Immunomodulatory Function of Polyvinylpyrrolidone (PVP)-Functionalized Gold Nanoparticles in Vibrio-Stimulated Sea Urchin Immune Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of Gold Nanoparticles Coated with Polyvinylpyrrolidone
2.2. Preparation of Bacteria
2.3. Sea Urchin, Immune Cell Exposure, and Cell Viability Assay
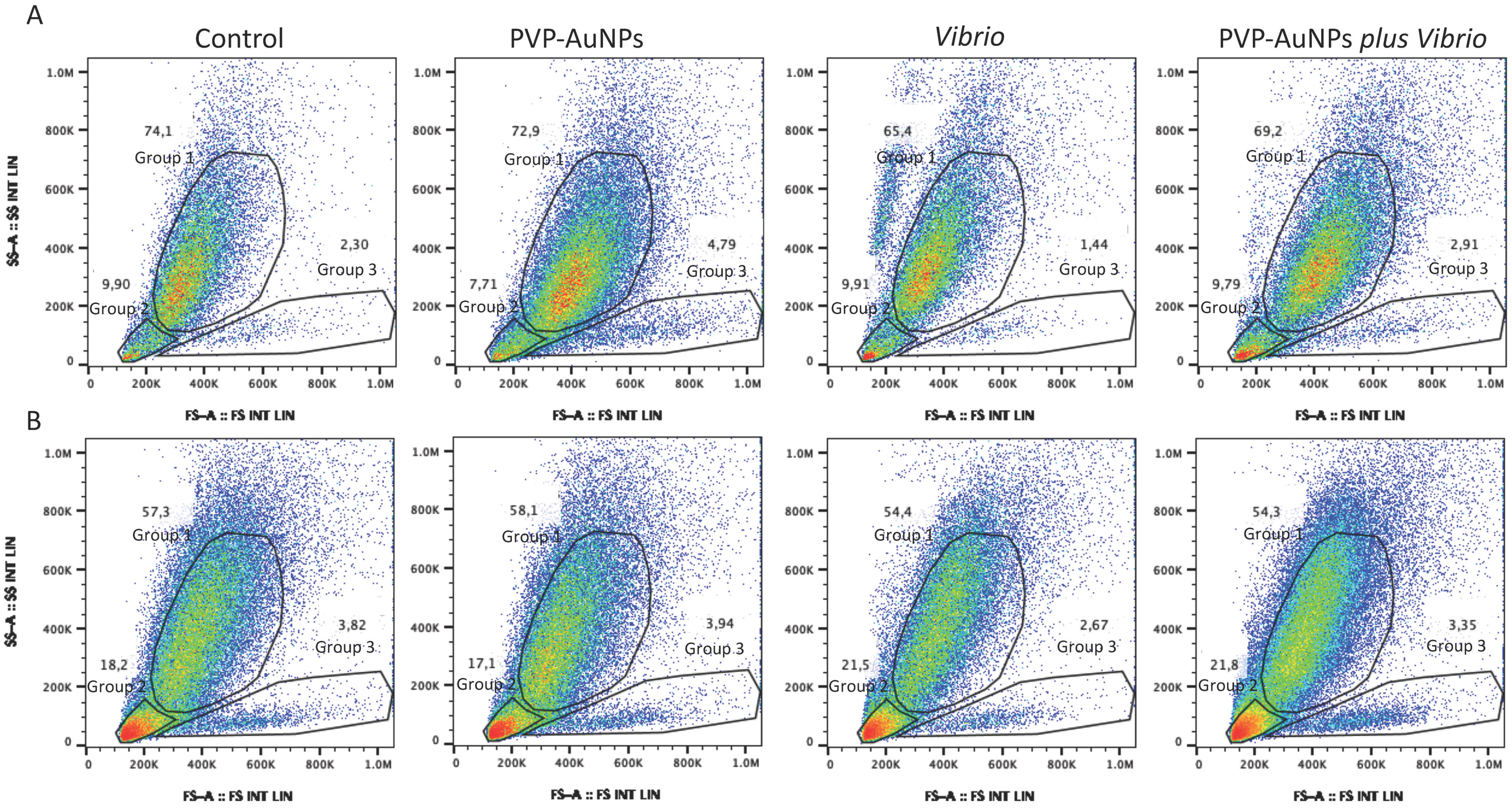
2.4. Flow Cytometry
2.5. SDS-PAGE and Immunoblotting
2.6. Statistics
3. Results and Discussion
3.1. Polyvinylpyrrolidone Leads to the Formation of Hetero-Aggregates Independently of the Concentration and Time Exposure of the Gold Particles in the Sea Urchin Coelomic Fluid
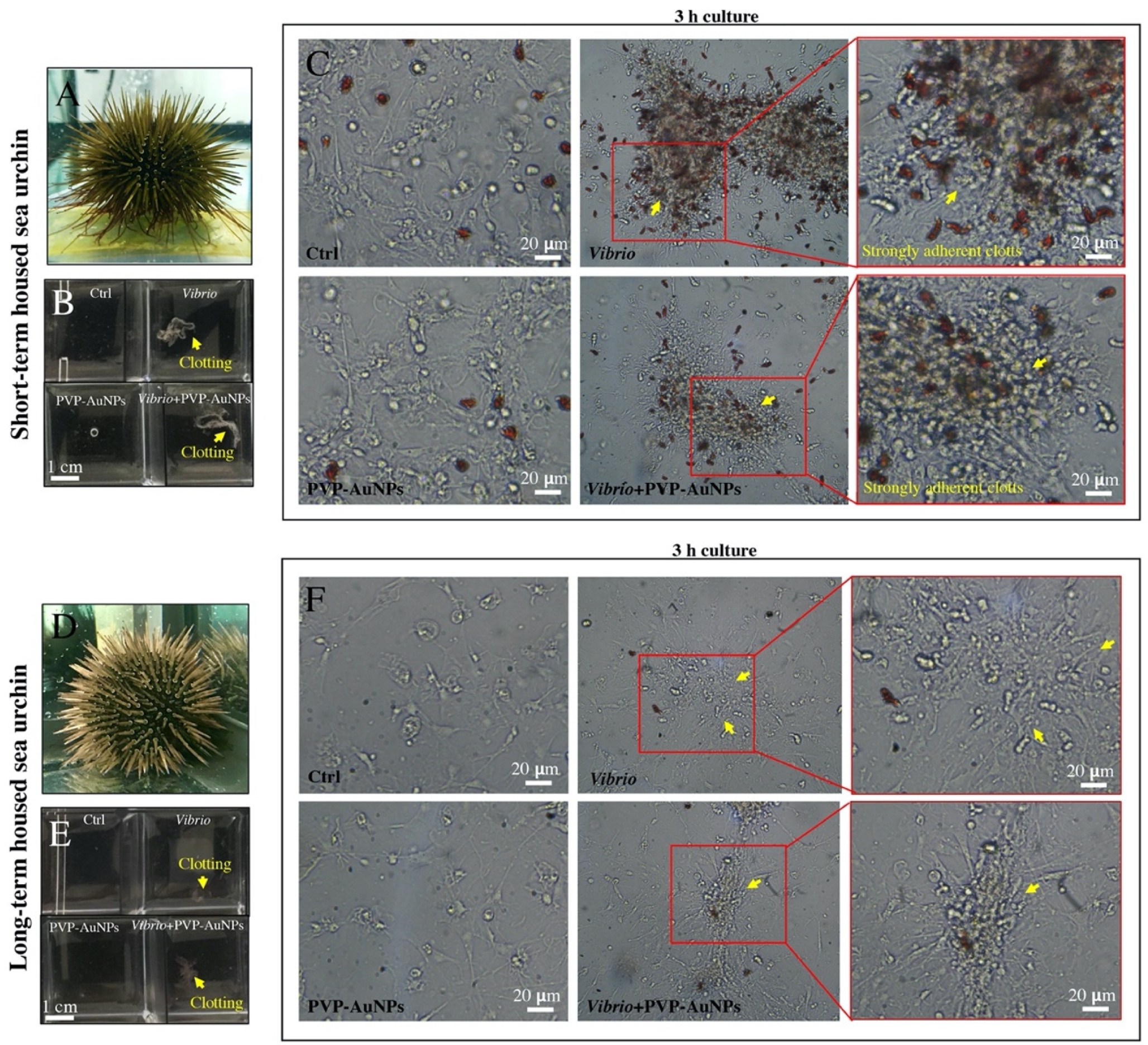
3.2. The In Vitro Particle–Bacteria–Immune Cell Interface: How Cells Behave Differently Based on the Baseline Immunological State of the Donor
3.3. Polyvinylpyrrolidone-Functionalized Gold Nanoparticles Try to Interfere with the Immunological State Affected by Vibrio Anguillarum
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Mou, L.; Jiang, X. Surface chemistry of gold nanoparticles for health-related applications. Chem. Sci. 2020, 11, 923–936. [Google Scholar] [CrossRef] [PubMed]
- Mahato, K.; Nagpal, S.; Shah, M.A.; Srivastava, A.; Maurya, P.K.; Roy, S.; Jaiswal, A.; Singh, R.; Chandra, P. Gold nanoparticle surface engineering strategies and their applications in biomedicine and diagnostics. 3 Biotech 2019, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- DeLong, R.K.; Reynolds, C.M.; Malcolm, Y.; Schaeffer, A.; Severs, T.; Wanekaya, A. Functionalized gold nanoparticles for the binding, stabilization, and delivery of therapeutic DNA, RNA, and other biological macromolecules. Nanotechnol. Sci. Appl. 2010, 3, 53. [Google Scholar] [CrossRef] [PubMed]
- Carnovale, C.; Bryant, G.; Shukla, R.; Bansal, V. Identifying trends in gold nanoparticle toxicity and uptake: Size, shape, capping ligand, and biological corona. ACS Omega 2019, 4, 242–256. [Google Scholar] [CrossRef]
