Photophysical and Electro-Optical Properties of Copolymers Bearing Blue and Red Chromophores for Single-Layer White OLEDs
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Synthetic Procedure
2.2. Ink Formulation
2.3. OLED Fabrication
2.4. Thin Film and Device Characterization
3. Results and Discussion
3.1. Copolymer Synthesis
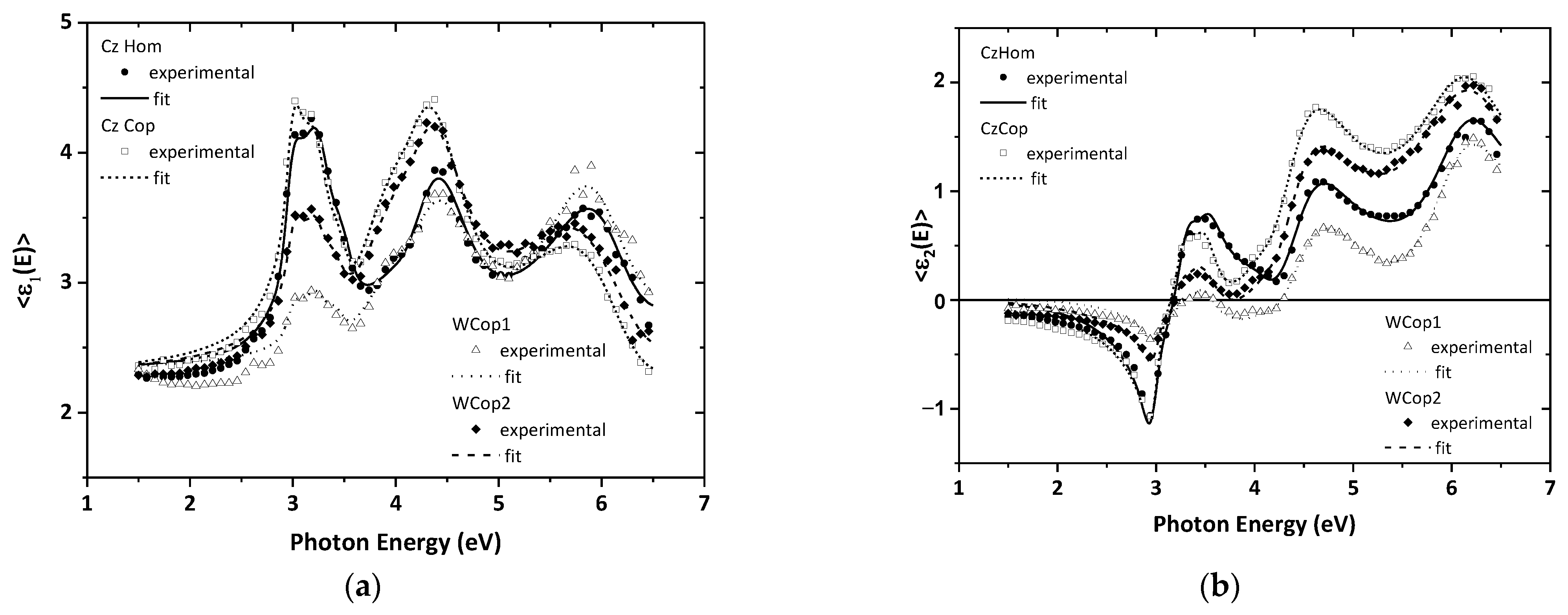
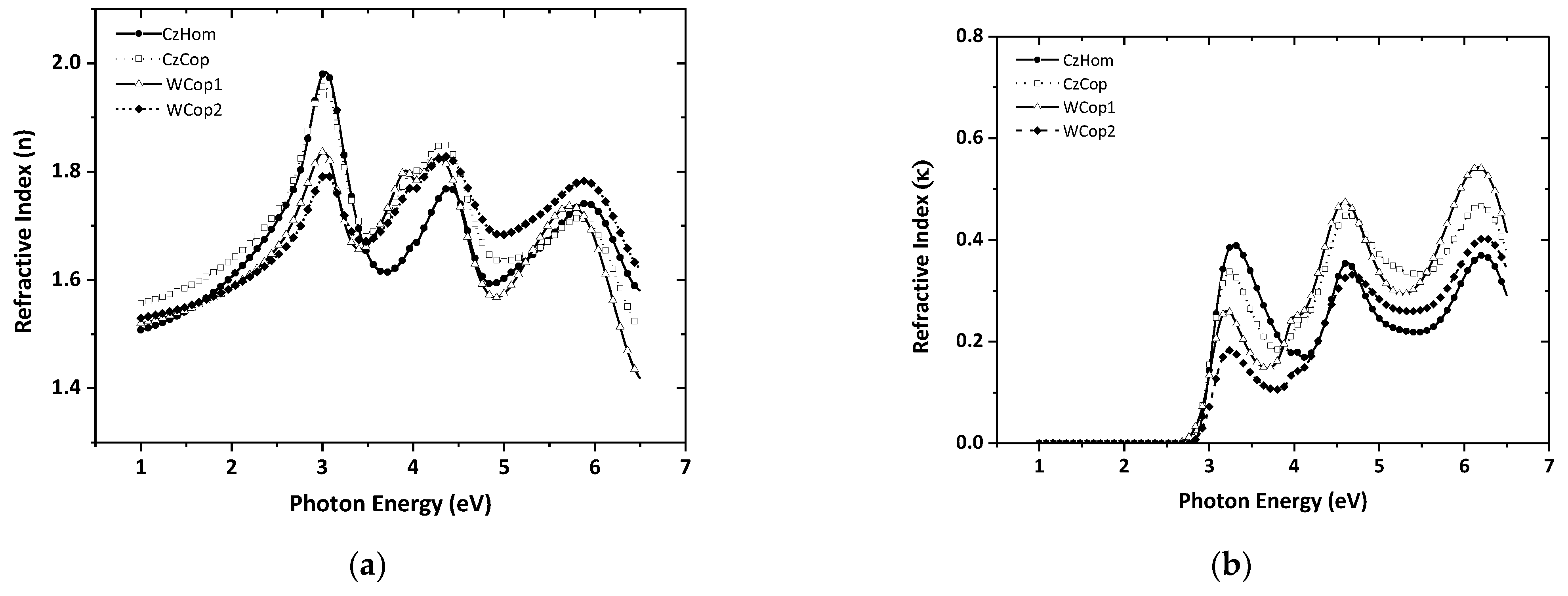
3.2. Spectroscopic Ellipsometry
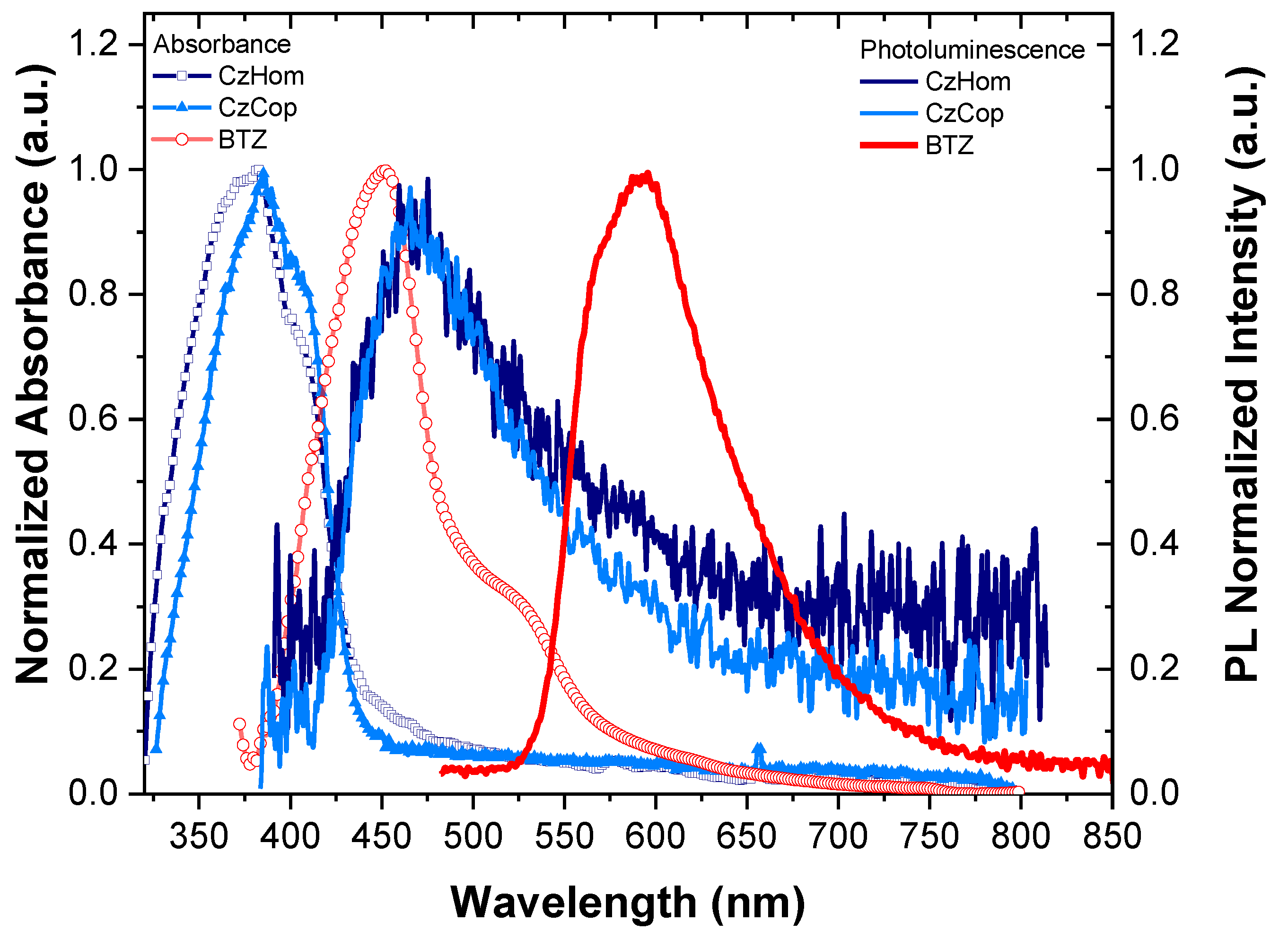
3.3. Absorption and Photoluminescence
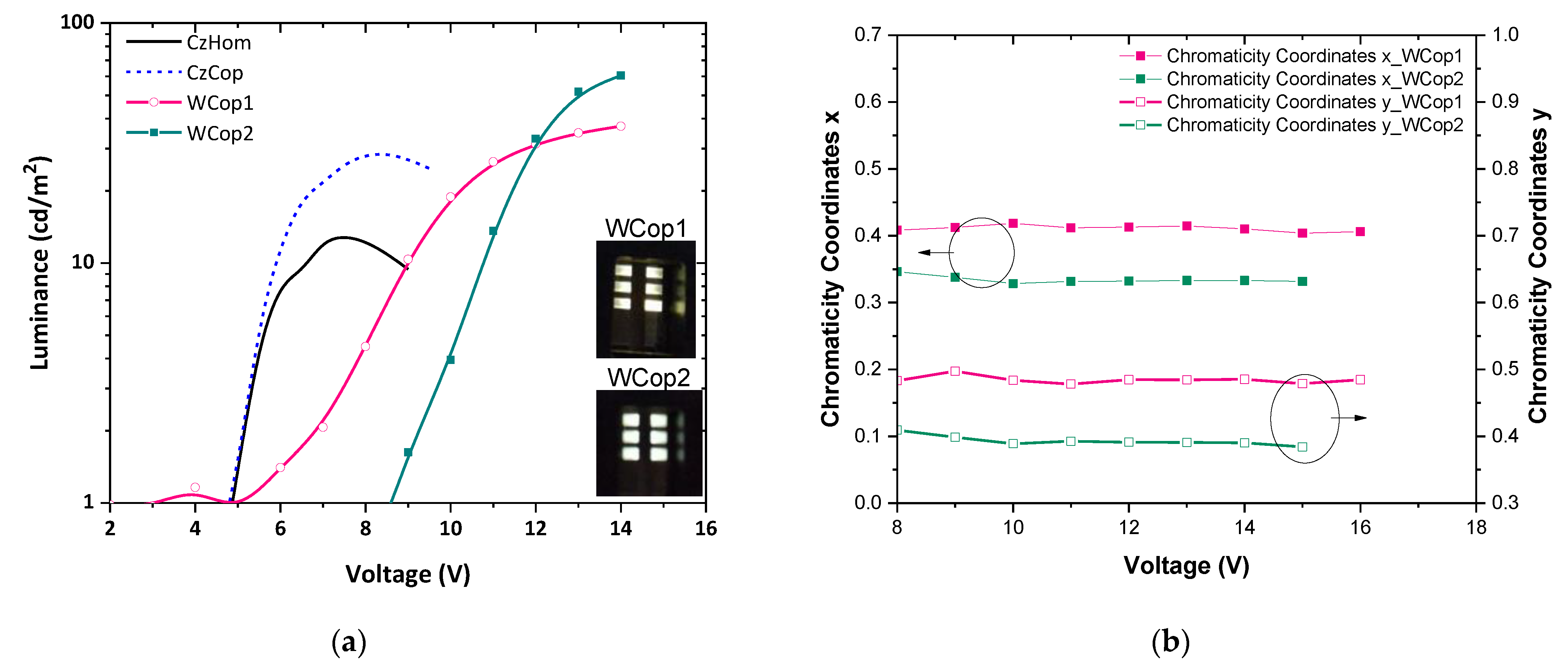
3.4. Electroluminescence
3.5. Electrical Characteristics of OLED Devices
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zou, S.J.; Shen, Y.; Xie, F.M.; Chen, J.D.; Li, Y.Q.; Tang, J.X. Recent advances in organic light-emitting diodes: Toward smart lighting and displays. Mater. Chem. Front. 2020, 4, 788–820. [Google Scholar] [CrossRef]
- Misra, A.; Kumar, P.; Kamalasanan, M.N.; Chandra, S. White organic LEDs and their recent advancements. Semicond. Sci. Technol. 2006, 21, 35–47. [Google Scholar] [CrossRef]
- Pode, R. Organic light emitting diode devices: An energy efficient solid state lighting for applications. Renew. Sustain. Energy Rev. 2020, 133, 110043. [Google Scholar] [CrossRef]
- Andreopoulou, A.K.; Gioti, M.; Kallitsis, J.K. Organic Light-emitting Diodes Based on Solution-Processable Organic Materials. In Solution-Processable Components for Organic Electronic Devices, 1st ed.; Łuszczynska, B., Matyjaszewski, K., Ulanski, J., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, German, 2019; Volume 8, pp. 413–482. [Google Scholar] [CrossRef]
- Reineke, S.; Thomschke, M.; Lussem, B.; Leo, K. White organic light-emitting diodes: Status and perspective. Rev. Mod. Phys. 2013, 85, 1245–1293. [Google Scholar] [CrossRef] [Green Version]
