Advances in Non-Animal Testing Approaches towards Accelerated Clinical Translation of Novel Nanotheranostic Therapeutics for Central Nervous System Disorders
Abstract
:1. Introduction
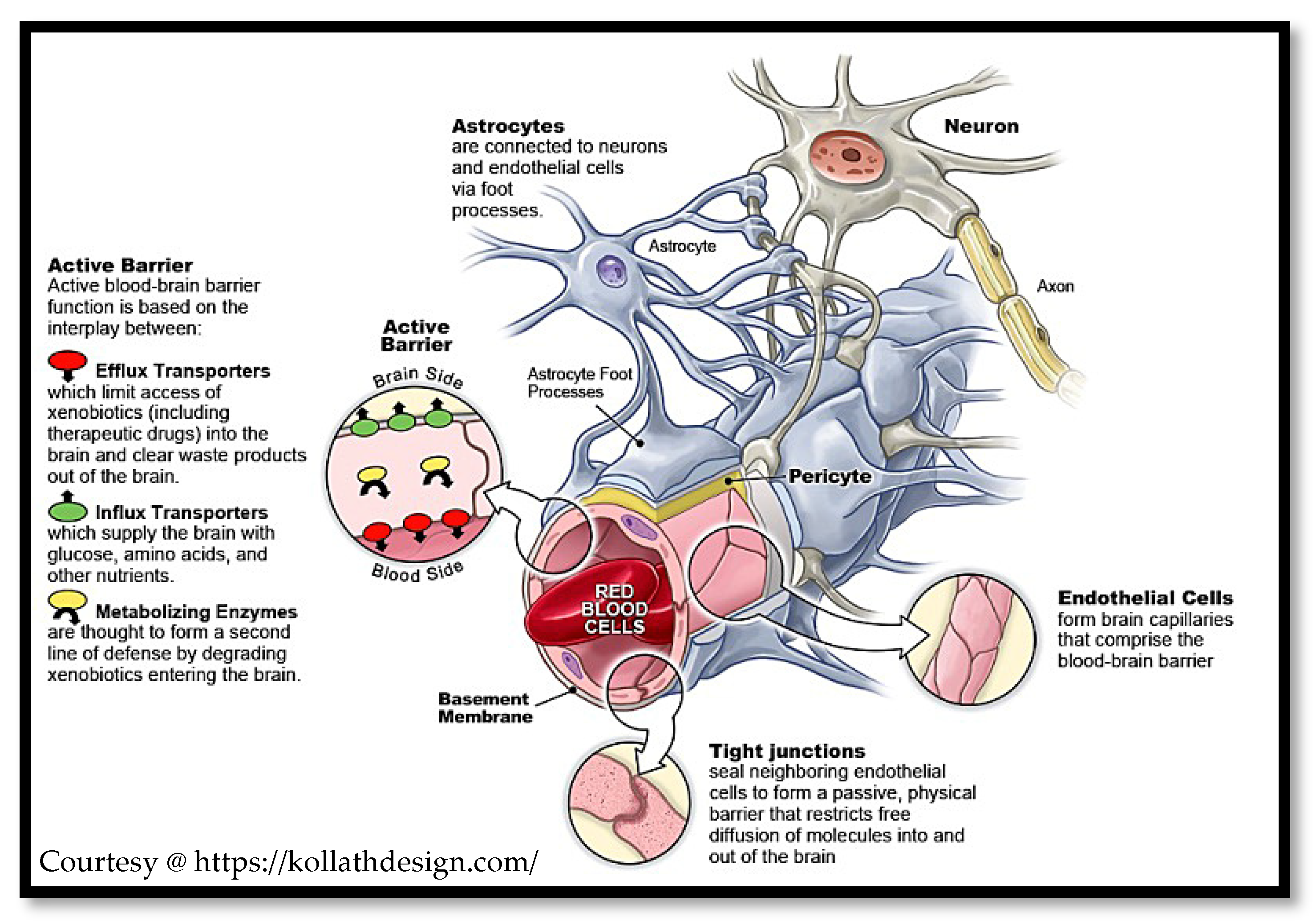
2. Physiological Aspects of the Brain and Blood–Brain Barrier
2.1. Modulating the BBB
2.2. Rationale for Nanotheranostics over Standard Therapies
2.3. Rationale for Modelling the BBB
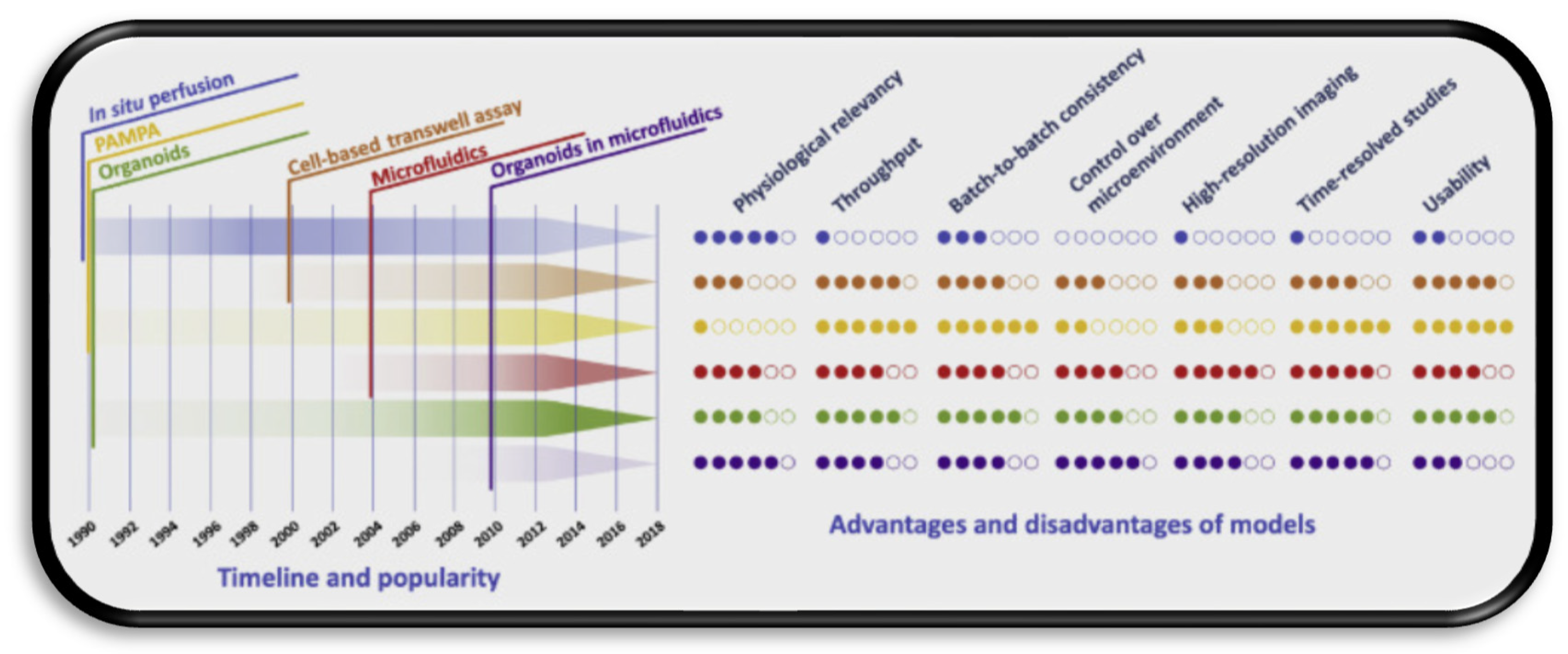
2.4. Overview of Current Modelling Approaches
3. NTP Delivery Approaches for Treating CNS Disorders
3.1. Adsorptive Endocytosis-Mediated NTP Delivery
3.2. Receptor-Mediated Transcytosis
3.2.1. Transferrin (TfR) Receptor-Mediated Transcytosis
3.2.2. Low-Density Lipoprotein (LDL) Receptor-Mediated Transcytosis
3.2.3. Other Notable Receptor-Mediated Approaches
3.3. Other Active Targeting Strategies
4. Towards Consolidated NTP Testing Using Validated BBB Models
4.1. Validation Markers for the Reviewed Models
4.2. Cell Culture Models
4.2.1. Monolayer Cell Culture
4.2.2. Co-Cultured Cell Cultures
4.2.3. Spheroid Cell Culture
5. Recent Trends and Future Directions for NTP Models
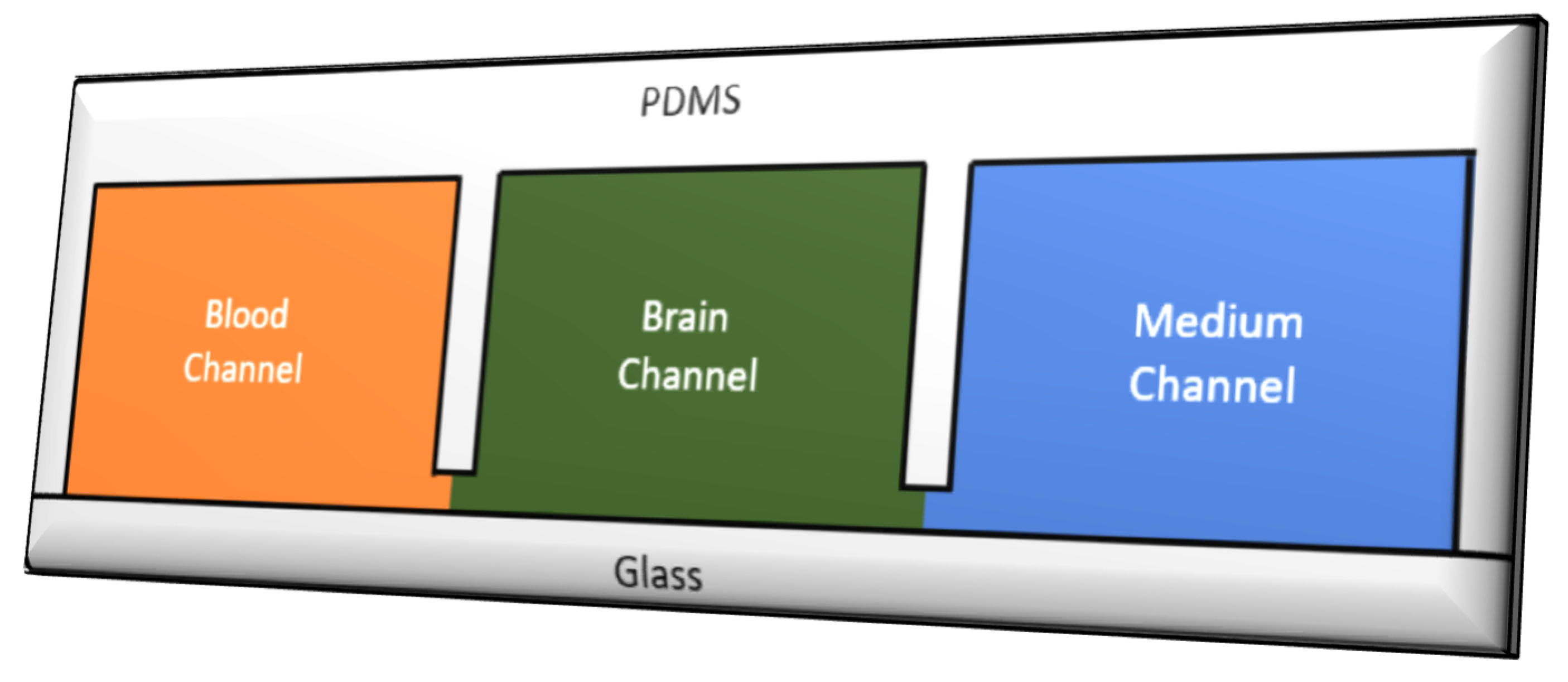
5.1. Microfluidic Organ-on-Chip Technology (µBBB)
5.2. In Silico Simulated NTP Transport Studies
6. Rational Nanotheranostic Design for Accelerated In Vitro Testing
6.1. Inorganic Nanotheranostic Clinical Pipeline
6.2. On Current Engineering and Rational Design
6.3. Unrealised Promise of Biomaterial-Inspired Rational NTP Design
7. Conclusions and Future Outlooks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Howes, O.D.; Mehta, M.A. Challenges in CNS drug development and the role of imaging. Psychopharmacology 2021, 238, 1229–1230. [Google Scholar] [CrossRef]
- Karami, Z.; Zanjani, M.R.S.; Hamidi, M. Nanoemulsions in CNS drug delivery: Recent developments, impacts and challenges. Drug Discov. Today 2019, 24, 1104–1115. [Google Scholar] [CrossRef]
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Feigin, V.L.; Nichols, E.; Alam, T.; Bannick, M.S.; Beghi, E.; Blake, N.; Culpepper, W.J.; Dorsey, E.R.; Elbaz, A.; Ellenbogen, R.G.; et al. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef] [Green Version]
- Borton, D.A.; Dawes, H.E.; Worrell, G.A.; Starr, P.A.; Denison, T.J. Developing Collaborative Platforms to Advance Neurotechnology and Its Translation. Neuron 2020, 108, 286–301. [Google Scholar] [CrossRef] [PubMed]
- Bors, L.A.; Erdő, F. Overcoming the Blood–Brain Barrier. Challenges and Tricks for CNS Drug Delivery. Sci. Pharm. 2019, 87, 6. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Shen, Z.; Anraku, Y.; Kataoka, K.; Chen, X. Nanomaterial-based blood-brain-barrier (BBB) crossing strategies. Biomaterials 2019, 224, 119491. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.L.; Wu, X.Y.; Bendayan, R. Nanotechnological advances for the delivery of CNS therapeutics. Adv. Drug Deliv. Rev. 2012, 64, 686–700. [Google Scholar] [CrossRef]
- Rashid, M.; Ahmad, Q.Z. Tajuddin Trends in Nanotechnology for Practical Applications. In Applications of Targeted Nano Drugs and Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Shi, Y.; Van Der Meel, R.; Chen, X.; Lammers, T. The EPR effect and beyond: Strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics 2020, 10, 7921–7924. [Google Scholar] [CrossRef] [PubMed]
- Wong, X.Y.; Sena-Torralba, A.; Álvarez-Diduk, R.; Muthoosamy, K.; Merkoçi, A. Nanomaterials for Nanotheranostics: Tuning Their Properties According to Disease Needs. ACS Nano 2020, 14, 2585–2627. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Xiao, Z.; Kamaly, N.; Farokhzad, O.C. Self-Assembled Targeted Nanoparticles: Evolution of Technologies and Bench to Bedside Translation. Acc. Chem. Res. 2011, 44, 1123–1134. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, D.E. Gartner’s hype cycle and information system research issues. Int. J. Account. Inf. Syst. 2008, 9, 240–252. [Google Scholar] [CrossRef]
- Alexander, A.A.; Jotterand, F. Market Considerations for Nanomedicines. In Cancer Theranostics; Academic Press: Cambridge, MA, USA, 2014; pp. 471–491. [Google Scholar] [CrossRef]
- Selvan, S.T.; Narayanan, K. Introduction to Nanotheranostics; Springer: Singapore, 2016. [Google Scholar] [CrossRef]
- Guo, D.; Ji, X.; Luo, J. Rational nanocarrier design towards clinical translation of cancer nanotherapy. Biomed. Mater. 2021, 16, 032005. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Miao, W.-S.; Zhang, Y.-D.; Gao, H.-J.; Hui, D. Mechanical properties of nanomaterials: A review. Nanotechnol. Rev. 2020, 9, 259–273. [Google Scholar] [CrossRef]
- Gharbavi, M.; Amani, J.; Kheiri-Manjili, H.; Danafar, H.; Sharafi, A. Niosome: A Promising Nanocarrier for Natural Drug Delivery through Blood-Brain Barrier. Adv. Pharmacol. Sci. 2018, 2018, 6847971. [Google Scholar] [CrossRef] [PubMed]
- Kuo, A.; Smith, M.T. Theoretical and practical applications of the intracerebroventricular route for CSF sampling and drug administration in CNS drug discovery research: A mini review. J. Neurosci. Methods 2014, 233, 166–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Upadhyay, R.K. Drug Delivery Systems, CNS Protection, and the Blood Brain Barrier. BioMed Res. Int. 2014, 2014, 869269. [Google Scholar] [CrossRef] [Green Version]
- Sharma, M.; Dube, T.; Chibh, S.; Kour, A.; Mishra, J.; Panda, J.J. Nanotheranostics, a future remedy of neurological disorders. Expert Opin. Drug Deliv. 2019, 16, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caracciolo, G.; Farokhzad, O.C.; Mahmoudi, M. Biological Identity of Nanoparticles In Vivo: Clinical Implications of the Protein Corona. Trends Biotechnol. 2017, 35, 257–264. [Google Scholar] [CrossRef]
- Zhou, Y.; Dai, Z. New Strategies in the Design of Nanomedicines to Oppose Uptake by the Mononuclear Phagocyte System and Enhance Cancer Therapeutic Efficacy. Chem. Asian J. 2018, 13, 3333–3340. [Google Scholar] [CrossRef] [PubMed]
- Bazak, R.; Houri, M.; El Achy, S.; Hussein, W.; Refaat, T. Passive targeting of nanoparticles to cancer: A comprehensive review of the literature. Mol. Clin. Oncol. 2014, 2, 904–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strazielle, N.; Ghersi-Egea, J.-F. Potential Pathways for CNS Drug Delivery Across the Blood-Cerebrospinal Fluid Barrier. Curr. Pharm. Des. 2016, 22, 5463–5476. [Google Scholar] [CrossRef] [Green Version]
- Dyrna, F.; Hanske, S.; Krueger, M.; Bechmann, I. The Blood-Brain Barrier. J. Neuroimmune Pharmacol. 2013, 8, 763–773. [Google Scholar] [CrossRef]
- Berndt, P.; Winkler, L.; Cording, J.; Breitkreuz-Korff, O.; Rex, A.; Dithmer, S.; Rausch, V.; Blasig, R.; Richter, M.; Sporbert, A.; et al. Tight junction proteins at the blood–brain barrier: Far more than claudin-5. Cell. Mol. Life Sci. 2019, 76, 1987–2002. [Google Scholar] [CrossRef]
- Kim, J.; Ahn, S.I.; Kim, Y. Nanotherapeutics engineered to cross the blood-brain barrier for advanced drug delivery to the central nervous system. J. Ind. Eng. Chem. 2019, 73, 8–18. [Google Scholar] [CrossRef] [PubMed]
- MacDougall, M.R.; Sharma, S. Physiology, Chemoreceptor Trigger Zone. StatPearls [Internet]. 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK537133/ (accessed on 29 April 2021).
- Shen, S.; Zhang, W. ABC Transporters and Drug Efflux at the Blood-Brain Barrier. Rev. Neurosci. 2010, 21, 29–53. [Google Scholar] [CrossRef] [PubMed]
- Eisenblätter, T.; Hüwel, S.; Galla, H.-J. Characterisation of the brain multidrug resistance protein (BMDP/ABCG2/BCRP) expressed at the blood–brain barrier. Brain Res. 2003, 971, 221–231. [Google Scholar] [CrossRef]
- Charabati, M.; Rabanel, J.-M.; Ramassamy, C.; Prat, A. Overcoming the Brain Barriers: From Immune Cells to Nanoparticles. Trends Pharmacol. Sci. 2020, 41, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-T.; Wei, K.-C.; Liu, H.-L. Theranostic Strategy of Focused Ultrasound Induced Blood-Brain Barrier Opening for CNS Disease Treatment. Front. Pharmacol. 2019, 10, 86. [Google Scholar] [CrossRef] [Green Version]
- Choi, M.R.; Bardhan, R.; Stanton-Maxey, K.J.; Badve, S.; Nakshatri, H.; Stantz, K.M.; Cao, N.; Halas, N.; Clare, S.E. Delivery of nanoparticles to brain metastases of breast cancer using a cellular Trojan horse. Cancer Nanotechnol. 2012, 3, 47–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casaos, J.; Gorelick, N.; Tyler, B. Neurosurgical Implant-Based Strategy for Brain Cancer Therapy. In Nanotherapy for Brain Tumor Drug Delivery; Humana: New York, NY, USA, 2021; pp. 225–244. [Google Scholar] [CrossRef]
- Viderman, D.; Brotfain, E.; Bilotta, F.; Zhumadilov, A. Risk Factors and Mechanisms of Postoperative Delirium after Intracranial Neurosurgical Procedures. Asian J. Anesthesiol. 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- Kumar, A.; Chaudhary, R.K.; Singh, R.; Singh, S.P.; Wang, S.-Y.; Hoe, Z.-Y.; Pan, C.-T.; Shiue, Y.-L.; Wei, D.-Q.; Kaushik, A.C.; et al. Nanotheranostic Applications for Detection and Targeting Neurodegenerative Diseases. Front. Neurosci. 2020, 14, 305. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, J.R.; Sriramoju, B.; Kanwar, R.K. Neurological disorders and therapeutics targeted to surmount the blood–brain barrier. Int. J. Nanomed. 2012, 7, 3259–3278. [Google Scholar] [CrossRef] [Green Version]
- Gklinos, P.; Papadopoulou, M.; Stanulovic, V.; Mitsikostas, D.; Papadopoulos, D. Monoclonal Antibodies as Neurological Therapeutics. Pharmaceuticals 2021, 14, 92. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Leak, R.; Thomson, A.W.; Yu, F.; Xia, Y.; Wechsler, L.R.; Chen, J. Promises and limitations of immune cell-based therapies in neurological disorders. Nat. Rev. Neurol. 2018, 14, 559–568. [Google Scholar] [CrossRef]