- Alijagic, A.; Barbero, F.; Gaglio, D.; Napodano, E.; Benada, O.; Kofroňová, O.; Puntes, V.F.; Bastús, N.G.; Pinsino, A. Gold nanoparticles coated with polyvinylpyrrolidone and sea urchin extracellular molecules induce transient immune activation. J. Hazard. Mater. 2021, 402, 123793. [Google Scholar] [CrossRef]
- Kennedy, L.C.; Bickford, L.R.; Lewinski, N.A.; Coughlin, A.J.; Hu, Y.; Day, E.S.; West, J.L.; Drezek, R.A. A new era for cancer treatment: Gold-nanoparticle-mediated thermal therapies. Small 2011, 7, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Huang, K.; Li, H.H.; Lu, Y.G.; Zheng, D.L. Antibacterial Properties of Functionalized Gold Nanoparticles and Their Application in Oral Biology. J. Nanomater. 2020, 2020, 5616379. [Google Scholar] [CrossRef]
- Boraschi, D.; Alijagic, A.; Auguste, M.; Barbero, F.; Ferrari, E.; Hernadi, S.; Mayall, M.; Michelini, S.; Navarro Pacheco, N.I.; Prinelli, A.; et al. Addressing nanomaterial immunosafety by evaluating innate immunity across living species. Small 2020, 16, 2000598. [Google Scholar] [CrossRef]
- Alijagic, A.; Gaglio, D.; Napodano, E.; Russo, R.; Costa, C.; Benada, O.; Kofroňová, O.; Pinsino, A. Titanium dioxide nanoparticles temporarily influence the sea urchin immunological state suppressing inflammatory-relate gene transcription and boosting antioxidant metabolic activity. J. Hazard. Mater. 2020, 384, 121389. [Google Scholar] [CrossRef]
- Hassanen, E.I.; Morsy, E.A.; Hussien, A.M.; Ibrahim, M.A.; Farroh, K.Y. The effect of different concentrations of gold nanoparticles on growth performance, toxicopathological and immunological parameters of broiler chickens. Biosci. Rep. 2020, 40, BSR20194296. [Google Scholar] [CrossRef] [PubMed]
- Le Guével, X.; Palomares, F.; Torres, M.J.; Blanca, M.; Fernandez, T.D.; Mayorga, C. Nanoparticle size influences the proliferative responses of lymphocyte subpopulations. RSC Adv. 2015, 5, 85305–85309. [Google Scholar] [CrossRef]
- Jiao, Q.; Li, L.; Mu, Q.; Zhang, Q. Immunomodulation of nanoparticles in nanomedicine applications. BioMed Res. Int. 2014, 2014, 426028. [Google Scholar] [CrossRef]