- Swayamprabha, S.S.; Dubey, D.K.; Shahnawaz, R.A.K.Y.; Nagar, M.R.; Sharma, A.; Tung, F.; Jou, J.H. Approaches for Long Lifetime Organic Light Emitting Diodes. Adv. Sci. 2021, 8, 2002254–2002283. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.H.; Hu, T.Q.; Zhou, Z.; Yang, M.; Quan, M.H.; Mei, Q.B.; Zhai, B.C.; Jia, Z.H.; Laia, W.Y.; Huang, W. Solution processed single-emission layer white polymer light-emitting diodes with high color quality and high performance from a poly(N-vinyl)carbazole host. Phys. Chem. Chem. Phys. 2015, 17, 8860–8869. [Google Scholar] [CrossRef]
- Duarte, L.G.T.A.; Germino, J.C.; Berbigier, J.F.; Barboza, C.A.; Faleiros, M.M.; Simoni, D.A.; Galante, M.T.; Holanda, M.S.; Rodembusch, F.S.; Atvars, T.D.Z. White-light generation from all-solution-processed OLEDs using a benzothiazole–salophen derivative reactive to the ESIPT process. Phys. Chem. Chem. Phys. 2019, 21, 1172–1182. [Google Scholar] [CrossRef]
- Gioti, M.; Kokkinos, D.; Chaidou, C.I.; Laskarakis, A.; Andreopoulou, A.K.; Kallitsis, J.K.; Logothetidis, S. A comprehensive study of the optical properties of emitting polymers for efficient flexible OLED devices. Phys. Status Solidi A 2016, 213, 2947–2953. [Google Scholar] [CrossRef]
- Gioti, M.; Kokkinos, D.; Stavrou, K.; Simitzi, K.; Andreopoulou, A.K.; Laskarakis, A.; Kallitsis, J.K.; Logothetidis, S. Fabrication and study of white-light OLEDs based on novel copolymers with blue, yellow, and red chromophores. Phys. Status Solidi RRL 2019, 1800419–1800424. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Y.; Tsuboi, T.; Xu, H.; Wua, Y.; Zhang, Z.; Miao, Y.; Hao, Y.; Liu, X.; Xu, B.; et al. Energy transfer in polyfluorene copolymer used for white-light organic light emitting device. Org. Electron. 2013, 14, 827–838. [Google Scholar] [CrossRef]
- Liu, J.; Guo, X.; Bu, L.; Xie, Z.; Cheng, Y.; Wang, Y.G.L.; Jing, X.; Wang, F. White Electroluminescence from a Single-Polymer System with Simultaneous Two-Color Emission: Polyfluorene Blue Host and Side-Chain-Located Orange Dopant. Adv. Funct. Mater. 2007, 17, 1917–1925. [Google Scholar] [CrossRef]