- Zottel, A.; Paska, A.V.; Jovčevska, I. Nanotechnology Meets Oncology: Nanomaterials in Brain Cancer Research, Diagnosis and Therapy. Materials 2019, 12, 1588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chodobski, A.; Zink, B.J.; Szmydynger-Chodobska, J. Blood–Brain Barrier Pathophysiology in Traumatic Brain Injury. Transl. Stroke Res. 2011, 2, 492–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, M.; Hanrahan, F.; Gobbo, O.; Kelly, M.E.; Kiang, A.-S.; Humphries, M.M.; Nguyen, A.T.; Ozaki, E.; Keaney, J.; Blau, C.W.; et al. Targeted suppression of claudin-5 decreases cerebral oedema and improves cognitive outcome following traumatic brain injury. Nat. Commun. 2012, 3, 849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, R.; Wood, T.R.; Nance, E. Nanotherapeutic modulation of excitotoxicity and oxidative stress in acute brain injury. Nanobiomedicine 2020, 7, 1849543520970819. [Google Scholar] [CrossRef]
- Torrice, M. Does Nanomedicine Have a Delivery Problem? ACS Cent. Sci. 2016, 2, 434–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aldape, K.; Brindle, K.M.; Chesler, L.; Chopra, R.; Gajjar, A.; Gilbert, M.R.; Gottardo, N.; Gutmann, D.; Hargrave, D.; Holland, E.C.; et al. Challenges to curing primary brain tumours. Nat. Rev. Clin. Oncol. 2019, 16, 509–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbott, N.J. Blood–brain barrier structure and function and the challenges for CNS drug delivery. J. Inherit. Metab. Dis. 2013, 36, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Pamies, D.; Hartung, T.; Hogberg, H.T. Biological and medical applications of a brain-on-a-chip. Exp. Biol. Med. 2014, 239, 1096–1107. [Google Scholar] [CrossRef] [PubMed]
- Olsson, I.A.S.; da Silva, S.P.; Townend, D.; Sandøe, P. Protecting Animals and Enabling Research in the European Union: An Overview of Development and Implementation of Directive 2010/63/EU. ILAR J. 2017, 57, 347–357. [Google Scholar] [CrossRef] [Green Version]
- Lacombe, O.; Videau, O.; Chevillon, D.; Guyot, A.-C.; Contreras, C.; Blondel, S.; Nicolas, L.; Ghettas, A.; Bénech, H.; Thévenot, E.A.; et al. In Vitro Primary Human and Animal Cell-Based Blood−Brain Barrier Models as a Screening Tool in Drug Discovery. Mol. Pharm. 2011, 8, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Gumbleton, M.; Audus, K.L. Progress and limitations in the use of in vitro cell cultures to serve as a permeability screen for the blood-brain barrier. J. Pharm. Sci. 2001, 90, 1681–1698. [Google Scholar] [CrossRef]
- Bagchi, S.; Chhibber, T.; Lahooti, B.; Verma, A.; Borse, V.; Jayant, R.D. In-vitro blood-brain barrier models for drug screening and permeation studies: An overview. Drug Des. Dev. Ther. 2019, 13, 3591–3605. [Google Scholar] [CrossRef] [Green Version]
- Oddo, A.; Peng, B.; Tong, Z.; Wei, Y.; Tong, W.Y.; Thissen, H.; Voelcker, N.H. Advances in Microfluidic Blood–Brain Barrier (BBB) Models. Trends Biotechnol. 2019, 37, 1295–1314. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, S.; Lawler, S.E.; Qu, Y.; Fadzen, C.M.; Wolfe, J.M.; Regan, M.S.; Pentelute, B.L.; Agar, N.Y.R.; Cho, C.-F. Blood–brain-barrier organoids for investigating the permeability of CNS therapeutics. Nat. Protoc. 2018, 13, 2827–2843. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Hou, Y.; Ai, X.; Sun, J.; Xu, B.; Meng, X.; Zhang, Y.; Zhang, S. Potential applications of microfluidics based blood brain barrier (BBB)-on-chips for in vitro drug development. Biomed. Pharmacother. 2020, 132, 110822. [Google Scholar] [CrossRef]
- Ng, T.S.; Garlin, M.A.; Weissleder, R.; Miller, M.A. Improving nanotherapy delivery and action through image-guided systems pharmacology. Theranostics 2020, 10, 968–997. [Google Scholar] [CrossRef]
- Aalinkeel, R.; Kutscher, H.L.; Singh, A.; Cwiklinski, K.; Khechen, N.; Schwartz, S.A.; Prasad, P.N.; Mahajan, S.D. Neuroprotective effects of a biodegradable poly(lactic-co-glycolic acid)-ginsenoside Rg3 nanoformulation: A potential nanotherapy for Alzheimer’s disease? J. Drug Target. 2017, 26, 182–193. [Google Scholar] [CrossRef]
- Irudayanathan, F.J.; Trasatti, J.P.; Karande, P.; Nangia, S. Molecular Architecture of the Blood Brain Barrier Tight Junction Proteins—A Synergistic Computational and In Vitro Approach. J. Phys. Chem. B 2016, 120, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, H.; Wu, Z.; Wang, T.; Li, W.; Tang, Y.; Liu, G. In Silico Prediction of Blood–Brain Barrier Permeability of Compounds by Machine Learning and Resampling Methods. ChemMedChem 2018, 13, 2189–2201. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Mei, S.; Jin, H.; Zhu, B.; Tian, Y.; Huo, J.; Cui, X.; Guo, A.; Zhao, Z. Identification of two immortalized cell lines, ECV304 and bEnd3, for in vitro permeability studies of blood-brain barrier. PLoS ONE 2017, 12, e0187017. [Google Scholar] [CrossRef]
- Ivask, A.; Pilkington, E.H.; Blin, T.; Kakinen, A.; Vija, H.; Visnapuu, M.; Quinn, J.F.; Whittaker, M.R.; Qiao, R.; Davis, T.P.; et al. Uptake and transcytosis of functionalized superparamagnetic iron oxide nanoparticles in an in vitro blood brain barrier model. Biomater. Sci. 2017, 6, 314–323. [Google Scholar] [CrossRef] [Green Version]
- Appelt-Menzel, A.; Oerter, S.; Mathew, S.; Haferkamp, U.; Hartmann, C.; Jung, M.; Neuhaus, W.; Pless, O. Human iPSC-Derived Blood-Brain Barrier Models: Valuable Tools for Preclinical Drug Discovery and Development? Curr. Protoc. Stem Cell Biol. 2020, 55, 122. [Google Scholar] [CrossRef] [PubMed]
- Dong, X. Current strategies for brain drug delivery. Theranostics 2018, 8, 1481. [Google Scholar] [CrossRef] [PubMed]
- Caffery, B.; Lee, J.S.; Alexander-Bryant, A.A. Vectors for Glioblastoma Gene Therapy: Viral & Non-Viral Delivery Strategies. Nanomaterials 2019, 9, 105. [Google Scholar] [CrossRef] [Green Version]
- Dal Magro, R.; Ornaghi, F.; Cambianica, I.; Beretta, S.; Re, F.; Musicanti, C.; Rigolio, R.; Donzelli, E.; Canta, A.R.; Ballarini, E.; et al. ApoE-modified solid lipid nanoparticles: A feasible strategy to cross the blood-brain barrier. J. Control. Release 2017, 249, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Lin, S. Double-Coated Poly (Butylcynanoacrylate) Nanoparticulate Delivery Systems for Brain Targeting of Dalargin via Oral Administration. J. Pharm. Sci. 2005, 94, 1343–1353. [Google Scholar] [CrossRef]
- Tam, V.H.; Sosa, C.; Liu, R.; Yao, N.; Priestley, R.D. Nanomedicine as a non-invasive strategy for drug delivery across the blood brain barrier. Int. J. Pharm. 2016, 515, 331–342. [Google Scholar] [CrossRef]
- Sharma, G.; Sharma, A.R.; Lee, S.-S.; Bhattacharya, M.; Nam, J.-S.; Chakraborty, C. Advances in nanocarriers enabled brain targeted drug delivery across blood brain barrier. Int. J. Pharm. 2019, 559, 360–372. [Google Scholar] [CrossRef]
- Lu, W.; Tan, Y.-Z.; Hu, K.-L.; Jiang, X.-G. Cationic albumin conjugated pegylated nanoparticle with its transcytosis ability and little toxicity against blood–brain barrier. Int. J. Pharm. 2005, 295, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Jain, A.; Jain, A.; Jain, A.K.; Garg, N.K.; Tekade, R.K.; Singh, T.R.R.; Iyer, A.K. Cationic bovine serum albumin (CBA) conjugated poly lactic-co-glycolic acid (PLGA) nanoparticles for extended delivery of methotrexate into brain tumors. RSC Adv. 2016, 6, 89040–89050. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Guo, K.; Lu, J.; Venkatraman, S.S.; Luo, D.; Ng, K.C.; Ling, E.-A.; Moochhala, S.; Yang, Y.-Y. Biologically active core/shell nanoparticles self-assembled from cholesterol-terminated PEG–TAT for drug delivery across the blood–brain barrier. Biomaterials 2008, 29, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Raval, N.; Mistry, T.; Acharya, N.; Acharya, S. Development of glutathione-conjugated asiatic acid-loaded bovine serum albumin nanoparticles for brain-targeted drug delivery. J. Pharm. Pharmacol. 2015, 67, 1503–1511. [Google Scholar] [CrossRef] [PubMed]
- Michaelis, M.; Hoffmann, M.M.; Dreis, S.; Herbert, E.; Alyautdin, R.; Kreuter, J.; Langer, K. Covalent Linkage of Apolipoprotein E to Albumin Nanoparticles Strongly Enhances Drug Transport into the Brain. J. Pharmacol. Exp. Ther. 2006, 317, 1246–1253. [Google Scholar] [CrossRef] [Green Version]
- Georgieva, J.V.; Kalicharan, D.; Couraud, P.-O.; Romero, I.; Weksler, B.; Hoekstra, D.; Zuhorn, I. Surface Characteristics of Nanoparticles Determine Their Intracellular Fate in and Processing by Human Blood–Brain Barrier Endothelial Cells In Vitro. Mol. Ther. 2011, 19, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Shang, T.; Zhang, X.; Ye, T.; Wang, D.; Rei, L. Passage of Magnetic Tat-Conjugated Fe3O4@SiO2 Nanoparticles Across In Vitro Blood-Brain Barrier. Nanoscale Res. Lett. 2016, 11, 451. [Google Scholar] [CrossRef] [Green Version]
- Mabrouk, O.S.; Falk, T.; Sherman, S.J.; Kennedy, R.T.; Polt, R. CNS penetration of the opioid glycopeptide MMP-2200: A microdialysis study. Neurosci. Lett. 2012, 531, 99–103. [Google Scholar] [CrossRef] [Green Version]
- Lv, H.; Zhang, S.; Wang, B.; Cui, S.; Yan, J. Toxicity of cationic lipids and cationic polymers in gene delivery. J. Control. Release 2006, 114, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Ingusci, S.; Verlengia, G.; Soukupova, M.; Zucchini, S.; Simonato, M. Gene Therapy Tools for Brain Diseases. Front. Pharmacol. 2019, 10, 724. [Google Scholar] [CrossRef] [PubMed]
- Zong, T.; Mei, L.; Gao, H.; Cai, W.; Zhu, P.; Shi, K.; Chen, J.; Wang, Y.; Gao, F.; He, Q. Synergistic Dual-Ligand Doxorubicin Liposomes Improve Targeting and Therapeutic Efficacy of Brain Glioma in Animals. Mol. Pharm. 2014, 11, 2346–2357. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, K.B.; Burkhart, A.; Thomsen, L.B.; Andresen, T.L.; Moos, T. Targeting the transferrin receptor for brain drug delivery. Prog. Neurobiol. 2019, 181, 101665. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Ahsan, S.M.; Kumar, J.M.; Kondapi, A.K.; Rao, N.M. Overcoming blood brain barrier with a dual purpose Temozolomide loaded Lactoferrin nanoparticles for combating glioma (SERP-17-12433). Sci. Rep. 2017, 7, 6602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, Y.-S.; Jung, H.-J.; Oh, J.-S.; Song, D.-Y. Use of PEGylated Immunoliposomes to Deliver Dopamine across the Blood-Brain Barrier in a Rat Model of Parkinson’s Disease. CNS Neurosci. Ther. 2016, 22, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.-Q.; Lv, Q.; Li, L.-M.; Tang, X.-J.; Li, F.-Z.; Hu, Y.-L.; Han, M. Glioma targeting and blood–brain barrier penetration by dual-targeting doxorubincin liposomes. Biomaterials 2013, 34, 5628–5639. [Google Scholar] [CrossRef]