- Herrmann, K.; Pistollato, F.; Stephens, M.L. Beyond the 3Rs: Expanding the use of human-relevant replacement methods in biomedical research. ALTEX-Altern. Anim. Exp. 2019, 36, 343–352. [Google Scholar] [CrossRef]
- Sea Urchin Genome Sequencing Consortium The genome of the sea urchin Strongylocentrotus purpuratus. Science 2006, 314, 941–952. [CrossRef]
- Gambardella, C.; Ferrando, S.; Gatti, A.M.; Cataldi, E.; Ramoino, P.; Aluigi, M.G.; Faimali, M.; Diaspro, A.; Falugi, C. Review: Morphofunctional and biochemical markers of stress in sea urchin life stages exposed to engineered nanoparticles. Environ. Toxicol. 2016, 31, 1552–1562. [Google Scholar] [CrossRef]
- Pikula, K.; Zakharenko, A.; Chaika, V.; Em, I.; Nikitina, A.; Avtomonov, E.; Tregubenko, A.; Agoshkov, A.; Mishakov, I.; Kuznetsov, V.; et al. Toxicity of Carbon, Silicon, and Metal-Based Nanoparticles to Sea Urchin Strongylocentrotus intermedius. Nanomaterials 2020, 10, 1825. [Google Scholar] [CrossRef] [PubMed]
- Pinsino, A.; Bastús, N.G.; Busquets-Fité, M.; Canesi, L.; Cesaroni, P.; Drobne, D.; Duschl, A.; Ewart, M.A.; Gispert, I.; Horejs-Hoeck, J.; et al. Probing the immune responses to nanoparticles across environmental species. A perspective of the EU Horizon 2020 project PANDORA. Environ. Sci. Nano 2020, 7, 3216–3232. [Google Scholar] [CrossRef]
- Franco, P.; De Marco, I. The Use of Poly (N-vinyl pyrrolidone) in the Delivery of Drugs: A Review. Polymers 2020, 12, 1114. [Google Scholar] [CrossRef]
- Javed, R.; Zia, M.; Naz, S.; Aisida, S.O.; ul Ain, N.; Ao, Q. Role of capping agents in the application of nanoparticles in biomedicine and environmental remediation: Recent trends and future prospects. J. Nanobiotechnol. 2020, 18, 1–15. [Google Scholar] [CrossRef]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227. [Google Scholar] [CrossRef] [PubMed]
- Frans, I.; Michiels, C.W.; Bossier, P.; Willems, K.A.; Lievens, B.; Rediers, H. Vibrio anguillarum as a fish pathogen: Virulence factors, diagnosis and prevention. J. Fish Dis. 2011, 34, 643–661. [Google Scholar] [CrossRef] [PubMed]