- Deus, J.F.; Faria, G.C.; Faria, R.M.; Iamazaki, E.T.; Atvars, T.D.Z.; Cirpan, A.; Akcelrud, L. White light emitting devices by doping polyfluorene with two red emitters. J. Photochem. Photobiol. A Chem. 2013, 253, 45–51. [Google Scholar] [CrossRef]
- Ding, L.; Karasz, F.E.; Lin, Y.; Pang, Y.; Liao, L. Photoluminescence and Electroluminescence Study of Violet-Blue and Green Emitting Polymers and Their Blend. Macromolecules 2003, 36, 7301–7307. [Google Scholar] [CrossRef]
- Lewińskaa, G.; Danel, K.S.; Wisła-Świder, A.; Usatenko, Z.; Kanak, J.; Walczak, L.; Kuterba, P.; Sanetr, J.; Marszalek, K.W. Photoelectrical properties and surface examination of luminescent copolymer compounds. Appl. Surf. Sci. 2020, 533, 147366–147377. [Google Scholar] [CrossRef]
- Xu, J.; Yu, L.; Sun, Z.; Li, T.; Chen, H.; Yang, W. Efficient, stable and high color rendering index white polymer light-emitting diodes by restraining the electron trapping. Org. Electron. 2020, 84, 105785–105792. [Google Scholar] [CrossRef]
- Beljonne, D.; Pourtois, G.; Silva, C.; Hennebicq, E.; Herz, L.M.; Friend, R.H.; Scholes, G.D.; Setayeshi, S.; Mullen, K.; Bredas, J.L. Interchain vs. intrachain energy transfer in acceptor-capped conjugated polymers. Proc. Natl. Acad. Sci. USA 2002, 99, 10982–10987. [Google Scholar] [CrossRef] [Green Version]
- Gioti, M.; Papadopoulos, K.; Kyriazopoulos, V.; Andreopoulou, A.K.; Kallitsis, J.K.; Laskarakis, A.; Logothetidis, S. Optical and emission properties of terpolymer active materials for white OLEDs (WOLEDs). Mater. Today Proc. 2021, 37, 141101. [Google Scholar] [CrossRef]
- Gourdoupi, N.; Andreopoulou, A.K.; Deimede, V.; Kallitsis, J.K. Novel Proton-Conducting Polyelectrolyte Composed of an Aromatic Polyether Containing Main-Chain Pyridine Units for Fuel Cell Applications. Chem. Mater. 2003, 15, 5044–5050. [Google Scholar] [CrossRef]
- Konstandakopoulou, F.D.; Kallitsis, J.K. Soluble rigid–flexible polyethers containing bis(biphenyl)anthracene or bis(styryl)anthracene units in the main chain for light-emitting applications. J. Polym. Sci. Polym. Chem. 1999, 37, 3826–3837. [Google Scholar] [CrossRef]