- Nam, L.; Coll, C.; Erthal, L.C.S.; De La Torre, C.; Serrano, D.; Martínez-Máñez, R.; Santos-Martínez, M.J.; Ruiz-Hernández, E. Drug Delivery Nanosystems for the Localized Treatment of Glioblastoma Multiforme. Materials 2018, 11, 779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tosi, G.; Pederzoli, F.; Belletti, D.; Vandelli, M.A.; Forni, F.; Duskey, J.T.; Ruozi, B. Nanomedicine in Alzheimer’s disease: Amyloid beta targeting strategy. Prog. Brain Res. 2019, 245, 57–88. [Google Scholar] [CrossRef]
- Johnsen, K.B.; Bak, M.; Kempen, P.J.; Melander, F.; Burkhart, A.; Thomsen, M.S.; Nielsen, M.S.; Moos, T.; Andresen, T.L. Antibody affinity and valency impact brain uptake of transferrin receptor-targeted gold nanoparticles. Theranostics 2018, 8, 3416. [Google Scholar] [CrossRef]
- Sonoda, H.; Morimoto, H.; Yoden, E.; Koshimura, Y.; Kinoshita, M.; Golovina, G.; Takagi, H.; Yamamoto, R.; Minami, K.; Mizoguchi, A.; et al. A Blood-Brain-Barrier-Penetrating Anti-human Transferrin Receptor Antibody Fusion Protein for Neuronopathic Mucopolysaccharidosis II. Mol. Ther. 2018, 26, 1366–1374. [Google Scholar] [CrossRef] [PubMed]
- Kida, S.; Kinoshita, M.; Tanaka, S.; Okumura, M.; Koshimura, Y.; Morimoto, H. Non-clinical evaluation of a blood-brain barrier-penetrating enzyme for the treatment of mucopolysaccharidosis type I. Mol. Genet. Metab. 2019, 126, S83–S84. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Q.Y.; Haqqani, A.S.; Leclerc, S.; Liu, Z.; Fauteux, F.; Baumann, E.; Delaney, C.E.; Ly, D.; Star, A.T.; et al. Differential expression of receptors mediating receptor-mediated transcytosis (RMT) in brain microvessels, brain parenchyma and peripheral tissues of the mouse and the human. Fluids Barriers CNS 2020, 17, 47. [Google Scholar] [CrossRef]
- Dehouck, B.; Fenart, L.; Dehouck, M.-P.; Pierce, A.; Torpier, G.; Cecchelli, R. A New Function for the LDL Receptor: Transcytosis of LDL across the Blood–Brain Barrier. J. Cell Biol. 1997, 138, 877–889. [Google Scholar] [CrossRef]
- Laskowitz, D.T.; Horsburgh, K.; Roses, A.D. Apolipoprotein E and the CNS response to injury. J. Cere. Blood Flow Metab. 1998, 18, 465–471. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.R.; Andrieux, K.; Gil, S.; Taverna, M.; Chacun, H.; Desmaële, D.; Taran, F.; Georgin, D.; Couvreur, P. Translocation of Poly(ethylene glycol-co-hexadecyl)cyanoacrylate Nanoparticles into Rat Brain Endothelial Cells: Role of Apolipoproteins in Receptor-Mediated Endocytosis. Biomacromolecules 2007, 8, 793–799. [Google Scholar] [CrossRef]
- Hartl, N.; Adams, F.; Merkel, O.M. From Adsorption to Covalent Bonding: Apolipoprotein E Functionalization of Polymeric Nanoparticles for Drug Delivery across the Blood–Brain Barrier. Adv. Ther. 2021, 4, 2000092. [Google Scholar] [CrossRef] [PubMed]
- Kafa, H.; Wang, J.T.-W.; Rubio, N.; Klippstein, R.; Costa, P.M.; Hassan, H.A.; Sosabowski, J.K.; Bansal, S.S.; Preston, J.E.; Abbott, N.J.; et al. Translocation of LRP1 targeted carbon nanotubes of different diameters across the blood–brain barrier in vitro and in vivo. J. Control. Release 2016, 225, 217–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ball, K.; Bouzom, F.; Scherrmann, J.-M.; Walther, B.; Decleves, X. Physiologically Based Pharmacokinetic Modelling of Drug Penetration Across the Blood–Brain Barrier—Towards a Mechanistic IVIVE-Based Approach. AAPS J. 2013, 15, 913–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uchida, Y.; Ohtsuki, S.; Katsukura, Y.; Ikeda, C.; Suzuki, T.; Kamiie, J.; Terasaki, T. Quantitative targeted absolute proteomics of human blood-brain barrier transporters and receptors. J. Neurochem. 2011, 117, 333–345. [Google Scholar] [CrossRef]
- di Polidoro, A.C.; Zambito, G.; Haeck, J.; Mezzanotte, L.; Lamfers, M.; Netti, P.; Torino, E. Theranostic Design of Angiopep-2 Conjugated Hyaluronic Acid Nanoparticles (Thera-ANG-cHANPs) for Dual Targeting and Boosted Imaging of Glioma Cells. Cancers 2021, 13, 503. [Google Scholar] [CrossRef]
- Engin, A.B.; Engin, A. Nanoparticles and neurotoxicity: Dual response of glutamatergic receptors. Prog. Brain Res. 2019, 245, 281–303. [Google Scholar] [CrossRef]
- Jensen, R.L.; Chkheidze, R. The Role of Glucose Transporter-1 (GLUT-1) in Malignant Gliomas. In Tumours of the Central Nervous System; Springer: Dordrecht, The Netherlands, 2011; Volume 1, pp. 99–108. [Google Scholar] [CrossRef]
- Wu, T.-T.; Zhou, S.-H. Nanoparticle-Based Targeted Therapeutics in Head-And-Neck Cancer. Int. J. Med. Sci. 2015, 12, 187–200. [Google Scholar] [CrossRef] [Green Version]
- Pardridge, W.M.; Kang, Y.-S.; Buciak, J.L.; Yang, J. Human insulin receptor monoclonal antibody undergoes high affinity binding to human brain capillaries in vitro and rapid transcytosis through the blood-brain barrier in vivo in the primate. Pharm. Res. 1995, 12, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, K.; Knobloch, T.; Kreuter, J. Targeting the insulin receptor: Nanoparticles for drug delivery across the blood–brain barrier (BBB). J. Drug Target. 2010, 19, 125–132. [Google Scholar] [CrossRef] [PubMed]
- McCord, E.; Pawar, S.; Koneru, T.; Tatiparti, K.; Sau, S.; Iyer, A.K. Folate Receptors’ Expression in Gliomas May Possess Potential Nanoparticle-Based Drug Delivery Opportunities. ACS Omega 2021, 6, 4111–4118. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Lv, L.; Wang, Z.; Zhao, Y.; Wu, L.; Fang, X.; Xu, Q.; Xin, H. Nanoparticles functionalized with Pep-1 as potential glioma targeting delivery system via interleukin 13 receptor α2-mediated endocytosis. Biomaterials 2014, 35, 5897–5907. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Bandla, A.; Chuan, C.K.; Magarajah, G.; Liao, L.-D.; Teh, D.B.L.; Kennedy, B.K.; Thakor, N.V.; Liu, B. Identifying glioblastoma margins using dual-targeted organic nanoparticles for efficient in vivo fluorescence image-guided photothermal therapy. Mater. Horiz. 2018, 6, 311–317. [Google Scholar] [CrossRef]
- Peng, L.; Liang, Y.; Zhong, X.; Liang, Z.; Tian, Y.; Li, S.; Liang, J.; Wang, R.; Zhong, Y.; Shi, Y.; et al. Aptamer-Conjugated Gold Nanoparticles Targeting Epidermal Growth Factor Receptor Variant III for the Treatment of Glioblastoma. Int. J. Nanomed. 2020, 15, 1363–1372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vashist, A.; Atluri, V.; Raymond, A.; Kaushik, A.; Parira, T.; Huang, Z.; Durygin, A.; Tomitaka, A.; Nikkhah-Moshaie, R.; Vashist, A.; et al. Development of Multifunctional Biopolymeric Auto-Fluorescent Micro- and Nanogels as a Platform for Biomedical Applications. Front. Bioeng. Biotechnol. 2020, 8, 315. [Google Scholar] [CrossRef]
- Salimi, H.; Klein, R.S. Disruption of the Blood-Brain Barrier during Neuroinflammatory and Neuroinfectious Diseases. In Neuroimmune Diseases: From Cells to the Living Brain; Springer: Cham, Switzerland, 2019; pp. 195–234. [Google Scholar] [CrossRef]
- Åslund, A.K.; Berg, S.; Hak, S.; Mørch, Ý.; Torp, S.H.; Sandvig, A.; Widerøe, M.; Hansen, R.; de Lange Davies, C. Nanoparticle delivery to the brain—By focused ultrasound and self-assembled nanoparticle-stabilized microbubbles. J. Control. Release 2015, 220, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Mainprize, T.; Lipsman, N.; Huang, Y.; Meng, Y.; Bethune, A.; Ironside, S.; Heyn, C.; Alkins, R.; Trudeau, M.; Sahgal, A.; et al. Blood-Brain Barrier Opening in Primary Brain Tumors with Non-invasive MR-Guided Focused Ultrasound: A Clinical Safety and Feasibility Study. Sci. Rep. 2019, 9, 321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, M.; Jayant, R.D.; Kaushik, A.; Sagar, V. Getting into the brain: Potential of nanotechnology in the management of NeuroAIDS. Adv. Drug Deliv. Rev. 2016, 103, 202–217. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.; Lee, S.; Park, S. iRGD Peptide as a Tumor-Penetrating Enhancer for Tumor-Targeted Drug Delivery. Polymers 2020, 12, 1906. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Feng, L.; Fan, L.; Zha, Y.; Guo, L.; Zhang, Q.; Chen, J.; Pang, Z.; Wang, Y.; Jiang, X.; et al. Targeting the brain with PEG–PLGA nanoparticles modified with phage-displayed peptides. Biomaterials 2011, 32, 4943–4950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batrakova, E.V.; Gendelman, H.E.; Kabanov, A. Cell-mediated drug delivery. Expert Opin. Drug Deliv. 2011, 8, 415–433. [Google Scholar] [CrossRef] [Green Version]
- D’Agata, F.; Ruffinatti, F.A.; Boschi, S.; Stura, I.; Rainero, I.; Abollino, O.; Cavalli, R.; Guiot, C. Magnetic nanoparticles in the central nervous system: Targeting principles, applications and safety issues. Molecules 2018, 23, 10009. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, M.; Castanho, M.A.R.B.; Serrano, I. In vitro blood-brain barrier models--latest advances and therapeutic applications in a chronological perspective. Mini-Rev. Med. Chem. 2010, 10, 263–271. [Google Scholar] [CrossRef]
- Helms, H.C.C.; Abbott, N.J.; Burek, M.; Cecchelli, R.; Couraud, P.-O.; Deli, M.; Förster, C.; Galla, H.J.; Romero, I.; Shusta, E.V.; et al. In vitro models of the blood–brain barrier: An overview of commonly used brain endothelial cell culture models and guidelines for their use. Br. J. Pharmacol. 2016, 36, 862–890. [Google Scholar] [CrossRef] [PubMed]
- Delsing, L.; Herland, A.; Falk, A.; Hicks, R.; Synnergren, J.; Zetterberg, H. Models of the blood-brain barrier using iPSC-derived cells. Mol. Cell. Neurosci. 2020, 107, 103533. [Google Scholar] [CrossRef]
- Chang, J.; Jallouli, Y.; Kroubi, M.; Yuan, X.-B.; Feng, W.; Kang, C.-S.; Pu, P.-Y.; Betbeder, D. Characterization of endocytosis of transferrin-coated PLGA nanoparticles by the blood–brain barrier. Int. J. Pharm. 2009, 379, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Cecchelli, R.; Berezowski, V.; Lundquist, S.; Culot, M.; Renftel, M.; Dehouck, M.-P.; Fenart, L. Modelling of the blood–brain barrier in drug discovery and development. Nat. Rev. Drug Discov. 2007, 6, 650–661. [Google Scholar] [CrossRef]
- Dehouck, M.-P.; Jolliet-Riant, P.; Brée, F.; Fruchart, J.-C.; Cecchelli, R.; Tillement, J.-P. Drug Transfer across the Blood-Brain Barrier: Correlation Between In Vitro and In Vivo Models. J. Neurochem. 1992, 58, 1790–1797. [Google Scholar] [CrossRef]