- Vezzulli, L.; Grande, C.; Reid, P.C.; Hélaouët, P.; Edwards, M.; Höfle, M.G.; Brettar, I.; Colwell, R.R.; Pruzzo, C. Climate influence on Vibrio and associated human diseases during the past half-century in the coastal North Atlantic. Proc. Natl. Acad. Sci. USA 2016, 113, E5062–E5071. [Google Scholar] [CrossRef] [PubMed]
- Sinatra, J.A.; Colby, K. Notes from the field: Fatal Vibrio anguillarum infection in an immunocompromised patient—Maine, 2017. Morb. Mortal. Wkly. Rep. 2018, 67, 962. [Google Scholar] [CrossRef]
- Di Gaudio, F.; Indelicato, S.; Indelicato, S.; Tricoli, M.R.; Stampone, G.; Bongiorno, D. Improvement of a rapid direct blood culture microbial identification protocol using MALDI-TOF MS and performance comparison with SepsiTyper kit. J. Microbiol. Methods 2018, 155, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Henson, J.H.; Svitkina, T.M.; Burns, A.R.; Hughes, H.E.; MacPartland, K.J.; Nazarian, R.; Borisy, G.G. Two components of actin-based retrograde flow in sea urchin coelomocytes. Mol. Biol. Cell 1999, 10, 4075–4090. [Google Scholar] [CrossRef] [PubMed]
- Pinsino, A.; Alijagic, A. Sea urchin Paracentrotus lividus immune cells in culture: Formulation of the appropriate harvesting and culture media and maintenance conditions. Biol. Open 2019, 8, bio039289. [Google Scholar] [CrossRef]
- Pinsino, A.; Thorndyke, M.; Matranga, V. Coelomocytes and post-traumatic response in the common sea star Asterias rubens. Cell Stress Chaperones 2007, 12, 331–341. [Google Scholar] [CrossRef]
- Zito, F.; Nakano, E.; Sciarrino, S.; Matranga, V. Regulative specification of ectoderm in skeleton disrupted sea urchin embryos treated with monoclonal antibody to Pl-nectin. Dev. Growth Differ. 2000, 42, 499–506. [Google Scholar] [CrossRef]
- Karakostis, K.; Costa, C.; Zito, F.; Matranga, V. Heterologous expression of newly identified galectin-8 from sea urchin embryos produces recombinant protein with lactose binding specificity and anti-adhesive activity. Sci. Rep. 2015, 5, 17665. [Google Scholar] [CrossRef]