- Kallitsis, J.K.; Andreopoulou, A.K.; Daletou, M.; Neophytides, S. Pyridine Containing Aromatic Polyether Membranes. In High Temperature Polymer Electrolyte Membrane Fuel Cells, 1st ed.; Li, Q., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 91–126. [Google Scholar] [CrossRef]
- Li, Q.; Aili, D.; Hjuler, H.; Jensen, J. Durability Issues and Status of PBI-Based Fuel Cells. In High Temperature Polymer Electrolyte Membrane Fuel Cells; Li, Q., Aili, D., Hjuler, H., Jensen, J., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 487–509. [Google Scholar] [CrossRef] [Green Version]
- Sasabe, H.; Seino, Y.; Kimura, M.; Kido, J. A m-Terphenyl-Modifed Sulfone Derivative as a Host Material for High-Efficiency Blue and Green Phosphorescent OLEDs. Chem. Mater. 2012, 24, 1404–1406. [Google Scholar] [CrossRef]
- Jellison, G.; Modine, F. Parameterization of the optical functions of amorphous materials in the interband region. Appl. Phys. Lett. 1996, 69, 371–373. [Google Scholar] [CrossRef]
- Azzam, R.; Bashara, N. Ellipsometry and Polarized Light; North-Holland Pub: Amsterdam, The Netherlands, 1977; ISBN 0720406943. [Google Scholar]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous Germanium. Phys. Status Solidi B 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Kundu, S.; Sk, B.; Pallavi, P.; Giri, A.; Patra, A. Molecular Engineering Approaches Towards All-Organic White Light Emitting Materials. Chem. A Eur. J. 2019, 26, 5557–5582. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Sun, J.; Liao, X.; Hou, M.; Chen, W.; Li, J.; Wang, H.; Li, L. Poly(9,9-dioctylfluorene) based hyperbranched copolymers with three balanced emission colors for solution-processable hybrid white polymer light-emitting devices. Dye Pigment 2017, 139, 611–618. [Google Scholar] [CrossRef]
- Georgiadou, D.G.; Vasilopoulou, M.; Pistolis, G.; Palilis, L.; Dimotikali, D.; Argitis, P. Energy transfer processes among emitters dispersed in a single polymer layer for colour tuning in OLEDs. Phys. Status Solidi 2008, 205, 2526–2531. [Google Scholar] [CrossRef]