- Qiao, R.; Jia, Q.; Hüwel, S.; Xia, R.; Liu, T.; Gao, F.; Galla, H.-J.; Gao, M. Receptor-Mediated Delivery of Magnetic Nanoparticles across the Blood–Brain Barrier. ACS Nano 2012, 6, 3304–3310. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.; Zensi, A.; Wien, S.L.; Tschickardt, S.E.; Maier, W.; Vogel, T.; Worek, F.; Pietrzik, C.U.; Kreuter, J.; Von Briesen, H. Uptake Mechanism of ApoE-Modified Nanoparticles on Brain Capillary Endothelial Cells as a Blood-Brain Barrier Model. PLoS ONE 2012, 7, e32568. [Google Scholar] [CrossRef]
- Martins, S.; Tho, I.; Reimold, I.; Fricker, G.; Souto, E.; Ferreira, D.; Brandl, M. Brain delivery of camptothecin by means of solid lipid nanoparticles: Formulation design, in vitro and in vivo studies. Int. J. Pharm. 2012, 439, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Gromnicova, R.; Davies, H.A.; Sreekanthreddy, P.; Romero, I.A.; Lund, T.; Roitt, I.M.; Phillips, J.B.; Male, D.K. Glucose-Coated Gold Nanoparticles Transfer across Human Brain Endothelium and Enter Astrocytes In Vitro. PLoS ONE 2013, 8, e81043. [Google Scholar] [CrossRef] [Green Version]
- Teow, H.M.; Zhou, Z.; Najlah, M.; Yusof, S.R.; Abbott, N.J.; D’Emanuele, A. Delivery of paclitaxel across cellular barriers using a dendrimer-based nanocarrier. Int. J. Pharm. 2013, 441, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Patabendige, A.; Skinner, R.A.; Abbott, N.J. Establishment of a simplified in vitro porcine blood–brain barrier model with high transendothelial electrical resistance. Brain Res. 2013, 1521, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rempe, R.; Cramer, S.; Qiao, R.; Galla, H.-J. Strategies to overcome the barrier: Use of nanoparticles as carriers and modulators of barrier properties. Cell Tissue Res. 2014, 355, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Cramer, S. The Influence of Silver Nanoparticles on the Blood-Brain and the Blood-Cerebrospinal Fluid Barrier in vitro. J. Nanomed. Nanotechnol. 2014, 5, 2157–7439. [Google Scholar] [CrossRef] [Green Version]
- Bramini, M.; Ye, D.; Hallerbach, A.; Nic Raghnaill, M.; Salvati, A.; Åberg, C.; Dawson, K.A. Imaging Approach to Mechanistic Study of Nanoparticle Interactions with the Blood–Brain Barrier. ACS Nano 2014, 8, 4304–4312. [Google Scholar] [CrossRef] [PubMed]
- Hanada, S.; Fujioka, K.; Inoue, Y.; Kanaya, F.; Manome, Y.; Yamamoto, K. Cell-Based in Vitro Blood–Brain Barrier Model Can Rapidly Evaluate Nanoparticles’ Brain Permeability in Association with Particle Size and Surface Modification. Int. J. Mol. Sci. 2014, 15, 1812–1825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakagawa, S.; Deli, M.A.; Kawaguchi, H.; Shimizudani, T.; Shimono, T.; Kittel, A.; Tanaka, K.; Niwa, M. A new blood–brain barrier model using primary rat brain endothelial cells, pericytes and astrocytes. Neurochem. Int. 2009, 54, 253–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shilo, M.; Sharon, A.; Baranes, K.; Motiei, M.; Lellouche, J.-P.M.; Popovtzer, R. The effect of nanoparticle size on the probability to cross the blood-brain barrier: An in-vitro endothelial cell model. J. Nanobiotechnol. 2015, 13, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dan, M.; Xu, L.; Shao, A.; Cheng, X.; Zhang, C.; Yokel, R.A.; Takemura, T.; Hanagata, N.; Niwa, M.; Watanabe, D. Silver nanoparticles induce tight junction disruption and astrocyte neurotoxicity in a rat blood–brain barrier primary triple coculture model. Int. J. Nanomed. 2015, 10, 6105–6119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Jong, E.; Williams, D.S.; Abdelmohsen, L.K.; Van Hest, J.C.; Zuhorn, I.S. A filter-free blood-brain barrier model to quantitatively study transendothelial delivery of nanoparticles by fluorescence spectroscopy. J. Control. Release 2018, 289, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Fan, J.; Li, G.; Yin, Z.; Fu, B.M. Transcellular Model for Neutral and Charged Nanoparticles Across an In Vitro Blood–Brain Barrier. Cardiovasc. Eng. Technol. 2020, 11, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, V.; Mekky, G.; Van Der Meer, S.B.; Seeds, M.C.; Atala, A.J.; Epple, M. Transport of ultrasmall gold nanoparticles (2 nm) across the blood–brain barrier in a six-cell brain spheroid model. Sci. Rep. 2020, 10, 18033. [Google Scholar] [CrossRef] [PubMed]
- Nzou, G.; Wicks, R.T.; Wicks, E.E.; Seale, S.A.; Sane, C.H.; Chen, A.; Murphy, S.V.; Jackson, J.D.; Atala, A.J. Human Cortex Spheroid with a Functional Blood Brain Barrier for High-Throughput Neurotoxicity Screening and Disease Modeling. Sci. Rep. 2018, 8, 7413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumarasamy, M.; Sosnik, A. Heterocellular spheroids of the neurovascular blood-brain barrier as a platform for personalized nanoneuromedicine. iScience 2021, 24, 102183. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER Measurement Techniques for In Vitro Barrier Model Systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef] [Green Version]
- Deli, M.A.; Ábrahám, C.S.; Kataoka, Y.; Niwa, M. Permeability Studies on In Vitro Blood–Brain Barrier Models: Physiology, Pathology, and Pharmacology. Cell. Mol. Neurobiol. 2005, 25, 59–127. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Han, L.; Bae, Y.; Manickam, D.S. Lucifer Yellow—A Robust Paracellular Permeability Marker in a Cell Model of the Human Blood-brain Barrier. J. Vis. Exp. 2019, 150, e58900. [Google Scholar] [CrossRef] [PubMed]
- Aday, S.; Cecchelli, R.; Hallier-Vanuxeem, D.; Dehouck, M.P.; Ferreira, L. Stem Cell-Based Human Blood–Brain Barrier Models for Drug Discovery and Delivery. Trends Biotechnol. 2016, 34, 382–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urich, E.; Lazic, S.E.; Molnos, J.; Wells, I.; Freskgård, P.-O. Transcriptional Profiling of Human Brain Endothelial Cells Reveals Key Properties Crucial for Predictive In Vitro Blood-Brain Barrier Models. PLoS ONE 2012, 7, e38149. [Google Scholar] [CrossRef] [Green Version]
- Ohtsuki, S.; Sato, S.; Yamaguchi, H.; Kamoi, M.; Asashima, T.; Terasaki, T. Exogenous expression of claudin-5 induces barrier properties in cultured rat brain capillary endothelial cells. J. Cell. Physiol. 2006, 210, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Czupalla, C.J.; Liebner, S.; Devraj, K. In Vitro Models of the Blood–Brain Barrier. In Cerebral Angiogenesis. Methods in Molecular Biology; Humana Press: New York, NY, USA, 2014; Volume 1135. [Google Scholar] [CrossRef]
- Grobstein, C. Morphogenetic Interaction between Embryonic Mouse Tissues separated by a Membrane Filter. Nat. Cell Biol. 1953, 172, 869–871. [Google Scholar] [CrossRef]
- Watanabe, T.; Dohgu, S.; Takata, F.; Nishioku, T.; Nakashima, A.; Futagami, K.; Yamauchi, A.; Kataoka, Y. Paracellular Barrier and Tight Junction Protein Expression in the Immortalized Brain Endothelial Cell Lines bEND.3, bEND.5 and Mouse Brain Endothelial Cell 4. Biol. Pharm. Bull. 2013, 36, 492–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaves, C.; Do, T.-M.; Cegarra, C.; Roudières, V.; Tolou, S.; Thill, G.; Rocher, C.; Didier, M.; Lesuisse, D. Non-Human Primate Blood–Brain Barrier and In Vitro Brain Endothelium: From Transcriptome to the Establishment of a New Model. Pharmaceutics 2020, 12, 967. [Google Scholar] [CrossRef] [PubMed]
- Leite, P.E.C.; Pereira, M.R.; Granjeiro, J.M. Hazard effects of nanoparticles in central nervous system: Searching for biocompatible nanomaterials for drug delivery. Toxicol. In Vitro 2015, 29, 1653–1660. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.S.E.; Siupka, P.; Georgian, A.; Preston, J.; Toth, A.E.; Yusof, S.R.; Abbott, N.J.; Nielsen, M.S. Improved Method for the Establishment of an In Vitro Blood-Brain Barrier Model Based on Porcine Brain Endothelial Cells. J. Vis. Exp. 2017, 127, e56277. [Google Scholar] [CrossRef] [PubMed]
- Poller, B.; Gutmann, H.; Krähenbühl, S.; Weksler, B.; Romero, I.; Couraud, P.-O.; Tuffin, G.; Drewe, J.; Huwyler, J. The human brain endothelial cell line hCMEC/D3 as a human blood-brain barrier model for drug transport studies. J. Neurochem. 2008, 107, 1358–1368. [Google Scholar] [CrossRef]
- Paolinelli, R.; Corada, M.; Ferrarini, L.; Devraj, K.; Artus, C.; Czupalla, C.J.; Rudini, N.; Maddaluno, L.; Papa, E.; Engelhardt, B.; et al. Wnt Activation of Immortalized Brain Endothelial Cells as a Tool for Generating a Standardized Model of the Blood Brain Barrier In Vitro. PLoS ONE 2013, 8, e70233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erickson, M.A.; Banks, W.A. Neuroimmune Axes of the Blood–Brain Barriers and Blood–Brain Interfaces: Bases for Physiological Regulation, Disease States, and Pharmacological Interventions. Pharmacol. Rev. 2018, 70, 278–314. [Google Scholar] [CrossRef] [PubMed]
- Bicker, J.; Alves, G.; Fortuna, A.; Falcão, A. Blood–brain barrier models and their relevance for a successful development of CNS drug delivery systems: A review. Eur. J. Pharm. Biopharm. 2014, 87, 409–432. [Google Scholar] [CrossRef]
- Hatherell, K.; Couraud, P.-O.; Romero, I.A.; Weksler, B.; Pilkington, G.J. Development of a three-dimensional, all-human in vitro model of the blood–brain barrier using mono-, co-, and tri-cultivation Transwell models. J. Neurosci. Methods 2011, 199, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Veszelka, S.; Bocsik, A.; Walter, F.; Hantosi, D.; Deli, M.A. Blood-brain carrier co-culture models to study nanoparticle penetration: Focus on co-culture systems. Acta Biol. Szeged. 2015, 59, 157–168. Available online: http://abs.bibl.u-szeged.hu/index.php/abs/article/view/2846 (accessed on 5 June 2021).