- Casals, E.; Gonzalez, E.; Puntes, V.F. Reactivity of inorganic nanoparticles in biological environments: Insights into nanotoxicity mechanisms. J. Phys. D Appl. Phys. 2012, 45, 443001. [Google Scholar] [CrossRef]
- Barbero, F.; Moriones, O.H.; Bastús, N.G.; Puntes, V. Dynamic Equilibrium in the Cetyltrimethylammonium Bromide–Au Nanoparticle Bilayer, and the Consequent Impact on the Formation of the Nanoparticle Protein Corona. Bioconjug. Chem. 2019, 30, 2917–2930. [Google Scholar] [CrossRef] [PubMed]
- Barbero, F.; Russo, L.; Vitali, M.; Piella, J.; Salvo, I.; Borrajo, M.L.; Busquets-Fité, M.; Grandori, R.; Bastús, N.G.; Casals, E.; et al. Formation of the protein corona: The interface between nanoparticles and the immune system. Semin. Immunol. 2017, 34, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Koczkur, K.M.; Mourdikoudis, S.; Polavarapu, L.; Skrabalak, S.E. Polyvinylpyrrolidone (PVP) in nanoparticle synthesis. Dalton Trans. 2015, 44, 17883–17905. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda, B.; Angelomé, P.C.; Lechuga, L.M.; Liz-Marzán, L.M. LSPR-based nanobiosensors. Nano Today 2009, 4, 244–251. [Google Scholar] [CrossRef]
- Chen, Y.; Xianyu, Y.; Jiang, X. Surface modification of gold nanoparticles with small molecules for biochemical analysis. Acc. Chem. Res. 2017, 50, 310–319. [Google Scholar] [CrossRef]
- Alijagic, A.; Benada, O.; Kofroňová, O.; Cigna, D.; Pinsino, A. Sea urchin extracellular proteins design a complex protein corona on titanium dioxide nanoparticle surface influencing immune cell behavior. Front. Immunol. 2019, 10, 2261. [Google Scholar] [CrossRef]
- Smith, L.C.; Arizza, V.; Hudgell, M.A.B.; Barone, G.; Bodnar, A.G.; Buckley, K.M.; Cunsolo, V.; Dheilly, N.M.; Franchi, N.; Fugmann, S.D.; et al. Echinodermata: The complex immune system in echinoderms. In Advances in Comparative Immunology; Cooper, E.L., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 409–501. Available online: https://link.springer.com/chapter/10.1007/978-3-319-76768-0_13 (accessed on 8 August 2018).