- Coya, C.; Álvarez, A.; Ramos, M.; Gómez, R.; Seoane, C.; Segura, J.L. Highly efficient solution-processed white organic light-emitting diodes based on novel copolymer single layer. Synth. Met. 2012, 161, 2580–2584. [Google Scholar] [CrossRef]
- Ying, L.; Ho, C.L.; Wu, H.; Cao, Y.; Wong, W.Y. White polymer light-emitting devices for solid-state lighting: Materials, devices, and recent progress. Adv. Mater. 2014, 26, 2459–2474. [Google Scholar] [CrossRef] [PubMed]
- Greiner, A. Design and synthesis of polymers for light-emitting diodes. Polym. Adv. Technol. 1998, 9, 371–389. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, J.; Cai, P.; Chen, J. Efficient Single-Layer White Light-Emitting Devices Based on Silole-Containing Polymers. J. Disp. Technol. 2013, 9, 490–496. Available online: https://www.osapublishing.org/jdt/abstract.cfm?URI=jdt-9-6-490 (accessed on 7 March 2013). [CrossRef]
- Luo, J.; Li, X.; Hou, Q.; Peng, J.; Yang, W.; Cao, Y. High-Efficiency White-Light Emission from a Single Copolymer: Fluorescent Blue, Green, and Red Chromophores on a Conjugated Polymer Backbone. Adv. Mater. 2007, 19, 1113–1117. [Google Scholar] [CrossRef]
- Azevedo, D.; Freitas, J.N.; Domingues, R.A.; Faleiros, M.M.; Atvars, T.D.Z. Correlation between the PL and EL emissions of polyfluorene-based diodes using bilayers or polymer blends. Synth. Met. 2017, 233, 28–34. [Google Scholar] [CrossRef]
- Huang, J.; Niu, Y.; Yang, W.; Mo, Y.; Yuan, M.; Cao, Y. Novel Electroluminescent Polymers Derived from Carbazole and Benzothiadiazole. Macromolecules 2002, 35, 6080–6082. [Google Scholar] [CrossRef]
- Yang, Y.; Peng, T.; Ye, K.Q.; Wu, Y.; Liu, Y.; Wang, Y. High-efficiency and high-quality white organic light-emitting diode employing fluorescent emitters. Org. Electron. 2011, 12, 29–33. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.L.; Luo, D.; Xiao, P.; Xiao, W.; Song, Y.; Ang, Q.; Liu, B. Strategies to Achieve High-Performance White Organic Light-Emitting Diodes. Materials 2017, 10, 1378. [Google Scholar] [CrossRef] [Green Version]