- Neuhaus, W.; Gaiser, F.; Mahringer, A.; Franz, J.; Riethmüller, C.; Förster, C. The pivotal role of astrocytes in an in vitro stroke model of the blood-brain barrier. Front. Cell. Neurosci. 2014, 8, 352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeshita, Y.; Obermeier, B.; Cotleur, A.; Sano, Y.; Kanda, T.; Ransohoff, R.M. An in vitro blood–brain barrier model combining shear stress and endothelial cell/astrocyte co-culture. J. Neurosci. Methods 2014, 232, 165–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Page, S.; Patel, R.; Raut, S.; Al-Ahmad, A. Neurological diseases at the blood-brain barrier: Stemming new scientific paradigms using patient-derived induced pluripotent cells. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2020, 1866, 165358. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.-F.; Wolfe, J.M.; Fadzen, C.M.; Calligaris, D.; Hornburg, K.; Chiocca, E.A.; Agar, N.Y.R.; Pentelute, B.L.; Lawler, S.E. Blood-brain-barrier spheroids as an in vitro screening platform for brain-penetrating agents. Nat. Commun. 2017, 8, 15623. [Google Scholar] [CrossRef]
- Logan, S.; Arzua, T.; Canfield, S.G.; Seminary, E.R.; Sison, S.L.; Ebert, A.D.; Bai, X. Studying Human Neurological Disorders Using Induced Pluripotent Stem Cells: From 2D Monolayer to 3D Organoid and Blood Brain Barrier Models. Compr. Physiol. 2019, 9, 565–611. [Google Scholar] [CrossRef]
- Szepesi, Z.; Manouchehrian, O.; Bachiller, S.; Deierborg, T. Bidirectional Microglia–Neuron Communication in Health and Disease. Front. Cell. Neurosci. 2018, 12, 323. [Google Scholar] [CrossRef]
- Song, L.; Yuan, X.; Jones, Z.; Vied, C.; Miao, Y.; Marzano, M.; Hua, T.; Sang, Q.-X.A.; Guan, J.; Ma, T.; et al. Functionalization of Brain Region-specific Spheroids with Isogenic Microglia-like Cells. Sci. Rep. 2019, 9, 11055. [Google Scholar] [CrossRef]
- Kumarasamy, M.; Sosnik, A. The Nose-To-Brain Transport of Polymeric Nanoparticles Is Mediated by Immune Sentinels and Not by Olfactory Sensory Neurons. Adv. Biosyst. 2019, 3, e1900123. [Google Scholar] [CrossRef]
- Burks, S.M.; Rosas-Hernandez, H.; Ramirez-Lee, M.A.; Cuevas, E.; Talpos, J.C. Can SARS-CoV-2 infect the central nervous system via the olfactory bulb or the blood-brain barrier? Brain, Behav. Immun. 2021, 95, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Shirure, V.S.; Hughes, C.C.; George, S.C. Engineering Vascularized Organoid-on-a-Chip Models. Annu. Rev. Biomed. Eng. 2021, 23, 141–167. [Google Scholar] [CrossRef]
- Mensch, J.; Melis, A.; Mackie, C.; Verreck, G.; Brewster, M.E.; Augustijns, P. Evaluation of various PAMPA models to identify the most discriminating method for the prediction of BBB permeability. Eur. J. Pharm. Biopharm. 2010, 74, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Li, S.; Zheng, J.; Li, Y.; Huang, H. Recent Progress in Microfluidic Models of the Blood-Brain Barrier. Micromachines 2019, 10, 375. [Google Scholar] [CrossRef] [Green Version]
- Holloway, P.M.; Willaime-Morawek, S.; Siow, R.; Barber, M.; Owens, R.M.; Sharma, A.D.; Rowan, W.; Hill, E.; Zagnoni, M. Advances in microfluidic in vitro systems for neurological disease modeling. J. Neurosci. Res. 2021, 99, 1276–1307. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, I.; Izzo, L.; Tunesi, M.; Comar, M.; Albani, D.; Giordano, C. Organ-On-A-Chip in vitro Models of the Brain and the Blood-Brain Barrier and Their Value to Study the Microbiota-Gut-Brain Axis in Neurodegeneration. Front. Bioeng. Biotechnol. 2020, 7, 435. [Google Scholar] [CrossRef] [PubMed]
- Wikswo, J.P.; Block, F.E.; Cliffel, D.E.; Goodwin, C.R.; Marasco, C.; Markov, D.A.; McLean, D.L.; McLean, J.A.; McKenzie, J.R.; Reiserer, R.S.; et al. Engineering Challenges for Instrumenting and Controlling Integrated Organ-on-Chip Systems. IEEE Trans. Biomed. Eng. 2013, 60, 682–690. [Google Scholar] [CrossRef]
- Park, T.-E.; Mustafaoglu, N.; Herland, A.; Hasselkus, R.; Mannix, R.; Fitzgerald, E.A.; Prantil-Baun, R.; Watters, A.; Henry, O.; Benz, M.; et al. Hypoxia-enhanced Blood-Brain Barrier Chip recapitulates human barrier function and shuttling of drugs and antibodies. Nat. Commun. 2019, 10, 2621. [Google Scholar] [CrossRef] [PubMed]
- Cong, Y.; Han, X.; Wang, Y.; Chen, Z.; Lu, Y.; Liu, T.; Wu, Z.; Jin, Y.; Luo, Y.; Zhang, X. Drug Toxicity Evaluation Based on Organ-on-a-chip Technology: A Review. Micromachines 2020, 11, 381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.A.; Kim, H.N.; Im, S.-K.; Chung, S.; Kang, J.Y.; Choi, N. Collagen-based brain microvasculature model in vitro using three-dimensional printed template. Biomicrofluidics 2015, 9, 024115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, B.; Tong, Z.; Tong, W.Y.; Pasic, P.J.; Oddo, A.; Dai, Y.; Luo, M.; Frescene, J.; Welch, N.G.; Easton, C.D.; et al. In Situ Surface Modification of Microfluidic Blood–Brain-Barriers for Improved Screening of Small Molecules and Nanoparticles. ACS Appl. Mater. Interfaces 2020, 12, 56753–56766. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Yoon, J.-Y. In situ sensors for blood-brain barrier (BBB) on a chip. Sens. Actuators Rep. 2021, 3, 100031. [Google Scholar] [CrossRef]
- Van Der Helm, M.W.; van der Meer, A.; Eijkel, J.C.; Berg, A.V.D.; Segerink, L.I. Microfluidic organ-on-chip technology for blood-brain barrier research. Tissue Barriers 2016, 4, e1142493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beckwitt, C.; Clark, A.; Wheeler, S.; Taylor, D.L.; Stolz, D.B.; Griffith, L.; Wells, A. Liver ‘organ on a chip’. Exp. Cell Res. 2018, 363, 15–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashammakhi, N.; Wesseling-Perry, K.; Hasan, A.; Elkhammas, E.; Zhang, Y.S. Kidney-on-a-chip: Untapped opportunities. Kidney Int. 2018, 94, 1073–1086. [Google Scholar] [CrossRef] [PubMed]
- Ashammakhi, N.; Nasiri, R.; de Barros, N.R.; Tebon, P.; Thakor, J.; Goudie, M.; Shamloo, A.; Martin, M.G.; Khademhosseini, A. Gut-on-a-chip: Current progress and future opportunities. Biomaterials 2020, 255, 120196. [Google Scholar] [CrossRef]
- Geraili, A.; Jafari, P.; Hassani, M.S.; Araghi, B.H.; Mohammadi, M.H.; Ghafari, A.M.; Tamrin, S.H.; Modarres, H.P.; Kolahchi, A.R.; Ahadian, S.; et al. Controlling Differentiation of Stem Cells for Developing Personalized Organ-on-Chip Platforms. Adv. Health Mater. 2017, 7, 1700426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jagadeesan, S.; Workman, M.J.; Herland, A.; Svendsen, C.N.; Vatine, G.D. Generation of a Human iPSC-Based Blood-Brain Barrier Chip. J. Vis. Exp. 2020, 157, e60925. [Google Scholar] [CrossRef]
- Raj, M.K.; Chakraborty, S. PDMS microfluidics: A mini review. J. Appl. Polym. Sci. 2020, 137, 48958. [Google Scholar] [CrossRef]
- Wang, Y.; Abaci, H.E.; Shuler, M.L. Microfluidic blood–brain barrier model provides in vivo-like barrier properties for drug permeability screening. Biotechnol. Bioeng. 2017, 114, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Bang, S.; Lee, S.-R.; Ko, J.; Son, K.; Tahk, D.; Ahn, J.; Im, C.; Jeon, N.L. A Low Permeability Microfluidic Blood-Brain Barrier Platform with Direct Contact between Perfusable Vascular Network and Astrocytes. Sci. Rep. 2017, 7, 8083. [Google Scholar] [CrossRef]
- Booth, R.; Kim, H. Characterization of a microfluidic in vitro model of the blood-brain barrier (μBBB). Lab Chip 2012, 12, 1784–1792. [Google Scholar] [CrossRef]
- Adriani, G.; Ma, D.; Pavesi, A.; Kamm, R.D.; Goh, E.L.K. A 3D neurovascular microfluidic model consisting of neurons, astrocytes and cerebral endothelial cells as a blood–brain barrier. Lab Chip 2016, 17, 448–459. [Google Scholar] [CrossRef]
- Campisi, M.; Shin, Y.; Osaki, T.; Hajal, C.; Chiono, V.; Kamm, R.D. 3D self-organized microvascular model of the human blood-brain barrier with endothelial cells, pericytes and astrocytes. Biomaterials 2018, 180, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Caballero, D.; Kaushik, S.; Correlo, V.; Oliveira, J.; Reis, R.; Kundu, S.C. Organ-on-chip models of cancer metastasis for future personalized medicine: From chip to the patient. Biomaterials 2017, 149, 98–115. [Google Scholar] [CrossRef] [PubMed]
- Vatine, G.D.; Barrile, R.; Workman, M.; Sances, S.; Barriga, B.K.; Rahnama, M.; Barthakur, S.; Kasendra, M.; Lucchesi, C.; Kerns, J.; et al. Human iPSC-Derived Blood-Brain Barrier Chips Enable Disease Modeling and Personalized Medicine Applications. Cell Stem Cell 2019, 24, 995–1005. [Google Scholar] [CrossRef]
- Bonakdar, M.; Graybill, P.M.; Davalos, R.V. A microfluidic model of the blood–brain barrier to study permeabilization by pulsed electric fields. RSC Adv. 2017, 7, 42811–42818. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.A.; Codreanu, S.G.; Shi, M.; Sherrod, S.D.; Markov, D.A.; Neely, M.D.; Britt, C.M.; Hoilett, O.S.; Reiserer, R.S.; Samson, P.C.; et al. Metabolic consequences of inflammatory disruption of the blood-brain barrier in an organ-on-chip model of the human neurovascular unit. J. Neuroinflamm. 2016, 13, 306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, S.I.; Sei, Y.J.; Park, H.-J.; Kim, J.; Ryu, Y.; Choi, J.J.; Sung, H.-J.; Macdonald, T.J.; Levey, A.I.; Kim, Y. Microengineered human blood–brain barrier platform for understanding nanoparticle transport mechanisms. Nat. Commun. 2020, 11, 175. [Google Scholar] [CrossRef]
- Yu, F.; Hunziker, W.; Choudhury, D. Engineering Microfluidic Organoid-on-a-Chip Platforms. Micromachines 2019, 10, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mensch, J.; Oyarzabal, J.; Mackie, C.; Augustijns, P. In vivo, in vitro and in silico methods for small molecule transfer across the BBB. J. Pharm. Sci. 2009, 98, 4429–4468. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, J.T.; Clark, D.E. In Silico Predictions of Blood-Brain Barrier Penetration: Considerations to “Keep in Mind”. J. Pharmacol. Exp. Ther. 2005, 315, 477–483. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Gallagher, E.; Jorgensen, C.; Troendle, E.P.; Hu, D.; Searson, P.C.; Ulmschneider, M.B. An experimentally validated approach to calculate the blood-brain barrier permeability of small molecules. Sci. Rep. 2019, 9, 6117. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, T.; Kirshner, D.A.; Lau, E.Y.; Wong, S.E.; Nilmeier, J.P.; Lightstone, F.C. A Method to Predict Blood-Brain Barrier Permeability of Drug-Like Compounds Using Molecular Dynamics Simulations. Biophys. J. 2014, 107, 630–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bendels, S.; Kansy, M.; Wagner, B.; Huwyler, J. In silico prediction of brain and CSF permeation of small molecules using PLS regression models. Eur. J. Med. Chem. 2008, 43, 1581–1592. [Google Scholar] [CrossRef] [PubMed]