- Pinsino, A.; Matranga, V. Sea urchin immune cells as sentinels of environmental stress. Dev. Comp. Immunol. 2015, 49, 198–205. [Google Scholar] [CrossRef]
- Alijagic, A.; Pinsino, A. Probing safety of nanoparticles by outlining sea urchin sensing and signaling cascades. Ecotoxicol. Environ. Saf. 2017, 144, 416–421. [Google Scholar] [CrossRef]
- Saptarshi, S.R.; Duschl, A.; Lopata, A.L. Interaction of nanoparticles with proteins: Relation to bio-reactivity of the nanoparticle. J. Nanobiotechnol. 2013, 11, 26. [Google Scholar] [CrossRef]
- Nair, S.V.; Del Valle, H.; Gross, P.S.; Terwilliger, D.P.; Smith, L.C. Macroarray analysis of coelomocyte gene expression in response to LPS in the sea urchin. Identification of unexpected immune diversity in an invertebrate. Physiol. Genom. 2005, 22, 33–47. [Google Scholar] [CrossRef]
- Valcourt, J.R.; Lemons, J.M.; Haley, E.M.; Kojima, M.; Demuren, O.O.; Coller, H.A. Staying alive: Metabolic adaptations to quiescence. Cell Cycle 2012, 11, 1680–1696. [Google Scholar] [CrossRef]
- Ganeshan, K.; Chawla, A. Metabolic regulation of immune responses. Annu. Rev. Immunol. 2014, 32, 609–634. [Google Scholar] [CrossRef]
- Herrick, S.E.; Allen, J.E. Surgical adhesions: A sticky macrophage problem. Science 2021, 371, 993–994. [Google Scholar] [CrossRef]
- Semeraro, N.; Lattanzio, A.; Montemurro, P.; Papanice, M.; De Lucia, O.; De Bellis, G.; Giordano, D. Mechanisms of blood clotting activation in inflammation: The role of mononuclear phagocytes. Int. J. Tissue React. 1985, 7, 313–320. [Google Scholar]
- Whittaker, C.A.; Bergeron, K.; Whittle, J.; Brandhorst, B.P.; Burke, R.D.; Hynes, R.O. The echinoderm adhesome. Dev. Biol. 2006, 300, 252–266. [Google Scholar] [CrossRef]
- Hibino, T.; Loza-Coll, M.; Messier, C.; Majeske, A.J.; Cohen, A.H.; Terwilliger, D.P.; Buckley, K.M.; Brockton, V.; Nair, S.V.; Berney, K.; et al. The immune gene repertoire encoded in the purple sea urchin genome. Dev. Biol. 2006, 300, 349–365. [Google Scholar] [CrossRef]
- Zindel, J.; Peiseler, M.; Hossain, M.; Deppermann, C.; Lee, W.Y.; Haenni, B.; Zuber, B.; Deniset, J.F.; Surewaard BG, J.; Candinas, D.; et al. Primordial GATA6 macrophages function as extravascular platelets in sterile injury. Science 2021, 371, eabe0595. [Google Scholar] [CrossRef]
- Romero, A.; Novoa, B.; Figueras, A. Cell mediated immune response of the Mediterranean sea urchin Paracentrotus lividus after PAMPs stimulation. Dev. Comp. Immunol. 2016, 62, 29–38. [Google Scholar] [CrossRef]
- Smith, L.C.; Rast, J.P.; Brockton, V.; Terwilliger, D.P.; Nair, S.V.; Buckley, K.M.; Majeske, A.J. The sea urchin immune system. Invertebr. Surviv. J. 2006, 3, 25–39. [Google Scholar]