- Hou, Q.; Xu, Y.S.; Yang, W.; Yuan, M.; Peng, J.B.; Cao, Y. Novel redemitting fluorene-based copolymers. J. Mater. Chem. 2002, 12, 2887–2892. [Google Scholar] [CrossRef]
- Gioti, M. Optical, photophysical, and electrooptical studies on slot-die polyfluorene-based flexible OLED devices. Opt. Mater. Express 2020, 11, 1442–1456. [Google Scholar] [CrossRef]
- Anthopoulos, T.D.; Markham, J.P.J.; Namdas, E.B.; Samuel, D.W. Highly efficient single-layer dendrimer light-emitting diodes with balanced charge transport. Appl. Phys. Lett. 2003, 82, 4824–4826. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Duan, L.; Zhang, D.; Qiao, J.; Dong, G.; Wang, L.; Qiu, Y. A Pyridine-Containing Anthracene Derivative with High Electron and Hole Mobilities for Highly Efficient and Stable Fluorescent Organic Light-Emitting Diodes. Adv. Funct. Mater. 2011, 21, 1881–1886. [Google Scholar] [CrossRef]
- Kim, S.K.; Yang, H.; Kim, Y.S. Control of carrier injection and transport in quantum dot light emitting diodes (QLEDs) via modulating Schottky injection barrier and carrier mobility. J. Appl. Phys. 2019, 126, 185702. [Google Scholar] [CrossRef]
- Ciobotaru, C.C.; Polosan, S.; Ciobotaru, I.C. Electroluminescence Properties of IrQ(ppy)2 Dual-Emitter Organometallic Compound in Organic Light-Emitting Devices. J. Electron. Mater. 2018, 47, 1490–1496. [Google Scholar] [CrossRef]
- Polosan, S.; Ciobotaru, I.C.; Ciobotaru, C.C. Organometallic Coatings for Electroluminescence Applications. Coatings 2019, 10, 277. [Google Scholar] [CrossRef] [Green Version]
- Ma, D.; Hummelgen, I.A.; Jing, X.; Hong, Z.; Wang, L.; Zhao, X.; Wang, F. Charge transport in a blue-emitting alternating block copolymer with a small spacer to conjugated segment length ratio. J. Appl. Phys. 2000, 87, 312. [Google Scholar] [CrossRef]
- Haneef, H.F.; Zeidell, A.M.; Jurchescu, O.A. Charge carrier traps in organic semiconductors: A review on the underlying physics and impact on electronic devices. J. Mater. Chem. C 2019, 8, 759–787. [Google Scholar] [CrossRef]
