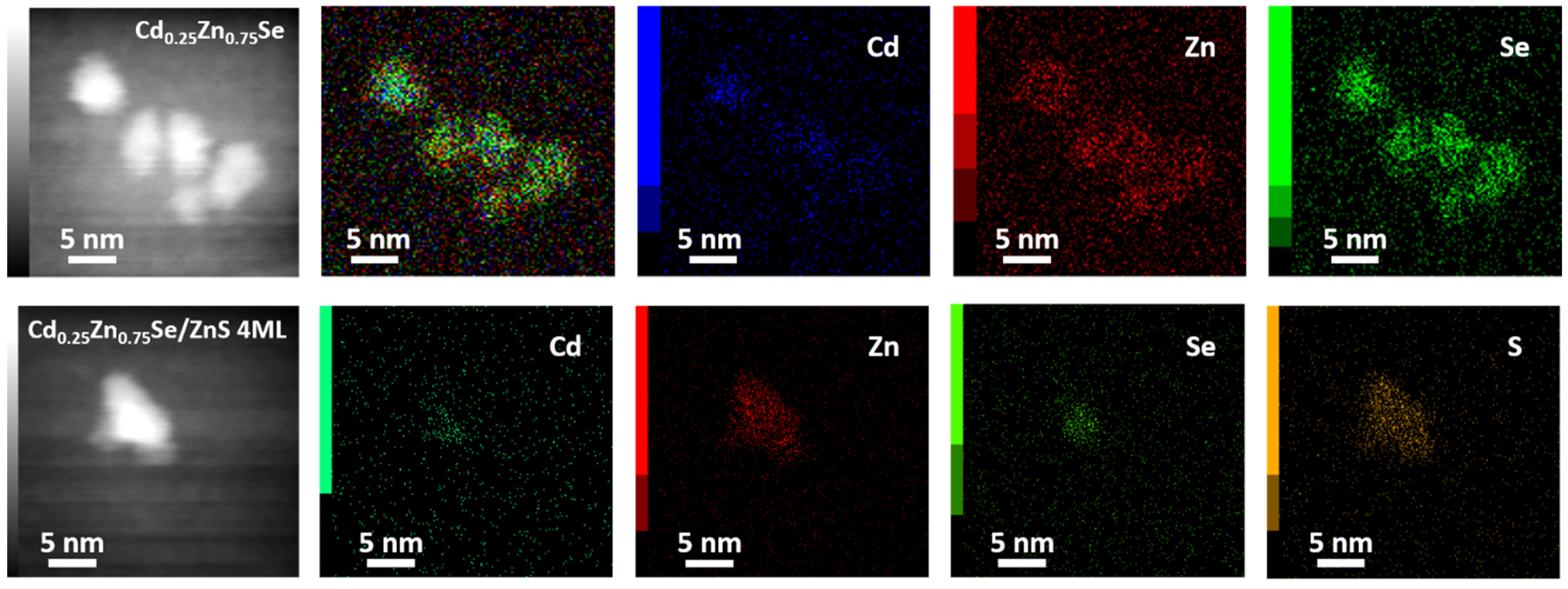
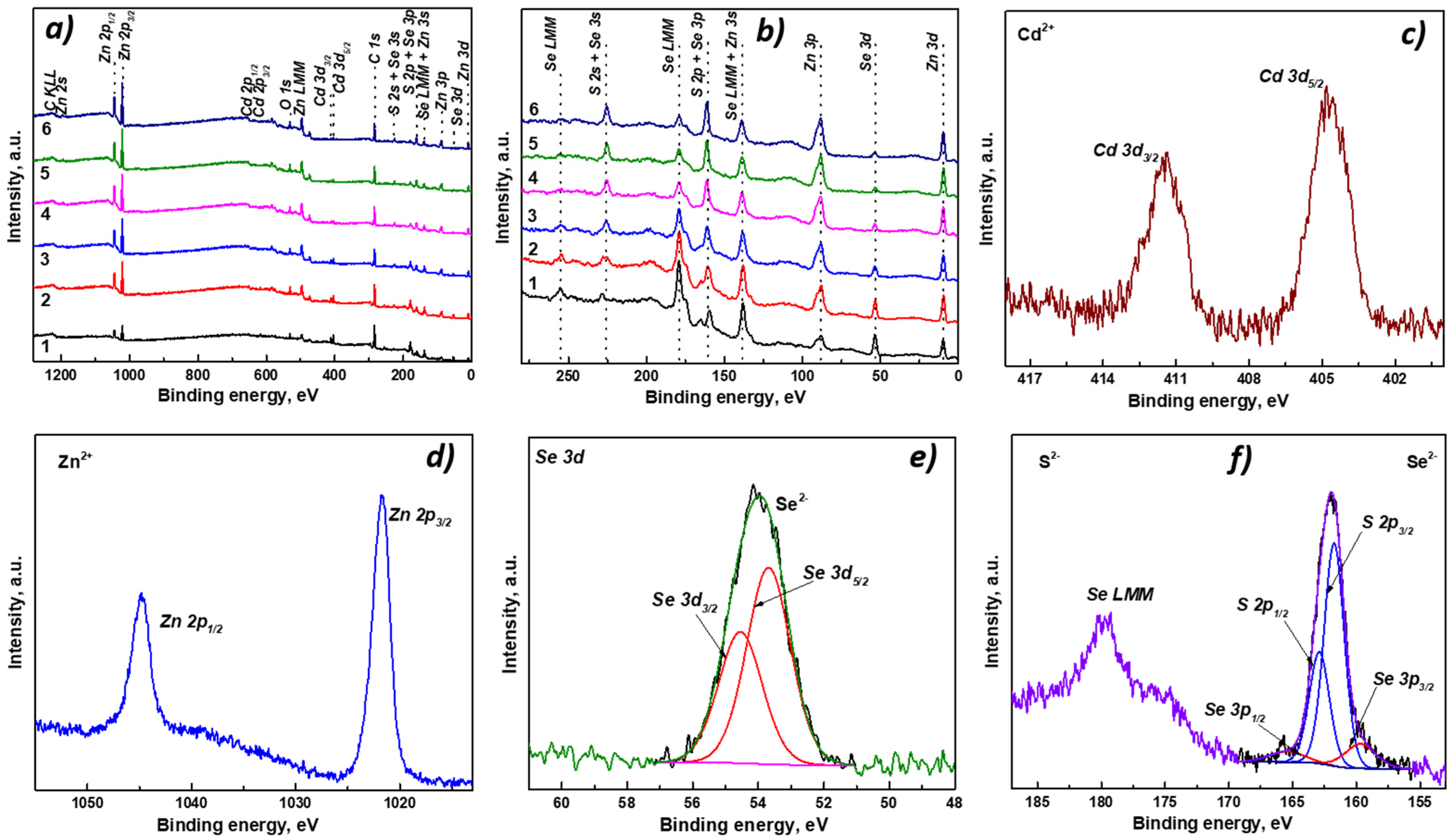
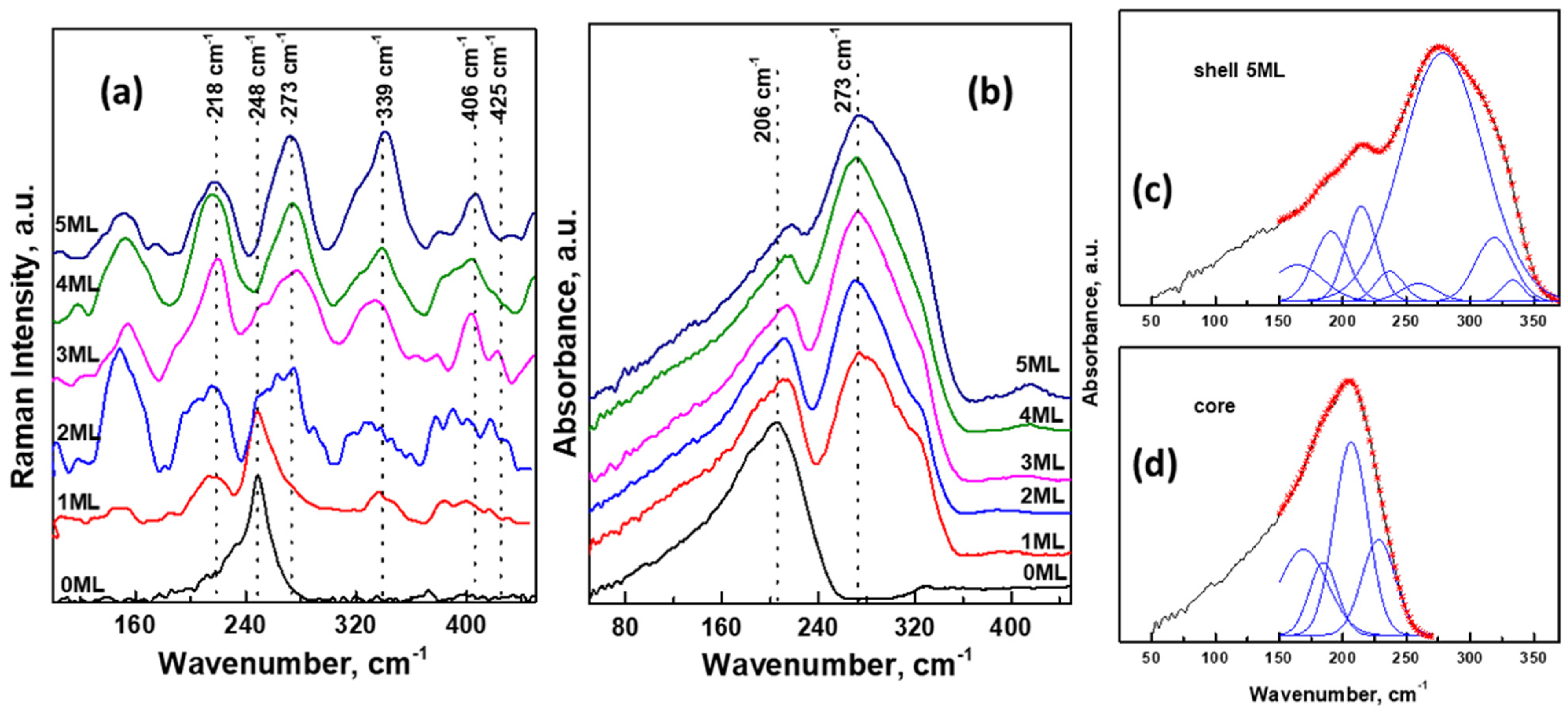

| Composition | EDS | XPS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cd, at.% | Zn, at.% | Se, at.% | S, at.% | Cd, at.% | Zn, at.% | Se, at.% | S, at.% | ||
| Cd0.25Zn0.75Se | 14.7 | 41.2 | 44.1 | - | 16.3 | 48.2 | 35.6 | - | |
| Cd0.25Zn0.75Se/ZnS 1 ML | 7.4 | 48.1 | 23.6 | 20.9 | 7.5 | 53.4 | 15.5 | 23.7 | |
| Cd0.25Zn0.75Se/ZnS 2 MLs | 5.8 | 48.5 | 15.9 | 29.8 | 4.3 | 54.5 | 10.4 | 30.9 | |
| Cd0.25Zn0.75Se/ZnS 3 MLs | 3.5 | 51.2 | 11.1 | 34.2 | 2.4 | 55.2 | 6.1 | 36.8 | |
| Cd0.25Zn0.75Se/ZnS 4 MLs | 2.5 | 52.3 | 8.1 | 37.1 | 1.6 | 55.9 | 4.0 | 38.5 | |
| Cd0.25Zn0.75Se/ZnS 5 MLs | 2.1 | 52.7 | 6.6 | 38.6 | 1.2 | 55.6 | 3.6 | 39.7 | |