- Ramírez-Gómez, F.; Aponte-Rivera, F.; Méndez-Castaner, L.; García-Arrarás, J.E. Changes in holothurian coelomocyte populations following immune stimulation with different molecular patterns. Fish Shellfish Immunol. 2010, 29, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Hohn, C.; Lee, S.R.; Pinchuk, L.M.; Petrie-Hanson, L. Zebrafish kidney phagocytes utilize macropinocytosis and Ca2+-dependent endocytic mechanisms. PLoS ONE 2009, 4, e4314. [Google Scholar] [CrossRef]
- Bossche, J.V.; Jangoux, M. Epithelial origin of starfish coelomocytes. Nature 1976, 261, 227–228. [Google Scholar] [CrossRef] [PubMed]
- Golconda, P.; Buckley, K.M.; Reynolds, C.R.; Romanello, J.P.; Smith, L.C. The axial organ and the pharynx are sites of hematopoiesis in the sea urchin. Front. Immunol. 2019, 10, 870. [Google Scholar] [CrossRef] [PubMed]
- Sharlaimova, N.; Shabelnikov, S.; Bobkov, D.; Martynova, M.; Bystrova, O.; Petukhova, O. Coelomocyte replenishment in adult Asterias rubens: The possible ways. Cell Tissue Res. 2021, 383, 1043–1060. [Google Scholar] [CrossRef] [PubMed]
- Fafanđel, M.; Bihari, N.; Smodlaka, M.; Ravlić, S. Hemocytes/coelomocytes DNA content in five marine invertebrates: Cell cycles and genome sizes. Biologia 2008, 63, 730–736. [Google Scholar] [CrossRef][Green Version]
- Hermiston, M.L.; Xu, Z.; Weiss, A. CD45: A critical regulator of signaling thresholds in immune cells. Annu. Rev. Immunol. 2003, 21, 107–137. [Google Scholar] [CrossRef]
- Saunders, A.E.; Johnson, P. Modulation of immune cell signalling by the leukocyte common tyrosine phosphatase, CD45. Cell. Signal. 2010, 22, 339–348. [Google Scholar] [CrossRef]
- Cross, J.L.; Kott, K.; Miletić, T.; Johnson, P. CD45 regulates TLR-induced proinflammatory cytokine and IFN-β secretion in dendritic cells. J. Immunol. 2008, 180, 8020–8029. [Google Scholar] [CrossRef]
- Arroyo-Espliguero, R.; Avanzas, P.; Jeffery, S.; Kaski, J.C. CD14 and toll-like receptor 4: A link between infection and acute coronary events? Heart 2004, 90, 983–988. [Google Scholar] [CrossRef]
- Nagasawa, T.; Kobayashi, H.; Aramaki, M.; Kiji, M.; Oda, S.; Izumi, Y. Expression of CD14, CD16 and CD45RA on monocytes from periodontitis patients. J. Periodontal Res. 2004, 39, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Pfau, J.C.; Walker, E.; Card, G.L. Monoclonal antibodies to CD45 modify LPS-induced arachidonic acid metabolism in macrophages. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2000, 1495, 212–222. [Google Scholar] [CrossRef]
- Gilles, K.W. Disease in sea urchins Strongylocentrotus purpuratus: Experimental infection and bacterial virulence. Dis. Aquat. Org. 1986, 1, 105–114. [Google Scholar] [CrossRef]
- Bonnin-Jusserand, M.; Copin, S.; Le Bris, C.; Brauge, T.; Gay, M.; Brisabois, A.; Grard, T.; Midelet-Bourdin, G. Vibrio species involved in seafood-borne outbreaks (Vibrio cholerae, V. parahaemolyticus and V. vulnificus): Review of microbiological versus recent molecular detection methods in seafood products. Crit. Rev. Food Sci. Nutr. 2019, 59, 597–610. [Google Scholar] [CrossRef]