- Shamloo, A.; Pedram, M.Z.; Heidari, H.; Alasty, A. Computing the blood brain barrier (BBB) diffusion coefficient: A molecular dynamics approach. J. Magn. Magn. Mater. 2016, 410, 187–197. [Google Scholar] [CrossRef]
- Nagpal, K.; Singh, S.K.; Mishra, D.N. Drug targeting to brain: A systematic approach to study the factors, parameters and approaches for prediction of permeability of drugs across BBB. Expert Opin. Drug Deliv. 2013, 10, 927–955. [Google Scholar] [CrossRef] [PubMed]
- Shityakov, S.; Roewer, N.; Broscheit, J.-A.; Förster, C. In silico models for nanotoxicity evaluation and prediction at the blood-brain barrier level: A mini-review. Comput. Toxicol. 2017, 2, 20–27. [Google Scholar] [CrossRef]
- Ozdemir-Kaynak, E.; Qutub, A.; Yesil-Celiktas, O. Advances in Glioblastoma Multiforme Treatment: New Models for Nanoparticle Therapy. Front. Physiol. 2018, 9, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.V.; Chandrasekar, V.; Janapareddy, P.; Mathews, D.E.; Laux, P.; Luch, A.; Yang, Y.; Garcia-Canibano, B.; Balakrishnan, S.; Abinahed, J.; et al. Emerging Application of Nanorobotics and Artificial Intelligence To Cross the BBB: Advances in Design, Controlled Maneuvering, and Targeting of the Barriers. ACS Chem. Neurosci. 2021, 12, 1835–1853. [Google Scholar] [CrossRef]
- Pedram, M.Z.; Shamloo, A.; Alasty, A.; Ghafar-Zadeh, E. Optimal Magnetic Field for Crossing Super-Para-Magnetic Nanoparticles through the Brain Blood Barrier: A Computational Approach. Biosensors 2016, 6, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Z.; Chen, Y.; Cai, X.; Xu, R. Predict drug permeability to blood–brain-barrier from clinical phenotypes: Drug side effects and drug indications. Bioinformatics 2016, 33, 901–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kisala, J.; Heclik, K.I.; Pogocki, K.; Pogocki, D. Essentials and Perspectives of Computational Modelling Assistance for CNS-oriented Nanoparticle-based Drug Delivery Systems. Curr. Med. Chem. 2019, 25, 5894–5913. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, G.; Kitchen, D.B. Computational models to predict blood-brain barrier permeation and CNS activity. J. Comput. Mol. Des. 2003, 17, 643–664. [Google Scholar] [CrossRef]
- Kumar, P.P.; Kumar, K.T.S.; Nainita, M.K.; Tarun, A.S.; Ramudu, B.G.R.; Deepika, K.; Pramoda, A.; Yasmeen, C. Cerebroprotective Potential of Hesperidin Nanoparticles Against Bilateral Common Carotid Artery Occlusion Reperfusion Injury in Rats and In silico Approaches. Neurotox. Res. 2020, 37, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, A.C.; Bharadwaj, S.; Kumar, S.; Wei, D.-Q. Nano-particle mediated inhibition of Parkinson’s disease using computational biology approach. Sci. Rep. 2018, 8, 9169. [Google Scholar] [CrossRef] [Green Version]
- Shityakov, S.; Förster, C. Multidrug resistance protein P-gp interaction with nanoparticles (fullerenes and carbon nanotube) to assess their drug delivery potential: A theoretical molecular docking study. Int. J. Comput. Biol. Drug Des. 2013, 6, 343–357. [Google Scholar] [CrossRef]
- Alsenan, S.; Al-Turaiki, I.; Hafez, A. A Recurrent Neural Network model to predict blood–brain barrier permeability. Comput. Biol. Chem. 2020, 89, 107377. [Google Scholar] [CrossRef] [PubMed]
- Gottardo, S.; Mech, A.; Drbohlavová, J.; Małyska, A.; Bøwadt, S.; Sintes, J.R.; Rauscher, H. Towards safe and sustainable innovation in nanotechnology: State-of-play for smart nanomaterials. NanoImpact 2021, 21, 100297. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, P.; Pullaiah, T. Future of Cellular and Molecular Diagnostics. In Advances in Cell and Molecular Diagnostics; Elsevier BV: Amsterdam, The Netherlands, 2018; pp. 203–270. [Google Scholar]
- Sherman, H.; Rossi, A.E. A Novel Three-Dimensional Glioma Blood-Brain Barrier Model for High-Throughput Testing of Tumoricidal Capability. Front. Oncol. 2019, 9, 351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeelani, S.; Reddy, R.J.; Maheswaran, T.; Asokan, G.S.; Dany, A.; Anand, B. Theranostics: A treasured tailor for tomorrow. J. Pharm. Bioallied Sci. 2014, 6 (Suppl. 1). [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, Y.; Sun, B.; Liu, S.; Liu, H. Two-Dimensional Nanomaterials for Cancer Nanotheranostics. Small 2017, 13, 1603446. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Guo, D.; Xiao, K.; Wang, X.; Wang, L.; Luo, J. A drug-specific nanocarrier design for efficient anticancer therapy. Nat. Commun. 2015, 6, 7449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohta, S.; Kikuchi, E.; Ishijima, A.; Azuma, T.; Sakuma, I.; Ito, T. Investigating the optimum size of nanoparticles for their delivery into the brain assisted by focused ultrasound-induced blood–brain barrier opening. Sci. Rep. 2020, 10, 18220. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.; Andreozzi, P.; Magro, R.D.; Fiordaliso, F.; Corbelli, A.; Talamini, L.; Chinello, C.; Raimondo, F.; Magni, F.; Tringali, M.; et al. Evolution of Nanoparticle Protein Corona across the Blood–Brain Barrier. ACS Nano 2018, 12, 7292–7300. [Google Scholar] [CrossRef] [PubMed]
- Sancey, L.; Kotb, S.; Truillet, C.; Appaix, F.; Marais, A.; Thomas, E.; van der Sanden, B.; Klein, J.-P.; Laurent, B.; Cottier, M.; et al. Long-Term in Vivo Clearance of Gadolinium-Based AGuIX Nanoparticles and Their Biocompatibility after Systemic Injection. ACS Nano 2015, 9, 2477–2488. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Yuan, D.; Nangia, S.; Xu, G.; Lam, K.S.; Luo, J. A Structure–Property Relationship Study of the Well-Defined Telodendrimers to Improve Hemocompatibility of Nanocarriers for Anticancer Drug Delivery. Langmuir 2014, 30, 6878–6888. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, W.; Xiao, K.; Berti, L.; Luo, J.; Tseng, H.P.; Fung, G.; Lam, K.S. Well-Defined, Reversible Boronate Crosslinked Nanocarriers for Targeted Drug Delivery in Response to Acidic pH Values andcis-Diols. Angew. Chem. 2012, 124, 2918–2923. [Google Scholar] [CrossRef]
- Wang, X.; Shi, C.; Zhang, L.; Bodman, A.; Guo, D.; Wang, L.; Hall, W.A.; Wilkens, S.; Luo, J. Affinity-controlled protein encapsulation into sub-30 nm telodendrimer nanocarriers by multivalent and synergistic interactions. Biomaterials 2016, 101, 258–271. [Google Scholar] [CrossRef] [Green Version]
- Huynh, L.; Neale, C.; Pomès, R.; Allen, C. Computational approaches to the rational design of nanoemulsions, polymeric micelles, and dendrimers for drug delivery. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 20–36. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Wang, X.; Wang, L.; Meng, Q.; Guo, D.; Chen, L.; Dai, M.; Wang, G.; Cooney, R.; Luo, J. A nanotrap improves survival in severe sepsis by attenuating hyperinflammation. Nat. Commun. 2020, 11, 3384. [Google Scholar] [CrossRef]
- Satapathy, M.; Yen, T.-L.; Jan, J.-S.; Tang, R.-D.; Wang, J.-Y.; Taliyan, R.; Yang, C.-H. Solid Lipid Nanoparticles (SLNs): An Advanced Drug Delivery System Targeting Brain through BBB. Pharmaceutics 2021, 13, 1183. [Google Scholar] [CrossRef]
- Lombardo, S.M.; Schneider, M.; Türeli, A.E.; Türeli, N.G. Key for crossing the BBB with nanoparticles: The rational design. Beilstein J. Nanotechnol. 2020, 11, 866–883. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Cao, B.; Li, Y.; Mao, C. Phage nanofibers in nanomedicine: Biopanning for early diagnosis, targeted therapy, and proteomics analysis. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1623. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Parayath, N.; Ganesh, S.; Wang, W.; Amiji, M. The role of apolipoprotein- and vitronectin-enriched protein corona on lipid nanoparticles for in vivo targeted delivery and transfection of oligonucleotides in murine tumor models. Nanoscale 2019, 11, 18806–18824. [Google Scholar] [CrossRef] [PubMed]
- Rajora, M.A.; Ding, L.; Valic, M.; Jiang, W.; Overchuk, M.; Chen, J.; Zheng, G. Tailored theranostic apolipoprotein E3 porphyrin-lipid nanoparticles target glioblastoma. Chem. Sci. 2017, 8, 5371–5384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorat, N.D.; Townley, H.E.; Patil, R.M.; Tofail, S.A.; Bauer, J. Comprehensive approach of hybrid nanoplatforms in drug delivery and theranostics to combat cancer. Drug Discov. Today 2020, 25, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Zhang, Y.; Li, Z.; Hou, X.; Feng, N. Red blood cell membrane-camouflaged nanoparticles: A novel drug delivery system for antitumor application. Acta Pharm. Sin. B 2019, 9, 675–689. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wu, B.; Wu, Y.; Song, X.; Zhang, S.; Liu, Z. Camouflaging Nanoparticles with Brain Metastatic Tumor Cell Membranes: A New Strategy to Traverse Blood–Brain Barrier for Imaging and Therapy of Brain Tumors. Adv. Funct. Mater. 2020, 30, 1909369. [Google Scholar] [CrossRef]
- Tosi, G.; Vilella, A.; Veratti, P.; Belletti, D.; Pederzoli, F.; Ruozi, B.; Vandelli, M.A.; Zoli, M.; Forni, F. Exploiting Bacterial Pathways for BBB Crossing with PLGA Nanoparticles Modified with a Mutated Form of Diphtheria Toxin (CRM197): In Vivo Experiments. Mol. Pharm. 2015, 12, 3672–3684. [Google Scholar] [CrossRef]
- Liang, T.; Zhang, R.; Liu, X.; Ding, Q.; Wu, S.; Li, C.; Lin, Y.; Ye, Y.; Zhong, Z.; Zhou, M. Recent advances in macrophage-mediated drug delivery systems. Int. J. Nanomed. 2021, 16, 2703. [Google Scholar] [CrossRef]
- Nowacek, A.S.; Balkundi, S.; McMillan, J.; Roy, U.; Martinez-Skinner, A.; Mosley, R.L.; Kanmogne, G.; Kabanov, A.V.; Bronich, T.; Gendelman, H.E. Analyses of nanoformulated antiretroviral drug charge, size, shape and content for uptake, drug release and antiviral activities in human monocyte-derived macrophages. J. Control. Release 2011, 150, 204–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piot-Grosjean, O.; Wahl, F.; Gobbo, O.; Stutzmann, J.-M. Assessment of Sensorimotor and Cognitive Deficits Induced by a Moderate Traumatic Injury in the Right Parietal Cortex of the Rat. Neurobiol. Dis. 2001, 8, 1082–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Strategy | Benefits | Limitations |
|---|---|---|