- Wang, D.; Fina, M.; Kim, S.; Zhang, C.; Zhang, T.; Deng, Y.; Chen, K.; Liang, L.; Mao, S.S.; Minor, M.A.; et al. Trap-Assisted Charge Injection into Large Bandgap Polymer Semiconductors. Materials 2019, 12, 2427. [Google Scholar] [CrossRef] [Green Version]
- Kokil, A.; Yang, K.; Kumar, J. Techniques for characterization of charge carrier mobility in organic semiconductors. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 1130–1144. [Google Scholar] [CrossRef]
- Kim, S.K.; Kim, Y.S. Charge carrier injection and transport in QLED layer with dynamic equilibrium of trapping/de-trapping carriers. J. Appl. Phys. 2019, 126, 035704. [Google Scholar] [CrossRef]

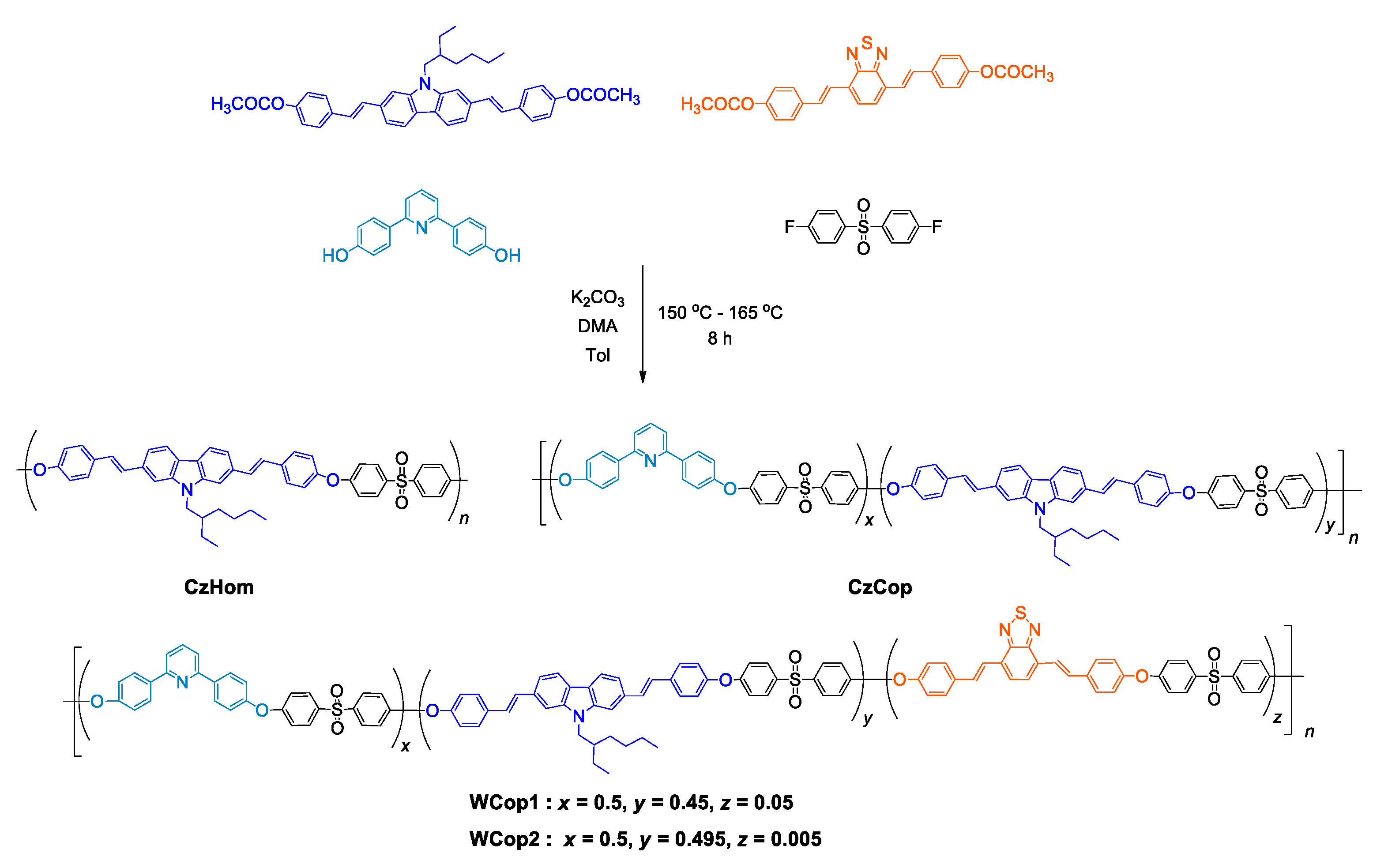




| Polymer | Co-Monomers Ratio a | ||
|---|---|---|---|
| Py (x) | Cz (y) | BTZ (z) | |
| CzHom | 0 | 1 | 0 |
| CzCop | 0.5 | 0.5 | 0 |
| WCop1 | 0.5 | 0.45 | 0.05 |
| Wcop2 | 0.5 | 0.495 | 0.005 |
| Polymer | Mp b | Mn c | PD d |
|---|---|---|---|
| CzCop | 59,300 | 34,700 | 1.50 |
| WCop1 | 74,900 | 49,800 | 1.77 |
| WCop2 | 53,100 | 34,900 | 1.62 |
| Thickness (nm) | Optical Band Gap (eV) | Electronic Transition Energy (eV) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| E01 | E02 | E03 | E04 | E05 | E06 | ||||
| CzHom | 23 | 2.78 | 3.00 | 3.05 | 4.02 | 4.57 | 5.28 | 6.20 | 9.12 |
| CzCop | 27 | 2.67 | 2.95 | 3.08 | 4.02 | 4.53 | 5.29 | 6.18 | 9.29 |
| WCop1 | 11 | 2.57 | 2.93 | 3.10 | 3.96 | 4.50 | 5.62 | 6.13 | 9.35 |
| WCop2 | 32 | 2.73 | 2.96 | 3.09 | 3.99 | 4.57 | 5.39 | 6.23 | 9.16 |
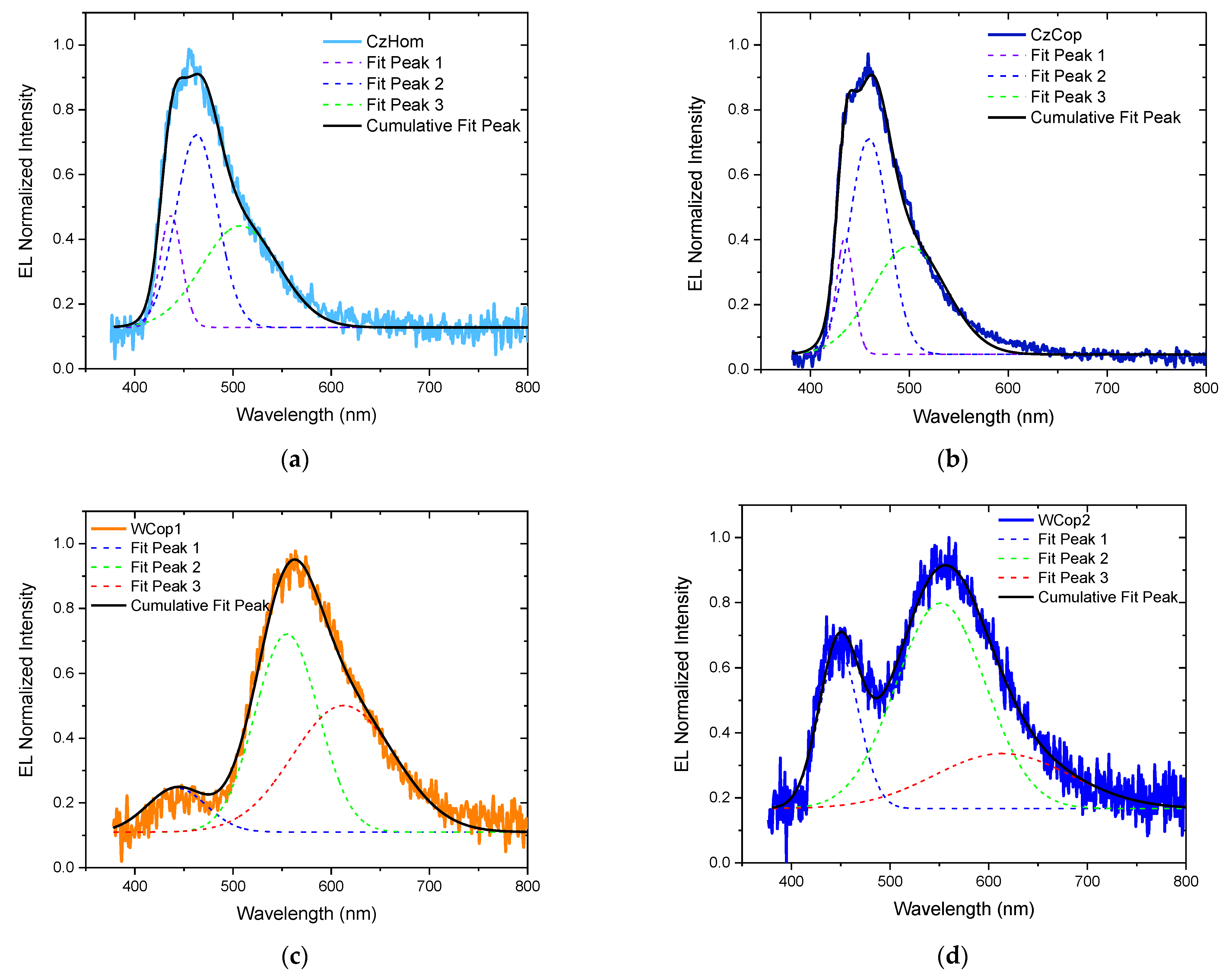
| PEAK 1 | PEAK 2 | PEAK 3 | |||||
|---|---|---|---|---|---|---|---|
| Spectrum | λmax (nm) | FWHM (nm) | λmax (nm) | FWHM (nm) | λmax (nm) | FWHM (nm) | |
| CzHom | PL | 466 | 57 | 531 | 119 | - | |
| EL | 437 | 22 | 463 | 42 | 507 | 79 | |
| CzCop | PL | 466 | 59 | 528 | 117 | - | - |
| EL | 435 | 18 | 460 | 40 | 500 | 75 | |
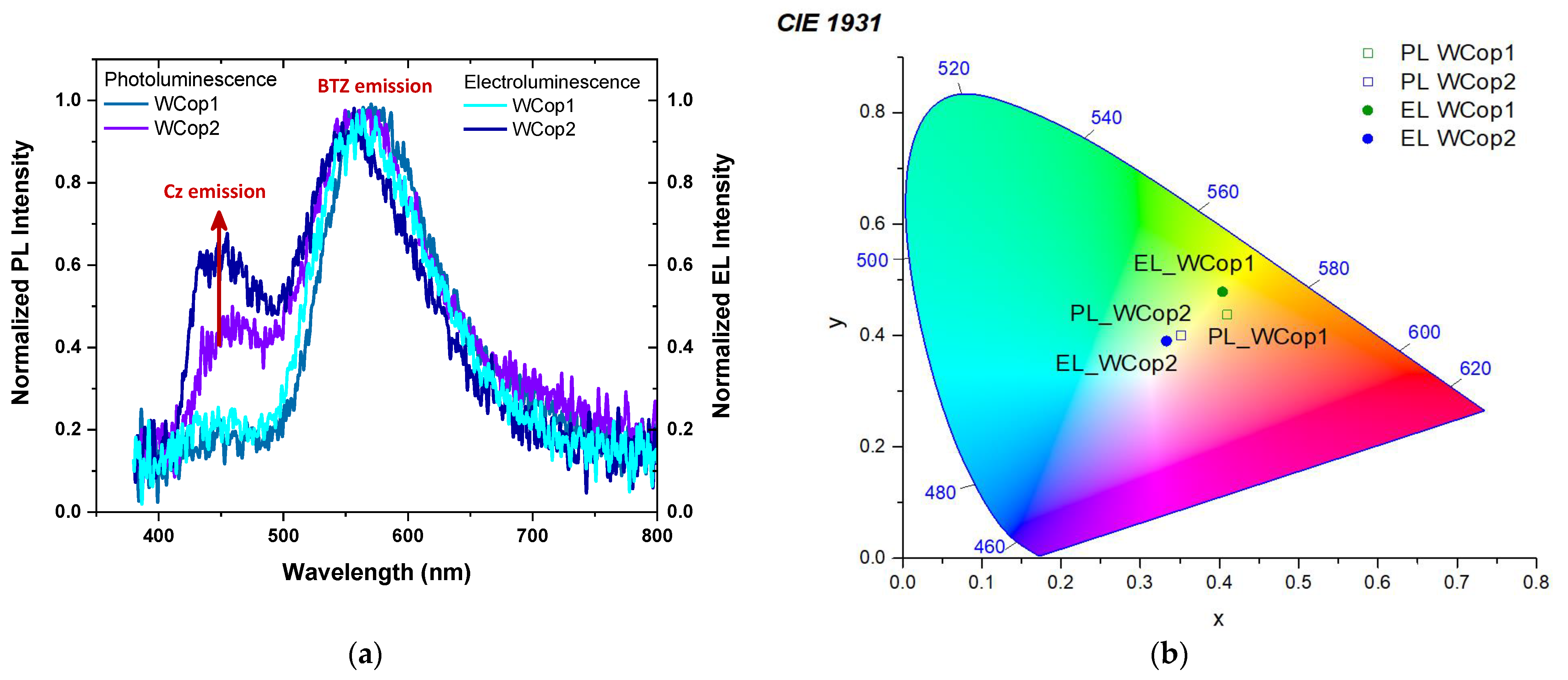
| BTZ monomer | PL | 563 | 27 | 592 | 56 | 638 | 105 |