- Gao, G. Nectin and nectin-like molecules: Immune regulator, adhesion molecule and virus receptors (P1001). J. Immunol. 2013, 1, 190. [Google Scholar]
- Hsu, R.Y.; Chan, C.H.; Spicer, J.D.; Rousseau, M.C.; Giannias, B.; Rousseau, S.; Ferri, L.E. LPS-induced TLR4 signaling in human colorectal cancer cells increases β1 integrin-mediated cell adhesion and liver metastasis. Cancer Res. 2011, 71, 1989–1998. [Google Scholar] [CrossRef] [PubMed]
- Khezerlou, A.; Alizadeh-Sani, M.; Azizi-Lalabadi, M.; Ehsani, A. Nanoparticles and their antimicrobial properties against pathogens including bacteria, fungi, parasites and viruses. Microb. Pathog. 2018, 123, 505–526. [Google Scholar] [CrossRef]
- Dykman, L.A. Gold nanoparticles for preparation of antibodies and vaccines against infectious diseases. Expert Rev. Vaccines 2020, 19, 465–477. [Google Scholar] [CrossRef]



| P. lividus Donor | Exposure Scenario | % of Cells after Doublet Exclusion | CD45+ (%) | CD14+ (%) |
|---|---|---|---|---|
| Quiescent 1 | Control | 2.09 | 17.83 | 20.43 |
| PVP-AuNPs | 3.33 | 14.53 | 32.03 | |
| Vibrio | 1.54 | 2.06 | 6.41 | |
| PVP-AuNPs plus Vibrio | 2.13 | 4.02 | 12.47 | |
| Quiescent 2 | Control | 11.84 | 1.08 | 2.43 |
| PVP-AuNPs | 11.68 | 1.54 | 3.28 | |
| Vibrio | 9.47 | 0.48 | 1.36 | |
| PVP-AuNPs plus Vibrio | 12.12 | 1.16 | 3.23 | |
| Immunologically active 1 | Control | 9.66 | 0.06 | 0.03 |
| PVP-AuNPs | 9.36 | 0.16 | 0.05 | |
| Vibrio | 8.82 | 1.31 | 0.16 | |
| PVP-AuNPs plus Vibrio | 8.60 | 1.95 | 0.32 | |
| Immunologically active 2 | Control | 10.10 | 2.44 | 1.15 |
| PVP-AuNPs | 11.70 | 4.25 | 0.43 | |
| Vibrio | 7.73 | 0.28 | 0.13 | |
| PVP-AuNPs plus Vibrio | 7.35 | 0.73 | 0.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alijagic, A.; Bonura, A.; Barbero, F.; Puntes, V.F.; Gervasi, F.; Pinsino, A. Immunomodulatory Function of Polyvinylpyrrolidone (PVP)-Functionalized Gold Nanoparticles in Vibrio-Stimulated Sea Urchin Immune Cells. Nanomaterials 2021, 11, 2646. https://doi.org/10.3390/nano11102646
Alijagic A, Bonura A, Barbero F, Puntes VF, Gervasi F, Pinsino A. Immunomodulatory Function of Polyvinylpyrrolidone (PVP)-Functionalized Gold Nanoparticles in Vibrio-Stimulated Sea Urchin Immune Cells. Nanomaterials. 2021; 11(10):2646. https://doi.org/10.3390/nano11102646
Chicago/Turabian StyleAlijagic, Andi, Angela Bonura, Francesco Barbero, Victor F. Puntes, Francesco Gervasi, and Annalisa Pinsino. 2021. "Immunomodulatory Function of Polyvinylpyrrolidone (PVP)-Functionalized Gold Nanoparticles in Vibrio-Stimulated Sea Urchin Immune Cells" Nanomaterials 11, no. 10: 2646. https://doi.org/10.3390/nano11102646
APA StyleAlijagic, A., Bonura, A., Barbero, F., Puntes, V. F., Gervasi, F., & Pinsino, A. (2021). Immunomodulatory Function of Polyvinylpyrrolidone (PVP)-Functionalized Gold Nanoparticles in Vibrio-Stimulated Sea Urchin Immune Cells. Nanomaterials, 11(10), 2646. https://doi.org/10.3390/nano11102646