| BBB disruption by focused ultrasound | Transient opening of the BBB facilitates increased concentration of NPs in brain | Inter-species limitations and variability of response between subjects’ limit findings [34] |
| Magnetic field-guided delivery | Enhanced imaging capabilities for diagnostics, in situ monitoring and follow-up of localisation and concentration and delivery guided by external device | Balance must be struck to attain efficient and specific hyperthermia while maintaining viability of healthy surrounding tissues in addition to observed development of thermotolerance in several subjects [62] |
| Active transporter-mediated delivery | Enhanced transport efficiency, active targeting and localisation of NPs administered intravenously | Homogenous surface functionalisation is difficult and requires additional characterisation, not applicable for larger NPs [64] |
| Viral vectors | Gene transfection efficiency high for delivery of siRNA and gene products | Safety concerns related to nature of delivery vector and dose optimisation issues in intravenous administration [65] |
| Delivery via altered permeability due to pathophysiological state of BBB | Improved probability of transport of NPs across the BBB due to leaky vasculature/altered endothelial cell morphology and confluency | Limited knowledge in relation to specific changes in the dynamic BBB environment in various brain disorders and pathophysiological states as well as dependence of response on disease model used limits predictive power [43] |
| Cell-mediated delivery | Ability to delivery NPs across exploiting natural products present in the body as a “Trojan horse”, thus improving circulation time, brain-targeting specificity and sustained delivery with reduced immunogenicity | Technical limitations pertaining to maintenance of viability during extraction, storage, formulation and administration and heterogeneity Incomplete characterisation of drug loading capacities and drug–macrophage interactions hampers clinical translation [35] |
| Non-intravenous administration | Can bypass the BBB by using alternative routes that are also less invasive e.g., nasal administration | Dose limitations and short residence time hamper nasal and pulmonary administration, in addition to propensity for localised irritation [18,66] Oral route largely precluded due to nature of NPs and addition of additional gastrointestinal barriers in addition to the BBB [67] |
| Study | Model Type | Cell Line | Validation Markers |
|---|---|---|---|
| Chang 2009 [120] | Co-Culture | Bovine brain endothelial cells Rat mixed glial cells (60% astrocytes, 20% oligodendrocytes, and 20% microglia) | Monolayer integrity-Fluorescence staining Occludin tightness-Not explicitly stated but tight junction, LDL, TfR and y-glutanyl transpeptidase (y-GT) activity considered to be retained as per Cecchelli and colleagues 2007 [121] Permeability-Transferrin receptor inhibitor pre-treatment to demonstrate the specific TfR mediated endocytosis In vitro/in vivo correlation-Not explicitly reported but referenced as method comparable to that described in Dehouck and colleagues 1992 [122] |
| Georgieva 2011 [75] | Plasma membrane | Human brain endothelial cells [hCMEC/D3 cells] | Monolayer integrity-Fluorescence staining Occludin tightness-TEER (50 Ω cm2) Permeability-Hydrophilic tracers (sucrose/inulin) PECAM, ZO-I and MRP-I expression-Laser scanning confocal microscopy |
| Qiao 2012 [123] | Monolayer cell culture | Porcine brain endothelial cells | Monolayer integrity-Fluorescence staining Occludin tightness-TEER (700 Ω cm2) Permeability-Lactoferrin blocker pre-treatment to demonstrate Lf dependent transcytosis Iron delivery efficiency by Fe3O4 nanoparticles measured by graphite furnace atomic absorption spectrometry |
| Wagner 2012 [124] | Monolayer cell culture | Mouse brain endothelial cells (bEnd3 cells) | Monolayer integrity- Fluorescence staining Occludin tightness-Not explicitly stated, but it was determined that incubation with nanoparticles did not adversely affect tightness and integrity was retained Permeability-Receptor associated protein (RAP) blocking by co-incubation to demonstrate the LDL/LRPI dependent uptake mechanism LRPI, LDL and Apo-E receptor expression-Laser confocal scanning microscopy In vitro/in vivo correlation-TEM investigations of ApoE modified nanoparticles confirm endocytosis both in-vitro and in-vivo is mediated by the same pit forming endocytosis mechanism |
| Martins 2012 [125] | Monolayer cell culture Macrophage cell culture | Porcine brain endothelial cells Macrophage cell line (from frozen human plasma) | Monolayer integrity-Fluorescence staining Occludin tightness-Not explicitly stated but similar in vitro and in vivo data and low cytotoxicity infers representative of maintained integrity Permeability-Confocal fluorescence microscopy revealed higher uptake in endothelial cell culture model than macrophage model Biocompatibility-Alamar Blue cell viability assay (MIT) following solid lipid nanoparticle incubation |
| Gromnicova 2013 [126] | (I)Monolayer cell Culture (2)3D astrocyte Co- culture model | Human brain endothelial cells (I-BEC) Primary human astrocytes and brain endothelial cells (hCMEC/D3 cells) | Monolayer integrity-Fluorescence staining, not affected by incubation with glucose coated gold nanoparticles for 24 hOccludin tightness-Not explicitly stated but suc hco-culture models using human tissue are considered the most representative to simulate the in-vivo environment with hCMEC/D3 models of the BBB Permeability-Demonstrated by glucose coated nanoparticle transport across the model. with negligible diffusion or sedimentation which could confound findings oi static 2D/3D models. Trans-endothelial movement not Glut-I dependent but more probably size and charge dependent (favouring non-ionic character imparted by glucose coating of AuNPs) Biocompatibility-assay showed low cytotoxicity and low immunogenicity |
| Teow 2013 [127] | Monolayer cell culture | Human adenocarcinoma cell line (Caco-2) Porcine brain endothelial | Monolayer integrity-Inverted light microscopy Occludin tightness-TEER (800–1000 Ω cm2 for Caco-2 cells and 200–300 Ω cm2 for PBEC cells) removal of serum and addition of hydrocortisone improved tightness of the models Occludin and claudin expression-Not explicitly studied, but considered to be similar to described in Patabendige and colleagues 2012. Papp measurements of paclitaxel in both directions demonstrated the expression of p-gp in the monolayer models [128] Permeability—TEER measurements before and after experiments/incubation. Apparent permeability coefficient (Pan) was calculated from the equation Papp (cm/s) − (dQ/dt)/(CoxA)dQ/dt. which constitutes a robust quantitative value which facilitates orthogonal comparisons with other studies Biocompatibility-LDH assay showed low cytotoxicity of the dendrimer nanocarriers, and converse high cytotoxicity (antitumour activity) when conjugated with paclitaxel |
| Rempe 2014 [129] | Monolayer cell culture | Porcine brain endothelial cells | Monolayer integrity-Fluorescence staining and immunocytochemical analysis Occludin tightness-TEER measurements, although stated as percentages rather than absolute values Permeability-Hydrophilic tracers NC-sucrose and fluorescein isothiocyanate labelled bovine serum albumin (FITC-BSA). Found maximal permeability after four hours due to decrease in TEER and maximum values of Papp (cm/s) P-gp, occludin expression-Immunocytochemical analysis and implied from experimental data showing disruption of model integrity after four hours when incubated with the poly(cyanobutylacrylate) NPs, following by recovery of integrity to 80 % baseline TEER values Biocompatibility-Critical solids content of 26.62 µg/mL led to irreversible monolayer disruption, while those below half this value i.e., <13.31 µg/mL led to complete recovery of barrier integrity |
| Cramer 2014 [130] | Monolayer cell culture | Porcine brain endothelial cells Capillary choroid plexus cells | Occludin tightness-TEER, being expressed in percentages than absolute values Occludin expression-Western blot and immunochemistry Permeability-TEER measurements before and after treatment with AgNPs, confirmed by FITC-dextran Papp measurements, which were in agreement Biocompatibility-Neutral red uptake assay and microscopy to monitor cell morphology after incubation with AgNPs. The ethylene oxide nanoparticles were notably more cytotoxic than their citrate counterparts, with a critical concentration dependence (75 µg/mL) of monolayer disruption Pro-inflammatory capacity-Reactive oxygen nitrogen species, MMP-2 and COX-2 activity measured by zymography which was upregulated by ethylene oxide AgNPs but not for citrate AgNPs at standard concentrations (25 µg/mL) |
| Bramini 2014 [131] | Monolayer cell culture | Human brain capillary microvascular endothelial cells (hCMEC/D3 cell line) | Monolayer integrity -Fluorescence staining Occludin tightness-TEER measurements and confocal microscopy, which found holes of total 200 µm2, and although these may have an exaggerating effect on the overall flux, they are accounted for in the mode. This would be consistent with those found in similar models, although this is frequently not investigated or reported Claudin expression Western blot and confocal microscopy Permeability-TEER measurements and fluorescent labelled permeability assay, Spinning disk confocal fluorescence microscopy and total internal reflection fluorescence microscopy (TIRFM) was used to quantify the translocation of the nanopartic1es in real time with 10 min exposure times of the carboxylated polystyrene NPs (40 nm and 100 nm sizes), demonstrating a preferential lysosomal accumulation within the model rather than true translocation |