| WCop1 | PL | 445 | 35 | 567 | 68 | 634 | 113 |
| EL | 443 | 60 | 555 | 64 | 613 | 104 | |
| WCop2 | PL | 454 | 41 | 560 | 84 | 638 | 168 |
| EL | 448 | 41 | 551 | 91 | 614 | 138 | |
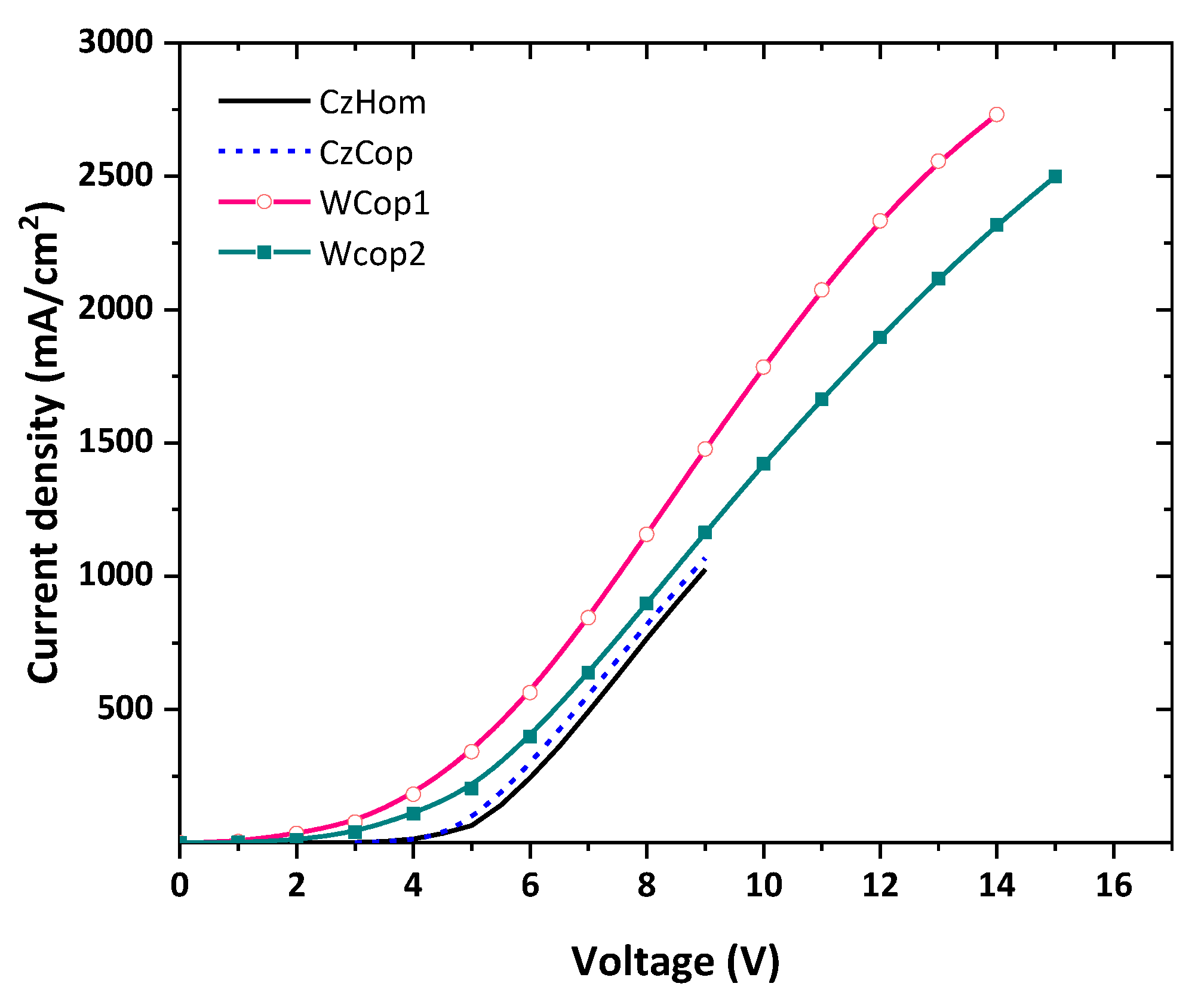
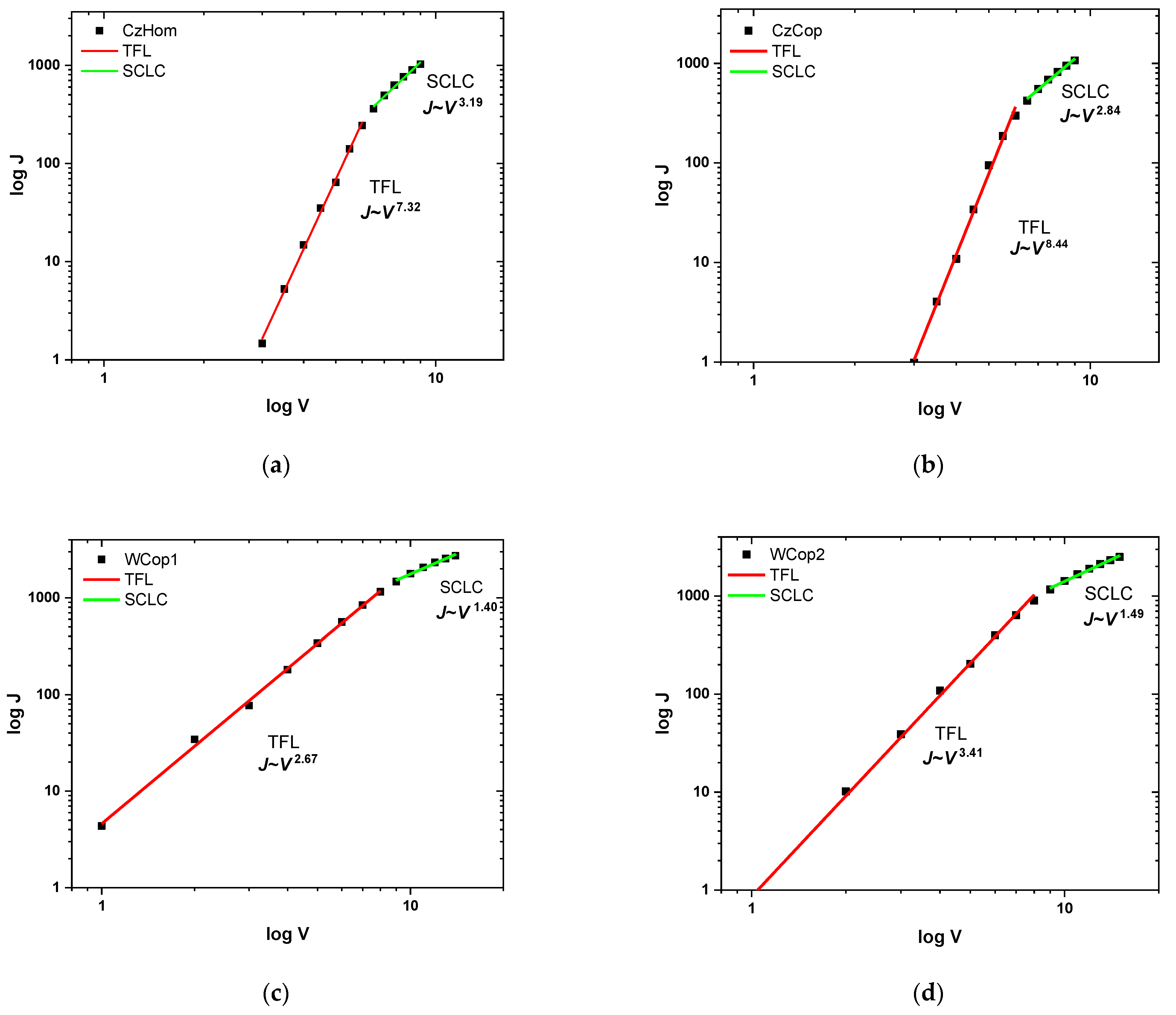
| Current Density Turn-on Voltage (V) | Luminance Turn-on Voltage (V) | Luminance (cd/m2) | CRI | CCT (K) | |
|---|---|---|---|---|---|
| CzHom | 4 | 5 | 12 | ||
| CzCop | 4 | 5 | 28 | ||
| Wcop1 | 2 | 5 | 40 | 60 | 4063 |
| Wcop2 | 2 | 8.8 | 60 | 70 | 5498 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tselekidou, D.; Papadopoulos, K.; Kyriazopoulos, V.; Andrikopoulos, K.C.; Andreopoulou, A.K.; Kallitsis, J.K.; Laskarakis, A.; Logothetidis, S.; Gioti, M. Photophysical and Electro-Optical Properties of Copolymers Bearing Blue and Red Chromophores for Single-Layer White OLEDs. Nanomaterials 2021, 11, 2629. https://doi.org/10.3390/nano11102629
Tselekidou D, Papadopoulos K, Kyriazopoulos V, Andrikopoulos KC, Andreopoulou AK, Kallitsis JK, Laskarakis A, Logothetidis S, Gioti M. Photophysical and Electro-Optical Properties of Copolymers Bearing Blue and Red Chromophores for Single-Layer White OLEDs. Nanomaterials. 2021; 11(10):2629. https://doi.org/10.3390/nano11102629
Chicago/Turabian StyleTselekidou, Despoina, Kyparisis Papadopoulos, Vasileios Kyriazopoulos, Konstantinos C. Andrikopoulos, Aikaterini K. Andreopoulou, Joannis K. Kallitsis, Argiris Laskarakis, Stergios Logothetidis, and Maria Gioti. 2021. "Photophysical and Electro-Optical Properties of Copolymers Bearing Blue and Red Chromophores for Single-Layer White OLEDs" Nanomaterials 11, no. 10: 2629. https://doi.org/10.3390/nano11102629
APA StyleTselekidou, D., Papadopoulos, K., Kyriazopoulos, V., Andrikopoulos, K. C., Andreopoulou, A. K., Kallitsis, J. K., Laskarakis, A., Logothetidis, S., & Gioti, M. (2021). Photophysical and Electro-Optical Properties of Copolymers Bearing Blue and Red Chromophores for Single-Layer White OLEDs. Nanomaterials, 11(10), 2629. https://doi.org/10.3390/nano11102629