| Number of Monolayers | Optical Parameters | Fitting Results of the PL Decay Curves | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| λexc, nm | λem, nm | QY,% | Eg, eV | τ1, ns | τ2, ns | τ3, ns | A1 | A1 | A1 | τavg, ns | ||
| 0 | 484 | 569 | 54 | 2.96 | 1.3 | 11.4 | 44.4 | 0.72 | 0.25 | 0.03 | 4.95 | |
| 1 | 474 | 571 | 72 | 3.59 | 1.4 | 11.0 | 26.8 | 0.42 | 0.45 | 0.13 | 9.11 | |
| 2 | 464 | 574 | 83 | 3.58 | 1.6 | 11.6 | 30.3 | 0.46 | 0.40 | 0.14 | 9.56 | |
| 3 | 393 | 568 | 78 | 3.08 | 2.1 | 14.1 | 69.9 | 0.64 | 0.34 | 0.02 | 7.31 | |
| 4 | 391 | 563 | 64 | 3.69 | 1.9 | 13.8 | 63.3 | 0.67 | 0.32 | 0.01 | 6.61 | |
| 5 | 358 | 507 | 57 | 3.55 | 1.7 | 13.1 | 86.1 | 0.71 | 0.28 | 0.01 | 5.80 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loghina, L.; Chylii, M.; Kaderavkova, A.; Slang, S.; Svec, P.; Rodriguez Pereira, J.; Frumarova, B.; Cieslar, M.; Vlcek, M. Highly Efficient and Controllable Methodology of the Cd0.25Zn0.75Se/ZnS Core/Shell Quantum Dots Synthesis. Nanomaterials 2021, 11, 2616. https://doi.org/10.3390/nano11102616
Loghina L, Chylii M, Kaderavkova A, Slang S, Svec P, Rodriguez Pereira J, Frumarova B, Cieslar M, Vlcek M. Highly Efficient and Controllable Methodology of the Cd0.25Zn0.75Se/ZnS Core/Shell Quantum Dots Synthesis. Nanomaterials. 2021; 11(10):2616. https://doi.org/10.3390/nano11102616
Chicago/Turabian StyleLoghina, Liudmila, Maksym Chylii, Anastasia Kaderavkova, Stanislav Slang, Petr Svec, Jhonatan Rodriguez Pereira, Bozena Frumarova, Miroslav Cieslar, and Miroslav Vlcek. 2021. "Highly Efficient and Controllable Methodology of the Cd0.25Zn0.75Se/ZnS Core/Shell Quantum Dots Synthesis" Nanomaterials 11, no. 10: 2616. https://doi.org/10.3390/nano11102616