| Hanada 2014 [132] | Co-Culture | Rat brain microvascular endothelial cells Rat brain pericytes | Monolayer Integrity-Fluorescence staining Occludin tightness-TEER measurements before permeability measurements (150–300 Ω cm2) Perrneability—Papp (cm/s) of 30 nm, 100 nm, 400 nm silica nanoparticles compared With Papp of tracer sulforhodamine B. Papp studies of quantum dots with different surface charge functionalisations Biocompatability—Histological data confirm some degree of BBB disruption implied by thinning of the endothelial cell layers following hematoxylin and eosin (H&E) staining, though long term permeability assays indicated negligible adverse effects on BBB functionality In vitro/in vivo correlation-Not explicitly investigated but commercial BBB model used which has been previously validated by Nakagawa and colleagues using a suite of drug molecules including known substrates of MRP-I and p-gp [133] |
| Shilo 2015 [134] | Monolayer cell culture | Mouse brain endothelial cells (bEnd3 cells) | Monolayer integrity-Fluorescence staining Occludin tightness-Most parameters were not explicitly investigated, but the bEnd3 monolayer is a validated and well established model, and imaging demonstrated it formed similarly to other studies Permeability-Flame atomic absorption spectrometry to quantify the AuNP uptake after 30 min incubation With various sizes of NPs, revealing preferential selection Of 70 nm barbiturate functionalized AuNPs for CT imaging applications (most total Au uptake), and 20 nm for drug loading (highest free surface area) In-vitro/ln-vivo correlation—Fluorescent confocal microscopy investigating interaction Of barbiturate loaded AuNPs with the model indicated specific pinocytosis mediated transport across the barrier, and some degree of association with the barrier itself |
| Xu 2015 [135] | Co-Culture | Rat microvascular endothelial cells Rat pericytes Rat astrocytes | Monolayer integrity—Fluorescence staining Occludin tightness-TEER (>200 Ω cm2) ZO-I, claudin 5 expression-Confocal microscopy Permeability-TEER measurements before and after incubation with AgNPs and polystyrene NPs as control, demonstrating BBB disruption by decreased resistance values after 24 h for the 10 µg/mL—AgNPs only (1 µg/mL AgNPs and control were unaffected) Biocompatibility—AgNPs at 10 µg/mL demonstrated reduced ZO-I expression, mitochondrial shrinkage, apoptosis and altered gene expression by immunostaining and microarray analysis of astrocytes In vitro/ln vivo correlation—Triple co-culture model gives high TJ protein expression and tightness for evaluating mechanisms of nanotoxicity and vasoactive compounds |
| De Jong 2018 [136] | Filter free monolayer cell culture | Human microvascular brain endothelial cells (hCMEC/D3 cells) | Monolayer integrity—Fluorescence staining Occludin tightness—Not explicitly stated but model validated with permeability measurements ZO-I expression—Fluorescence microscopy Permeability—Model validated with Papp (cm/min) measurements for 4 kDa and 2000 kDa dextran, which were in agreement with 3D microfluidic organ on a chip models of the BBB. Also validated by collagenase A digestion of apical, cellular and basolateral fractions facilitating quantitative assessment of active LDL mediated transcytosis by fluorescence spectroscopy illustrating the quantitative mode of the model In vitro/ln vivo correlation—Filter-free model in a human cell line allowing quantitative and real time imaging of nanoparticle transport across the membrane |
| Zhang 2020 [137] | Transcellular monolayer cell culture | Mouse brain endothelial cells (bEnd3 cells) | Monolayer integrity—Fluorescence staining Occludin tightness—Not explicitly investigated, but permeability measurements used to validate the model and same protocols used as other studies which generated confluent monolayers with high TJ protein expression Permeability—Papp measurements of neutral nanoparticles used to validate the model quantitatively by fluorescence spectroscopy In vitro/in vivo correlation—Model mathematically expressed as a 2D barrier in terms of its bending rigidity, surface tension, viscoelasticity and surface charge, as well as ion concentration of the medium and size and charge properties of nanoparticles. Therefore, recapitulates several key aspects of electrochemical gradient driven endocytosis rather than receptor mediated targeting, which allows elucidation of key rational design properties for NP delivery to the brain |
| Sokolova 2020 [138] | Spheroid model | Human brain microvascular endothelial cells Human brain pericytes Human astrocytes Human microglia (iPSC derived) Human oligodendrocytes (iPSC derived) Human cortical neurons (iPSC derived) | Monolayer integrity-Fluorescence staining, confocal scanning microscopy Occludin tightness-Not explicitly investigated, but characterisation was conducted as per Zhou and colleagues who have extensively established and Validated this model (140] ZO-1, claudin-5, CD31, P-gp. GLUT-I expression—immunohistochemistry Biocompatibility-ATP production as a cell viability assay following incubation with dye (FAM-Alkyne) conjugated AuNPs for up to 24 h. showing negligible change demonstrating lack of clinically significant cytotoxicity In vitro/in vivo correlation-3D Model employing six types of human or human related tissues which comprise the NVU, Hypoxia condition e.g. following ischaemic stroke recapitulated to determine influence of pathophysiology on nanoparticle behaviour and distribution |
| Kumarasamy 2021 [141] | Spheroid model | Human brain microvascular endothelial cells Human brain vascular pericytes Human astrocytes Rat neurons Rat microglia | Monolayer integrity-Fluorescence STEM Occludin tightness-Confocal laser scanning fluorescence microscopy, RNA-sequencing VE-cadherin, claudin-5, NG2 proteoglycan, GFAP β-III tubulin, iNOS, MAP-2 expression-Immunohistochemistry, Western Blot, SDS PAGE, ABC, GLUT 1,3,5, SLC, p-gp expression—RNA sequencing and PCA Permeability—FITC labelling and incubation Of nanoparticles with model for 24 h, followed by imaging Biocompatibility—Metabolic and morphological studies on endothelial and epithelial cells following incubation with different classes Of nanocarriers including graphene nanoplatesr carbon dots, polymeric and metallic nanoparticles In vitro/in vivo correlation-3D Model employing five cell types encompassing the BBB and associated microglia and neural networks with exquisite audio-visual data and extensive characterisation of an essentially ex-vivo NVU is the most biomimetic model type to date, though using rat and human derived cells can limit the translation and reproduction of results |
| Product | Nanoplatform | Diagnostic Component | Therapeutic Component | Phase | Prospective Indication |
|---|---|---|---|---|---|
| AGuIX AGuIX | Polysiloxane matrix with gadolinium chelate radiosensitiser “Nano-Rad” Polysiloxane matrix with gadolinium chelate | Gadolinium Gadolinium | Gadolinium as radiotherapy adjuvant Temozolomide * | I Completed I/II Recruiting 2021 | Whole brain radiation therapy in metastases Glioblastoma |
| Abraxane | Nano-albumin bound paclitaxel | In-situ MRI * | Paclitaxel, carboplatin and darvalumab * | II Recruiting 2017 | Metastatic cancer of the head and neck |
| MM398 | Nanoliposomal irinotecan | In-situ MRI * following Convection enhanced delivery | Irinotecan | I Active (Recruiting) 2013–2021 | High grade Glioma |
| RXDX-107 | Human serum albumin bound bendamustine | In-situ MRI * | Bendamustine (as dodecanol alkyl ester | I/1b (Terminated 2015) | Solid tumour (Locally advanced or metastatic) |
| SNB-101 | SN-38 nanoparticles | In-situ MRI * | SN-38 (Active metabolite of irinotecan) | I Recruiting 2020 | Metastatic head and neck cancer |
| NBTXR3 NBTXR3 | Crystalline halfnium (HfO2) nanoparticles Crystalline halfnium (HfO2) nanoparticles | Halfnium nanoparticle as radiosensitiser Halfnium nanoparticle as radiosensitiser | Halfnium as radiotherapy adjuvant and anti—PD1 * (pembrolizumab) Halfnium as radiotherapy adjuvant and anti—PD1 * (pembrolizumab) with radiotherapy | I Recruiting 2018 II Recruiting 2021 | Malignant high grade solid tumour Metastatic Head and neck cancer |
| Feraheme | Iron oxide nanoparticle with functionalised coating | Ferumoxytol as MRI enhancer | Macrophage polarisation by ferumoxytol * | I Recruiting 2017 | Childhood brain neoplasm |
| Feraheme | Iron oxide nanoparticle with functionalised coating | Ferumoxytol as MRI enhancer Ferumoxytol for dynamic susceptibility contrast-enhanced MRI Ferumoxytol for neuroinflammatory imaging Ferumoxytol and gadolinium MRI | Magnetic guided Therapy * Gadolinium for dynamic contrast enhanced MRI/as radiosensitiser * Magnetic guided therapy * Magnetic guided therapy * | II Recruiting 2017 N/A 2018 I (Early) Completed II Recruiting 2021 | Brain neoplasm Recurrent childhood brain neoplasm Primary brain neoplasm CNS degenerative disorder CNS infectious disorder CNS vascular malformation Haemorrhagic and ischaemic brain accident CNS neoplasm Cranial nerve disorder Metastatic malignant neoplasm in the brain |
| MM398 plus Feraheme | Nanoliposomal irinotecan and iron oxide nanoparticles | Ferumoxytol | Irinotecan | I Completed | Solid tumours Breast cancer wit hactive brain metastasis |
| CPC634 | Polymeric micelles of docetaxel | In-Situ MRI * | Docetaxel | I Completed | Metastatic cancer Solid tumours |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lynch, M.J.; Gobbo, O.L. Advances in Non-Animal Testing Approaches towards Accelerated Clinical Translation of Novel Nanotheranostic Therapeutics for Central Nervous System Disorders. Nanomaterials 2021, 11, 2632. https://doi.org/10.3390/nano11102632
Lynch MJ, Gobbo OL. Advances in Non-Animal Testing Approaches towards Accelerated Clinical Translation of Novel Nanotheranostic Therapeutics for Central Nervous System Disorders. Nanomaterials. 2021; 11(10):2632. https://doi.org/10.3390/nano11102632
Chicago/Turabian StyleLynch, Mark J., and Oliviero L. Gobbo. 2021. "Advances in Non-Animal Testing Approaches towards Accelerated Clinical Translation of Novel Nanotheranostic Therapeutics for Central Nervous System Disorders" Nanomaterials 11, no. 10: 2632. https://doi.org/10.3390/nano11102632
APA StyleLynch, M. J., & Gobbo, O. L. (2021). Advances in Non-Animal Testing Approaches towards Accelerated Clinical Translation of Novel Nanotheranostic Therapeutics for Central Nervous System Disorders. Nanomaterials, 11(10), 2632. https://doi.org/10.3390/nano11102632







